This study demonstrates substantial, melanopsin-driven, post-illumination pupilloconstriction in normal subjects.
Abstract
Purpose.
A sustained pupilloconstriction is often observed after the cessation of a bright visual stimulus. This post-illumination pupil response (PIPR) is produced by the intrinsically photosensitive retinal ganglion cells (ipRGCs). The present study was designed to examine the characteristics of the PIPR in a normal population without ocular disease.
Methods.
Thirty-seven subjects (mean age, 48.6 years) were tested by presenting a 60°, 10-second light stimulus (13 log quanta/cm2/s retinal irradiance) and recording pupillary responses for 50 seconds after light cessation. The light stimuli (470 [blue] and 623 [red] nm) were presented by an optical system to one eye after dilation, while the consensual pupil response of the fellow, undilated eye was recorded by infrared pupillometry.
Results.
A positive PIPR was seen in all subjects tested. The population average of the PIPR for 470-nm light was 1.5 mm (SEM 0.10, P < 0.05) and the net PIPR (blue PIPR minus red PIPR) was 1.4 mm (SEM 0.09, P < 0.0001). The net PIPR correlated positively with baseline pupil diameter (P < 0.05), but not significantly with age, race, or sex (P > 0.05) in the test population.
Conclusions.
All normal subjects displayed a significant PIPR for a 10-second, 470-nm light stimulus, but not a 623-nm stimulus, which is consistent with the proposed melanopsin-mediated response. In most normal individuals, the amplitude of the PIPR was substantial. This test has the potential to be used as a tool in evaluating subjects with inner retinal dysfunction or melanopsin-related disorders.
The pupillary light reflex (PLR), a reflexive constriction of the pupil in response to an increase in ocular illumination, is an indispensable clinical measure of visual, neurologic, and autonomic function. Until recently, it was thought that the PLR was driven by rods and cones.1,2 In 2000, a novel photopigment, melanopsin, was discovered in the inner retina of both primates and rodents that was later determined to be expressed by a unique class of retinal ganglion cells (RGCs).3–8 These melanopsin-containing cells, in addition to receiving rod and cone inputs, are intrinsically photosensitive and have therefore been termed ipRGCs.9
Intracellular recordings of ipRGCs in both rodents and macaques have shown that these cells exhibit a characteristic transient burst of firing at stimulus onset that decays to a plateau of sustained firing that continues well past stimulus cessation.9–12 Dacey et al.,11 identified rod-driven, cone-driven, and intrinsic responses in the ipRGCs and determined the relative contribution of these inputs to the firing pattern of these cells. The melanopsin-driven, intrinsic responses of ipRGCs were present at higher irradiances (>11 log quanta/cm2/s), and the spectral sensitivity of this response closely matched a single pigment action spectrum with a peak at 483 nm.11 It has been reported that, in humans lacking rods and cones, these ipRGCs may contribute to rudimentary visual awareness, thus challenging the assumption that rod- and cone-based photoreception mediates all visual responses to light.13 Preliminary data in humans suggest that under photopic conditions, cones contribute only to the initial light-evoked pupillary constriction, whereas rods and intrinsic activation of the ipRGCs contribute substantially to sustained pupilloconstriction.14 In behaving macaques, after pharmacologic blockade of the classic photoreceptors, significant melanopsin-driven pupillary responses are present both during continuous (10 seconds) light stimuli (light-evoked response) and after light cessation (henceforth termed the post-illumination pupil response [PIPR]).4 Indeed, since the PIPR was not significantly diminished after pharmacologic blockade in these studies, it must be produced by the melanopsin-driven, intrinsic photoresponse of the ipRGCs.4
The present study was conducted to determine the characteristics of the PIPR in a sample of normal individuals without ocular disease. This study represents an initial step in the potential use of the PIPR as a clinical test that allows differentiation between disorders affecting photoreceptors and those affecting the RGCs.10
Materials and Methods
Subject Selection
Normal subjects for the study were recruited as part of ongoing studies within the Glaucoma Service of the Department of Ophthalmology at the University of Alabama at Birmingham. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board. Normal subjects between the ages of 19 and 80 years were enrolled with the understanding and written consent of each subject. All individuals had best corrected visual acuity of 20/30 or better in each eye and normal findings in slit lamp, Goldmann applanation tonometry, gonioscopy, and dilated fundus examinations. All subjects had refraction no worse than ±5 D spherical and ±2 D cylinder. Standard visual field testing was performed with a retinal perimeter (Humphrey field Analyzer, model 750; Carl Zeiss Meditec, Dublin, CA, with the SITA [Swedish Interactive Threshold Algorithm] standard 24-2 program). A visual field was considered reliable when the fixation losses were less than 20% and false-negatives and -positive rates were less than 25%.15 A normal visual field was defined as a mean deviation (MD) and pattern standard deviation (PSD) within 95% confidence limits, with fewer than three non–edge-contiguous points identified as significant (P < 0.05) on the same side of the horizontal meridian in the pattern-deviation plot, and within the normal limits of the glaucoma hemifield test (Carl Zeiss Meditec). Only individuals with repeatable and reliable visual fields were included in the study. Fundus photographs were obtained and reviewed by a fellowship-trained ophthalmologist (CAG) in masked fashion, to verify normality. An additional 11 subjects used in the study were volunteers recruited from the clinic and laboratory. These individuals had had an eye examination within the past year with normal results.
Testing Apparatus
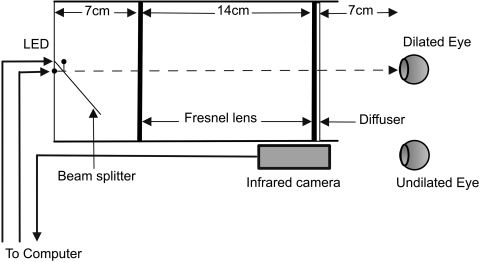
The optical system used for the study was an extended Maxwellian-view system consisting of LED light sources imaged in the pupil plane of the eye via two Fresnel lenses (Edmund Optics, Barrington, NJ), each of a 10-cm diameter a 7-cm focal length, and a 14-cm separation.16 The two lenses were mounted within an enclosure. At one end of the enclosure the blue (470 nm) and red (623 nm) LEDs (25 nm, full width at half maximum; Optek Technology, Inc., Carrollton, TX) were positioned at the focal point of the first Fresnel lens with a beam splitter (50 × 50 × 1 mm; Edmund Optics; Fig. 1). At the other end of the enclosure, a 5° light-shaping diffuser (Physical Optics Corp., Torrance, CA) was placed in front of the second Fresnel lens. The effective field of view of this optical system was ±30°. One eye of the subject was dilated with 1% tropicamide (Myrdal; Bausch & Lomb, Rochester, NY) and 2.5% phenylephrine (Neofrin; Bausch & Lomb). In all subjects, we achieved full pupil dilation before we ran the test. Based on previous studies, dilated pupil diameter would be expected to be 7.8 mm (0.7, SD).17 This small variation in pupil size would result in a standard deviation in retinal irradiance of approximately 0.08 log units. After full dilation, the light stimulus was presented to this eye, while the consensual pupil response in the undilated eye was recorded via infrared camera (Digivue EC-PC-Cam; Elyssa Corp., Briarcliff Manor, NY) and computer. The LEDs had a diameter of 2 mm, which yielded an image source area of 3.14 mm2. The area of the image at the plane of the pupil was 3.14 cm2.
Figure 1.
The optical system used in the study. Two Fresnel lenses (10-cm diameter) were placed twice their focal length apart within an enclosure. The two LEDs (470 and 623 nm) were placed at one end of the enclosure. The subject's dilated eye was aligned at the other end, while the undilated eye was recorded by the infrared camera attached to the computer.
Stimulus Presentation
The experiment was conducted with commercial graphics programming software (LabView; National Instruments, Austin, TX). Light stimuli were adjusted to present a retinal irradiance of 13 log quanta/cm2/s, assuming normal prereceptoral filtering.4,18 Retinal irradiance was corrected for a lens transmission based on an average age of 40 years.19 Each test was run as two epochs (either a blue or red stimulus), each of 80 seconds' duration and separated by up to 5 minutes. During each epoch, after a 20-second fixation period, the stimuli were presented to the dilated eye for a period of 10 seconds. The red stimulus primarily served as a control for such nonspecific influences on the PIPR as fatigue. A total of three or four tests were conducted, and the duration of the entire session was approximately 45 minutes.
Data Analyses
Data from all tests were stored and analyzed off-line. Traces showing pupillary diameter were displayed, regions of the data were selected for further analysis, (Excel, Microsoft Corp., Redmond, WA; SAS, SAS, Inc., Cary, NC; and MatLab, The MathWorks, Natick, MA), and data plots were generated (SigmaPlot; SPSS, Chicago, IL).
For the analyses, we defined the following measures. Baseline pupil diameter was the average pupil diameter, over a 7-second period, before light stimulus. Initial pupil response was the average pupil diameter over a 4-second period, starting 1 second after the light stimulus onset. Sustained pupil diameter was the average pupil diameter over a period of 30 seconds, starting 10 seconds after light offset. The variables used for the subsequent analyses were:
Descriptive statistics (mean and SE) were calculated for baseline, sustained, initial response, and the PIPR measures. A test for normalcy was performed with the Shapiro-Wilk test. Statistical analyses were performed with linear regression models, and statistical significance was obtained with a nominal P ≤ 0.05. The blue- and red-light responses were compared by using Student's t-test and the slopes for regression were tested with the F test. Relationships between baseline pupil sizes, subject characteristics, and the PIPR parameters were investigated by means of Pearson's correlation coefficients and analysis of covariance (ANCOVA).
Results
A total of 45 subjects were tested for the study. Eight interviewed subjects were excluded due to detected ocular abnormality (n = 5) or unusable data (n = 3). Hence, a total of 37 subjects were used for the analyses (9 men and 9 women of European ancestry and 8 men and 11 women of African ancestry). The subjects' average age was 48.6 years and ranged from 26 to 80 years.
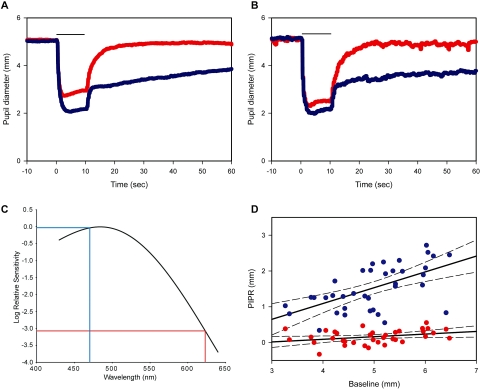
Average pupil diameters of the subjects were plotted against time for both control and test stimuli (623 and 470 nm), respectively (Figs. 2A, 2B). As expected, given the spectral sensitivity of melanopsin (Fig. 2C), the 470-nm stimulus evoked a substantial PIPR after light offset. However, despite producing substantial light-evoked pupillary responses at both 13 and 13.5 log quanta/cm2/s (Figs. 2A, 2B), the 623-nm stimulus produced no significant PIPR. The PIPRs to both the blue and red stimuli were plotted against the baseline pupil diameters (Fig. 2D; blue light, R2 =0.354; red light R2 =0.157). The slope of the PIPR as a function of baseline pupil diameter, for blue light, was significantly different from 0 (P < 0.05).
Figure 2.
Time trace plots of the pupillary response to the control (red) and test (blue) LEDs (n = 37). Bar, light stimulus duration. The red and blue traces depict the pupil diameter for the red and blue lights, respectively. (A) Average pupil diameter plotted against time in all subjects. (B) Maximum response for red light generated by increasing the retinal irradiance by half a log unit. Average pupil diameter plotted against time in five subjects. (C) Spectral sensitivity nomogram for melanopsin showing the expected relative PIPR sensitivity for the 470 nm (blue trace) and 623 nm (red trace) stimuli. (D) Linear regression plots for the control (red circles, R2 = 0.157) and test (blue circles, R2 = 0.354) with 95% confidence interval (CI; dashed lines). Baseline pupil diameters are plotted against the PIPRs (n = 37).
The values for the baseline pupil diameter, sustained pupil diameter, and PIPR measures for red (control) and blue (test) lights are shown in Table 1. The mean response for the blue stimulus was 1.5 mm (SEM 0.10, P < 0.05) with a net PIPR of 1.4 mm (SEM 0.09, P < 0.001).
Table 1.
Pupil Measurements in Test Subjects
| Red (R) | Blue (B) | Net (B − R) | |
|---|---|---|---|
| Baseline | 5.0 (0.15) | 5.0 (0.14) | 0.0 (0.05) |
| Sustained | 4.8 (0.15) | 3.4 (0.11) | 1.4 (0.11) |
| PIPR | 0.2 (0.03) | 1.5 (0.10)* | 1.4 (0.09)† |
n = 37. The baseline measure is the average pupil diameter over a 7-second period before light onset. The sustained measure is the average pupil diameter over a period of 30 seconds, starting 10 seconds after light offset. The difference between the two measures provides the PIPR value. The SEM for all the measures are indicated in parentheses.
Statistically significant P < 0.05.
P < 0.001. Values rounded to 1 decimal point.
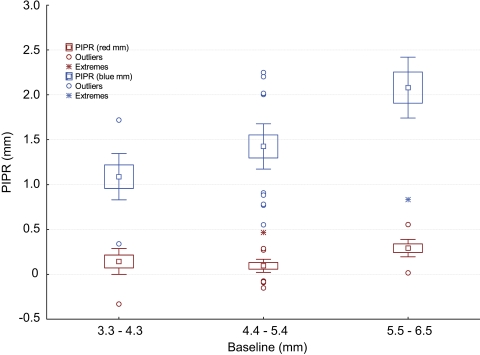
To investigate the influence of baseline pupil diameter on the PIPR values, we grouped the study population into tertiles (3.3–4.3 mm [group 1], 4.4–5.4 mm [group 2], and 5.5–6.5 mm [group 3]), and plotted them against the PIPR as box plots with error bars (Fig. 3). An ANCOVA, autoregressive model, showed that the PIPR was significantly different between the test and control in all the groups (P < 0.05), although the power of the test substantially increased (90%, P < 0.001) when the baseline pupil diameters were greater than 4.4 mm (groups 2 and 3).
Figure 3.
Box plot of tertile groups with error bars. The baseline pupil diameter response to red light grouped into tertiles, 3.3 to 4.3, 4.4 to 5.4, and 5.5 to 6.5 mm, and plotted against the PIPR. Boxes indicate the mean and 1 SE; whiskers, 2 SE.
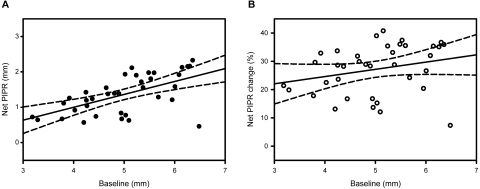
We calculated the difference between the test and control measures (net PIPR) and used this for further analyses. To control for the influence of baseline pupil diameter on the PIPR amplitude, we calculated the change in the PIPR as a percentage of baseline pupil diameter (PIPR change). The PIPR change for the red and blue stimuli was calculated, with the difference between the two measures being the net PIPR change. The mean of the net PIPR change showed a reduction of 27% (SEM 1.44, P < 0.001), for the test population. Figure 4 demonstrates that, whereas the net PIPR (Fig. 4A, R2 = 0.345, P < 0.05) correlated positively with baseline pupil diameter, the net PIPR change (Fig. 4B, R2 = 0.066, P > 0.05) did not.
Figure 4.
Net PIPR plotted against baseline pupil diameters, with linear regression lines (solid line) and 95% CI (dashed lines). (A) Net PIPRs (R2 = 0.345, P < 0.05) = −0.465 + (0.366 × baseline). (B) Net PIPR change (R2 = 0.066, P > 0.05) = 14.37 + (2.566 × baseline).
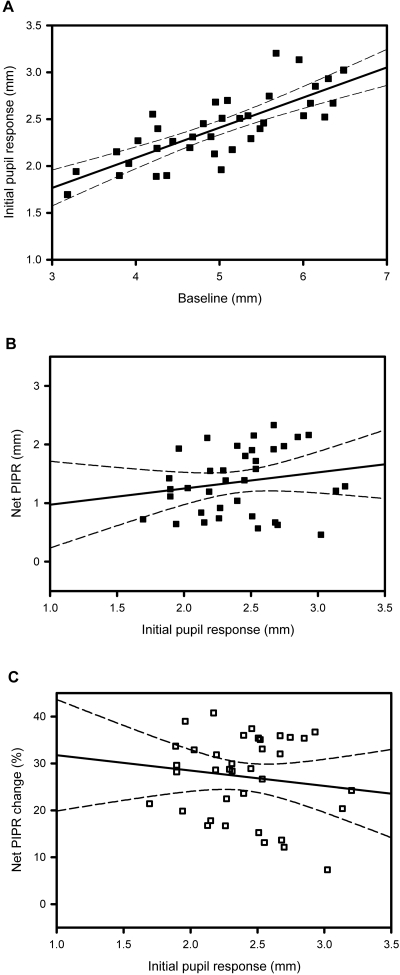
We investigated the relationship between the initial pupil response, baseline pupil diameter, and net PIPR characteristics. Figure 5A demonstrates the relation between initial pupil response and baseline pupil diameter (R2 = 0.612, P < 0.001). The net PIPR (Fig. 5B, R2 = 0.033, P > 0.05) and net PIPR change (Fig. 5C, R2 = 0.018, P > 0.05) plotted against initial pupil response to light stimulus did not correlate significantly.
Figure 5.
Correlation between baseline pupil diameter, net PIPR, and the initial pupil responses. Regression plots show linear trend lines (solid line) and 95% CI (dashed lines). (A) Initial pupil response plotted against baseline pupil diameter (R2 = 0.612, P < 0.001) = 0.419 + (1.901 × initial pupil response). (B) Net PIPR plotted as a function of initial pupil response (R2 = 0.033, P > 0.05) = 0.697 + (0.276 × initial pupil response). (C) Net PIPR change plotted against initial pupil response (R2 = 0.018, P > 0.05) =35.0 + (−3.271 × initial pupil response). P values are for the slopes of the regression lines.
To examine the reliability of the test, we repeated the experiment in five subjects. We found a very high correlation (R2 = 0.98) of both the baseline pupil diameters and the PIPR values, measured on different days. In all subjects, we also calculated area measures of the PIPR and proportional area change in the PIPR. The results were similar to those found with linear measures.
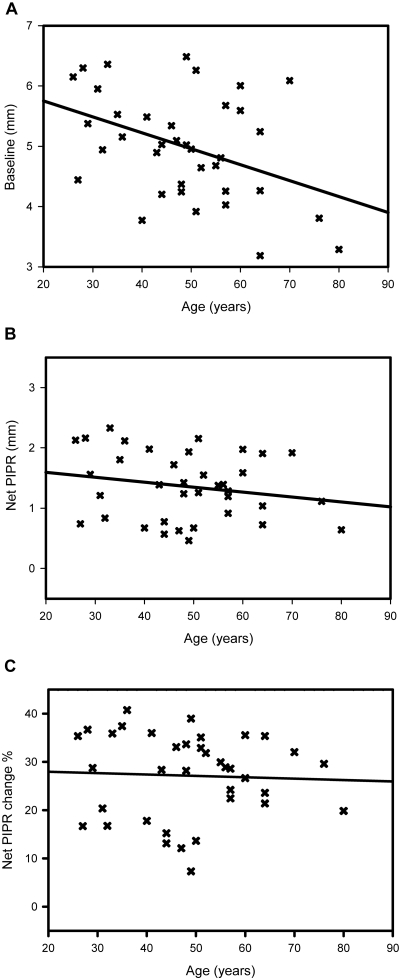
To examine the effect of age, we plotted baseline pupil diameter, net PIPR, and net PIPR change against age (in years; Fig. 6). The baseline pupil diameters decreased significantly with increasing age (R2 = 0.166, P < 0.05; Fig. 6A), but there was no correlation of the net PIPR (R2 = 0.04, P > 0.05; Fig. 6B) or net PIPR change (R2 = 0.002, P > 0.05; Fig. 6C) with age when adjusted for baseline pupillary diameter.
Figure 6.
Influence of age on baseline pupil and the PIPR. (A) Average baseline pupil diameter plotted against age in years (R2 = 0.166, P < 0.05). (B) Net PIPR plotted as a function of age (R2 = 0.04, P > 0.05). (C) Net PIPR change plotted against age (R2 = 0.002, P > 0.05). P values are for the slopes of the regression lines.
There was no statistically significant difference between the men and women (P > 0.05) or between the individuals of European and African ancestry (P > 0.05) in the net PIPR values (results not shown).
Discussion
The goal of this study was to investigate the PIPR in a sample of individuals with no ocular disease. Gamlin et al.4 have reported a melanopsin-driven PIPR in a small sample of macaques and humans. Our results demonstrate a positive melanopsin-driven PIPR in all 37 subjects tested. Overall, we found the average PIPR for a 470-nm stimulus to be 1.5 mm (SEM = 0.10 mm), which is consistent with the results of the original study.4 More specifically, the stimulus used in the present study was 13 log quanta/cm2/s and had an area 0.44 log units greater than that used in the original study. Assuming linear summation, this result corresponds to a retinal irradiance of ∼13.5 log quanta/cm2/s in the original study; this retinal irradiance resulted in an average PIPR to a 470-nm stimulus of 1.7 mm in humans and 1.5 mm in monkeys.4
We found that the magnitude of the net PIPR varied from 0.5 to 2.3 mm in our sample population. The small PIPRs in some of the subjects were not the result of unresponsive pupils, since these subjects showed substantial light-evoked pupilloconstriction (Initial pupil response). However, our initial analyses showed that the subjects with smaller baseline pupils tended to display smaller PIPRs. To remove this interaction from our subsequent analyses, we found that the data could be normalized by plotting the percentage of PIPR change as a function of the baseline pupil measure. Even with this correction, we observed a significant range in the percentages of PIPR change. In particular, a few subjects displayed minimal PIPRs. It is possible that these subjects represent a small percentage of the general population that possesses a reduced PIPR. Such reduced responses could be seen in individuals with defects in the OPN4 gene, which codes for melanopsin.20 They could also be seen in the subjects in whom the intrinsic photoresponse was reduced in magnitude because of a reduced expression of melanopsin. In either of these cases, given such reduced melanopsin-driven pupillary responses, the possibility that these subjects could also display circadian or sleep/wake abnormalities would be interesting to explore.
It is well known that baseline pupil diameters tend to get smaller with age,17–19,21–24 and this age-related miosis is seen over a wide range of illuminance levels.21 Furthermore, it has been suggested that the magnitude of light-evoked pupillary responses is reduced with age.22 Our results indicate that, after adjustment for baseline pupil diameter, age was not a significant contributing factor to the magnitude of the PIPR, independent of variation in pupil size. However, a recent study of light-induced melatonin suppression showed an age-related loss in sensitivity to short-wavelength light.24 It is thought that such light-induced melatonin suppression is driven by the ipRGCs,25 which therefore suggests that there is an age-related reduction in ipRGC signaling. Of note, such a decrease in ipRGC signaling may be expected to result in age-related mydriasis rather than the miosis that is actually seen. It is clear that more extensive study is needed to investigate the effect of age on ipRGC mediated pupillary responses. Although there have been some previous reports of racial differences, particularly as they relate to iris color, in pupillary response magnitude,25,26 we found no effect of race or sex on the net PIPR.
In conclusion, using a newly developed, wide-field optical system, we have demonstrated that all normal subjects display a PIPR with wavelength sensitivities consistent with melanopsin-mediated mechanism. This response is substantial in most normal individuals. Therefore, this system may have potential to be used as a clinical tool in evaluating patients with inner retinal dysfunction or melanopsin-related disorders.
Acknowledgments
The authors thank all the subjects for participating; Bobbie Hill (Glaucoma Service, EFH) for invaluable help in recruitment; and Jerry Millican, David McDougal, Abidin Yildrim, and Mark Bolding for help in the development of the test apparatus.
Footnotes
Supported by National Institutes of Health Grants EY09380 (PDG) and P30 EY03039 (PDG); The EyeSight Foundation of Alabama; and a Research to Prevent Blindness Physician Scientist Award (CAG).
Disclosure: L. Kankipati, None; C.A. Girkin, None; P.D. Gamlin, None
References
- 1.Alpern M, Campbell FW. The spectral sensitivity of the consensual light reflex. J Physiol 1962;164:479–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loewenfeld IE, Lowenstein O. The Pupil Anatomy, Physiology, and Clinical Applications Vol. 1.Ames, IA: Iowa State University Press; 1993. [Google Scholar]
- 3.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci 2003;23(18):7093–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res 2007;47:946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci 2001;4(6):621–626 [DOI] [PubMed] [Google Scholar]
- 6.Oyster CW. The iris and the pupil. In: Oyster CW. ed. The Human Eye: Structure and Function Sunderland, MA: Sinauer Associates; 1999:411–446 [Google Scholar]
- 7.Kardon RH. Anatomy and Physiology of the pupil. In: Walsh FB, Hoyt WF. eds. Clinical Neuro-ophthalmology Baltimore: Williams & Wilkins; 1998:847–897 [Google Scholar]
- 8.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci 2000;20(2):600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002;295(5557):1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol 2007;27(3):195–204 [DOI] [PubMed] [Google Scholar]
- 11.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature 2005;433(7027):749–754 [DOI] [PubMed] [Google Scholar]
- 12.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Sci Ophthalmol 2002;106(1):98–99 [DOI] [PubMed] [Google Scholar]
- 13.Zaidi FH, Hull JT, Peirson SN, et al. Wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol 2007;17(24):2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDougal DH, Gamlin PD. The influence of intrinsically photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res 2010;50(1):72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojima T, Tanabe T, Hangai M, Yu S, Morishita S, Yoshimura N. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol 2007;51(3):197–203 [DOI] [PubMed] [Google Scholar]
- 16.Beer RD, Macleod DI, Miller TP. The extended Maxwellian view (BIGMAX): a high-intensity, high-saturation color display for clinical diagnosis and vision research. Behav Res Methods 2005;37(3):513–521 [DOI] [PubMed] [Google Scholar]
- 17.Hammond CJ, Snieder H, Spector TD, Gilbert CE. Factors affecting pupil size after dilatation: the Twin Eye Study. Br J Ophthalmol 2000;84(10):1173–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winn B, Whitaker D, Elliott DB, et al. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci 1994;35(3):1132–1137 [PubMed] [Google Scholar]
- 19.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt 1987;26(8):1437–1440 [DOI] [PubMed] [Google Scholar]
- 20.Newsome DA. After image and pupillary activity following strong light exposure. Vision Res 1971;11(3):275–288 [DOI] [PubMed] [Google Scholar]
- 21.Fatiou DF, Brozou CG, Tsiptsios DJ, et al. Effect of age on pupillary light reflex: evaluation of pupil mobility for clinical practice and research. Electroyogr Clin Neurophysiol 2007;47(1):11–22 [PubMed] [Google Scholar]
- 22.Herljevic M, Middleton B, Thapan K, et al. Light-induced melatonin suppression: age-related reduction in response to short wavelength. Exp Gerontol 2005;40(3):237–242 [DOI] [PubMed] [Google Scholar]
- 23.Higuchi S, Motohashi Y, Ishibashi K, et al. Influence of eye colors of Caucasians and Asians on suppression of melatonin secretion by light. Am J Physiol Regul Integr Comp Physiol 2007;292(6):R2352–R2356 [DOI] [PubMed] [Google Scholar]
- 24.Ijspeert J, deWaard PW, van den Berg TJ, et al. The intraocular stray light function in 129 healthy volunteers; dependence on angle, age and pigmentation. Vision Res 1990;30:699–707 [DOI] [PubMed] [Google Scholar]
- 25.Pickard GE, Baver SB, Ogilvie MD, Sollars PL. Light-induced fos expression in intrinsically photosensitive retinal ganglion cells in melanopsin knockout (opn4) mice. PLoS One 2009;4(3):e4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Rico C, de la Villa P, Blanco R, et al. Alterations in nocturnal melatonin levels in patients with optic neuropathies (in Spanish). Arch Soc Esp Oftalmol 2009;84(5):251–257 [DOI] [PubMed] [Google Scholar]