Abstract
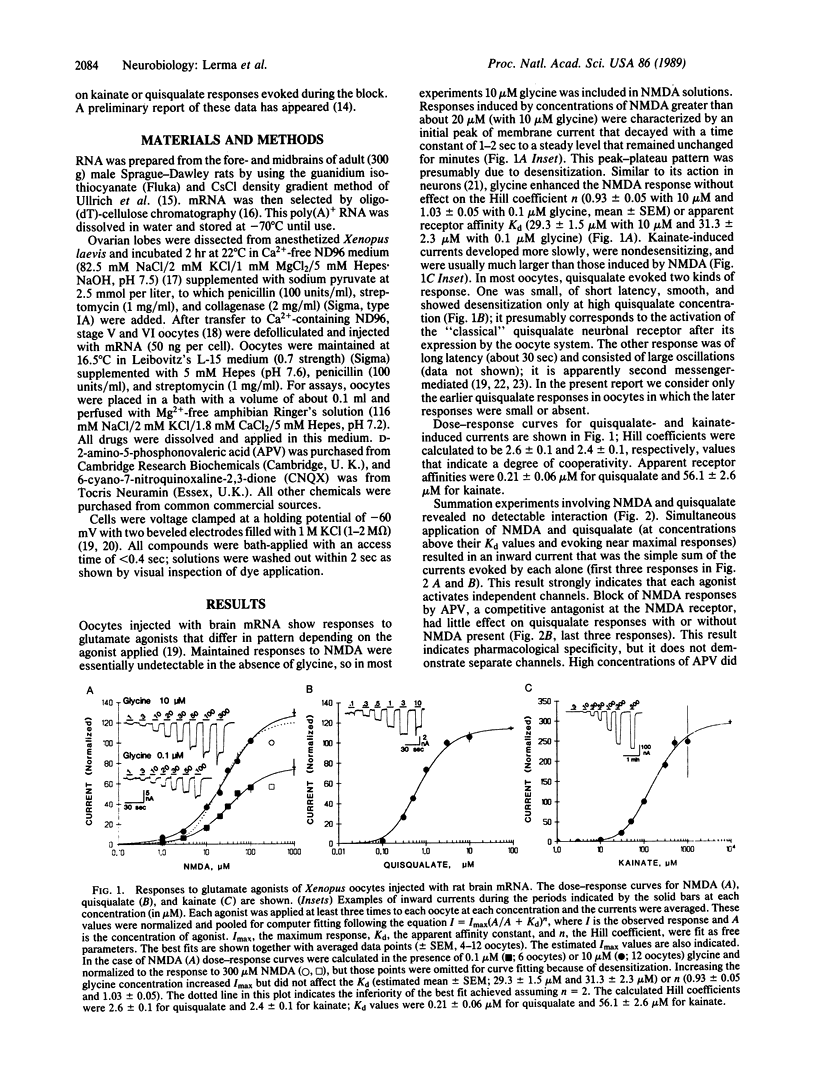
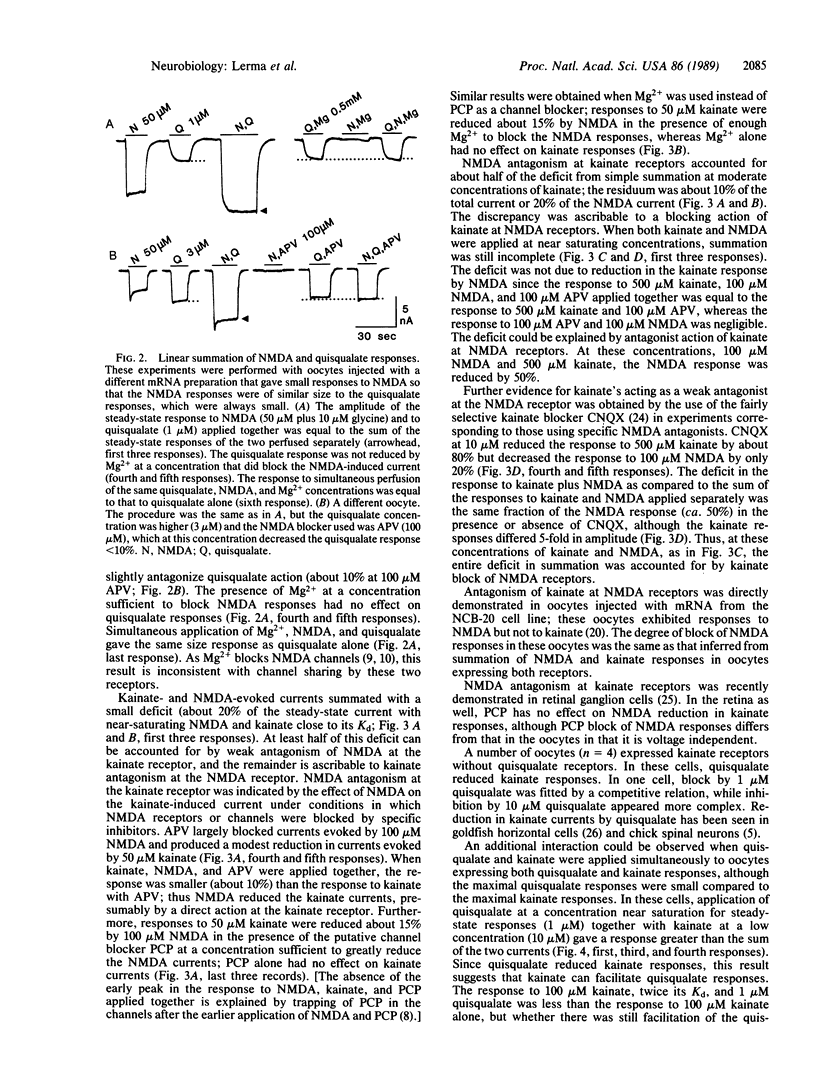
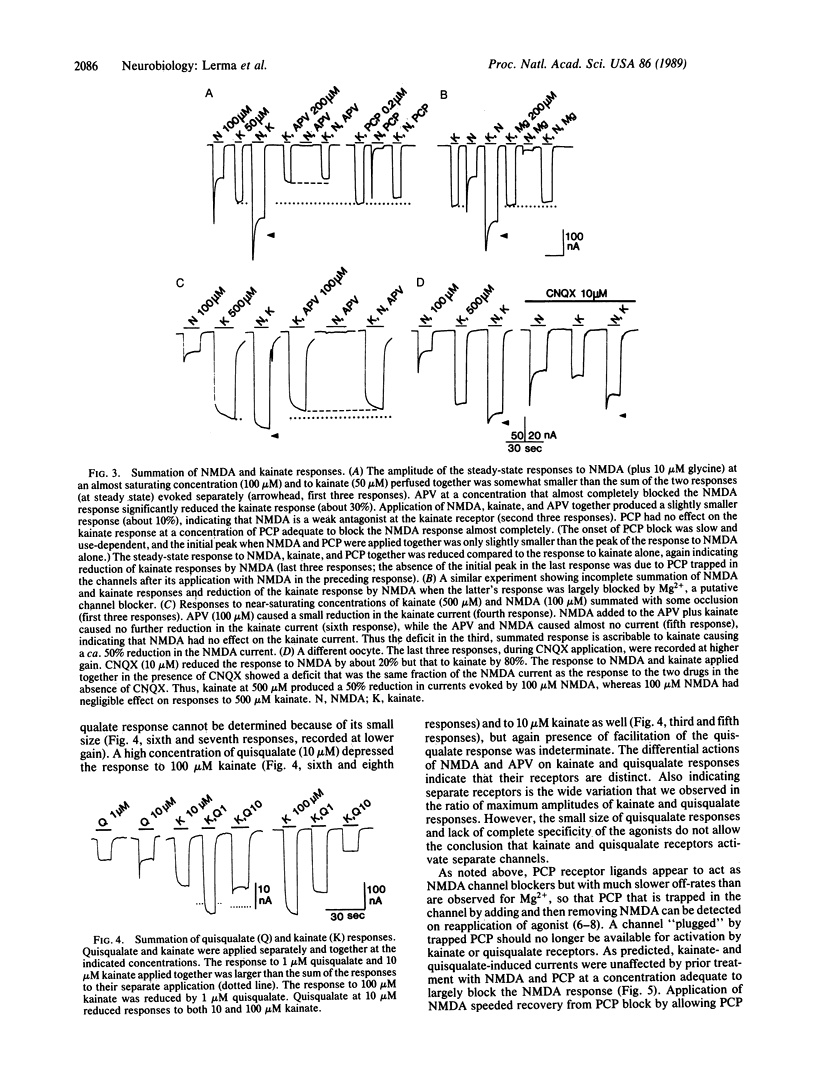
In the mammalian central nervous system, the excitatory amino acid transmitter L-glutamate activates three pharmacologically distinguishable receptors, the N-methyl-D-aspartate (NMDA), kainate, and quisqualate receptors. The present paper addresses the issue of whether these three receptors operate independent channels or whether they share channels that may have several conductance substates. The Xenopus oocyte provides a system for expression of exogenous mRNAs that permits detailed study of receptor structure and function. In oocytes injected with rat brain mRNA, NMDA has a stoichiometry of channel activation different from that for kainate and quisqualate. NMDA activates its own channels as indicated by simple summation or near-summation of currents evoked by NMDA with those evoked by quisqualate or kainate. Deviations from summation are ascribable to lack of selectivity in which an agonist at one receptor acts as a weak antagonist at another receptor. A further indication of separate channels is that block of NMDA channels by Mg2+ or phencyclidine has no effect on kainate or quisqualate responses evoked during the block. Interactions of kainate and quisqualate are more complex, but they can be explained by lack of complete specificity of these agonists for their own receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. T., Neyton J. Quisqualate and L-glutamate inhibit retinal horizontal-cell responses to kainate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1837–1841. doi: 10.1073/pnas.82.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Karschin A., Aizenman E., Lipton S. A. The interaction of agonists and noncompetitive antagonists at the excitatory amino acid receptors in rat retinal ganglion cells in vitro. J Neurosci. 1988 Aug;8(8):2895–2906. doi: 10.1523/JNEUROSCI.08-08-02895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner L., Lerma J., Zukin R. S., Bennett M. V. Coexpression of N-methyl-D-aspartate and phencyclidine receptors in Xenopus oocytes injected with rat brain mRNA. Proc Natl Acad Sci U S A. 1988 May;85(9):3250–3254. doi: 10.1073/pnas.85.9.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Spray D. C., Bennett M. V., Zukin R. S. mRNA from NCB-20 cells encodes the N-methyl-D-aspartate/phencyclidine receptor: a Xenopus oocyte expression study. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1708–1711. doi: 10.1073/pnas.86.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mayer M. Two channels reduced to one. Nature. 1987 Feb 5;325(6104):480–481. doi: 10.1038/325480a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vlachová V., Vyklický L., Vyklický L., Jr, Vyskocil F. The action of excitatory amino acids on chick spinal cord neurones in culture. J Physiol. 1987 May;386:425–438. doi: 10.1113/jphysiol.1987.sp016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J. AMPA, kainate, and quisqualate activate a common receptor-channel complex on embryonic chick motoneurons. J Neurosci. 1988 Nov;8(11):4277–4286. doi: 10.1523/JNEUROSCI.08-11-04277.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


