Abstract
Rationale: Patients with asthma exhibit variable response to inhaled corticosteroids (ICS). Vitamin D is hypothesized to exert effects on phenotype and glucocorticoid (GC) response in asthma.
Objectives: To determine the effect of vitamin D levels on phenotype and GC response in asthma.
Methods: Nonsmoking adults with asthma were enrolled in a study assessing the relationship between serum 25(OH)D (vitamin D) concentrations and lung function, airway hyperresponsiveness (AHR), and GC response, as measured by dexamethasone-induced expression of mitogen-activated protein kinase phosphatase (MKP)-1 by peripheral blood mononuclear cells.
Measurements and Main Results: A total of 54 adults with asthma (FEV1, 82.9 ± 15.7% predicted [mean ± SD], serum vitamin D levels of 28.1 ± 10.2 ng/ml) were enrolled. Higher vitamin D levels were associated with greater lung function, with a 22.7 (±9.3) ml (mean ± SE) increase in FEV1 for each nanogram per milliliter increase in vitamin D (P = 0.02). Participants with vitamin D insufficiency (<30 ng/ml) demonstrated increased AHR, with a provocative concentration of methacholine inducing a 20% fall in FEV1 of 1.03 (±0.2) mg/ml versus 1.92 (±0.2) mg/ml in those with vitamin D of 30 ng/ml or higher (P = 0.01). In ICS-untreated participants, dexamethasone-induced MKP-1 expression increased with higher vitamin D levels, with a 0.05 (±0.02)-fold increase (P = 0.02) in MKP-1 expression observed for each nanogram per milliliter increase in vitamin D, a finding that occurred in the absence of a significant increase in IL-10 expression.
Conclusions: In asthma, reduced vitamin D levels are associated with impaired lung function, increased AHR, and reduced GC response, suggesting that supplementation of vitamin D levels in patients with asthma may improve multiple parameters of asthma severity and treatment response.
Clinical trials registered with www.clinicaltrials.gov (NCT00495157, NCT00565266, and NCT00557180).
Keywords: asthma, vitamin D, glucocorticoids, treatment
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Patients with asthma exhibit variable response to inhaled corticosteroids. Vitamin D may exert effects on phenotype and glucocorticoid (GC) response in asthma.
What This Study Adds to the Field
In asthma, reduced vitamin D levels are associated with impaired lung function, increased airway hyperresponsiveness, and reduced GC response, suggesting that supplementation of vitamin D levels in patients with asthma may improve asthma severity and treatment response.
Inhaled corticosteroids (ICS) are glucocorticoid (GC) preparations widely employed, on the basis of national and international guidelines (1, 2), as the principal controller therapy for patients with persistent asthma. Although studies suggest that the majority of patients with asthma are successfully treated with ICS-containing regimens (3), there is variability in response to ICS, with a substantial proportion of patients not achieving optimal asthma control despite even high-dose ICS treatment (3–6). In addition to impairing response to treatment, as measured by improved asthma control, steroid resistance has been associated with alterations in the long-term prognosis of asthma, as indicated by accelerated deterioration in lung function (7). Given this, alternative treatment strategies need to be considered for patients who continue to experience suboptimal asthma control after initiation of ICS, and understanding the mechanisms that underlie the lack of universal response to ICS in asthma is critical for improving strategies for optimizing asthma treatment.
A number of potential explanations exist for the variable clinical response to ICS, and insensitivity to the anti-inflammatory effects of GCs must be considered as one of them in patients who do not respond optimally. The mechanisms of GC insensitivity are complex, reflecting the multiple steps involved in GC action. For example, GCs have been reported to increase expression of a key regulator of mitogen-activated protein kinase (MAPK) inactivation, MAPK phosphatase (MKP)-1 (8–10), and augmented inflammatory responses in GC-insensitive subjects with asthma could be hypothesized to be due to a lack of ability of steroids to induce expression of specific phosphatases, such as MKP-1, which then allows persistent MAPK activation and a more proinflammatory immune cell phenotype (8).
Some published data (11) suggest that vitamin D interacts with GC signaling pathways in ways that are clinically relevant, although little experimental work has been published in this area. In 2006, Xystrakis and colleagues (11) reported that supplementation with the active pharmacological form of vitamin D3 enhanced dexamethasone (DEX)-induced expression of IL-10 by T regulatory cells. Furthermore, they demonstrated that allergen-induced T helper type 2 cell cytokine production was inhibited by allergen-induced IL-10–secreting T regulatory cells in an IL-10–dependent manner, and that, in subjects with steroid-resistant asthma, preincubation of T cells with both IL-10 and vitamin D3 overcame defects in DEX-induced CD4+ T cell IL-10 production, perhaps by a mechanism in which vitamin D3 reversed ligand-induced down-regulation of the GC receptor. Complementing these experimental data, a recent population study suggested an association between lower vitamin D levels and increased ICS requirements in children, with a reduced need for anti-inflammatory controller therapy as vitamin D levels increased (12). To assess the association between vitamin D levels, asthma phenotype, and steroid response in adults, we tested the hypothesis that reduced serum vitamin D concentrations would be associated with worsening of a number of relevant asthma biomarkers, including lung function, airway hyperresponsiveness (AHR), and in vitro GC response.
METHODS
Participants
Participants in two ongoing trials (NCT00495157 and NCT00565266) of the National Heart, Lung, and Blood Institute–sponsored Asthma Clinical Research Network were enrolled in a separate cross-sectional ancillary study evaluating predictors of GC response in vivo and in vitro (NCT00557180). Participants were enrolled from the common run-in period of these two trials at a single Asthma Clinical Research Network center (National Jewish Health, Denver, CO). All participants underwent cross-sectional phenotyping and phlebotomy for vitamin D and steroid response outcomes within 2 weeks of enrollment (after meeting inclusion criteria, but before randomization or allocation to the parent trials). All studies were reviewed and approved by the National Jewish Health institutional review board, all participants provided written informed consent, and both the parent trials and the ancillary study were registered at http://www.clinicaltrials.gov.
We enrolled nonsmoking adults (18 yr of age or older) with a clinical history of asthma confirmed by either bronchodilator responsiveness (BDR; as defined by an improvement in the FEV1 of ≥ 200 ml and 12% after administration of four puffs [360 μg] albuterol by metered-dose inhaler) or AHR (defined as the concentration of methacholine in mg/ml inducing a 20% fall in FEV1 [PC20 FEV1] of ≤ 8 mg/ml if not currently treated with ICS or ≤ 16 mg/ml if currently treated with ICS). Participants with significant medical illness other than asthma, a history of respiratory tract infection, or asthma exacerbation within the previous 4 weeks, or a history of life-threatening asthma within the past 5 years, were excluded.
Measurements
Assessment of AHR and prebronchodilator lung function were performed at the time of enrollment in the common run-in period, according to published guidelines and interpreted according to reference values (13–16). Body mass index (BMI; kg/m2) was calculated from measured height and weight, and ICS use at the time of enrollment was assessed both dichotomously (yes/no) and with regard to concomitant long-acting β-agonist (LABA) use. Serum 25(OH)D (hereafter “vitamin D”) concentrations were assayed by liquid chromatography–tandem mass spectrometry at Mayo Clinical Laboratories (Rochester, MN).
Real-time PCR was used to assess baseline expression of MKP-1 (which inactivates p38 and is up-regulated by DEX), TNF-α, and IL-10 by unstimulated peripheral blood mononuclear cells (PBMCs) isolated from heparinized blood by Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ) gradient centrifugation (17). After isolation, RNA was extracted from PBMCs, transcribed into cDNA, and analyzed by real-time PCR via the dual-labeled fluorigenic probe method (ABI Prism 7000; Applied Biosystems, Carlsbad, CA) (18). Primers and probes for human MKP-1, TNF-α, and IL-10 were used, and standard curves were generated with data from twofold serial dilutions of total RNA of the highest expression sample. Data were normalized to the corresponding levels of 18s rRNA in each sample (ng/ml per ng/ml of 18s). To assess induction of MKP-1 by DEX, 2 × 106 PBMCs were treated with 10−7 M DEX or culture medium for 4 hours. RNA was isolated from DEX-treated cells, with PCR performed as above, and fold change in expression of targets of interest determined by dividing post-DEX treatment MKP-1 expression by baseline expression (19).
To assess suppression of mitogen-induced cell proliferation by DEX, PBMCs were stimulated with 1 μg/ml anti-CD3 in the presence of a range of DEX concentrations (10−10 to 10−6 M), as well as without DEX (20). Cultures were incubated for 72 hours, and 6 hours before harvesting, 3H-thymidine was added to a final concentration of 1 μCi/well, and cell proliferation was assessed based on scintillation counting of radionucleotide incorporation. Steroid sensitivity of cells as a function of response to DEX (% inhibition of cell proliferation by 10−7 M DEX, the highest physiologically relevant dose achieved in vivo) was calculated.
Statistical Analyses
Unadjusted between-group comparisons were performed using Student's t test or Chi-square, as appropriate, with simple linear regression used to evaluate unadjusted correlations between continuous variables. Log transformation was used when data were not normally distributed. To determine the association between serum vitamin D concentrations and physiologic, inflammatory, and GC response biomarkers, least squares regression was used, with models adjusted for the potentially confounding effects of age and sex, with BMI included as a confounder in analyses of lung function and MKP-1 expression. Due to the potential effects of current ICS treatment on certain prespecified variables of interest (e.g., in vitro GC response assays), the study population was dichotomized into ICS-treated and ICS-untreated groups for stratified analyses. All analyses were performed using JMP 8.0.1 (SAS Institute, Cary, NC).
RESULTS
Subjects
A total of 54 adult participants with persistent asthma were enrolled (Table 1). Mean age of participants with asthma was 38.3 (±11.2) years (mean [SD]), with FEV1 of 82.9 (±15.7)% predicted, PC20 of 2.2 (±1.9) mg/ml, and BMI of 28.8 (±7.3) kg/m2. Mean serum vitamin D concentration was 28.1 (±10.2) ng/ml. A total of 24 of the 54 subjects were using ICS at the time of enrollment, with 13 of the 24 ICS recipients (54%) receiving medium- or high-dose ICS (1) or an ICS/LABA combination. When participants with asthma were stratified based on current ICS use, the two groups differed only with regard to age and degree of AHR, with a mean age of 34.4 (±10.7) years in those not receiving ICS versus 43.3 (±9.6) years in those receiving ICS (P = 0.003 for the comparison) and PC20 FEV1 of 1.7 (±1.4) versus 2.8 (±2.2), respectively (P = 0.04).
TABLE 1.
PHYSIOLOGIC AND DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS
| Characteristics | Entire Cohort (n = 54) | ICS Untreated (n = 30) | ICS Treated (n = 24) | P Value |
|---|---|---|---|---|
| Age, years | 38.3 ± 11.2 | 34.4 ± 10.7 | 43.3 ± 9.6 | 0.003 |
| Percent female | 57.4 | 46.7 | 71.0 | 0.07 |
| BMI, kg/m2 | 28.8 ± 7.3 | 30.4 ± 8.1 | 26.8 ± 5.5 | 0.07 |
| FEV1, L | 2.9 ± 1.0 | 3.1 ± 1.1 | 2.7 ± 0.8 | 0.1 |
| FEV1 % predicted | 82.9 ± 15.7 | 84.0 ± 16.8 | 81.5 ± 14.4 | 0.6 |
| FEV1/FVC, % | 69 ± 7.9 | 69.6 ± 8.2 | 68.9 ± 7.5 | 0.8 |
| PC20 FEV1, mg/ml | 2.2 ± 1.9 | 1.7 ± 1.4 | 2.8 ± 2.2 | 0.04 |
| 25(OH)D, ng/ml | 28.1 ± 10.2 | 28.2 ± 11.4 | 28.0 ± 8.5 | 0.9 |
| LABA use, n | 8 | 0 | 8 | — |
Definition of abbreviations: 25(OH)D = vitamin D; BMI = body mass index; ICS = inhaled corticosteroids; LABA = long-acting β-agonist; PC20 FEV1 = provocative concentration of methacholine inducing a 20% fall in FEV1.
Data reported as mean ± SD or percent. All P values for comparison of ICS-untreated versus ICS-treated subjects with asthma.
Vitamin D Levels, Lung Function, and AHR
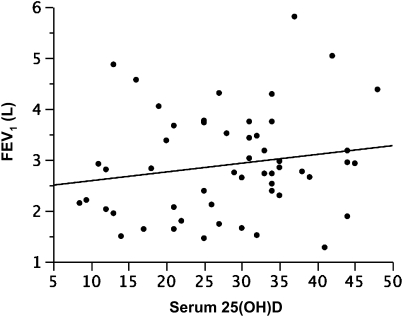
In participants with asthma, increasing serum vitamin D concentrations were associated with greater lung function in continuous analyses. Least squares regression of the relationship between lung function (FEV1, L) and serum vitamin D (Figure 1, Table 2), adjusted for age, sex, and dichotomous BMI (lean versus overweight/obese), indicated that there was a 22.7 (±9.3) ml (mean [SE]) increase in FEV1 for each nanogram per milliliter increase in vitamin D (P = 0.02; adjusted r = 0.8). When participants were stratified into ICS-treated and ICS-untreated populations, a stronger relationship between FEV1 and vitamin D was observed in those subjects with asthma not currently receiving ICS (n = 30) versus those who were (n = 24), with an adjusted effect estimate of +34.6 (±11.3) ml for each unit increase in vitamin D (P = 0.005; adjusted r = 0.8) in ICS-untreated subjects with asthma versus −12.0 (±17.4) ml (P = 0.5) in those receiving ICS.
Figure 1.
Plot of serum vitamin D (25[OH]D, ng/ml) versus prebronchodilator FEV1 (L), representing an increase of 22.7 (±9.3) ml in FEV1 for each nanogram per milliliter increase in vitamin D (P = 0.02; covariate adjusted r = 0.8).
TABLE 2.
MODELED EFFECT ESTIMATE, P VALUES, AND r VALUES FOR RELATIONSHIP BETWEEN INCREASING SERUM 25(OH)D AND PARAMETERS OF INTEREST
| Parameter | Entire Cohort (n = 54) | ICS Untreated (n = 30) | ICS Treated (n = 24) |
|---|---|---|---|
| FEV1, L | +22.7 ± 9.3 | +34.6 ± 11.3 | −12.0 ± 17.4 |
| P = 0.02 | P = 0.005 | P = 0.5 | |
| r = 0.8 | r = 0.8 | ||
| BMI, kg/m2 | −0.71 ± 0.17 | −0.86 ± 0.23 | −0.56 ± 0.34 |
| P = 0.0001 | P = 0.0009 | P = 0.1 | |
| r = −0.5 | r = −0.6 | ||
| DEX-induced MKP-1 expression, fold change | +0.03 ± 0.01 | +0.05 ± 0.02 | −0.01 ± 0.03 |
| P = 0.04 | P = 0.02 | P = 0.8 | |
| r = 0.4 | r = 0.5 | ||
| DEX-mediated suppression of mitogen-induced PBMC proliferation | −0.27 ± 0.23 | NS | NS |
| P = 0.2 | — | — | |
| Baseline TNF-α expression | −0.06 ± 0.02 | −0.03 ± 0.02 | −0.09 ± 0.04 |
| P = 0.01 | P = 0.2 | P = 0.05 | |
| r = −0.3 | r = −0.5 | ||
| DEX-induced IL-10 expression, fold change | 0.004 ± 0.006 | 0.01 ± 0.007 | −0.02 ± 0.01 |
| P = 0.5 | P = 0.1 | P = 0. 1 |
Definition of abbreviations: BMI = body mass index; DEX = dexamethasone; ICS = inhaled corticosteroids; MKP = mitogen-activated protein kinase phosphatase; NS = not significant; PBMC = peripheral blood mononuclear cell.
Modeled effect estimate values (±SE) are presented. Except for BMI, covariate adjusted r values are presented (if P value is significant) for relationship between increasing serum 25(OH)D (per ng/ml increase).
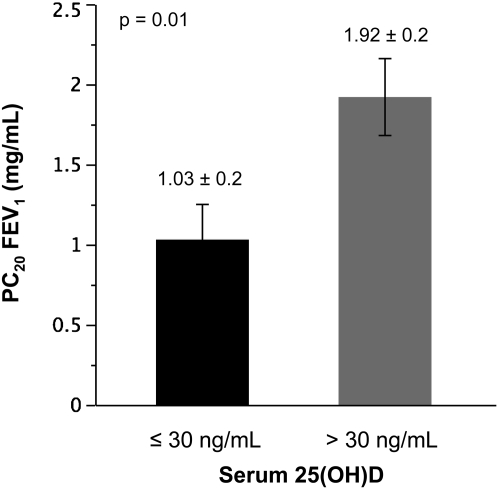
Among subjects with AHR to methacholine, as defined by American Thoracic Society criteria (13) of a PC20 FEV1 to methacholine of 4 mg/ml or less (n = 41), reduced serum vitamin D concentrations were associated with a greater degree of AHR. When subjects were grouped by a serum vitamin D threshold of 30 ng/ml, a threshold corresponding to the serum vitamin D concentration proposed by many investigators as the lower end of the optimal range for both skeletal and other outcomes (21–23), those participants with vitamin D concentrations less than 30 ng/ml demonstrated a PC20 FEV1 of 1.03 (±0.2) mg/ml (mean [SE]) compared with a PC20 FEV1 of 1.92 (±0.2) in those with vitamin D concentrations 30 ng/ml or greater (n = 22; P = 0.01 for the comparison; Figure 2). A similar categorical relationship was observed when PC20 FEV1 was log transformed (log PC20, −0.43 ± 0.22 versus 0.45 ± 0.2; P = 0.005), and in the adjusted least squares model, a significant continuous relationship between serum vitamin D and PC20 was also observed (effect estimate, +0.03 ± 0.02; P = 0.05; adjusted r = 0.4).
Figure 2.
Airway hyperresponsiveness to methacholine, stratified by serum vitamin D (25[OH]D, ng/ml) concentrations of 30 ng/ml. PC20 FEV1 = provocative concentration of methacholine inducing a 20% fall in FEV1.
Serum Vitamin D Levels and Body Mass
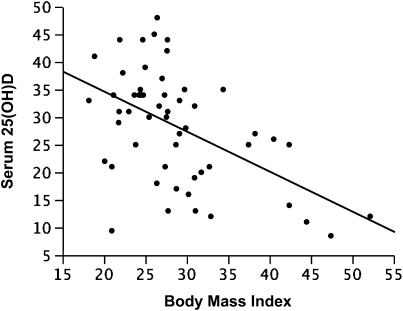
Strong inverse correlations between BMI (kg/m2) and serum vitamin D concentrations (ng/ml) were observed. In all participants, unadjusted analyses indicated that there was a 0.71 (±0.17) ng/ml decrease in serum vitamin D for each unit increase in BMI (P = 0.001; r = −0.5; Figure 3), a relationship that was also observed in analyses adjusted for age, sex, and physiologic impairment (FEV1 % predicted), with an effect estimate of −0.71 (±0.17) (P = 0.0001; adjusted r = −0.5). Although the inverse relationship between vitamin D and BMI remained significant when current ICS use (dichotomized) was added to the model (effect estimate, −0.76 ± 0.18; P < 0.0001), when participants were stratified into ICS-treated and ICS-untreated populations, the overall inverse relationship was found to be stronger and significant only in those subjects with asthma not currently receiving ICS (n = 30; effect estimate, −0.86 ± 0.23; P = 0.0009; adjusted r = −0.6) as compared with those currently receiving ICS (n = 24; effect estimate, −0.56 ± 0.34; P = 0.1; adjusted r = −0.2).
Figure 3.
Plot of body mass index (BMI; kg/m2) versus serum vitamin D (25[OH]D, ng/ml), representing a reduction of 0.71 (±0.17) ng/ml in vitamin D for each unit increase in BMI (P = 0.0001; r = −0.5).
Serum Vitamin D Levels and Expression of TNF-α by PBMCs at Baseline
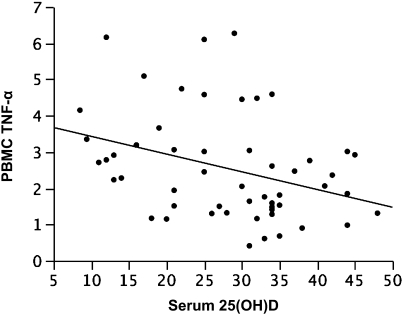
Higher serum vitamin D concentrations were associated with decreased baseline expression of TNF-α by PBMCs (Figure 4), with the adjusted model (age, sex, BMI) indicating an reduction in normalized TNF-α expression of 0.06 (±0.02) units for each unit increase in vitamin D (P = 0.01; adjusted r = −0.3). This inverse association was stronger in ICS-treated participants (0.09 ± 0.04 unit reduction; P = 0.05; adjusted r = −0.5) than in ICS-untreated participants (0.03 ± 0.02 unit reduction; P = 0.2). However, when analyzed with regard to dichotomous ICS use (yes/no), baseline expression of TNF-α was not significantly modified by current use of ICS, with mean normalized TNF-α expression of 2.2 (±0.3) units in ICS-untreated and 3.0 (±0.4) units in ICS-treated individuals (P = 0.09 for the comparison).
Figure 4.
Plot of serum vitamin D (25[OH]D, ng/ml) versus baseline TNF-α, representing a reduction of 0.06 (±0.02) units TNF-α expression for each nanogram per milliliter increase in vitamin D (P = 0.01; covariate adjusted r = −0.3). PBMC = peripheral blood mononuclear cell.
Serum Vitamin D Levels and Effects of DEX on Expression of MKP-1 and Suppression of Mitogen-Induced Cell Proliferation
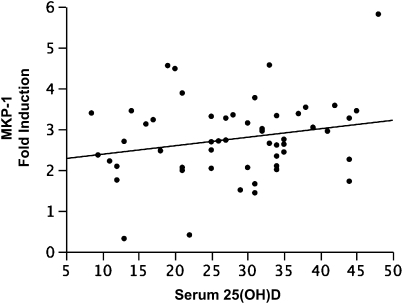
Increasing serum vitamin D concentrations were associated with enhanced GC response in vitro. In analyses restricted to those individuals not treated with ICS (designed to exclude the effect of exogenous GC exposure on response to DEX), adjusted (age, sex, BMI) least squares regression demonstrated a positive correlation between increasing vitamin D concentrations and increasing DEX-induced MKP-1 expression, with a 0.05 (±0.02)-fold increase (P = 0.02; r = 0.5) in MKP-1 expression observed for each unit increase in vitamin D concentration. The relationship between vitamin D and MKP-1 remained (Figure 5), but was weaker, when the entire study population was analyzed irrespective of ICS use (effect of 0.03 ± 0.01; P = 0.04; r = 0.4), and was not observed solely in ICS-treated subjects (−0.01 ± 0.03; P = 0.8).
Figure 5.
Plot of serum vitamin D (25[OH]D, ng/ml) versus dexamethasone-induced mitogen-activated protein kinase phosphatase (MKP)-1 expression, representing an increase of MKP-1 of 0.03 (±0.01)-fold for each nanogram per milliliter increase in vitamin D (P = 0.04; covariate adjusted r = 0.4).
When DEX-mediated suppression of mitogen-induced cell proliferation was analyzed, significant relationships with vitamin D were not observed in either of the two ICS treatment strata (data not shown), or in the study population as a whole (effect estimate, −0.27 ± 0.23; P = 0.2).
Serum Vitamin D Levels, IL-10 Expression, and Effects of DEX
Baseline expression of IL-10 by PBMCs was low, with a mean standardized value of 0.01 (±0.001). There was not an association between serum vitamin D concentrations and baseline IL-10 expression in the entire study population, with an effect estimate of 1.9 × 10−6 (±0.0001) (P = 1.0), a relationship that was equally weak in ICS-treated and ICS-untreated participants. In vitro exposure to DEX did not significantly enhance IL-10 production by PBMCs, either in the study population as a whole (effect estimate, 0.004 ± 0.006; P = 0.5), or in ICS treated (−0.02 ± 0.01; P = 0. 1) or untreated (0.01 ± 0.007; P = 0.1) participants.
DISCUSSION
We report data demonstrating that, in adults with persistent asthma, there is a significant and deleterious association between reduced serum vitamin D levels and lung function, AHR, and GC sensitivity, which together constitute three important biomarkers of asthma severity, impairment, and prognosis (1, 7, 24). In addition, reduced vitamin D levels are associated with increased expression of TNF-α, suggesting that enhanced expression of this proinflammatory cytokine is one potential pathway by which reduced vitamin D levels could exert a proinflammatory effect in asthma (25–27). These observations suggest that evaluation of serum vitamin D concentrations should be considered in adult patients with asthma that responds suboptimally to ICS, and they raise the underlying possibility that vitamin D supplementation could result in improvement of these phenotypic variables in the subset of subjects with asthma who are vitamin D deficient.
Given that our study population incorporated both ICS-untreated and ICS-treated (with concomitant LABA use in some cases) participants, we were able to assess the impact of ICS exposure on the relationship between vitamin D and the aforementioned parameters. In the case of lung function, the correlation between higher vitamin D levels and improved lung function was even stronger in those who were ICS untreated than in the study population as a whole. In addition, the relationship between vitamin D levels and in vitro GC response was stronger in ICS-untreated participants than that observed in all subjects with asthma, independent of treatment. This suggests that, although the relationship between low vitamin D levels and impaired GC response is pertinent to subjects with asthma, independent of treatment, those who are not treated with ICS appear to be more responsive to the beneficial effects of higher vitamin D levels, and that vitamin D supplementation could further enhance GC response in patients with asthma, at least in vitro. It is interesting to note that, in our study population, there was not a significant relationship between vitamin D levels and baseline expression of IL-10 by PBMCs, nor did DEX further enhance production of IL-10 by PBMCs, indicating that IL-10 is not involved in pathways by which vitamin D enhances of DEX-induced MKP-1 expression in PBMCs.
Also of interest is the inverse relationship between BMI and vitamin D levels observed in our study, a finding previously reported in adults without asthma (28–30). Although obesity has been reported to increase asthma risk (31), one of the most significant effects of obesity on asthma relates to phenotype, particularly its association with reduced response to GCs, both clinically (32–34) and in vitro (19). This report suggests that vitamin D, the levels of which are reduced in overweight and obese subjects with asthma, may be one pathway by which obesity and reduced steroid response are related. Finally, we did not observe a relationship between vitamin D levels and mitogen-induced cell proliferation, in contrast to previous reports in which vitamin D has been reported to have inhibitory effects on T cell proliferation (35, 36). However, these previous data were generated in healthy human subjects, and it is possible that this effect of vitamin D is attenuated in asthma.
In summary, in adults with persistent asthma, higher vitamin D levels are associated with improved lung function, reduced AHR, and improved in vitro response to GCs. These findings suggest that supplementation of vitamin D in patients with asthma, when appropriate, may result in improvements in multiple parameters of asthma severity and treatment response.
Supported by National Institutes of Health grants HL090982, HL074073 and AI070140.
Conflict of Interest Statement: E.R.S. received $1,001–$5,000 from Schering Plough (as a Data and Safety Monitoring Board member), up to $1,000 from Meda, up to $1,000 from GlaxoSmithKline (as a DSMB member), up to $1,000 from Archimedes, Inc. (as a consultant) and $1,001–$5,000 from Teva in consultancy fees, $10,001–$50,000 from Dey and $5,001–$10,000 from GlaxoSmithKline for serving on an advisory board, and $10,001–$50,000 from Novartis and $10,001–$50,000 from Boehringer Ingelheim in grants. E.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.P.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.Y.M.L. received $1,001–$5,000 from Sanofi-Aventis, $1,001–$5,000 from Schering, up to $1,000 from Merck, and up to $1,000 from Actelion in advisory board fees, and more than $100,001 from Genentech and $10,001–$50,000 from Novartis in industry-sponsored grants.
References
- 1.U.S. Department of Health and Human Services. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program; 2007. NIH Publication No. 07-4051.
- 2.Global Initiative for Asthma. Global strategy for asthma management and prevention: 2008 update. Bethesda, MD: National Heart, Lung, and Blood Institute [updated 2009; accessed November, 2009]. Available from: http://www.ginasthma.org/guidelineitem.asp??l1=2&l2=1&intId=1561.
- 3.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836–844. [DOI] [PubMed] [Google Scholar]
- 4.Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest 2008;134:394–401. [DOI] [PubMed] [Google Scholar]
- 5.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487–495. [DOI] [PubMed] [Google Scholar]
- 6.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, Craig TJ, Dolovich M, Drazen JM, Fagan JK, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002;109:410–418. [DOI] [PubMed] [Google Scholar]
- 7.Goleva E, Hauk PJ, Boguniewicz J, Martin RJ, Leung DY. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. J Allergy Clin Immunol 2007;120:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J Endocrinol 2003;178:5–12. [DOI] [PubMed] [Google Scholar]
- 9.Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J 2001;20:7108–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase–1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem 2005;280:8101–8108. [DOI] [PubMed] [Google Scholar]
- 11.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, et al. Reversing the defective induction of IL-10–secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 2006;116:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med 2000;161:309–329. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 17.Sher ER, Leung DY, Surs W, Kam JC, Zieg G, Kamada AK, Szefler SJ. Steroid-resistant asthma: cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest 1994;93:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003;171:3262–3269. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DYM. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med 2008;178:682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LB, Goleva E, Hall CF, Ou LS, Leung DY. Superantigen-induced corticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal–regulated kinase (MEK–ERK) pathway. J Allergy Clin Immunol 2004;114:1059–1069. [DOI] [PubMed] [Google Scholar]
- 21.Lamberg-Allardt CJ, Outila TA, Karkkainen MU, Rita HJ, Valsta LM. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res 2001;16:2066–2073. [DOI] [PubMed] [Google Scholar]
- 22.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317–322. [DOI] [PubMed] [Google Scholar]
- 23.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–716. [DOI] [PubMed] [Google Scholar]
- 24.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351. [DOI] [PubMed] [Google Scholar]
- 25.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor-α in refractory asthma. N Engl J Med 2006;354:697–708. [DOI] [PubMed] [Google Scholar]
- 26.Peterson CA, Heffernan ME. Serum tumor necrosis factor-α concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mora JR, Iwata M, von Andriano UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 2003;88:157–161. [DOI] [PubMed] [Google Scholar]
- 29.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D–endocrine system in obese subjects. J Clin Invest 1985;76:370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 2004;89:1196–1199. [DOI] [PubMed] [Google Scholar]
- 31.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007;175:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med 2007;101:2240–2247. [DOI] [PubMed] [Google Scholar]
- 33.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006;27:495–503. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol 2009;123:1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 Alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest 1984;74:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest 1984;74:1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]