Abstract
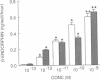
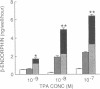
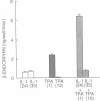
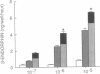
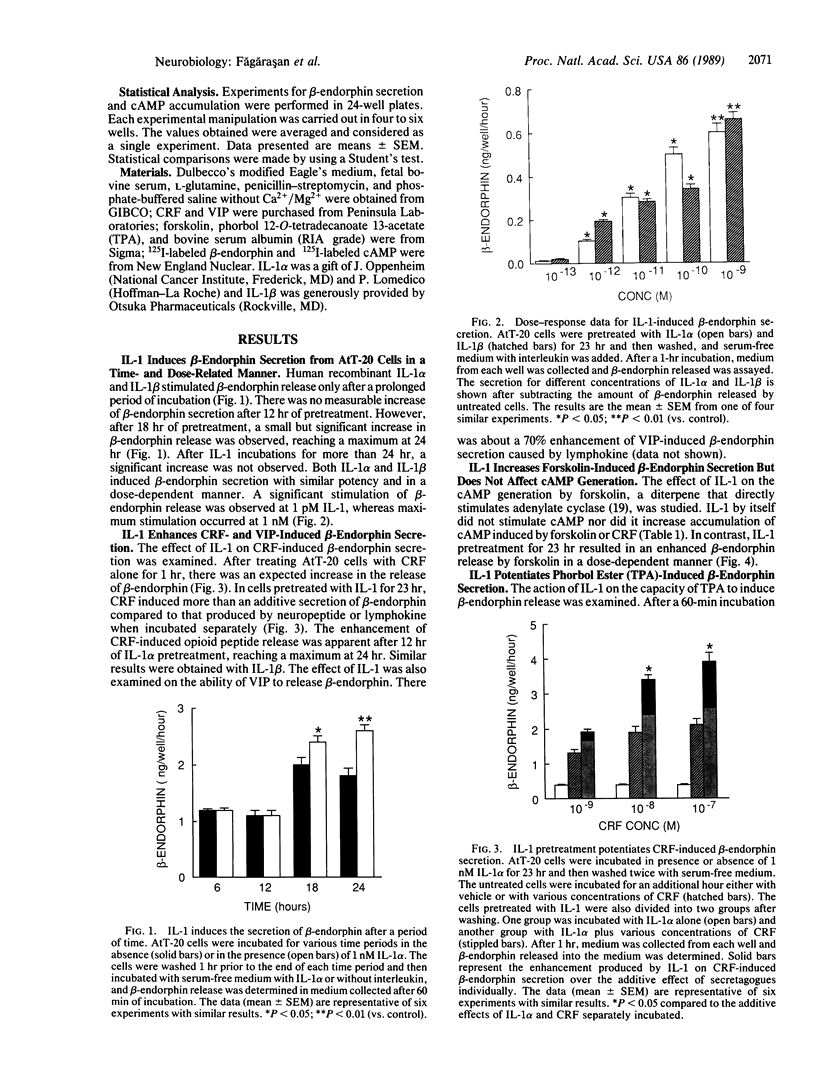
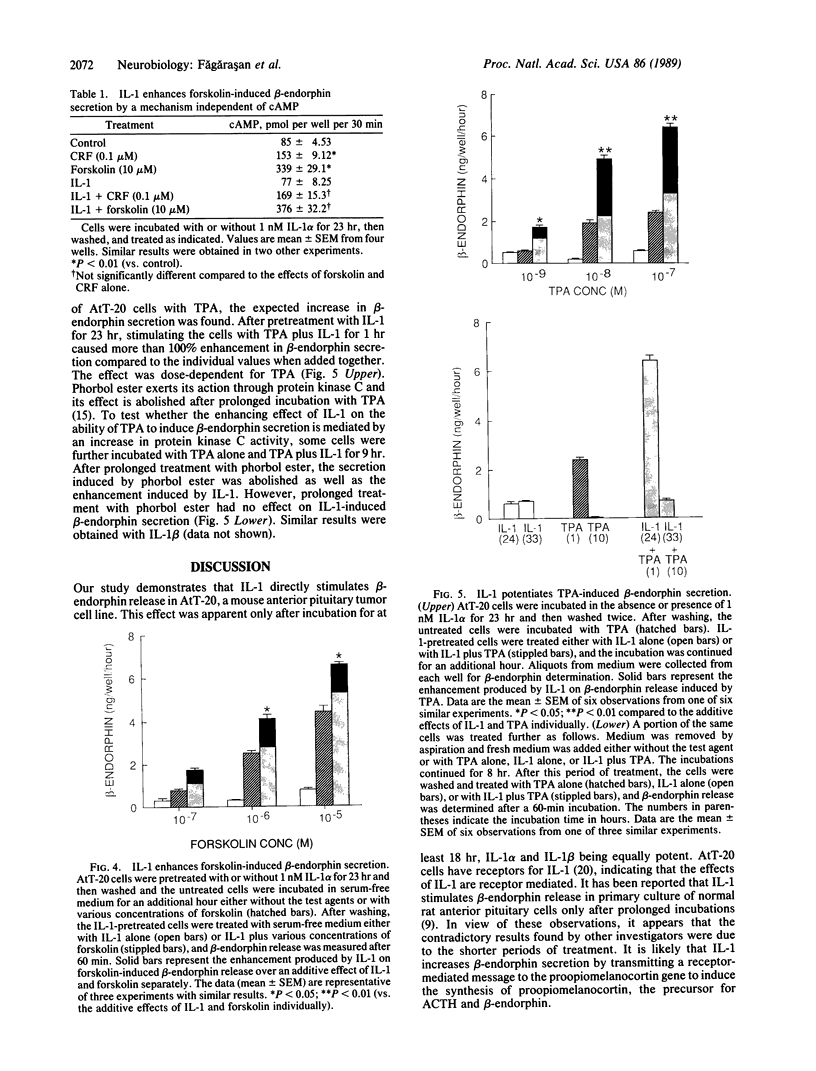
Previous work has shown that corticotropin releasing factor, vasoactive intestinal peptide, phorbol ester, and forskolin cause the secretion of adrenocorticotropic hormone and beta-endorphin from the AtT-20 mouse pituitary cell line. Human recombinant interleukin 1 alpha and 1 beta also stimulated adrenocorticotropic hormone and beta-endorphin secretion from AtT-20 cells in a time- and dose-related manner. The effect appeared only after pretreatment with interleukin 1 (IL-1) for at least 18 hr and was maximum at 24 hr. After pretreatment of the cells over a period of time with IL-1, the secretion induced by corticotropin releasing factor and vasoactive intestinal peptide was increased in more than an additive manner. The enhancement of corticotropin releasing factor-induced beta-endorphin release produced by IL-1 was apparent after 12 hr and reached a maximum at 24 hr. IL-1 did not affect forskolin-induced cAMP generation but enhanced the effect of forskolin on beta-endorphin secretion. This suggests that IL-1 does not induce adenylate cyclase and that forskolin causes the secretion of beta-endorphin by a mechanism independent of cAMP. IL-1 enhanced phorbol ester-induced beta-endorphin secretion. After prolonged treatment with phorbol ester (an activator of protein kinase C), the secretion induced by phorbol ester was abolished as well as the enhancement induced by IL-1. However, prolonged treatment with phorbol ester had no effect on IL-1-induced beta-endorphin secretion. These observations suggest that IL-1 enhances peptide-generated secretion of beta-endorphin by inducing protein kinase C.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Catt K. J., Aguilera G. Involvement of protein kinase C in the regulation of adrenocorticotropin release from rat anterior pituitary cells. Endocrinology. 1986 Jan;118(1):212–217. doi: 10.1210/endo-118-1-212. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F., van Oers J., del Rey A., Tilders F., Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238(4826):524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Beach J. E., Holaday J. W., Smallridge R. C., Fein H. G. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Boyle M., Yamamoto G., Chen M., Rivier J., Vale W. Interleukin 1 prevents loss of corticotropic responsiveness to beta-adrenergic stimulation in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5556–5560. doi: 10.1073/pnas.85.15.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Axelrod J. Interleukin 1 amplifies receptor-mediated activation of phospholipase A2 in 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6306–6309. doi: 10.1073/pnas.85.17.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave J. R., Rubinstein N., Eskay R. L. Evidence that beta-endorphin binds to specific receptors in rat peripheral tissues and stimulates the adenylate cyclase-adenosine 3',5'-monophosphate system. Endocrinology. 1985 Oct;117(4):1389–1396. doi: 10.1210/endo-117-4-1389. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Ellinwood W. E., Brenner R. M., Hess D. L., Resko J. A. Testosterone synthesis in rhesus fetal testes: comparison between middle and late gestation. Biol Reprod. 1980 May;22(4):955–963. doi: 10.1095/biolreprod22.4.955. [DOI] [PubMed] [Google Scholar]
- Heisler S., Reisine T. Forskolin stimulates adenylate cyclase activity, cyclic AMP accumulation, and adrenocorticotropin secretion from mouse anterior pituitary tumor cells. J Neurochem. 1984 Jun;42(6):1659–1666. doi: 10.1111/j.1471-4159.1984.tb12757.x. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Kehrer P., Turnill D., Dayer J. M., Muller A. F., Gaillard R. C. Human recombinant interleukin-1 beta and -alpha, but not recombinant tumor necrosis factor alpha stimulate ACTH release from rat anterior pituitary cells in vitro in a prostaglandin E2 and cAMP independent manner. Neuroendocrinology. 1988 Aug;48(2):160–166. doi: 10.1159/000125004. [DOI] [PubMed] [Google Scholar]
- Kilian P. L., Kaffka K. L., Stern A. S., Woehle D., Benjamin W. R., Dechiara T. M., Gubler U., Farrar J. J., Mizel S. B., Lomedico P. T. Interleukin 1 alpha and interleukin 1 beta bind to the same receptor on T cells. J Immunol. 1986 Jun 15;136(12):4509–4514. [PubMed] [Google Scholar]
- Phillips M. A., Jaken S. Specific desensitization to tumor-promoting phorbol esters in mouse pituitary cells. Evidence that desensitization is a two-step process. J Biol Chem. 1983 Mar 10;258(5):2875–2881. [PubMed] [Google Scholar]
- Reisine T. D., Heisler S., Hook V. Y., Axelrod J. Activation of beta 2-adrenergic receptors on mouse anterior pituitary tumor cells increases cyclic adenosine 3':5'-monophosphate synthesis and adrenocorticotropin release. J Neurosci. 1983 Apr;3(4):725–732. doi: 10.1523/JNEUROSCI.03-04-00725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol S. L. Storage and secretion of beta-endorphin and related peptides by mouse pituitary tumor cells: regulation by glucocorticoids. Arch Biochem Biophys. 1980 Aug;203(1):37–48. doi: 10.1016/0003-9861(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L., Kunos G. Synergistic regulation of pulmonary beta-adrenergic receptors by glucocorticoids and interleukin-1. J Biol Chem. 1988 Nov 5;263(31):15876–15879. [PubMed] [Google Scholar]
- Uehara A., Gottschall P. E., Dahl R. R., Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987 Oct;121(4):1580–1582. doi: 10.1210/endo-121-4-1580. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Gola M. Forskolin interaction with voltage-dependent K channels in Helix is not mediated by cyclic nucleotides. Neurosci Lett. 1987 Jul 22;78(2):211–216. doi: 10.1016/0304-3940(87)90635-5. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Phillips M. A., Schonbrunn A. Vasoactive intestinal peptide stimulates hormone release from corticotropic cells in culture. Endocrinology. 1983 Feb;112(2):550–557. doi: 10.1210/endo-112-2-550. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]