Abstract
Nalbuphine, a mixed mu-/kappa-opioid analgesic, may have potential as a new medication for the treatment of cocaine abuse. Kappa-opioid agonists functionally antagonize some abuse-related and locomotor effects of cocaine, and both kappa-selective and mixed mu-/kappa-opioids reduce cocaine self-administration by rhesus monkeys. Because cocaine’s interactions with the hypothalamic-pituitary-adrenal and (HPA) hypothalamic-pituitary-gonadal (HPG) axes may contribute to its reinforcing properties, we examined the effects of cocaine alone and in combination with nalbuphine. Neuroendocrine effects of a single dose of cocaine alone (0.2 mg/kg, IV), with nalbuphine (5 mg/70 kg, IV) + cocaine (0.2 mg/kg, IV) in combination were compared in seven adult men (ages 18-35) who met DSM-IV criteria for current cocaine abuse. Cocaine alone, and in combination with nalbuphine was administered on separate test days under placebo-controlled, double blind conditions. Cocaine stimulated ACTH, cortisol, and LH, whereas cocaine + nalbuphine in combination produced a smaller increase in ACTH, and decreased cortisol and LH. Thus it appears that nalbuphine attenuated cocaine’s effects on ACTH, cortisol, and LH. These data are consistent with our earlier report that nalbuphine modestly attenuated cocaine’s positive subjective effects, and that the subjective and cardiovascular effects of cocaine + nalbuphine in combination were not additive.
Keywords: Cocaine, nalbuphine, ACTH, cortisol, LH
Background
Cocaine abuse continues to be a major public health problem, and the adverse medical consequences of cocaine abuse and dependence on cardiovascular, cerebrovascular, pulmonary, and immune function contribute to the social and economic costs (Mendelson and Mello, 2008; SAMHSA, 2006). As yet, no effective pharmacotherapies have been developed, but advances in understanding the neurobiological bases of cocaine abuse have led to identification of some novel pharmacological approaches to treatment. There is emerging evidence from preclinical studies that the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes may be important for cocaine’s abuse-related effects (Goeders, 1997, 2002a, b). The intriguing possibility that rapid changes in anterior pituitary,adrenal and gonadal hormonal levels may be related to cocaine’s reinforcing effects is suggested by clinical and preclinical studies (see (Goeders, 1997, 2002a, b; Mello and Mendelson, 2008 in press) for review). For example, the IV cocaine-induced increase in adrenocorticotropin hormone (ACTH) parallels increases in plasma cocaine levels and reports of positive subjective effects (Mendelson et al., 2002; Sholar et al., 1998).
ACTH stimulation after cocaine administration has been consistently observed in both humans and animals (see (Mello and Mendelson, 2008 in press) for review). Several lines of evidence suggest that cocaine’s effects on ACTH may reflect stimulation of endogenous hypothalamic corticotropin-releasing factor (CRF). The amplitude of ACTH pulsatile release is controlled by CRF, and the frequency of ACTH pulses appears to reflect an intrinsic secretory rhythm of the anterior pituitary corticotrophs (Carnes et al., 1990; Gambacciani et al., 1987; Mershon et al., 1992). In preclinical studies, adrenalectomized rats did not learn to self-administer cocaine, and pharmacological blockade of corticosterone synthesis by metyrapone significantly decreased cocaine self-administration (Goeders and Guerin, 1996). Moreover, corticosterone administration facilitated cocaine self-administration (Mantsch et al., 1998), and corticosterone levels after cocaine self-administration sessions were cocaine dose-dependent (Goeders and Guerin, 1996; Mantsch et al., 2000). Importantly, administration of a CRF-1 antagonist decreased IV cocaine self-administration by rats with minimal effects on food-maintained responding (Goeders and Guerin, 2000).
Similarly, cocaine consistently stimulates LH release in male and female subjects in numerous preclinical and clinical studies. For example, significant IV cocaine-induced dose-dependent increases in LH levels were observed in male rhesus monkeys (Mello et al., 2004; Mello et al., 1993b), early follicular-phase female rhesus monkeys (Mello et al., 1989; Mello et al., 1990; Mello et al., 1993b), and cocaine abusers (Mendelson et al., 2003; Mendelson et al., 2001). Transient elevations in LH levels were observed in luteal-phase rhesus monkeys and maximal effects occurred after repetitive cocaine administration (Evans and Foltin, 2006). Incremental changes in LH levels were documented under exogenous progesterone administration in ovariectomized rhesus monkeys (Mello et al., 2004). When women were studied in both follicular and luteal phases of the menstrual cycle, only a high cocaine dose significantly augmented LH levels compared to baseline (Mendelson et al., 2001).
No changes in testosterone levels have been detected in clinical studies after acute IV (Mendelson et al., 1989; Mendelson et al., 2003), intranasal (Heesch et al., 1996), or chronic cocaine administration (Mendelson et al., 1988). However, persistent cocaine use was shown to negatively affect sexual functioning in cocaine abusers and cocaine-dependent individuals (Saso, 2002). In preclinical studies, the effects of cocaine on testosterone have been inconsistent. For example, in rhesus monkeys, a 50% increase in testosterone levels from the average baseline was observed after high dose IV cocaine administration (Mello et al., 1993b). In another study, however, the same dose of IV cocaine did not produce any significant changes in testosterone (Mello et al., 2004). Similarly, no alterations in serum testosterone levels were observed after a higher dose of i.p. cocaine administered acutely to male rats (Walker et al., 2001). Acute high doses of i.p. cocaine were shown to significantly increase serum testosterone levels with subsequent significant serum testosterone decrement persisting for 2 hrs (Berul and Harclerode, 1989). However, in another study, the same dose of cocaine was shown to decrease testosterone levels in a single administration (Festa et al., 2003). “Binge-like” patterns of cocaine administration decreased testosterone levels in male rats (Festa et al., 2003). Repetitive low dose, but not high dose, cocaine injections have also been reported to elevate serum testosterone levels in male rats (Rodriguez et al., 1992). However, testosterone levels declined after chronic administration of high dose cocaine, and lighter weight seminal vesicles and epididymis were documented in this group (Berul and Harclerode, 1989). In another study, higher cocaine doses administered IP in a “binge-like” pattern over a period of several weeks also reduced plasma testosterone levels in rats (Sarnyai et al., 1998). Significant damage to male gonads, including marked reduction in testicular weight and seminiferous tubule size/diameter, were observed beginning after two weeks of daily high dose IP cocaine administration (Barroso-Moguel et al., 1994; Yang et al., 2006). In cocaine-dependent population, a “ greater number of abnormal sperm, lower sperm counts and instances of lower motility” were reported (George et al., 1995; Bracken et al., 1990).
As discussed above, cocaine-induced hypogonadism in cocaine abusers and dependent individuals may produce a significant impairment in their daily lives. Therefore, restoring normal gonadal functioning should be considered as one of the critical therapeutic objectives while treating cocaine abuse/dependence. It is especially important since gonadal steroids are known not only to regulate reproductive function but also to exert a crucial influence on plasticity and overall activity of CNS (Quinones-Jehab et al., 2006). The existing evidence in the literature suggests that cocaine interacts with sex hormones on three major levels: via affecting steroids’ secretion, metabolism and clearance rates (Quinones-Jehab et al., 2006), via alteration of “rapid non-genomic mechanisms”, including “modulation of extracellular monoamines and opioids” (Quinones-Jehab et al., 2006; Chen et al., 2003; Becker et al., 1999) and via slower genomic mechanisms, such as synthesis of new proteins (early genes for e.g c-fos, dynorphin and/or late genes for e.g. opioid and monoamine receptors (Quinones-Jehab et al., 2006). As a result, cocaine may significantly alter “biological and long-term adaptive changes in neuronal function” and overall brain plasticity as it was demonstrated in a number of preclinical studies (Quinones-Jehab et al., 2006; Chen et al., 2003; Chin et al., 2002; Quinones-Jehab et al., 2000a; Quinones-Jehab et al., 2000b; Quinones-Jehab et al., 2000c; Becker et al., 1999).
Major neurotransmitter systems are involved in pathogenesis of cocaine abuse, such as dopaminergic, noradrenergic and serotonergic (Johanson and Fischman, 1989; Dackis and Gold, 1988; Gawin et al., 1988; Kleber and Gawin, 1984; Woolverton and Johnson,1992; Spealman et. al., 1992; Bergman et. al., 1990; Millman et al.,1988). Interestingly, HPA and HPG axes hormones are documented to share the same modulatory monoaminergic mechanisms as most stimulants, including cocaine (Weiner et. al., 1988, Barraclough et. al., 1984, Taleisnik and Sawyer,1986). These systems also play a vital role in modulating HPA and HPG axes under stressful conditions. For example, activation of the hypothalamic alpha-1 and beta- receptors was reported to be stimulatory to CRF release, while stimulation of alpha-2 receptors was shown to inhibit CRF levels (Plotsky et. al., 1989). Moreover, tyrosine-hydroxylase–positive terminals were demonstrated to innervate both GnRH perikarya and dendrites in the medial preoptic area of the hypothalamus. (Chen et. al., 1989). Additionally, stimulation or inhibition of LH release was observed within catecholamine–GnRH axis (Rivier and Rivest, 1991). Specifically, stimulation of alpha -1 receptors was reported to be responsible for a preovulatory LH surge (Condon et al., 1989), while activation of the noradrenergic system resulted in either increase or decrease of LH release depending on presence or absence of steroids, respectively (Taleisnik and Sawyer, 1986). Therefore, potentially “CRF could activate the noradrenergic system during stress”, in turn acting “directly on GnRH neurons” (Rivier, and Rivest ,1991). Specifically, the locus coeruleus was reported to be a site of direct stimulation of cathecholaminergic neurons by CRF (Rivier, and Rivest, 1991, Butler et al.,1990). Additionally, biphasic feedback of HPG axis in response to stressful stimuli was documented as being stimulatory under acute circumstances and inhibitory under chronic/sufficient magnitude stress (Grey et al., 1978, Rivier, and Rivest, 1991). Finally, evidence exists that the above-mentioned anti-reproductive effects of EOPs are mediated via monoaminergic-dependent pathways (Leadman et al., 1985, Gopalan et al., 1989, Johnson et al.,1986). For example, it was documented that systemic injections of mu-agonists such as morphine stimulated dopaminergic and serotonergic systems in hypothalamus, while inhibiting hypothalamic “norepinephrine concentrations and plasma LH levels” (Gopalan et al.,1989, Rivier and Rivest, 1991). In contrast, selective kappa-agonists such as tifluadom were shown to decrease LH levels but increase “norepinephrine and 5-HT turnover” in the hypothalamus (Gopalan et al., 1989, Rivier and Rivest ,1991). Furthermore, serotonergic influence on the hypothalamus from the dorsal raphe nucleus was documented to directly modulate 5-HT receptors on GnRH neurons, possibly exerting an inhibitory influence on GnRH secretion under acute stress (Assenmacher et al., 1987, Rivier and Rivest, 1991).
The role of the HPA and HPG axes in the effects of medications on cocaine’s reinforcing effects is poorly understood. One approach to the medication-based treatment of cocaine abuse has been to study compounds that can modulate dopaminergic activity indirectly by acting on other receptor systems, for example opioid analgesics. Kappa-opioid agonists are one novel candidate medication for cocaine abuse treatment. Opioid analgesics are classified as full agonists, partial agonists, or mixed agonist-antagonists, depending on the specific receptors to which they bind and their intrinsic activity at that receptor. Full agonists do not have a ceiling to their analgesic efficacy and will not reverse or antagonize the effects of other opioids within this class given simultaneously. Partial agonists have relatively low intrinsic efficacy at the opioid receptor in comparison to full opioid agonists and display a ceiling effect to analgesia. Mixed agonist-antagonists have an analgesic ceiling and block opioid analgesia at one type of opioid receptor or are neutral at this receptor while simultaneously activating a different opioid receptor.
Furthermore, as Archer S et al., (1996) reported, “since both mu-antagonists and kappa-agonists prevent dopamine release in nucleus accumbens, these properties should be additive if present in one compound”. For example, mixed mu-antagonist/kappa-agonists such as L- cyclorphan and cyclazocine were suggested to be useful for treatment cocaine abuse in humans (Neumeyer et al., 2000a; Neumeyer et al., 2000b, Preston et al., 2004; Archer et al., 1996).
In previous studies by our group, the mixed kappa-agonist/mu-antagonist and analgesic nalbuphine produced significant and sustained reductions in cocaine self-administration by rhesus monkeys without altering food-maintained responding (Mello et al., 1993a). Nalbuphine also produced dose-dependent downward shifts in the cocaine self-administration dose-effect curve (Negus and Mello, 2002). In clinical studies, nalbuphine attenuated the positive subjective effects of cocaine, and had no additive effects on cardiovascular measures (Mello et al., 2005). This study was designed to examine the effects of nalbuphine on the pharmacokinetic profile of cocaine and on cocaine-induced changes in ACTH, cortisol, and LH. The present study was conducted under double blind, placebo-controlled conditions in men who met DSM-IV criteria for current cocaine abuse. Each subject was studied as his own control across conditions.
Methods
Subjects
Seven male volunteers who fulfilled DSM-IV criteria for current cocaine abuse (305.6) (American Psychiatric Association, DSM-IV-TR, 2000) provided written informed consent for participation in this study. All subjects were recruited via local newspaper advertisements and were paid for their participation as described in the Informed Consent Form. This study was approved by the Institutional Review Board of McLean Hospital. Volunteers with any lifetime DSM-IV Axis I disorder other than cocaine abuse and nicotine dependence (305.1) were excluded (American Psychiatric Association, DSM-IV-TR, 2000). These subjects had no history of any mental illness, including Axis II personality disorders and were not functionally impaired in society. Selected subjects had normal physical and laboratory screening profiles, and were in good health. Subject confidentiality was protected by a Confidentiality Certificate from the National Institute on Drug Abuse (NIDA), and by compliance with Health Insurance Portability and Accountability Act (HIPAA) health privacy regulations. Particpants did not differ significantly with respect to age (26.14 +/-1.58 years), body mass index (25.07 +/- 1.06), education (13 +/- 0.69 years), and reported history of cocaine abuse (6 +/- 0.9 years).
Subjects received 0.2 mg/kg of IV cocaine alone and in combination with 5 mg/70 kg IV nalbuphine. Subjects received 0.2 mg/kg IV cocaine alone and the same dose of cocaine in combination with 5 mg/70 kg IV nalbuphine.
Rationale for Drug Dose Selection
The analgesic potency of nalbuphine is essentially equivalent to that of morphine and the usual recommended adult analgesic dose is 5 mg/70 kg or 10 mg/70 kg (PDR, 2005). In normal healthy volunteers, these doses of nalbuphine and morphine have been shown to produce similar subjective and physiological effects (Zacny et al., 1997). However, 10 mg/70 kg nalbuphine produced emesis and sedation as previously reported by our group (Goletiani et al., 2007), therefore, a lower dose of 5 mg/70 kg was used for all 7 subjects. Nalbuphine doses of 5 or 6 mg/70 kg have proven to be safe and to induce changes in positive subjective effects (e.g., High, Like Drug Effects, Feel Drug Effect) (Preston and Bigelow, 2000; Zacny et al., 1997). The cocaine dose of 0.2 mg/kg was determined to be safe and tolerable. In previous studies, this dose induced changes in positive subjective effects (e.g., High, Like Drug Effects, Feel Drug Effect) on a Visual Analog Scale (VAS) as previously reported by our group (Mendelson et al., 1998).
Nalbuphine Dose Preparation
The nalbuphine dose was prepared by diluting Nubain® (20 mg/ml, Endo Pharmaceuticals Inc., Chadds Ford, PA) with 0.9% sterile saline. The dose was based on body weight and is expressed as milligrams of the salt weight per 70-kg body weight.
Cocaine Hydrochloride Dose Preparation
Cocaine hydrochloride was acquired from the National Institute on Drug Abuse in powder form and was dissolved in sterile water for the intravenous injection by the McLean Hospital Pharmacy. Sterility was ensured by passing the solution through a 0.22 micron Millipore filter and subjecting it to a Limulus Amebocyte Lysate (LAL) test for detection of gram negative bacterial endotoxins. The test kit is manufactured by Whittaker Bioproducts, Walkersville, MD. The cocaine dose was prepared immediately prior to the study and hand-delivered to the Clinical Unit shortly thereafter.
Drug Abstinence Requirements
Subjects were tested for recent drug use before nalbuphine and cocaine administration. On the morning of each study, subjects provided a urine sample for analysis with the Triage® Drugs of Abuse (DOA) Panel (Biosite Diagnostics, San Diego, CA). The Triage® DOA Panel qualitatively detects the presence of the following drugs of abuse (or their metabolites) in urine at the designated cut-off concentrations recommended by the Substance Abuse and Mental Health Services Administration: phencyclidine (25 ng/mL), benzodiazepines (300 ng/mL), benzylecgonine (a metabolite of cocaine) (300 ng/mL), amphetamines (1000 ng/mL), tetrahydrocannabinol (50 ng/mL), opiates (300 ng/mL), and barbiturates (300 ng/mL). No subject tested positive for any of these drugs or metabolites. Subjects were also given a breath alcohol test (Alco-Sensor IV, Intoximeters, Inc., St. Louis, MO) to ensure that they had not been drinking alcoholic beverages recently. Participants were asked to abstain from smoking and caffeinated beverages after midnight prior to the study, however caffeine abstinence was not independently confirmed. Subjects were also asked not to eat food or drink any non-clear liquids for 4 h, and not to drink clear liquids for 2 h before the study session. Carbon monoxide (CO) levels were measured with a Vitalograph Breath CO Monitor from Vitalograph, Inc. (Lenexa, KS) to assess compliance with the smoking abstinence requirement. Subjects with a CO level above 10 ppm were excluded.
Experimental Conditions
All studies were carried out on a clinical research ward used exclusively for investigation of substance abuse. Only one subject was studied on each experimental day, and studies were conducted at approximately the same time on each morning. Subjects sat in a comfortable chair in front of a computer that was used to collect subjective responses during the test session. Subjective responses (Mello et al., 2005) and physiological data were collected before and after nalbuphine and cocaine administration. Each test session lasted for 150 min.
Fifteen minutes after completion of the baseline subjective-effects questionnaire, nalbuphine (or placebo) was administered intravenously over a 15-s interval into the subject’s antecubital vein. Fifteen minutes later, cocaine was administered intravenously as a bolus over a 60-s interval into the subject’s antecubital vein in the arm opposite that used for the collection of samples for hormone analysis. All men were studied in a semi-supine position, and heart rate, blood pressure, and electrocardiograms (ECG) were continuously monitored for 30 min prior to intravenous cocaine administration, as well as throughout the study session, with a noninvasive patient monitor model (Scholar II/507E, Criticare Systems, Waukesha, WI). Systolic and diastolic blood pressures were measured periodically at baseline, as well as at 10, 20, 25, 30, 35, 40, 45, 60, 75, 105 and 135 min after nalbuphine injection. Vital signs were monitored for at least 4 h after completion of the study. A physician certified in cardiopulmonary resuscitation was present during each study, and a cardiac defibrillator and appropriate emergency treatment medications were located in the study room.
Sample Collection Procedures
All samples for nalbuphine, cocaine, and hormone analysis were collected from an intravenous catheter placed in the antecubital vein of the arm opposite the arm used for nalbuphine and cocaine injection. Baseline blood samples for analysis of serum drug levels were collected 15 min before nalbuphine injection and 5 min before cocaine administration. Samples for serum/plasma analysis of nalbuphine, cocaine, ACTH, cortisol, and LH were obtained 15 min prior to nalbuphine administration, 10 min after nalbuphine administration, 5 min before cocaine administration and at 17, 19, 23, 27, 31, 35, 40, 45, 60, 75, 105, and 135 min after nalbuphine injection. Blood samples for hormone analysis were collected in heparinized tubes, tubes containing K3 EDTA (for ACTH analysis), and SST gel and clot activator (for analysis of LH and cortisol). Blood samples for plasma cocaine analysis were collected and transferred to heparinized Vacutainer tubes containing sodium fluoride and ascetic acid (to prevent the cocaine hydrolysis), which were immediately iced. After centrifuge, the plasma was removed and frozen at -70° C for cocaine and endocrine analysis.
Assay procedures
Adrenocorticotropin Hormone Assay
Plasma ACTH was determined in duplicate by IRMA method using Alegro kits (Cat. #: 40-2195) purchased from Nichols Institute Diagnostics (San Juan Capistrano, CA). The assay sensitivity was 1.7 pg/ml and the intra- and interassay C.V.’s were 3.9% and 5.6%, respectively.
Cortisol Assay
Serum cortisol was determined in duplicate by the GammaCoat RIA method, using kits (Cat. #: CA-1529) purchased from DiaSorin Corporation (Stillwater, MN). The assay sensitivity was 0.2 pg/dl and the intra- and interassay C. V.’s were 5.9% and 9.6%, respectively.
Luteinizing Hormone Assay
Serum LH was determined in a duplicate by a direct, double antibody RIA method, using kits purchased from Incstar Corporation (Stillwater, MN). The assay sensitivity was 9.2 ng/ml, and the intra- and interassay coefficients of variation were 2.9 and 5.3%, respectively.
Testosterone Assay
Serum total testosterone was determined n duplicate by Coat-A-Count RIA method, using kits purchased from Diagnostic Products Corporation. The e assay sensitivity was 2.3 ng/ml, and the intra- and interassay coefficients of variation were 3.0 and 5.9%, respectively
Serum Nalbuphine Analysis
Serum concentrations of nalbuphine were measured in duplicate using a solid phase ELISA purchased from Neogen Corporation (Lexington, KY), with nalbuphine hydrochloride dehydrate standard from Sigma-Aldrich (St. Louis, MO). The assay sensitivity was 0.06 ng/ml, and the intra- and interassay C.V.s were 5.4% and 6.5%, respectively.
Plasma Cocaine GC/MS analysis
Plasma cocaine levels were measured in duplicate using a solid phase extraction method, described by SPEC Instruction Manual by Ansys. Levels were determined by utilizing a Hewlett-Packard Model 5890 Series II gas chromatograph equipped with a capillary column, and a Hewlett-Packard 5971 Series Mass Selective detector (Abusada et al., 1993). The assay sensitivity was 10 ng/ml, and intra- and interassay C.V.’ s of variations were 1.6% and 4.6%, respectively.
Data Analysis
Data were analyzed by using a two-factor repeated measure ANOVA. If significant main effects were detected, one-way ANOVAs were performed to identify the time points that differed significantly from baseline within each group. Comparisons between the effects of 0.2 mg/kg cocaine alone and in combination with 5 mg nalbuphine/70 kg were also analyzed with ANOVA for repeated measures.
Results
Cocaine Plasma Levels After Cocaine Alone and Nalbuphine
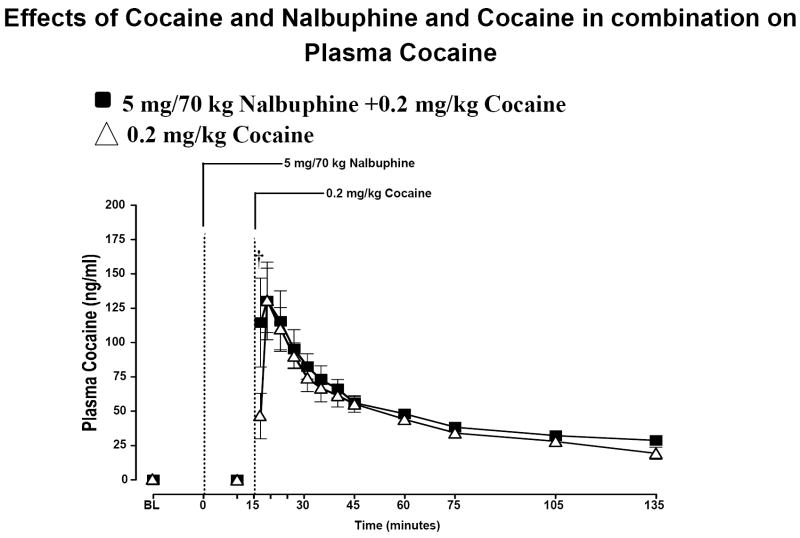
When cocaine alone was administered, peak cocaine plasma levels of 131 ± 29.46 ng/ml were measured at 4 min (Figure 1). When cocaine was administered 15 min after pretreatment with nalbuphine, plasma cocaine levels were maximal within 4 min after cocaine injection and averaged 130 ± 28 ng/ml. However, at 2 min after cocaine administration, plasma cocaine levels were significantly higher after nalbuphine administration than after cocaine alone (P=0.005). Plasma cocaine levels gradually decreased over the remainder of the sampling period and averaged 19 ± 4.4 ng/ml at 135 min. The calculated half-life (t1/2) of cocaine in plasma was 49.4 ± 7.7 min after nalbuphine administration. Importantly, pretreatment with nalbuphine did not affect cocaine pharmacokinetics. Plasma cocaine levels gradually decreased over the sampling period and averaged 29.0 ± 1.7 ng/ml at 120 min after cocaine administration. The calculated half-life (t1/2) of cocaine in plasma was 51.5 ± 6.1 min. No cocaine was detected in plasma after placebo-cocaine injection.
Fig. 1.
Plasma cocaine levels after i.v. administration of 5 mg /70 kg and 0.2 mg/kg cocaine. Significant changes from baseline are indicated by daggers (†, P=0.005).
Effect of Cocaine and Nalbuphine + Cocaine Combination On ACTH Levels
As shown in Figure 2, cocaine alone (0.2 mg/kg IV) increased ACTH significantly from baseline within 8 min. ACTH remained significantly above baseline until 30 min after cocaine injection (P=0.001-0.02). However, cocaine + nalbuphine in combination resulted in a lower increase in ACTH, although the overall time course was similar (Figure 2).
Fig. 2.
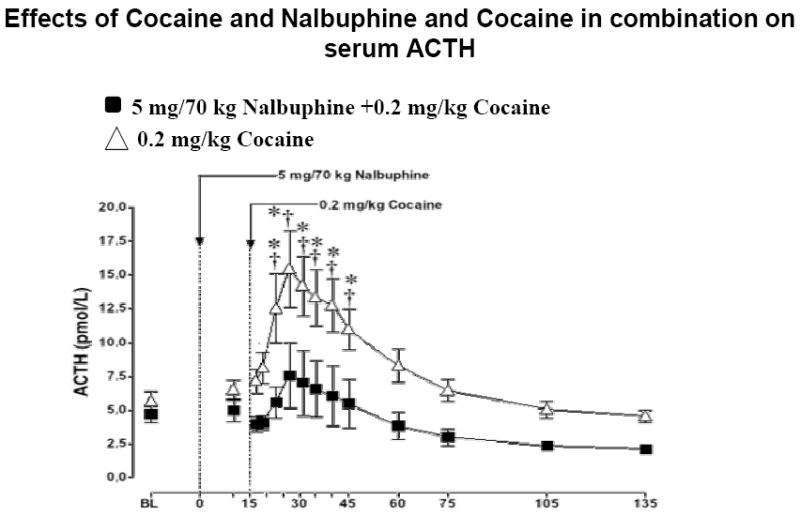
Plasma ACTH levels after i.v. administration of 5 mg/70kg nalbuphine and of 0.2 mg/kg cocaine. Significant changes from the baseline are indicated by daggers (†, P=0.001-0.02). Asterisks (*, P<0.05) indicate points at which ACTH levels differed between subjects who received 0.2 mg/kg cocaine or a combination of 5 mg/70 kg nalbuphine and 0.2 mg/kg cocaine.
Effect of Cocaine and Nalbuphine + Cocaine Combination On Cortisol Levels
Cortisol increased significantly from baseline at 12 min after 0.2 mg/kg cocaine injection, and remained significantly above baseline until 45 min after cocaine injection (P=0.001-0.02). After administration of cocaine + nalbuphine in combination, cortisol decreased significantly from baseline at 75,105 and 135 min post-nalbuphine IV administration (P=0.007-0.03) (Figure 3).
Fig. 3.
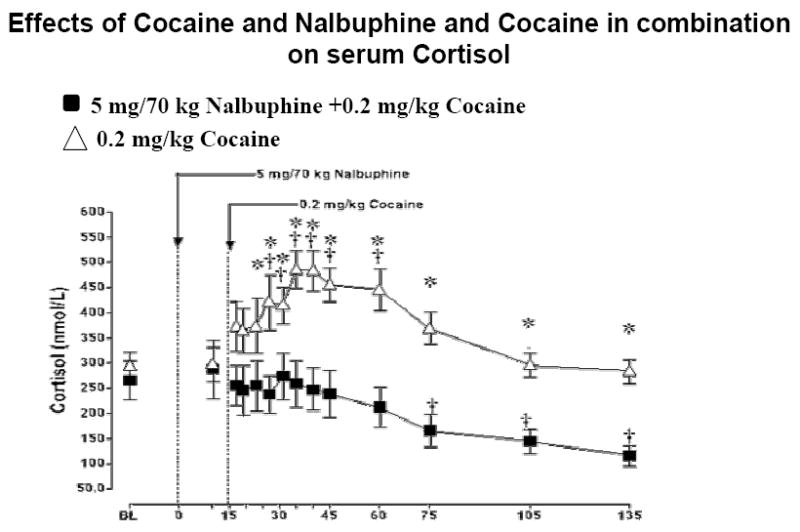
Plasma Cortisol levels after i.v. administration of 5 mg/70kg nalbuphine and of 0.2 mg/kg cocaine. Significant changes from the baseline are indicated by daggers (†, P P=0.001-0.02). Asterisks (*, P<0.05) indicate points at which cortisol levels differed between subjects who received 0.2 mg/kg cocaine or a combination of 5 mg/70 kg nalbuphine and 0.2 mg/kg cocaine.
Effect of Cocaine and Nalbuphine + Cocaine Combination On LH Levels
LH increased significantly from baseline at 2 min after 0.2 mg/kg cocaine injection to peak levels of 6.3 ng/ml. LH remained significantly above baseline until 45 min post-cocaine injection (P=0.16-0.049). In contrast, following administration of cocaine + nalbuphine, there was a small increase in LH, followed by a significant decrease at 75, 105, and 135 min after nalbuphine injection (P=0.02-0.04) (Figure 4).
Fig. 4.
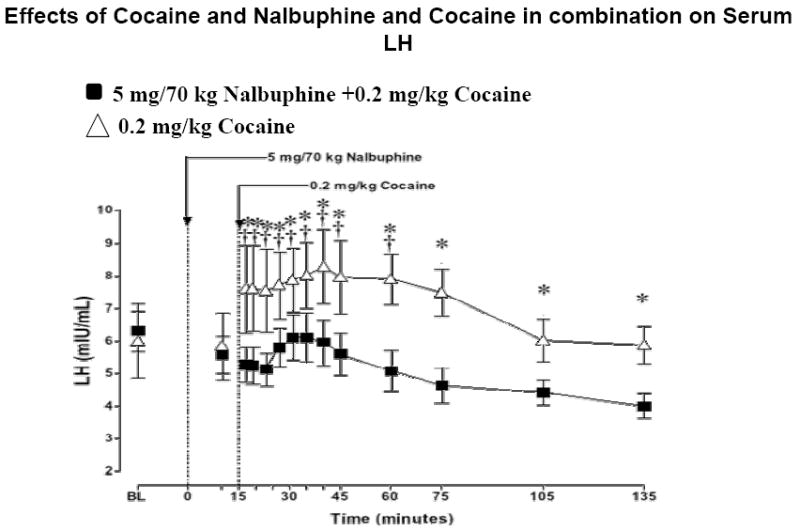
Serum LH levels after i.v. administration of 5mg /70 kg nalbuphine and 0.2 mg/kg cocaine. Significant changes from the baseline are indicated by daggers (†, P<0.05). Asterisks (*, P<0.05) indicate points at which LH levels differed between subjects who received 0.2 mg/kg cocaine or a combination of 5 mg/70 kg nalbuphine and 0.2 mg/kg cocaine.
Effect of Cocaine and Nalbuphine + Cocaine Combination On Serum Testosterone Levels
Testosterone levels showed a slight, non-significant increase from baseline levels of 570 nmol/L up to 135 min after 0.2 mg/kg cocaine injection. There was a small decrease in testosterone at 135 min following administration of cocaine + nalbuphine, although this difference was also not significant. Overall, no significant change was observed in testosterone levels after cocaine + nalbuphine in combination, or after cocaine alone (Data not shown).
Discussion
This is the first clinical study to examine the interactions between nalbuphine, a mixed mu-antagonist/kappa-agonist, and cocaine on the HPA and HPG axes in male cocaine abusers. One major finding was that the endocrine effects of cocaine alone were attenuated by administration of nalbuphine + cocaine in combination. However, cocaine’s pharmacokinetic profile did not change after nalbuphine administration. Cocaine stimulated release of ACTH, cortisol and LH, whereas cocaine + nalbuphine in combination produced a minimal increase in ACTH, and decreased cortisol and LH. The overall time course for ACTH changes was similar after administration of nalbuphine + cocaine in combination, and cocaine alone. In our previous studies, using similar methodology, IV administration of the vehicle alone did not result in a significant change in ACTH, cortisol, or LH levels compared to baseline (Mendelson et al., 2002). However, one limitation of the current study was that there was no vehicle control condition. The endocrine effects of cocaine alone were also consistent with our previous reports (Mendelson et al., 2002).
The observed diminution of cocaine-related stimulation of the HPA axis by nalbuphine is consistent with the hypothesis that stimulation of ACTH may be important for cocaine’s reinforcing effects (see (Goeders, 1997, 2002a, b; Mello and Mendelson, 2008 in press) for review). These data are also consistent with our previous clinical report that nalbuphine attenuated cocaine’s positive subjective effects in cocaine abusers (Mello et al., 2005). Nalbuphine also selectively reduced cocaine self-administration by nonhuman primates (Mello et al., 1993a; Negus and Mello, 2002). Mixed mu/kappa agonists appear to be more effective in selectively reducing cocaine self-administration by rhesus monkeys than kappa agonists alone (Bowen et al., 2003; Mello and Negus, 2000).
The regulation of the HPA and the HPG axis is very complex, and there is no simple explanation for the differences in the effects of cocaine and cocaine + nalbuphine on ACTH, cortisol and LH. There are many inconsistencies in the literature that further complicate interpretation of these data. A summary of selected clinical and preclinical studies follows.
Opioids and CRF/ACTH/Cortisol
Several systems are involved in regulation of ACTH and cortisol release. Adrenergic, serotonergic and muscarinic systems exert predominantly stimulatory effects, whereas GABAergic system exerts inhibitory effects in both humans and rodents (Al-Damluji et al., 1987; Coiro et al., 1990; Fuller, 1992; Giordano et al., 2006; Rubin et al., 2006). Hypothalamic control of ACTH release is mediated by the secretion of corticotropin-releasing factor (CRH). CRH release is in turn influenced by several systems/peptides, such as NPY, angiotensin II, and opioids (Aguilera et al., 1995; Dimitrov et al., 2007; Koenig, 1989; Suda et al., 1993). Endogenous opioid peptides (EOPs) released as a stress defense (Amir et al., 1980) inhibit CRH release into the hypothalamo-hypophyseal portal system, resulting in downstream decrease in ACTH/cortisol release (Koenig, 1989; Kuhn and Saltiel, 1986; Morley, 1983). This effect varies between species.
Some controversy exists with respect to neuroendocrine response/receptor specificity in preclinical studies. For example, one study reported a tonic inhibition of CRF-secretion by beta-endorphin (beta-E) and dynorphin (DYN), acting via mu- and kappa-opioid receptors, respectively (Plotsky, 1986). In a later study, the mu–agonist morphine and kappa-agonist MR-2034 significantly stimulated ACTH release in rats (Pfeiffer et al., 1985). Dose-dependent ACTH/corticosterone stimulation by morphine and kappa-agonists MR-2034, MRZ 2549, and MR-2266BS was also reported when these opioids were infused centrally and peripherally to rats (Pfeiffer et al., 1985; Pfeiffer et al., 1987; Xu et al., 1989). A robust increase in ACTH secretion was found after a single injection of morphine to a healthy rat (Buckingham and Cooper, 1984).
While preclinical studies have reported inconsistent results of opioid agonist action on CRF, ACTH, and cortisol, opioid suppression of secretory activity of the HPA axis has been uniformly observed in human studies with both normal controls and former heroin dependent subjects (Adrian et al., 1988; Allolio et al., 1987; Degli Uberti et al., 1985; Delitala et al., 1983; Delitala et al., 1991; Pfeiffer et al., 1986; Pfeiffer et al., 1987).
For example, in one study, a group of 25 otherwise healthy patients with orthopedic injuries had attenuated levels of ACTH and cortisol after intrathecal administration of low doses of morphine (Adrian et al., 1988). Moreover, a slow-release preparation of oral morphine inhibited ACTH and cortisol production in normal human subjects (Allolio et al., 1987). IV administration of a more potent mu-receptor agonist, dermorphine, was shown to diminish plasma cortisol levels and produce a decrease in ACTH levels in a naloxone-reversible manner. In another study, five different opioid drugs with mu-, kappa- and delta-receptor affinity [morphine, methadone, pentazocine, nalorphine and DAMME (met-enkephalin analogue)] inhibited cortisol secretion in six normal male subjects (Delitala et al., 1983). The kappa-opioid agonist MR 2033 also attenuated ACTH and cortisol in clinical trials (Pfeiffer et al., 1986).
ACTH and cortisol secretion was diminished in 20 buprenorphine-maintained heroin addicts after metyrapone challenge (Kakko et al., 2008) Metyrapone is used in diagnostic testing of the HPA axis (Yen, 2004). ACTH secretion was significantly attenuated in heroin-addicted individuals in comparison to healthy controls after a metyrapone challenge (Vescovi et al., 1990). Adrenergic system involvement was postulated when plasma cortisol levels declined throughout the session after IV clonidine administration in eight heroin abusers (Folli et al., 1992). Abstinent heroin-dependent subjects had higher basal levels of ACTH/cortisol than healthy controls (Gerra et al., 2003).
Opioid antagonists, such as naloxone, naltrexone and nalmefene, modulate tonic inhibitory control of the HPA axis via the EOP system (Schluger et al., 1998). Nalmefene is more potent than naloxone at the mu-receptors, and has higher affinity at kappa- and delta-receptors. Nalmefene injection produced a greater increase in ACTH and cortisol than naloxone, possibly indicating kappa-opioid receptor involvement in the regulation of the HPA axis (Schluger et al., 1998). A dose-dependent relationship and diurnal variation of ACTH and cortisol release involving an alpha-2 adrenergic mechanism (Geer et al., 2005) were also observed after naloxone administration (al-Damluji et al., 1990; Coiro et al., 1985; Delitala et al., 1994; Ehrenreich et al., 1987; Hernandez-Avila et al., 2003; Martin del Campo et al., 1994), but ACTH levels were not significantly elevated after naloxone infusion in 7 male high-dose methadone-maintained addicts. However, there was a significant and robust post-naloxone increase in ACTH levels in healthy controls (Gold et al., 1981). Taken together, these findings suggest that there may be a chronic impairment of the EOP system with subsequent maladaption of the HPA axis in opioid-addicted individuals.
Nalbuphine is classified as a kappa-agonist/mu-antagonist analgesic (PDR, 2005). Its mixed actions may account for the attenuation of cocaine’s effects on ACTH and cortisol observed in the present study. As previously reported, cocaine induces a rapid increase in corticotropin secretion in humans (Mendelson et al., 1992) and rhesus monkeys (Sarnyai et al., 1996) and its effects have been antagonized by administration of antiserum to corticotropin-releasing hormone (CRH) in rodents (Rivier and Vale, 1987). This “increased secretion of corticotropin maybe associated in humans with the reinforcing properties of cocaine and its rapid enhancement of perceived pleasure and diminution of concern about environmental stressors” (Mendelson J and Mello K, 1996; Mendelson et al., 1992). Therefore, based on nalbuphine-induced neuroendocrine effects as demonstrated in our study, it is feasible to conclude that nalbuphine may attenuate cocaine-induced reinforcement.
Opioids and LH
LH regulation is influenced by several factors, including hypothalamic (GnRH), intrapituitary (peptides, activin and follistatin), and gonadal feedback (both steroidal and peptide) (Hall, 2004). The intricate interplay between LH and various neurotransmitter/neuromodulator systems can be stimulatory, inhibitory, or both, depending on physiological state. It is well accepted that EOP, which are widely distributed throughout the CNS, play an inhibitory role in LH release. In most species, LH levels are decreased by opioid agonists and stimulated by antagonists (Grossman, 1983; Pfeiffer and Herz, 1984). The secretion of more bioactive LH was documented in eugonadal men when endogenous GnRH release was enhanced by the opioid-receptor antagonist naltrexone (Veldhuis et al., 1983). LH pulse was also shown to correlate positively with mu-receptor binding in the amygdala of 10 heathy women (Smith et al., 1998) in early follicular phase. A temporary decline in opioidergic activity was hypothesized to contribute to the ovulatory LH surge in 12 normally-cycling women (Rossmanith et al., 1988). Mu-receptors were demonstrated to directly control the excitability of hypothalamic neurons via activation of potassium channels and the inhibition of calcium channels (Zheng et al., 2005). Furthermore, opioidergic inhibition was suggested to account for diurnal fluctuation of LH pulse and frequency after naloxone administration in early follicular phase women (Rossmanith and Wirth, 1993; Rossmanith and Yen, 1987).
Administration of mu- and kappa-opioid agonist/antagonists modulate LH release in several species. For example, the mu-opioid agonists morphine and levorphanol, and kappa-agonists such as cyclazosine, ketocyclazosine, and tifluadom inhibit LH, while naloxone stimulates LH release in intact rats (Gopalan et al., 1989). Similarly, diamorphine and kappa-agonist U-50488H injected IP into rats suppressed LH secretion via modulation of hypothalamic NA concentrations (Yilmaz and Gilmore, 1999b). In the same study, naloxone and the kappa-antagonist MR2266 administered IP successfully abolished opioid-induced suppression of LH release. Another study showed a similar dose-dependent effect of LH suppression after acute s.c. administration of morphine and kappa-agonist bremazocine to female rats. This inhibitory action of morphine, but not bremazocine, was reversed by naloxone. Moreover, the kappa-agonist U-50488H administered intraventricularly also blocked the LH surge and ovulation, while norbinaltorphimine, a specific kappa-receptor antagonist, reversed this effect (Zhang and Gallo, 2002).
Analogous results have also been reported in human studies. Acute morphine administration was shown to suppress LH secretion via tonic inhibition of GnRH (Genazzani et al., 1993). Mu- and kappa-opioid receptor agonists also reduced LH when low doses of IV morphine, methadone, pentazocine, nalorphine, met-enkephalin analogue DAMME, and buprenorphine (Delitala et al., 1983; Pende et al., 1986) were administered to both men and luteal phase women (Petraglia et al., 1986; Spremovic-Radjenovic et al., 1997). A GABA-ergic mechanism was proposed to play an important role in LH regulation and the GABA-agonist valproate produced a robust reduction in LH levels in the luteal phase females (Spremovic-Radjenovic et al., 1997).
Most studies report that mu opioid antagonists induce an increase in LH levels, but involvement of different mechanisms have been postulated. Serotonergic system invfluence in LH secretion was postulated after naloxone administration (Foresta et al., 1986; Foresta et al., 1985). Naltrexone, another opioid antagonist, also markedly increased the mean serum concentration of LH levels and the absolute amplitude of LH peaks in 7 adult males (Ellingboe et al., 1982). Dose-dependent, naloxone-induced LH stimulation with an increase in rate and amplitude was also documented in normal young male (Veldhuis et al., 1981) and female volunteers in luteal phase (Coiro et al., 1994; Petraglia et al., 1986; Ropert et al., 1981; Snowden et al., 1984), late follicular phase, and midluteal phases (Blankstein et al., 1981; Rossmanith et al., 1989) compared to placebo (Delitala et al., 1981; Delitala et al., 1980; Esposti et al., 1988; Grossman et al., 1986; Grossman et al., 1981; Limone et al., 1997; Petraglia et al., 1986). Similar effects were observed after naloxone infusion to 6 normal male subjects before and after pretreatment with valproic acid, suggesting that opioidergic mechanisms can override the GABA influence on LH regulation (Elias et al., 1986). In another study, naloxone-induced LH stimulation in 5 normal males was further amplified by clomiphene, suggesting an attenuation of inhibitory opioid tone in estrogen-receptor blocked environment (Foresta et al., 1983). A naloxone-induced increase in LH levels was inhibited by progesterone in 16 normal women in mid-follicular phase, suggesting that progesterone has a positive modulatory effect on inhibitory opioidergic tone (Steele and Judd, 1986).
In earlier studies by our group, incremental doses of buprenorphine, a mixed opioid agonist-antagonist, were found to suppress plasma LH levels in male heroin addicts after 12 days of consecutive s.c. administration, reflecting buprenorphine’s agonist-like effects (Mendelson et al., 1982). However, administration of naltrexone resulted in a marked increase in LH levels involved 20 buprenorphine-maintained heroin addicts (Kakko et al., 2008).
As described earlier, a significant decrease in LH levels from baseline was observed following the administration of cocaine + nalbuphine in combination, while administration of cocaine alone produced an elevation in LH levels. The mixed action of nalbuphine at mu- and kappa-receptors may account for this. Cocaine is known to stimulate “the secretion of LH in rhesus monkeys (Mello et al.,1993; Mello et al.,1990) and humans (Mendelson et al.,1992). This effect may be related to the perception of enhanced sexual interest and responsivity after the drug is used” (Mendelson et al.,1992) and therefore contribute to rewarding properties of cocaine.
Nalbuphine is generally regarded to be a mu-receptor antagonist, and mu opioid antagonists stimulate LH release under many conditions (Mendelson and Mello, 2008). However, recent in vitro studies of the activation of the cloned mu-receptor suggest that nalbuphine is a potent agonist at mu-receptors, with an efficacy similar to that of morphine (Gharagozlou et al., 2003). As discussed above, there is also considerable evidence that both mu- and kappa-agonists can inhibit the LH surge (Gopalan et al., 1989; Leadem and Kalra, 1985; Leadem and Yagenova, 1987; Marko and Romer, 1983; Pfeiffer et al., 1987; Siegel and Revesz, 1989; Yilmaz and Gilmore, 1999a). Therefore, the above-discussed effects of nalbuphine may diminish cocaine’s rewarding and reinforcing properties as documented in our study and previously by our group (Mello et al., 2005).
In contrast, mu- and kappa-antagonists increased LH levels in rats (Siegel and Revesz, 1989) and humans (Mendelson, 1991; Mendelson et al., 1986; Mendelson et al., 1979; Mendelson et al., 1980; Mendelson and Mello, 2008; Mendelson et al., 1987; Mendelson et al., 2001; Mendelson et al., 1991; Teoh et al., 1988).
Opioids and Testosterone
Despite reported alterations in LH levels, no significant changes have been observed in testosterone (T) levels after cocaine + nalbuphine administration. Reports of studies examining cocaine’s effect on testosterone have been inconsistent.
In preclinical studies, acute s.c. administration of mu-receptor agonists, such as morphine and fentanyl, dose-dependently suppressed testosterone levels in the brain and plasma of rats (Adams et al., 1993; Ceccarelli et al., 2006). There was a significant decline in serum and spinal cord testosterone levels after acute s.c. injection of a low dose of morphine (Amini and Ahmadiani, 2005). Plasma testosterone levels also declined significantly after methadone administration, with subsequent reversal of this effect by L-dopa suggesting DA input in GnRH inhibition (Singh et al., 1982). Acute opioid receptor antagonists such as naloxone, naltrexone, and nalmefene significantly elevated testosterone levels in rats and rhesus monkeys (Adams et al., 1997; Anand and Vijayan, 1998; Emanuele et al., 1998; Kostic et al., 1997; Leposavic et al., 1991; Mello et al., 1988; Mello et al., 2000).
In chronic pain patients and opioid-dependent subjects, consistent but temporary suppression of serum free (FT) and total testosterone (TT) levels were observed (Abs et al., 2000; Mendelson et al., 1980; Rajagopal et al., 2003; Roberts et al., 2002). In addition, methadone dose-dependently reduced testosterone levels in methadone-maintained heroin addicts (Bliesener et al., 2005; Hallinan et al., 2008; Mendelson et al., 1976) and in chronic heroin users (Celani et al., 1984; Mendelson et al., 1980; Mendelson and Mello, 1982, 1975; Mendelson et al., 1975a; Mendelson et al., 1975b; Mirin et al., 1980; Sikharulidze et al., 2006). Acute administration of opioid antagonists such as naltrexone did not significantly alter plasma testosterone levels (Mirin et al., 1976).
In addition, buprenorphine, a partial agonist at mu- and antagonist at kappa-receptors, dose-dependently produced a decline or no change in testosterone levels in men treated with buprenorphine, but to a lesser extent than methadone (Bliesener et al., 2005), in methadone-and buprenorphine-maintained patients (Hallinan et al., 2008), in heroin and buprenorphine addicts (Sikharulidze et al., 2006). Since kappa-receptor stimulation appears to inhibit the activity of the HPA/HPG axes (Bliesener et al., 2005; Cicero et al., 1988), kappa-antagonism of buprenorphine may overcome its mu-suppressive influence on the HPA/HPG axes. Similarly, in the present study, the mu-antagonist properties of nalbuphine stimulation of the HPA/HPG axes may counteract the inhibitory influence of its kappa-agonism on the HPA/HPG axes. This may explain why no appreciable change in testosterone levels was observed despite a robust elevation in LH levels.
Finally, some studies documented “no effect of castration on cocaine self-administration (Jackson et al., 2006; Hu et al., 2004), behavioral sensitization to cocaine (Hu and Becker, 2003) after acute or chronic cocaine administration” (van Haaren and Meyer, 1991). In contrast, other studies have observed behavioral response differences when castrated animals were compared to intact males (Chin et al., 2002). Furthermore, when testosterone was administered exogenously reduced sensitization to cocaine was reported in castrated animals (Chen et al., 2003). Another study also investigated possible rewarding effects of testosterone examining conditioned place preference (CPP) mediated by dopaminergic function (Schroeder et al., 2000). Both D1 and D2 receptors were proposed to be involved in the acquisition of a testosterone CPP (Schroeder et al., 2000). Additionally, therapeutic doses of testosterone were reported to induce euphoria and increased energy in young and elderly population (Chestnut et al., 1979; Samuels et al., 1942; Sherwin et al.,1985; Taylor et al., 1987). Therefore, based on the above-mentioned evidence and fact that there was no change observed in testosterone levels in our study after nalbuphine+cocaine administration, it is reasonable to conclude that testosterone may have some mild rewarding properties, similar to less powerful stimulants such as caffeine, nicotine or benzodiazepines (Wood et al., 2004).
Conclusions
Taken together, these data suggest that nalbuphine administration may attenuate cocaine-induced stimulation of ACTH, cortisol and LH release, not only via its kappa-agonist component, but through its mu-agonist activity as well. In conclusion, the results of this study are consistent with our earlier report that nalbuphine modestly attenuated cocaine’s positive subjective effects, and that the subjective and cardiovascular effects of cocaine + nalbuphine in combination were not additive (Mello et al., 2005).
Acknowledgments
This research was supported by grants P01-DA14528, T32-DA07252, K05-DA00064 and K05-DA00101 from the National Institute on Drug Abuse. We are grateful to Alicja Skupny for excellent technical assistance in conducting hormone and cocaine analyses, Christine Bettis and Haley Duncanson for outstanding editorial input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, Verlooy J, Van Havenbergh T, Smet M, Van Acker K. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215–2222. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- Abusada GM, Abukhalaf IK, Alford DD, Vinzon-Bautista I, Pramanik AK, Ansari NA, Manno JE, Manno BR. Solid-phase extraction and GC/MS quantitation of cocaine, ecgonine methyl ester, benzoylecgonine, and cocaethylene from meconium, whole blood, and plasma. J Anal Toxicol. 1993;17:353–358. doi: 10.1093/jat/17.6.353. [DOI] [PubMed] [Google Scholar]
- Adams ML, Meyer ER, Cicero TJ. Interactions between alcohol- and opioid-induced suppression of rat testicular steroidogenesis in vivo. Alcohol Clin Exp Res. 1997;21:684–690. [PubMed] [Google Scholar]
- Adams ML, Sewing B, Forman JB, Meyer ER, Cicero TJ. Opioid-induced suppression of rat testicular function. J Pharmacol Exp Ther. 1993;266:323–328. [PubMed] [Google Scholar]
- Adrian JR, Sanz Lipuzcoa J, Sanz Fernandez J, Olmos M, Ayesa MA, Arroyo JL. A bupivacaine-morphine combination by intrathecal route: correlation between pain relief and postoperative neuroendocrine response. Rev Med Univ Navarra. 1988;32:35–39. [PubMed] [Google Scholar]
- Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology. 1995;61:437–444. doi: 10.1159/000126866. [DOI] [PubMed] [Google Scholar]
- al-Damluji S, Bouloux P, White A, Besser M. The role of alpha-2-adrenoceptors in the control of ACTH secretion; interaction with the opioid system. Neuroendocrinology. 1990;51:76–81. doi: 10.1159/000125319. [DOI] [PubMed] [Google Scholar]
- Al-Damluji S, Perry L, Tomlin S, Bouloux P, Grossman A, Rees LH, Besser GM. Alpha-adrenergic stimulation of corticotropin secretion by a specific central mechanism in man. Neuroendocrinology. 1987;45:68–76. doi: 10.1159/000124705. [DOI] [PubMed] [Google Scholar]
- Allolio B, Schulte HM, Deuss U, Kallabis D, Hamel E, Winkelman W. Effect of oral morphine and naloxone on pituitary-adrenal response in man induced by human corticotropin-releasing hormone. Acta Endocrinol (Copenh) 1987;114:509–514. doi: 10.1530/acta.0.1140509. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Schulz R, Herz A. Paradoxical LH and prolactin responses to naloxone after chronic treatment with morphine. J Endocrinol. 1986;108:181–189. doi: 10.1677/joe.0.1080181. [DOI] [PubMed] [Google Scholar]
- Amini H, Ahmadiani A. In vivo evidence for an increase in 5alpha-reductase activity in the rat central nervous system following morphine exposure. Int J Dev Neurosci. 2005;23:621–626. doi: 10.1016/j.ijdevneu.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Amir S, Brown ZW, Amit Z. The role of endorphins in stress: evidence and speculations. Neurosci Biobehav Rev. 1980;4:77–86. doi: 10.1016/0149-7634(80)90027-5. [DOI] [PubMed] [Google Scholar]
- Anand LN, Vijayan E. Studies on the effect of intratesticular administration of opioid peptides, naloxone or N-acetyl beta-endorphin antiserum on some testicular parameters in rats. Indian J Physiol Pharmacol. 1998;42:107–112. [PubMed] [Google Scholar]
- Assenmacher I, Szafar A, Alonso G, Ixart G, Barbanel G. Phyisology of neural pathways affecting CRH secretion. Ann NY Acad Sci. 1987;512:149–161. doi: 10.1111/j.1749-6632.1987.tb24957.x. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Riepl RL, Schopohl J, Lehnert P, Muller OA, Stalla GK. In man the mu-opiate agonist loperamide specifically inhibits ACTH secretion induced by the cholecystokinin-like peptide ceruletide. Neuroendocrinology. 1994;60:16–22. doi: 10.1159/000126715. [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Wise PM, Selmanoff MK. A Role for hypothalamic catecholamines in the regulation of gonadotropin secretion. Rec Prog Horm Res. 1984;40:487–529. doi: 10.1016/b978-0-12-571140-1.50016-5. [DOI] [PubMed] [Google Scholar]
- Barroso-Moguel R, Mendez-Armenta M, Villeda-Hernandez J. Testicular lesions by chronic administration of cocaine in rats. J Appl Toxicol. 1994;1:37–41. doi: 10.1002/jat.2550140108. [DOI] [PubMed] [Google Scholar]
- Berul CI, Harclerode JE. Effects of cocaine hydrochloride on the male reproductive system. Life Sci. 1989;45:91–95. doi: 10.1016/0024-3205(89)90440-2. [DOI] [PubMed] [Google Scholar]
- Blankstein J, Reyes FI, Winter JS, Faiman C. Endorphins and the regulations of the human menstrual cycle. Clin Endocrinol (Oxf) 1981;14:287–294. doi: 10.1111/j.1365-2265.1981.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Bliesener N, Albrecht S, Schwager A, Weckbecker K, Lichtermann D, Klingmuller D. Plasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependence. J Clin Endocrinol Metab. 2005;90:203–206. doi: 10.1210/jc.2004-0929. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of mixed-action kappa-mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38:411–417. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neuroscience. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes M, Lent SJ, Goodman B, Mueller C, Saydoff J, Erisman S. Effects of immunoneutralization of corticotropin-releasing hormone on ultradian rhythms of plasma adrenocorticotropin. Endocrinology. 1990;126:1904–1913. doi: 10.1210/endo-126-4-1904. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, De Padova AM, Fiorenzani P, Massafra C, Aloisi AM. Single opioid administration modifies gonadal steroids in both the CNS and plasma of male rats. Neuroscience. 2006;140:929–937. doi: 10.1016/j.neuroscience.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Celani MF, Carani C, Montanini V, Baraghini GF, Zini D, Simoni M, Ferretti C, Marrama P. Further studies on the effects of heroin addiction on the hypothalamic-pituitary-gonadal function in man. Pharmacol Res Commun. 1984;16:1193–1203. doi: 10.1016/s0031-6989(84)80084-3. [DOI] [PubMed] [Google Scholar]
- Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neurosci Lett. 2003;51(3):161–164. doi: 10.1016/j.neulet.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, et al. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Chen W-P, Witkin JW, Silverman A-J. Gonadotropin releasing hormone (GnRH) neurons are directly innervated by catholamine terminals. Synapse. 1989;3:288–290. doi: 10.1002/syn.890030314. [DOI] [PubMed] [Google Scholar]
- Chesnut CH, Baylink DJ, Nelp WB. Stanozolol therapy in postmenopausal osteoporosis: preliminary results. Clin Res. 1979;26:363A. [Google Scholar]
- Cicero TJ, Meyer ER, Miller BT, Bell RD. Age-related differences in the sensitivity of serum luteinizing hormone to prototypic mu, kappa and delta opiate agonists and antagonists. J Pharmacol Exp Ther. 1988;246:14–20. [PubMed] [Google Scholar]
- Ciechanowska MO, Lapot M, Malewski T, Mateusiak K, Misztal T, Przekop F. The Central Effect of beta-Endorphin and Naloxone on the Expression of GnRH Gene and GnRH Receptor (GnRH-R) Gene in the Hypothalamus, and on GnRH-R Gene in the Anterior Pituitary Gland in Follicular Phase Ewes. Exp Clin Endocrinol Diabetes. 2008;116:40–46. doi: 10.1055/s-2007-990299. [DOI] [PubMed] [Google Scholar]
- Coiro V, Chiodera P, Rossi G, Volpi R, Salvi M, Camellini L, Roti E. Effect of naloxone on oxytocin-induced cortisol decrease in normal men. Acta Endocrinol (Copenh) 1985;108:261–265. doi: 10.1530/acta.0.1080261. [DOI] [PubMed] [Google Scholar]
- Coiro V, Cigarini C, Volpi R, Capretti L, Bacchi-Modena A, Chiodera P. Naloxone enhances angiotensin II-induced increase in serum luteinizing hormone concentrations in normal women. Regul Pept. 1994;51:161–167. doi: 10.1016/0167-0115(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Coiro V, Volpi R, Capretti L, Speroni G, Bianconi L, Cavazzini U, Marcato A, Buonanno G, Caiazza A, Chiodera P. 5-HT1-, but not 5-HT2-serotonergic, M1-, M2-muscarinic cholinergic or dopaminergic receptors mediate the ACTH/cortisol response to metoclopramide in man. Horm Res. 1990;33:233–238. doi: 10.1159/000181523. [DOI] [PubMed] [Google Scholar]
- Condon TP, Ronnekleiv OK, Kelly MJ. Estrogen modulation of the alpha-1-adrenergic response of hypothalamic neurons. Neuroendocrinology. 1989;50:51–58. doi: 10.1159/000125201. [DOI] [PubMed] [Google Scholar]
- Degli Uberti EC, Petraglia F, Trasforini G, Salvadori S, Margutti A, Bianconi M, Teodori V, Facchinetti F, Tomatis R, Genazzani AR, et al. Dermorphin reduces the metyrapone-evoked release of adrenocorticotropin, beta-endorphin, and beta-lipotropin in man. J Clin Endocrinol Metab. 1985;61:1018–1022. doi: 10.1210/jcem-61-6-1018. [DOI] [PubMed] [Google Scholar]
- Delitala G, Devilla L, Arata L. Opiate receptors and anterior pituitary hormone secretion in man. Effect of naloxone infusion. Acta Endocrinol (Copenh) 1981;97:150–156. doi: 10.1530/acta.0.0970150. [DOI] [PubMed] [Google Scholar]
- Delitala G, Devilla L, Di Biaso D. Dopamine inhibits the naloxone induced gonadotrophin rise in man. Clin Endocrinol (Oxf) 1980;13:515–518. doi: 10.1111/j.1365-2265.1980.tb03418.x. [DOI] [PubMed] [Google Scholar]
- Delitala G, Grossman A, Besser M. Differential effects of opiate peptides and alkaloids on anterior pituitary hormone secretion. Neuroendocrinology. 1983;37:275–279. doi: 10.1159/000123558. [DOI] [PubMed] [Google Scholar]
- Delitala G, Palermo M, Tomasi P, Besser M, Grossman A. Adrenergic stimulation of the human pituitary-adrenal axis is attenuated by an analog of met-enkephalin. Neuroendocrinology. 1991;53:41–46. doi: 10.1159/000125695. [DOI] [PubMed] [Google Scholar]
- Delitala G, Trainer PJ, Oliva O, Fanciulli G, Grossman AB. Opioid peptide and alpha-adrenoceptor pathways in the regulation of the pituitary-adrenal axis in man. J Endocrinol. 1994;141:163–168. doi: 10.1677/joe.0.1410163. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148:3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Kolmar C, Muller OA, Goebel FD. Potentiation of the hCRF-induced release of ACTH in man by an opioid antagonist. Klin Wochenschr. 1987;65:453–457. doi: 10.1007/BF01712837. [DOI] [PubMed] [Google Scholar]
- Elias AN, Kyaw T, Stone S, Weathersbee P, Iyer K, Ascher MS. Interaction between gabaergic and opioid pathways in the regulation of gonadotropin secretion in males. Horm Metab Res. 1986;18:838–841. doi: 10.1055/s-2007-1012452. [DOI] [PubMed] [Google Scholar]
- Ellingboe J, Veldhuis JD, Mendelson JH, Kuehnle JC, Mello NK. Effect of endogenous opioid blockade on the amplitude and frequency of pulsatile luteinizing hormone secretion in normal men. J Clin Endocrinol Metab. 1982;54:854–857. doi: 10.1210/jcem-54-4-854. [DOI] [PubMed] [Google Scholar]
- Emanuele MA, LaPaglia N, Steiner J, Jabamoni K, Hansen M, Kirsteins L, Emanuele NV. Reversal of ethanol-induced testosterone suppression in peripubertal male rats by opiate blockade. Alcohol Clin Exp Res. 1998;22:1199–1204. [PubMed] [Google Scholar]
- Esposti D, Lissoni P, Mauri R, Rovelli F, Orsenigo L, Pescia S, Vegetti G, Esposti G, Fraschini F. The pineal gland-opioid system relation: melatonin-naloxone interactions in regulating GH and LH releases in man. J Endocrinol Invest. 1988;11:103–106. doi: 10.1007/BF03350114. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of repeated doses of intravenous cocaine across the menstrual cycle in rhesus monkeys. Pharmacol Biochem Behav. 2006;83:56–66. doi: 10.1016/j.pbb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Grasso A, Petraglia F, Parrini D, Volpe A, Genazzani AR. Impaired circadian rhythmicity of beta-lipotrophin, beta-endorphin and ACTH in heroin addicts. Acta Endocrinol (Copenh) 1984;105:149–155. doi: 10.1530/acta.0.1050149. [DOI] [PubMed] [Google Scholar]
- Festa ED, Jenab S, Chin J, Gazi FM, Wu HB, Russo SJ, Quinones-Jenab V. Frequency of cocaine administration affects behavioral and endocrine responses in male and female Fischer rats. Cell Mol Biol (Noisy-le-grand) 2003;49:1275–1280. [PubMed] [Google Scholar]
- Folli D, Mutti A, Van der Venne MT, Berlin A, Gerra G, Cavazzini S, Maninetti L, Caccavari R, Vescovi P. Neuroendocrine response to psychological performance testing. Psychoneuroendocrinology. 1992;17:467–474. doi: 10.1016/0306-4530(92)90005-r. [DOI] [PubMed] [Google Scholar]
- Foresta C, Marra S, Scanelli G, Scandellari C. Gonadal steroids and opioid control of gonadotropin secretion in man. Fertil Steril. 1983;40:798–801. doi: 10.1016/s0015-0282(16)47482-0. [DOI] [PubMed] [Google Scholar]
- Foresta C, Mioni R, Scandellari C. Evidence for serotoninergic system involvement in opioid control of luteinizing hormone secretion in man. Clin Endocrinol (Oxf) 1986;25:573–578. doi: 10.1111/j.1365-2265.1986.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Foresta C, Scanelli G, Tramarin A, Scandellari C. Serotonin but not dopamine is involved in the naloxone-induced luteinizing hormone release in man. Fertil Steril. 1985;43:447–450. doi: 10.1016/s0015-0282(16)48447-5. [DOI] [PubMed] [Google Scholar]
- Fuller RW. The involvement of serotonin in regulation of pituitary-adrenocortical function. Front Neuroendocrinol. 1992;13:250–270. [PubMed] [Google Scholar]
- Gambacciani M, Liu JH, Swartz WH, Tueros VS, Rasmussen DD, Yen SS. Intrinsic pulsatility of ACTH release from the human pituitary in vitro. Clin Endocrinol (Oxf) 1987;26:557–563. doi: 10.1111/j.1365-2265.1987.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Geer EB, Landman RE, Wardlaw SL, Conwell IM, Freda PU. Stimulation of the hypothalamic-pituitary-adrenal axis with the opioid antagonist nalmefene. Pituitary. 2005;8:115–122. doi: 10.1007/s11102-005-5227-6. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Genazzani AD, Volpogni C, Pianazzi F, Li GA, Surico N, Petraglia F. Opioid control of gonadotrophin secretion in humans. Hum Reprod. 1993;8(Suppl 2):151–153. doi: 10.1093/humrep/8.suppl_2.151. [DOI] [PubMed] [Google Scholar]
- Gerra G, Baldaro B, Zaimovic A, Moi G, Bussandri M, Raggi MA, Brambilla F. Neuroendocrine responses to experimentally-induced emotions among abstinent opioid-dependent subjects. Drug Alcohol Depend. 2003;71:25–35. doi: 10.1016/s0376-8716(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Gharagozlou P, Demirci H, David Clark J, Lameh J. Activity of opioid ligands in cells expressing cloned mu opioid receptors. BMC Pharmacol. 2003;3:1. doi: 10.1186/1471-2210-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano R, Pellegrino M, Picu A, Bonelli L, Balbo M, Berardelli R, Lanfranco F, Ghigo E, Arvat E. Neuroregulation of the hypothalamus-pituitary-adrenal (HPA) axis in humans: effects of GABA-, mineralocorticoid-, and GH-Secretagogue-receptor modulation. ScientificWorldJournal. 2006;6:1–11. doi: 10.1100/tsw.2006.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002a;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002b;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AL, Extein I, Martin DA, Finn LB, Sweeney DR, Kleber HD. Evidence for an endorphin dysfunction in methadone addicts: lack of ACTH response to naloxone. Drug Alcohol Depend. 1981;8:257–262. doi: 10.1016/0376-8716(81)90069-7. [DOI] [PubMed] [Google Scholar]
- Goletiani NV, Mendelson JH, Sholar MB, Siegel AJ, Skupny A, Mello NK. Effects of nalbuphine on anterior pituitary and adrenal hormones and subjective responses in male cocaine abusers. Pharmacol Biochem Behav. 2007;86:667–677. doi: 10.1016/j.pbb.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan C, Gilmore DP, Brown CH. Effects of different opiates on hypothalamic monoamine turnover and on plasma LH levels in pro-oestrous rats. J Neurol Sci. 1989;94:211–219. doi: 10.1016/0022-510x(89)90231-1. [DOI] [PubMed] [Google Scholar]
- Grey GD, Smith ER, Damassa DA, Ehrenkranz JRL, Davidson JM. Neuroendocrine mechanisms mediating the suppression of circulating testosterone levels associated with chronic stress in male rats. Neuroendocrinology. 1978;25:247–256. doi: 10.1159/000122746. [DOI] [PubMed] [Google Scholar]
- Grossman A. Brain opiates and neuroendocrine function. Clin Endocrinol Metab. 1983;12:725–746. doi: 10.1016/s0300-595x(83)80062-0. [DOI] [PubMed] [Google Scholar]
- Grossman A, Moult PJ, Cunnah D, Besser M. Different opioid mechanisms are involved in the modulation of ACTH and gonadotrophin release in man. Neuroendocrinology. 1986;42:357–360. doi: 10.1159/000124463. [DOI] [PubMed] [Google Scholar]
- Grossman A, Moult PJ, Gaillard RC, Delitala G, Toff WD, Rees LH, Besser GM. The opioid control of LH and FSH release: effects of a met-enkephalin analogue and naloxone. Clin Endocrinol (Oxf) 1981;14:41–47. doi: 10.1111/j.1365-2265.1981.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Hall JE. Neuroendocrine Control of the Menstrual Cycle. In: Strauss JF 3rd, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia: Elsevier Saunders; 2004. pp. 195–211. [Google Scholar]
- Hallinan R, Byrne A, Agho K, McMahon C, Tynan P, Attia J. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684–692. doi: 10.1111/j.1743-6109.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Bost JE, Keffer JH, Snyder RW, 2nd, Eichhorn EJ. Effects of cocaine on anterior pituitary and gonadal hormones. J Pharmacol Exp Ther. 1996;278:1195–1200. [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am J Med Genet B Neuropsychiatr Genet. 2003;118:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Heybach JP, Vernikos J. Naloxone inhibits and morphine potentiates the adrenal steroidogenic response to ACTH. Eur J Pharmacol. 1981;75:1–6. doi: 10.1016/0014-2999(81)90337-x. [DOI] [PubMed] [Google Scholar]
- Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman MF. Intermittent morphine administration induces dependence and is a chronic stressor in rats. Neuropsychopharmacology. 2003;28:1960–1972. doi: 10.1038/sj.npp.1300271. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Kim HS, Wood PL. Kappa opiate agonists modulate the hypothalamic-pituitary-adrenocortical axis in the rat. J Pharmacol Exp Ther. 1986;238:429–436. [PubMed] [Google Scholar]
- Johnson MD, Crowley WR, Carroll Bl. Role of central serotonin systems in the stimulatory effects of ovarian hormones and naloxone on luteinizing hormone relase in female rats. Endocrinology. 1986;118:1180–1186. doi: 10.1210/endo-118-3-1180. [DOI] [PubMed] [Google Scholar]
- Kakko J, von Wachenfeldt J, Svanborg KD, Lidstrom J, Barr CS, Heilig M. Mood and neuroendocrine response to a chemical stressor, metyrapone, in buprenorphine-maintained heroin dependence. Biol Psychiatry. 2008;63:172–177. doi: 10.1016/j.biopsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kaminski T, Siawrys G, Bogacka I, Okrasa S, Przala J. The regulation of steroidogenesis by opioid peptides in porcine theca cells. Anim Reprod Sci. 2003;78:71–84. doi: 10.1016/s0378-4320(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Koenig JI. Pituitary gland: neuropeptides, neurotransmitters and growth factors. Toxicol Pathol. 1989;17:256–265. doi: 10.1177/019262338901700204. [DOI] [PubMed] [Google Scholar]
- Kostic T, Andric S, Kovacevic R, Maric D. The effect of opioid antagonists in local regulation of testicular response to acute stress in adult rats. Steroids. 1997;62:703–708. doi: 10.1016/s0039-128x(97)00071-8. [DOI] [PubMed] [Google Scholar]
- Kuhn JM, Saltiel H. Role of endogenous opioid peptides in the regulation of pituitary secretions. Ann Endocrinol (Paris) 1986;47:97–105. [PubMed] [Google Scholar]
- Leadem CA, Crowley WR, Simpkins JW, KAlra SP. Effects of naloxone on catecholamine and LHRN release from the perifused hypothalamus of the steroid primed rat. Neuroendocrinology. 1985;40:497–500. doi: 10.1159/000124121. [DOI] [PubMed] [Google Scholar]
- Leadem CA, Kalra SP. Effects of endogenous opioid peptides and opiates on luteinizing hormone and prolactin secretion in ovariectomized rats. Neuroendocrinology. 1985;41:342–352. doi: 10.1159/000124199. [DOI] [PubMed] [Google Scholar]
- Leadem CA, Yagenova SV. Effects of specific activation of mu-, delta- and kappa-opioid receptors on the secretion of luteinizing hormone and prolactin in the ovariectomized rat. Neuroendocrinology. 1987;45:109–117. doi: 10.1159/000124712. [DOI] [PubMed] [Google Scholar]
- Leposavic G, Cover PO, Buckingham JC. In vivo and in vitro studies on the opioidergic control of the secretion of gonadotrophin-releasing hormone and luteinizing hormone in sexually immature and adult male rats. Neuroendocrinology. 1991;53:579–588. doi: 10.1159/000125777. [DOI] [PubMed] [Google Scholar]
- Limone P, Calvelli P, Altare F, Ajmone-Catt P, Lima T, Molinatti GM. Evidence for an interaction between alpha-MSH and opioids in the regulation of gonadotropin secretion in man. J Endocrinol Invest. 1997;20:207–210. doi: 10.1007/BF03346904. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Saphier D, Goeders NE. Corticosterone facilitates the acquisition of cocaine self-administration in rats: opposite effects of the type II glucocorticoid receptor agonist dexamethasone. J Pharmacol Exp Ther. 1998;287:72–80. [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000;294:239–247. [PubMed] [Google Scholar]
- Marko M, Romer D. Inhibitory effect of a new opioid agonist on reproductive endocrine activity in rats of both sexes. Life Sci. 1983;33:233–240. doi: 10.1016/0024-3205(83)90381-8. [DOI] [PubMed] [Google Scholar]
- Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology (Berl) 1994;114:583–590. doi: 10.1007/BF02244988. [DOI] [PubMed] [Google Scholar]
- Mello NK, Kamien JB, Lukas SE, Drieze J, Mendelson JH. The effects of nalbuphine and butorphanol treatment on cocaine and food self-administration by rhesus monkeys. Neuropsychopharmacology. 1993a;8:45–55. doi: 10.1038/npp.1993.6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, Hormones and Behavior: Clinical and Precliinical Studies. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. second edition. Elsevier, Inc.; 2008. in press. [Google Scholar]
- Mello NK, Mendelson JH. Drug therapy: management of cocaine abuse and dependence. The New England J of Medicine. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Kelly ML, Drieze JM. Cocaine stimulates LH and decreases PRL in female rhesus monkeys. NIDA Res Monogr. 1989;95:337–338. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Skupny A. Naltrexone effects on pituitary and gonadal hormones in male and female rhesus monkeys. Pharmacol Biochem Behav. 1988;31:683–691. doi: 10.1016/0091-3057(88)90248-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Drieze J, Kelly M. Cocaine effects on luteinizing hormone-releasing hormone-stimulated anterior pituitary hormones in female rhesus monkey. J Clin Endocrinol Metab. 1990;71:1434–1441. doi: 10.1210/jcem-71-6-1434. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kelly M. Acute effects of nalmefene on LH, prolactin, and testosterone in male rhesus monkeys. Pharmacol Biochem Behav. 2000;66:275–283. doi: 10.1016/s0091-3057(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Drieze J, Kelly M. Acute effects of cocaine on prolactin and gonadotropins in female rhesus monkey during the follicular phase of the menstrual cycle. J Pharmacol Exp Ther. 1990;254:815–823. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Negus SS, Kelly M, Knudson I, Roth ME. The effects of cocaine on gonadal steroid hormones and LH in male and female rhesus monkeys. Neuropsychopharmacology. 2004;29:2024–2034. doi: 10.1038/sj.npp.1300511. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sholar MB, Jaszyna-Gasior M, Goletiani N, Siegel AJ. Effects of the mixed mu/kappa opioid nalbuphine on cocaine-induced changes in subjective and cardiovascular responses in men. Neuropsychopharmacology. 2005;30:618–632. doi: 10.1038/sj.npp.1300631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Interactions between kappa opioid agonists and cocaine: Preclinical studies. In: Glick SD, Maisonneuve IM, editors. The Archer Conference on Drug Abuse: New Medications. New York: New York Academy of Sciences; 2000. pp. 104–132. [DOI] [PubMed] [Google Scholar]
- Mello NK, Sarnyai Z, Mendelson JH, Drieze JM, Kelly M. Acute effects of cocaine on anterior pituitary hormones in male and female rhesus monkeys. J Pharmacol Exp Ther. 1993b;266:804–811. [PubMed] [Google Scholar]
- Mendelson JH. Plasma prolactin levels and cocaine abuse. Am J Psychiatry. 1991;148:397. doi: 10.1176/ajp.148.3.aj1483397. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Cristofaro P, Mello NK, Skupny AS, Ellingboe J, Benedikt R. Endogenous opioid modulation of luteinizing hormone, prolactin, and estradiol in women: interactions with ethanol. NIDA Res Monogr. 1986;67:112–118. [PubMed] [Google Scholar]
- Mendelson JH, Ellingboe J, Judson BA, Goldstein A. Plasma testosterone and luteinizing hormone levels during levo-alpha-acetylmethadol maintenance and withdrawal. Clin Pharmacol Ther. 1984;35:545–547. doi: 10.1038/clpt.1984.75. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Ellingboe J, Kuehnle J, Mello NK. Heroin and naltrexone effects on pituitary-gonadal hormones in man: tolerance and supersensitivity. NIDA Res Monogr. 1979;27:302–308. [PubMed] [Google Scholar]
- Mendelson JH, Ellingboe J, Kuehnle JC, Mello NK. Heroin and naltrexone effects on pituitary-gonadal hormones in man: interaction of steroid feedback effects, tolerance and supersensitivity. J Pharmacol Exp Ther. 1980;214:503–506. [PubMed] [Google Scholar]
- Mendelson JH, Ellingboe J, Mello NK, Kuehnle J. Buprenorphine effects on plasma luteinizing hormone and prolactin in male heroin addicts. J Pharmacol Exp Ther. 1982;220:252–255. [PubMed] [Google Scholar]
- Mendelson JH, Inturrisi CE, Renault P, Senay EC. Effects of acetylmethadol on plasma testosterone. Clin Pharmacol Ther. 1976;19:371–374. doi: 10.1002/cpt1976193371. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Cocaine and Other Commonly Abused Drugs. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 17. New York: The McGraw-Hill Companies, Inc.; 2008. pp. 2733–2736. [Google Scholar]
- Mendelson JH, Mello NK. Hormones and psycho-sexual development in young men following chronic heroin use. Neurobehav Toxicol Teratol. 1982;4:441–445. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Plasma testosterone levels during chronic heroin use and protracted astinence. A study of Hong Kong addicts. Clin Pharmacol Ther. 1975;17:529–533. doi: 10.1002/cpt1975175529. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Cristofaro P, Ellingboe J, Skupny A, Palmieri SL, Benedikt R, Schiff I. Alcohol effects on naloxone-stimulated luteinizing hormone, prolactin and estradiol in women. J Stud Alcohol. 1987;48:287–294. doi: 10.15288/jsa.1987.48.287. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinology. 2002;27:71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Teoh SK, Ellingboe J, Cochin J. Cocaine effects on pulsatile secretion of anterior pituitary, gonadal, and adrenal hormones. J Clin Endocrinol Metab. 1989;69:1256–1260. doi: 10.1210/jcem-69-6-1256. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mendelson JE, Patch VD. Plasma testosterone levels in heroin addiction and during methadone maintenance. J Pharmacol Exp Ther. 1975a;192:211–217. [PubMed] [Google Scholar]
- Mendelson JH, Meyer RE, Ellingboe J, Mirin SM, McDougle M. Effects of heroin and methadone on plasma cortisol and testosterone. J Pharmacol Exp Ther. 1975b;195:296–302. [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18:263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone and prolactin in men. J Pharmacol Exp Ther. 2003;307:339–348. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Siegel AJ, Mello NK. Effects of cocaine on luteinizing hormone in women during the follicular and luteal phases of the menstrual cycle and in men. J Pharmacol Exp Ther. 2001;296:972–979. [PubMed] [Google Scholar]
- Mendelson JH, Teoh SK, Lange U, Mello NK, Weiss R, Skupny A, Ellingboe J. Anterior pituitary, adrenal, and gonadal hormones during cocaine withdrawal. Am J Psychiatry. 1988;145:1094–1098. doi: 10.1176/ajp.145.9.1094. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Teoh SK, Mello NK, Ellingboe J, Rhoades E. Acute effects of cocaine on plasma adrenocorticotropic hormone, luteinizing hormone and prolactin levels in cocaine-dependent men. J Pharmacol Exp Ther. 1992;263:505–509. [PubMed] [Google Scholar]
- Mendelson JH, Weiss R, Griffin M, Mirin SM, Teoh SK, Mello NK, Lex BW. Some special considerations for treatment of drug abuse and dependence in women. NIDA Res Monogr. 1991;106:313–327. [PubMed] [Google Scholar]
- Mershon JL, Sehlhorst CS, Rebar RW, Liu JH. Evidence of a corticotropin-releasing hormone pulse generator in the macaque hypothalamus. Endocrinology. 1992;130:2991–2996. doi: 10.1210/endo.130.5.1572307. [DOI] [PubMed] [Google Scholar]
- Mirin SM, Mendelson JH, Ellingboe J, Meyer RE. Acute effects of heroin and naltrexone on testosterone and gonadotropin secretion: A pilot study. Psychoneuroendocrinology. 1976;1:359–369. [Google Scholar]
- Mirin SM, Meyer RE, Mendelson JH, Ellingboe J. Opiate use and sexual function. Am J Psychiatry. 1980;137:909–915. doi: 10.1176/ajp.137.8.909. [DOI] [PubMed] [Google Scholar]
- Morley JE. Neuroendocrine effects of endogenous opioid peptides in human subjects: a review. Psychoneuroendocrinology. 1983;8:361–379. doi: 10.1016/0306-4530(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Muraki T, Tokunaga Y, Nakadate T, Kato R. Suppressive effect of morphine on serum gonadotropin levels in castrated rats. Arch Int Pharmacodyn Ther. 1980;246:324–334. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of mu-agonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Bidlack JM, Zong R, Bakthavachalam V, Gao P, Cohen DJ, Negus SS, Mello NK. Synthesis and opioid receptor affinity of morphinan and bezomorphan derivatives: mixed kappa agonists and mu agonists/antagonists as potential pharmacotherapeutics for cocaine dependence. J Med Chem. 2000:114–22. doi: 10.1021/jm9903343. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Mello NK, Negus SS, Bidlack JM. Kappa Opioid agonists as targets for pharmacotherapies in cocaine abuse. Pharm Acta Helv. 2000:337–44. doi: 10.1016/s0031-6865(99)00044-8. [DOI] [PubMed] [Google Scholar]
- Nikolarakis K, Pfeiffer A, Stalla GK, Herz A. The role of CRF in the release of ACTH by opiate agonists and antagonists in rats. Brain Res. 1987;421:373–376. doi: 10.1016/0006-8993(87)91311-4. [DOI] [PubMed] [Google Scholar]
- Patey G, Rossier J. Discovery, anatomical mapping and biosynthesis of various families of endogenous opioid peptides. Ann Endocrinol (Paris) 1986;47:71–87. [PubMed] [Google Scholar]
- PDR. Physicians Desk Reference. 58. Montvale, NJ: Thomson PDR; 2005. [Google Scholar]
- Pende A, Musso NR, Montaldi ML, Pastorino G, Arzese M, Devilla L. Evaluation of the effects induced by four opiate drugs, with different affinities to opioid receptor subtypes, on anterior pituitary LH, TSH, PRL and GH secretion and on cortisol secretion in normal men. Biomed Pharmacother. 1986;40:178–182. [PubMed] [Google Scholar]
- Petraglia F, Locatelli V, Facchinetti F, Bergamaschi M, Genazzani AR, Cocchi D. Oestrous cycle-related LH responsiveness to naloxone: effect of high oestrogen levels on the activity of opioid receptors. J Endocrinol. 1986;108:89–94. doi: 10.1677/joe.0.1080089. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Herz A. Endocrine actions of opioids. Horm Metab Res. 1984;16:386–397. doi: 10.1055/s-2007-1014801. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Herz A, Loriaux DL, Pfeiffer DG. Central kappa- and mu-opiate receptors mediate ACTH-release in rats. Endocrinology. 1985;116:2688–2690. doi: 10.1210/endo-116-6-2688. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Knepel W, Braun S, Meyer HD, Lohmann H, Brantl V. Effects of a kappa-opioid agonist on adrenocorticotropic and diuretic function in man. Horm Metab Res. 1986;18:842–848. doi: 10.1055/s-2007-1012453. [DOI] [PubMed] [Google Scholar]
- Pfeiffer DG, Pfeiffer A, Almeida OF, Herz A. Opiate suppression of LH secretion involves central receptors different from those mediating opiate effects on prolactin secretion. J Endocrinol. 1987;114:469–476. doi: 10.1677/joe.0.1140469. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Opioid inhibition of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation of rats. Regul Pept. 1986;16:235–242. doi: 10.1016/0167-0115(86)90022-4. [DOI] [PubMed] [Google Scholar]
- Plotsky P, Cunningham ETJ, Widmaiere Ep. Catecholamineregic modulation of coricotropin-releasing factor and andrenocorticotropin secretion. Endocrine Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Effects of Agonist-Antagonist Opioids in Humans Trained in a Hydromorphone/Not Hydromorphone Discrimination. J Pharmacol Exp Ther. 2000;295:114–124. [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Schroeder JR, abreu ME, Epstein DH, Pickworth WB. Cyclazocine: comparison to hydromorphone and interaction with cocaine. Behavioural Pharmacology. 2004:91–102. doi: 10.1097/00008877-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Hypogonadism and sexual dysfunction in male cancer survivors receiving chronic opioid therapy. J Pain Symptom Manage. 2003;26:1055–1061. doi: 10.1016/s0885-3924(03)00331-2. [DOI] [PubMed] [Google Scholar]
- Ramirez D, Feder H, Saywer C. The role of brain catecholamines in the regulation of LH secretion: a critical inquiry. In: Martini L, Ganong W, editors. Frontiers in Neuroendocrinology. New York: Raven Press; 1984. pp. 27–84. [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biology of Reproduction. 1991:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Finch PM, Pullan PT, Bhagat CI, Price LM. Sex hormone suppression by intrathecal opioids: a prospective study. Clin J Pain. 2002;18:144–148. doi: 10.1097/00002508-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Sanchez-Yague J, Paniagua R. Effects of cocaine on testicular structure in the rat. Reprod Toxicol. 1992;6:51–55. doi: 10.1016/0890-6238(92)90020-t. published erratum appears in Reprod. Toxicol., 1992; 6(3):283. [DOI] [PubMed] [Google Scholar]
- Ropert JF, Quigley ME, Yen SS. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab. 1981;52:583–585. doi: 10.1210/jcem-52-3-583. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Mortola JF, Yen SS. Role of endogenous opioid peptides in the initiation of the midcycle luteinizing hormone surge in normal cycling women. J Clin Endocrinol Metab. 1988;67:695–700. doi: 10.1210/jcem-67-4-695. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Wirth U. Effects of sleep on gonadotropin secretion. Geburtshilfe Frauenheilkd. 1993;53:735–741. doi: 10.1055/s-2007-1023619. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Wirth U, Sterzik K, Yen SS. The effects of prolonged opioidergic blockade on LH pulsatile secretion during the menstrual cycle. J Endocrinol Invest. 1989;12:245–252. doi: 10.1007/BF03349974. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Yen SS. Sleep-associated decrease in luteinizing hormone pulse frequency during the early follicular phase of the menstrual cycle: evidence for an opioidergic mechanism. J Clin Endocrinol Metab. 1987;65:715–718. doi: 10.1210/jcem-65-4-715. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Rhodes ME, Miller TH, Jakab RL, Czambel RK. Sequence of pituitary-adrenal cortical hormone responses to low-dose physostigmine administration in young adult women and men. Life Sci. 2006;79:2260–2268. doi: 10.1016/j.lfs.2006.07.023. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration Results from the 2006 National Survey on Drug Use and Health: National Findings. Washington, DC: 2006. [Google Scholar]
- Samuels LT, Henschel AF, Keys A. Influence of methyltestosterone on muscular work and creatine metabolism in normal young men. J Clin Endocrinol. 1942;2:649–654. [Google Scholar]
- Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, ‘binge’ cocaine administration: diminished individual differences in stress-induced corticosterone response. Neuroendocrinology. 1998;68:334–344. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Mello NK, Mendelson JH, Eros-Sarnyai M, Mercer G. Effects of cocaine on pulsatile activity of the hypothalamic-pituitary-adrenal axis in male rhesus monkeys: neuroendocrine and behavioral correlates. J Pharmacol Exp Ther. 1996 [PubMed] [Google Scholar]
- Saso L. Effects of drug abuse on sexual response. Ann Ist Super Sanita. 2002;38:289–296. [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Role of dopamine receptor subtypes in the acquisition of a testosterone conditioned place preference in rats. Neurosci Lett. 2000;282(1-2):17–20. doi: 10.1016/s0304-3940(00)00839-9. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. Differential symptom response to parenteral estrogen and/or androgen administration in the surgical menopause. Am J Obstet Gynecol. 1985;151:153–160. doi: 10.1016/0002-9378(85)90001-8. [DOI] [PubMed] [Google Scholar]
- Sholar MB, Mendelson JH, Mello NK, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Concurrent pharmacokinetic analysis of plasma cocaine and adrenocorticotropic hormone in men. J Clin Endocrinol Metab. 1998;83:966–968. doi: 10.1210/jcem.83.3.4654. [DOI] [PubMed] [Google Scholar]
- Short RE, Brooks AN, Peters AR, Lamming GE. Opioid modulation of LH secretion during the oestrous cycle of heifers. J Reprod Fertil. 1987;80:213–219. doi: 10.1530/jrf.0.0800213. [DOI] [PubMed] [Google Scholar]
- Siegel RA, Revesz L. Effects of the novel opiate antagonist, SDZ 210-096, on luteinizing hormone secretion in the rat. J Pharmacol Exp Ther. 1989;249:264–270. [PubMed] [Google Scholar]
- Sikharulidze ZD, Kopaliani MG, Kilasoniia LO. Comparative evaluation of clinical symptoms and status of bone metabolism in patients with heroin and buprenorphine addiction in the period of withdrawal. Georgian Med News. 2006:72–76. [PubMed] [Google Scholar]
- Singh HH, Purohit V, Ahluwalia BS. Methadone blocks dopamine-mediated release of gonadotropins in rat hypothalamus. Neuroendocrinology. 1982;34:347–352. doi: 10.1159/000123326. [DOI] [PubMed] [Google Scholar]
- Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, Frost JJ. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metab. 1998;83:4498–4505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- Snowden EU, Khan-Dawood FS, Dawood MY. The effect of naloxone on endogenous opioid regulation of pituitary gonadotropins and prolactin during the menstrual cycle. J Clin Endocrinol Metab. 1984;59:298–302. doi: 10.1210/jcem-59-2-298. [DOI] [PubMed] [Google Scholar]
- Spremovic-Radjenovic S, Popovic V, Matijasevic S, Lazovic G, Petkovic S. Effect of opioids and gamma-aminobutyric acid on ovulation. Srp Arh Celok Lek. 1997;125:329–332. [PubMed] [Google Scholar]
- Steele PA, Judd SJ. Role of endogenous opioids in reducing the frequency of pulsatile luteinizing hormone secretion induced by progesterone in normal women. Clin Endocrinol (Oxf) 1986;25:669–674. doi: 10.1111/j.1365-2265.1986.tb03622.x. [DOI] [PubMed] [Google Scholar]
- Suda T, Tozawa F, Iwai I, Sato Y, Sumitomo T, Nakano Y, Yamada M, Demura H. Neuropeptide Y increases the corticotropin-releasing factor messenger ribonucleic acid level in the rat hypothalamus. Brain Res Mol Brain Res. 1993;18:311–315. doi: 10.1016/0169-328x(93)90094-6. [DOI] [PubMed] [Google Scholar]
- Archer Sydney, G Stanley, Bidlack Jean. Cyclazocine Revisited. Neurochemical Research. 1996:1369–1373. doi: 10.1007/BF02532378. [DOI] [PubMed] [Google Scholar]
- Taleisnik S, Sawyer CH. Activation of the CNS noradrenergic system may inhibit as well as faciliatate pituitary luteinzing hormone release. Neuroendocrinology. 1986;44:265–268. doi: 10.1159/000124655. [DOI] [PubMed] [Google Scholar]
- Taylor WN. Synthetic anabolic androgenic steroids: a plea for controlled substance status. Phys Sports Med. 1987;15:140–151. doi: 10.1080/00913847.1987.11709356. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Mello NK, Skupny A. Alcohol effects on naltrexone-induced stimulation of pituitary, adrenal, and gonadal hormones during the early follicular phase of the menstrual cycle. J Clin Endocrinol Metab. 1988;66:1181–1186. doi: 10.1210/jcem-66-6-1181. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Beha. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Rogol AD, Johnson ML. Endogenous opiates modulate the pulsatile secretion of biologically active luteinizing hormone in man. J Clin Invest. 1983;72:2031–2040. doi: 10.1172/JCI111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Worgul TJ, Monsaert R, Hammond JM. A possible role of endogenous opioids in the control of prolactin and luteinizing-hormone secretion in the human. J Endocrinol Invest. 1981;4:31–36. doi: 10.1007/BF03349410. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Gerra G, Maninetti L, Pedrazzoni M, Michelini M, Pioli G, Girasole G, Caccavari R, Maestri D, Passeri M. Metyrapone effects on beta-endorphin, ACTH and cortisol levels after chronic opiate receptor stimulation in man. Neuropeptides. 1990;15:129–132. doi: 10.1016/0143-4179(90)90143-m. [DOI] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM. Effect of ovarian hormones and estrous cycle on stimulation of the hypothalamo-pituitary-adrenal axis by cocaine. J Pharmacol Exp Ther. 2001;297:291–298. [PubMed] [Google Scholar]
- Weiner RI, Findell PR, Kordon C. Role of classic and peptide neuromediators in the neuroendocrine regulation of LH and prolactin. In: Knobil E, Neill J, editors. The Physiology of Reprodeuction. New York: Raven Press; 1988. pp. 1235–1281. [Google Scholar]
- Xu RK, Chen L, Li H, Yang SJ, Huang XY. Effects of kappa-opiate receptor antagonist MR-2266-BS on ACTH and prolactin release. Sheng Li Xue Bao. 1989;41:395–401. [PubMed] [Google Scholar]
- Woods RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83(2):279–89. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Yang GS, Wang W, Wang YM, Chen ZD, Wang S, Fang JJ. Effect of cocaine on germ cell apoptosis in rats at different ages. Asian J Androl. 2006;8:569–575. doi: 10.1111/j.1745-7262.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Yen Samuel SC. The Synthesis and Metabolism of Steroid Hormones. In: Strauss JF 3rd, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia: Elsevier Saunders; 2004. p. 149. [Google Scholar]
- Yilmaz B, Gilmore DP. Effects of mu, kappa, and delta opioid receptor agonists and antagonists on rat hypothalamic noradrenergic neurotransmission. Brain Res Bull. 1999a;48:491–495. doi: 10.1016/s0361-9230(99)00020-9. [DOI] [PubMed] [Google Scholar]
- Yilmaz B, Gilmore DP. Opioid modulation of hypothalamic catecholaminergic neurotransmission and the pre-ovulatory LH surge in the rat. Neuro Endocrinol Lett. 1999b;20:115–121. [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–1197. [PubMed] [Google Scholar]
- Zhang Q, Gallo RV. Effect of prodynorphin-derived opioid peptides on the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine. 2002;18:27–32. doi: 10.1385/ENDO:18:1:27. [DOI] [PubMed] [Google Scholar]
- Zheng SX, Bosch MA, Ronnekleiv OK. mu-opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol. 2005;487:332–344. doi: 10.1002/cne.20557. [DOI] [PubMed] [Google Scholar]