Abstract
We investigated the effects of operator-applied force on diffuse optical spectroscopy (DOS) by integrating a force transducer into the handheld probe. Over the typical range of contact forces measured in the breasts of eight patients, absorption and reduced scattering coefficients (650 to 1000 nm) variance was 3.1 ± 1.0% and 1.0 ± 0.4%. For trained operators, we observed <5% variation in hemoglobin and <2% variation in water and lipids. Contact force is not a significant source of variation, most likely because of a relatively wide probe surface area and the stability of the DOS method for calculating tissue optical properties.
1. Introduction
A. Quality Control with a Handheld Optical Probe
There are several optical imaging and/or spectroscopic instruments used in breast tissue characterization that employ a handheld probe design [1–6]. Handheld probes potentially offer several advantages compared to fixed imaging geometries. A breast lesion may be easily and rapidly localized with multiple views, as with conventional ultrasound, and probes can be placed in difficult-to-access areas such as the chest wall. In addition, handheld probe instrumentation can be engineered to be office-based and portable.
However, concerns arise with the potential for operator-dependent errors. Despite extensive training, variations in measurement technique between individuals can lead to disparities in data collection and calibration [7,8]. Most studies involving research-grade optical instruments, including ours, are the products of university laboratories that typically stress scientific results and initial translational studies, but have not emphasized standardization.
Over the years we have standardized many aspects of data collection, including calibration, patient positioning/posture, and probe handling. In general, these concerns can be addressed by providing careful operator training. However, the impact of probe contact pressure has not been well-characterized. Variations in probe pressure can potentially impact hemodynamics, optical coupling, and interrogation volume. As a result, this study investigates the effects of operator-applied force on diffuse optical spectroscopy (DOS) measurements by integrating a small force transducer into the handheld probe.
B. Diffuse Optical Spectroscopy
DOS measures tissue optical properties (i.e., absorption coefficient, μa, and reduced scattering coefficient, μs′) using well-characterized methods that combine broadband frequency-domain and steady-state spectroscopies [4,9,10]. Typically, diffusion-based models are used to calculate absolute absorption and scattering optical properties from discrete-wavelength frequency-domain (FD) phase and amplitude measurements. Quantitative absorption and scattering spectra are calculated by combining broadband steady-state reflectance with FD data [10]. By combining FD with broadband reflectance, complete coverage of the NIR spectral range can be achieved.
Within the near-infrared (NIR) spectral range (650–1000 nm) where DOS is routinely employed, the tissue concentrations of oxygenated (ctO2Hb) and deoxygenated hemoglobins (ctHHb), water (ctH2O), and bulk lipid are calculated from tissue absorption spectra [9]. We distinguish DOS from the more generic near infrared spectroscopy (NIRS) in that, generally, NIRS does not employ spatial, time-, or frequency-domain methods to separate absorption from scattering [11]. The DOS method combines the quantitative accuracy of frequency-domain photon migration with the broadband spectral content of NIRS.
C. Study Goals
DOS has shown promise in the detection of breast tumors and in monitoring the effects of neoadjuvant chemotherapy. In particular, DOS has been used in long-term monitoring of breast tumor response to therapy, where many measurements are taken over the course of weeks to months [5,12–16]. To standardize these DOS measurements, it is necessary to determine how a DOS probe operator might influence measurements by varying tissue-probe contact force.
The overall purpose of this study was to characterize the effects of probe-tissue contact force on DOS measurements of breast tissue optical properties. A simple force transducer was attached to the bottom of our DOS handheld probe and the contact force between the breast tissue and the probe was measured. Breast tissue optical properties were measured as a function of relative contact force in both normal and lesion-containing breasts. The forces used in this study were significantly smaller than those used in mammographic compression. Rather, our main concern was to determine limits on the repeatability of DOS measurements in terms of the sensitivity of our results to contact force. These results are important to consider in view of potential DOS applications in longitudinal patient monitoring [8,12].
2. Methods
A. Diffuse Optical Spectroscopic Imaging Instrument
Specific details of our DOS instrument, the laser breast scanner (LBS), have been previously described in detail [4]. The current LBS instrument is a bedside-capable, multiwavelength device that integrates both broadband frequency-domain and broadband steady-state approaches to provide complete tissue absorption and scattering spectra from 650–1000 nm. Briefly, the frequency-domain component employs six fiber-coupled diode lasers (658, 682, 785, 810, 830, and 850 nm) and an avalanche photodiode (APD) detector. Each laser diode is swept in modulation frequency from 50 to 500 MHz in 401 steps [9]. The steady-state component employs a fiber-coupled broadband lamp and a fiber-coupled back-illuminated CCD spectrometer (BW Tek, Newark, Delaware). A handheld probe integrates both frequency-domain and steady-state approaches, and was designed to interrogate overlapping regions of breast tissue (Fig. 1). The source-detector separation distance was fixed at 28.5 mm in a reflectance geometry. Measurement time at a single spatial location was less than 10 s for the combined frequency-domain and steady-state measurements. Calibrations for the frequency-domain and steady-state instruments were performed using a tissue phantom and an integrating sphere, respectively.
Fig. 1.
(Color online) Photograph of the bottom of the DOS handheld probe with the force transducer attached near the APD. The transducer (FlexiForce) was attached as close as possible to the optical viewing field without interfering with the measurement. The transducer 9.53 mm2 active area could measure up to 4.4 N.
Simple calculations of tissue chromophore concentrations were performed using the Beer–Lambert law and known absorption extinction coefficients. We assumed that the main near-infrared absorbers in breast tissue were ctHHb (µM), ctO2Hb (µM), ctH2O (%), and lipids (%) [17–19]. We report ctH2O as the concentration of measured tissue water divided by pure water concentration (55.6 M), whereas tissue bulk lipids are reported as the percentage lipid measured relative to an assumed “pure” lipid density of 0.9 g mL−1. Thus, reported water and lipid percentages are relative amounts compared to pure solutions of the substance and are not expected to add to 100%.
B. Measurement of Probe-Breast Contact Force
Probe-to-breast contact force was measured using a commercial tactile sensor (FlexiForce Model A201, Tekscan, Boston, Massachusetts) that was integrated into the DOS handheld probe (Fig. 1). Force was recorded in real time using a second computer connected to the transducer. The transducer active area was 9.53 mm2, with a thickness of 0.203 mm, and was positioned at one point on the bottom surface of the DOS handheld probe as close as possible to the region of tissue interrogated by the light. The maximum applied force that can be registered by the particular transducer was 4.4 N (1 lb). The transducer was calibrated using a series of weight standards as per the manufacturer specifications, with the FlexiForce transducer resting on a hard flat surface. Approximately 3000 mm2 of a total 5400 mm2 of the DOS probe bottom surface area made contact with breast tissue during a typical measurement.
Contact forces are reported as normalized percentages of the maximum applied force (i.e., a scale of 0 to 100%). In this study, 0% was chosen by the operator (S.S.) to be a “significantly lighter than typical” force that was barely in contact with the breast, yet displayed adequate optical coupling. In similar fashion, 100% was chosen to be a “much harder than typical” force that did not result in patient discomfort. We stress that the 100% force reading varied from patient-to-patient in terms of the absolute force. In general, DOS operators found on this relative scale that their typical measurements would have ranged from about 20 to 50% in terms of relative contact force. A single probe operator (S.S.) performed all measurements.
C. Study Patient Characteristics
Eight subjects were recruited for this study. Subjects provided informed written consent to participate in this research study which was approved by the University of California, Irvine, Institutional Review Board (Protocol #95–563). There were three subject categories in this study: Lesion, Normal Premenopausal, and Normal Postmenopausal. The Lesion category included three subjects who each had a palpable lesion (2 malignant and 1 benign). The lesion sizes were 12 × 10 × 6 mm, 10 × 6.4 × 4.1 mm, and 41 × 32 × 64 mm. The Normal Premenopausal category included two subjects with regular menstrual cycles and no history of breast lesions. The Normal Postmenopausal category included three subjects with no periods for at least one year and no history of breast lesions.
D. Diffuse Optical Spectroscopic Imaging Measurement Technique
Subjects laid supine with their arm at their side on a recliner at about 30° from horizontal for all DOS measurements. For subjects in the normal category (N = 5), DOS measurements were taken at two spatial locations. 12 o’clock and 9 o’clock positions of the breast. In the lesion subject category (N = 3), a point at the center of the lesion was measured. In all cases, the probe operator applied a series of different relative probe–breast contact forces ranging from 10–100% at the measurement location. The operator started with the lightest force and moved towards the maximum in approximately equal steps. For each force level, five repetitions of DOS measurements were taken consecutively at constant force, without lifting the probe off the tissue. The operator then increased the pressure and repeated five repetitions. The operator regulated the contact force using the constant updates from the computer readout.
Force-dependent variation in a given DOS parameter was calculated for each measurement location in normal subjects using the CV% (coefficient of variation), which was defined as the standard deviation of a DOS parameter value measured at all forces divided by the average DOS parameter value measured at all forces. To obtain an overall force-dependent variation value for each normal subject, the CV%’s of both locations were averaged. For each lesion subject, one CV% was obtained for the one location that was measured.
3. Results
A. Impact of Relative Contact Force on Measured Spectra
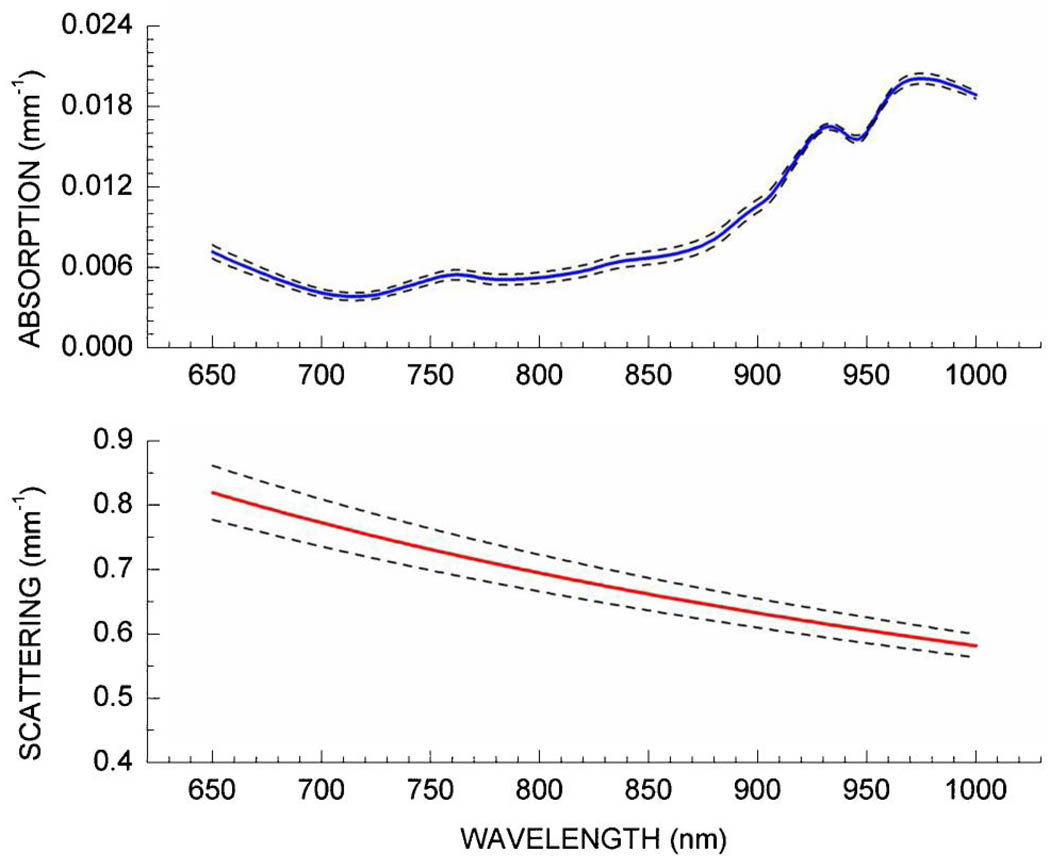
Figure 2 displays the DOS-measured absorption and reduced scattering spectra from a single normal subject, measured as a function of probe–breast application force. The solid lines represent the average spectra measured at seven different forces; the dashed lines represent the standard deviation of these measurements. Five repeat measurements were obtained and averaged at each force level to represent the spectrum for a given force level. On average across the entire wavelength range, the coefficient of variance in μa and μs′ was 5.7 ± 2.4% and 4.0 ± 0.6% for all relative contact forces. If we restrict the relative contact force range to the range most likely used by the operator (i.e., 20–50%), these values decrease to 3.1 ± 1.0% and 1.0 ± 0.4% for μa and μs′, respectively. The variation of μa and μs′ with respect to contact force is very similar to the variation in μa and μs′ seen in repeated measurements by picking up the probe and replacing it at the same location.
Fig. 2.
(Color online) DOS absorption and reduced scattering spectra measured as a function of probe–breast contact force in a single normal subject. The solid lines represent the average spectrum for all pressures (N = 7), while the dashed lines represent the standard deviation of the average. Note that over the typical range of contact forces measured in the breasts of eight patients, absorption and reduced scattering coefficients (650 to 1000 nm) variance was 3.1 ± 1.0% and 1.0 ± 0.4%, respectively.
B. Relative Force Measurements in Normal Tissues
Figure 3 displays typical variation of DOS parameters measured as a function of probe–breast application force in a single subject (i.e., for the same subject as Fig. 2). The error bars represent the standard deviation of five repeated measurements at each force level for a single spatial location. The minimum and maximum measurement values for each parameter for all individual measurements (i.e., all repeated measures at each force) were: 10.2 to 16.5 μM (ctO2Hb), 3.8 to 5.2 μM (ctHHb), 17.5 to 18.6% (ctH2O), and 59.9 to 65.8% (bulk lipid). If we average the repeated measures for each force, the DOS-measured parameters did not differ appreciably: 5.1% for ctO2Hb, 3.4% for ctHHb, 1.4% for ctH2O, and 1.9% for lipid. These variances are consistent with placement errors observed within a given measurement session. Further, if we restrict the relative contact force range to the range most likely used by the operator (i.e., 20–50%), these values decrease to 1.8% for ctO2Hb, 2.9% for ctHHb, 0.2% for ctH2O, and 1.9% for lipid. Thus, this variance does not represent significant dependence of DOS parameters resulting from probe–breast contact force.
Fig. 3.
(Color online) DOS parameters measured as a function of probe–breast contact force in a single subject. The error bars represent the variance of five repeated measurements at a given force (0% being “very light” and 100% being “pretty hard”). Typical contact force is approximately 30% on this scale.
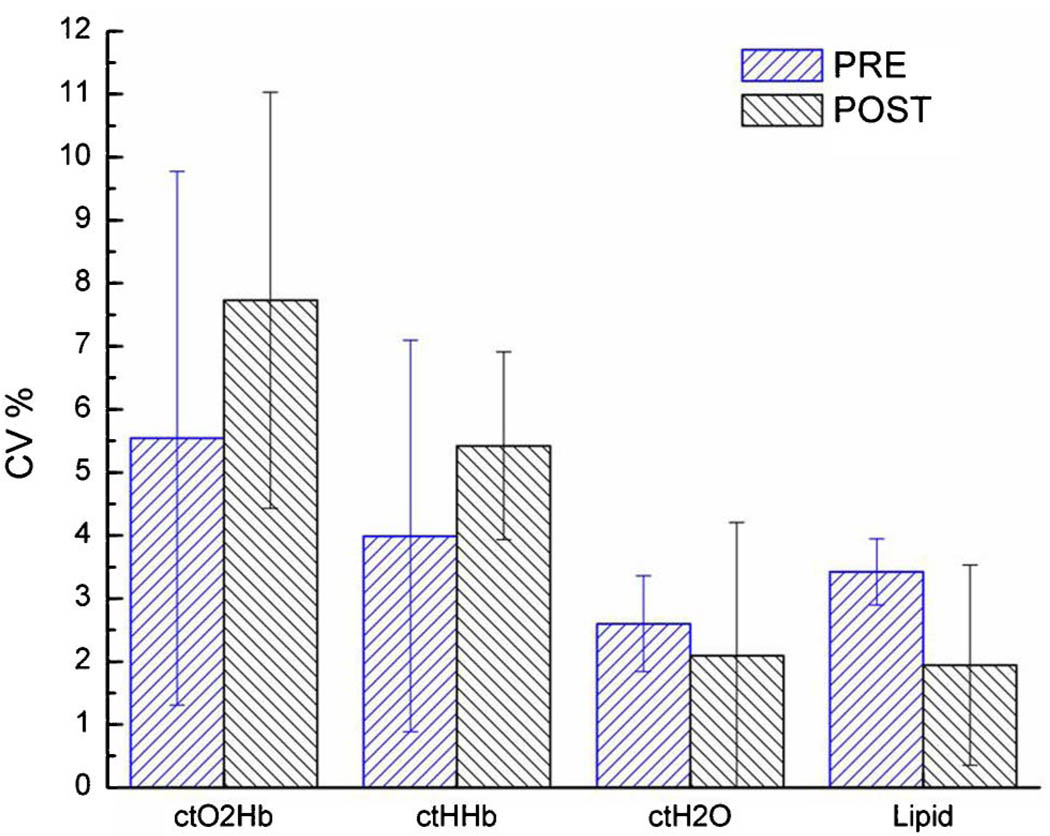
The overall force-dependent (full range) DOS variation in terms of CV% for Pre- and Postmenopausal subject groups is shown in Fig. 4. The error bars represent the population variation between subjects within each group. In both groups, ctO2Hb varied the most, followed by ctHHb; ctH2O and lipid varied the least. Although not statistically significant, postmenopausal breast tissue exhibited more force-dependent variation in ctO2Hb and ctHHb than premenopausal breast; presumably this is due to the generally lower concentrations of these components in postmenopausal breast tissues [4,20]. There was a slightly higher force-dependent variation (though not significant) in lipid in premenopausal breast than in postmenopausal breast. If we again restrict the applied force range to that commonly used by trained operators, the variance in measurements (CV%) decreases similarly to the previous example (Table 1).
Fig. 4.
(Color online) Comparison of relative variance in DOS measurements for all probe–breast contact forces in eight normal subjects. CV% is defined as the standard deviation of the population, divided by the average value of the population. Differences between the pre- and postmenopausal groups are not significant.
Table 1.
Comparison of CV%’s When Using Full (0–100%) Versus Restricted (20–50%) Forces
| CV% | |||||
|---|---|---|---|---|---|
| Category | Force Range | ctO2Hb | ctHHb | ctH2O | Lipid |
| Pre (N = 5) | Full | 5.5 ± 4.2 | 4.0 ± 3.1 | 2.6 ± 0.8 | 3.4 ± 0.5 |
| Restricted | 5.0 ± 2.2 | 3.6 ± 1.5 | 1.9 ± 1.3 | 2.0 ± 1.0 | |
| Post (N = 3) | Full | 7.7 ± 3.3 | 5.4 ± 1.5 | 2.1 ± 2.1 | 1.9 ± 1.6 |
| Restricted | 7.0 ± 2.2 | 5.0 ± 1.3 | 1.3 ± 0.7 | 1.9 ± 0.5 | |
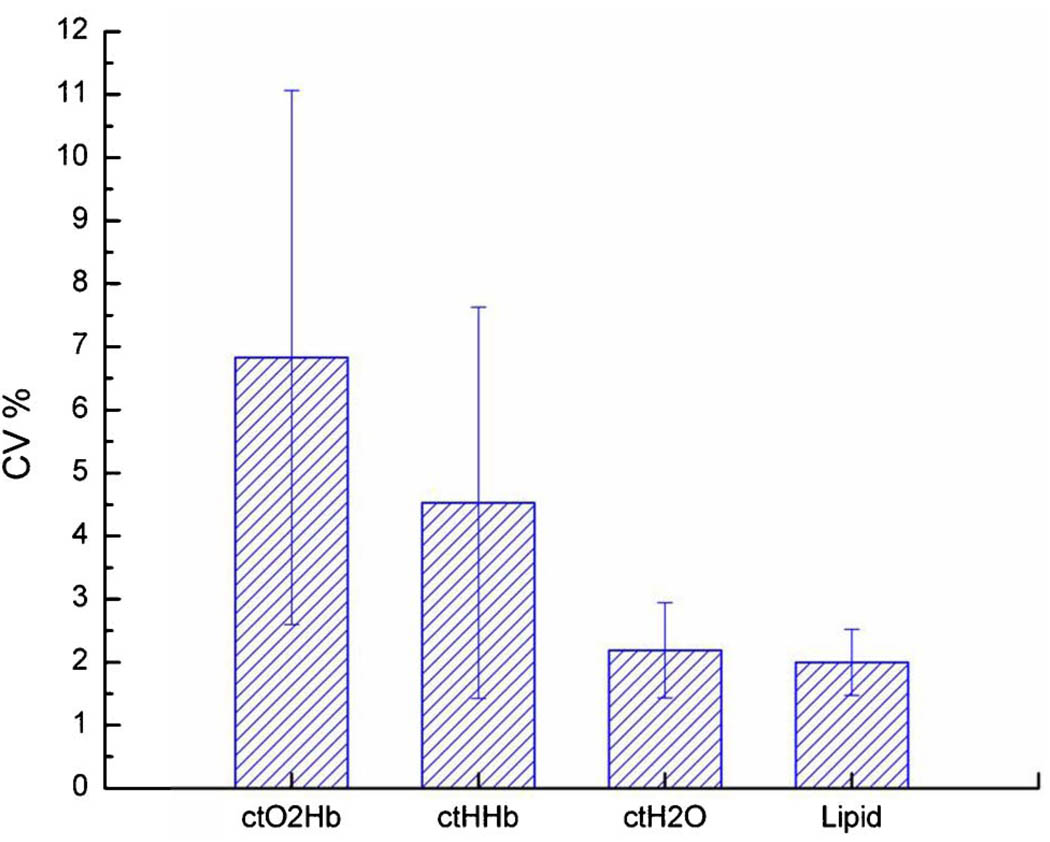
| Lesion (N = 3) | Full | 6.8 ± 4.2 | 4.5 ± 3.1 | 2.2 ± 0.8 | 2.0 ± 0.5 |
| Restricted | 5.0 ± 2.3 | 3.7 ± 2.7 | 1.4 ± 0.5 | 1.3 ± 0.8 | |
| All (N = 11) | Full | 6.5 ± 3.1 | 4.6 ± 2.0 | 2.5 ± 1.5 | 2.3 ± 1.1 |
| Restricted | 5.6 ± 2.7 | 4.1 ± 1.7 | 1.5 ± 0.8 | 1.7 ± 0.8 | |
C. Relative Force Measurements in Lesion-Containing Tissues
Figure 5 displays the variation in DOS-measured values as a function of probe–breast force in a single subject with a malignant lesion (29 × 20 × 23 mm, 43 years old). As before, the error bars represent the variation between five repeated measurements at each force level at the measured location on the breast. As before, the minimum to maximum range measured for each parameter was: 13.7 to 20.4 μM (ctO2Hb), 7.2 to 9.2 μM (ctHHb), 22.6 to 24.4% (ctH2O), and 53.8 to 61.1% (lipid). Also as before, DOS-measured parameters did not differ appreciably over the probe–breast force range: 7.2% for ctO2Hb, 5.5% for ctHHb, 2.7% for ctH2O, and 2.5% for lipid. Reducing the range to the force range used by our operators slightly decreases these numbers to 6.7% for ctO2Hb, 5.5% for ctHHb, 1.9% for ctH2O, and 2.2% for lipid. While higher than those reported in the previous example, these numbers are still representative of the placement variations in repeated measurements, and thus likely do not represent significant force dependence.
Fig. 5.
(Color online) DOS Parameters measured as a function of probe–breast contact force in three patients with lesions. The error bars represent the variance of five repeated measurements at a given force (0% being “very light” and 100% being “pretty hard”). Typical contact force is approximately 30% on this scale.
DOS parameter variation as a function of contact force for lesion subjects showed a similar trend as with normal subjects (Fig. 6). Error bars represent the population variance. As before, we observed that ctO2Hb varied the most, followed by ctHHb, ctH2O, and lastly lipid. There were no significant differences between the CV%’s between each population for any DOS variable, as verified by standard ANOVA. However, the small number of subjects makes precise statistical assertions tenuous. The least significant number of subjects needed to demonstrate statistically significant differences in the CV%’s between premenopausal, postmenopausal, and lesion subjects is 56 for ctO2Hb. Demonstrating the existence of force-dependent tissue optical properties is important but is better measured using significantly higher forces than the ones we have employed in this study.
Fig. 6.
(Color online) Comparison of relative variance in DOS measurements for all probe–breast contact forces in three subjects with breast lesions. CV% is defined as the standard deviation of the population, divided by the average value of the population. The CV%’s are not significantly different from those in the normal patient population (see Fig. 4).
Overall, the force-dependent DOS variation averaged for all eight subjects in all categories of breast tissue was found to be 6.6 ± 3.0% in ctO2Hb, 4.6 ± 2.0% in ctHHb, 2.4 ± 1.5% in ctH2O, and 2.3 ± 1.1% in lipid. These results were averaged over the entire ranges of contact forces (0 to 100%). If we consider only the typical limited 20 to 50% pressure range, the population variation for all subjects becomes 5.6 ± 2.7% for ctHHbO2, 4.1 ± 1.7% for ctHHb, 1.5 ± 0.8% for ctH2O, and 1.7 ± 0.8% for lipids. The full comparison between force ranges for all patient categories is provided in Table 1.
4. Discussion
A. Limitations on the Quantification of Probe-Breast Forces
The use of a handheld probe in tissue optical spectroscopy raises potential concerns with respect to both intra- and interoperator variability. As a result, we have attempted to quantify the impact that relative changes in contact force and/or pressure have on a typical DOS measurement. It was difficult to provide a precise numerical value of the force and/or pressure because of several factors. First, the maximum force used for each patient was not the same. Thus, the 100% force level for each patient represented different absolute pressures, though in each case 100% represented a force the operator could apply without patient discomfort. Second, the distribution of force over the entire contact area was not measured because only a single force transducer was used. An array would be more suitable to determine the spatial dependence of the force. In addition, the contact area between probe and breast was not measured because only a fraction of the probe bottom was in contact with the patient. In general we expect the contact area to be similar in most cases. For these reasons, a simple, yet precise quantitative value for probe–breast contact force or pressure is unreliable.
Despite these limitations, the data strongly imply that the precision (i.e., repeatability) of the spectroscopy measurements from a single operator was not affected by variations in probe–breast contact force. Thus, absolute force quantitation is unnecessary because different forces for a given patient yielded very similar optical properties. In our experience, probe orientation, patient positioning, and spatial location repeatability are significantly more likely to produce inter- and/or intraoperator variations. These sources of variation can be minimized through training, whereas contact force/pressure is harder to standardize precisely. However, our results demonstrated that for all DOS measurements with trained operators, we can expect at worst 6–7% variation as a result of contact force or pressure variance.
The meaning of these numbers can be contextualized. We have observed changes in hemoglobin concentrations and water >50% and on the order of 20% in bulk lipids [4]. We have observed average changes in deoxy hemoglobin, oxy-hemoglobin and water of 27%, 33%, and 11%, respectively, from one week after baseline in tumors that responded to adriamycin/-cytoxan [21]. These changes are significantly larger than the variance observed as a function of contact force. Of course the variances will at some point mask physiological changes if they are small enough (i.e., small tumors, weak response). In the above-mentioned studies, only stage II/III lesions were considered.
One last consideration is that the range of forces used in this study is artificial. Based upon our experience, trained DOS operators typically use somewhere between 20 to 50% of the maximum contact forces presented in this study. Although the force-dependent variance is already low, it is further reduced if we consider only this typical 20% to 50% pressure range. Under these conditions, the population variation for all subjects becomes 5.6 ± 2.7% for ctHHbO2 (down from 6.5%), 4.1 ± 1.7% for ctHHb (down from 4.6%), 1.5 ± 0.8% for ctH2O (down from 2.5%), and 1.7 ± 0.8% for lipids (down from 2.3%).
B. Implications for Optical Imaging and Spectroscopic Handheld Probes
There are several disadvantages to handheld probes that should be addressed. These limitations have been discussed in the ultrasound literature and, in certain cases, may have relevance to optical probes. For example, the real-time nature of ultrasound scanning means that operator image interpretation factors heavily into the diagnostic process [22]. In contrast, other radiological exams are performed in prescribed protocols by technicians and later interpreted by a radiologist. The tissue/probe coupling is also a concern and artifacts may appear due to improper probe handling. Sampled volume differences between scans and operators offer another challenge. Some of these variations are minimized because of the extensive training and attention to standards issued by the medical ultrasound community. Interoperator variations have been reduced somewhat by the introduction of 3D scanning, and also by introducing criteria on the reporting of lesion features [23,24].
In the case of the handheld DOS probes, quantitative objective criteria (i.e., optical properties) are used to characterize tissue. Thus, the impact of operator interpretation on scan variation is likely to be minimal. Importantly, our absence of force sensitivity implies that quantitation can be maintained without careful regulation of the contact force and/or pressure. This has important implications for the reliability of longitudinal measurements [12,13].
C. Comparison with Other Research
Our studies should not be confused with efforts to deliberately compress the breast to alter blood volume [6,25–27]. While the exact compression force in our system is not easily calculated, it is well below forces used in studies that involve full compression or localized compression. For example, Carp et al. employed a mammography-type compression using 0, 3, 6, and 12 lbs, whereas our compression force was well below 1 lb [26]. Xu et al. used a handheld probe with a compression range of approximately 2 to 14 N, whereas ours was in the 1–2 N range [6]. In both cases our applied contact forces were well below those used in the cited compression studies.
Our results should also not be taken to mean that there is no force/pressure sensitivity for breast tissue; rather this effect, if any, is minimized by the way we perform DOS. We believe this is mainly due to the following factors: (1) the overall contact force is very small; (2) the probe surface area and source-detector separation are large, resulting in a relatively small force that is distributed over a large interrogated tissue volume; (3) the combined frequency-domain and broadband steady-state technique is generally insensitive to the absolute optical power, so that significant variance in coupling has a minimal effect on the measured optical properties.
Other optical imaging handheld probes present their own design-specific challenges. Most are limited in the number of detection channels: for example, one combined optical imaging and ultrasound probe has 8 channels and 2 wavelengths [3], another probe has 8 channels with 3 wavelengths [1], a flow probe has 1 wavelength and 1–4 channels [2], a compression probe has 8 channels and 2 wavelengths [6], and our DOS probe has 1 channel but acquires a continuous spectrum (1024 wavelengths) [4]. Investigators have commented on the great care that must be used to ensure good optical coupling to eliminate artifacts [1]. In particular, small-area fiber-based probes can introduce distortions in the tissue surface that can change recovered optical properties [28]. Although the performance of other devices can potentially vary with probe force, our results suggest that the use of a wide probe contact area, large-source-detector separation, and robust data analysis methods can help minimize this effect.
5. Conclusion
The standardization of measurements is of critical importance, especially when a handheld probe is employed that could induce operator-dependent measurements. Our combined frequency-domain and broadband steady-state DOS instrument uses a handheld probe; however, we have demonstrated that only small changes in DOS parameters are observed despite large variations in contact force. We therefore conclude that the effects of force and/or pressure upon our DOS measurements are small. Based upon our published measurements in breast tissues, the variance in force would not have masked the presence of stage II/III lesions or mask changes resulting from therapeutic intervention. For a trained operator, we can expect less than 5% variation in hemoglobin (ctO2Hb and ctHHb) and less than 2% variation in ctH2O and lipids.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) under grants P41-RR01192 (Laser Microbeam and Medical Program: LAMMP) and U54-CA105480 (Network for Translational Research in Optical Imaging: NTROI), the California Breast Cancer Research Program, and the Chao Family Comprehensive Cancer Center (P30-CA62203). BLI programmatic support from the Beckman Foundation and the Air Force Research Laboratory under agreement number FA9550-04-1-0101 is acknowledged. The authors thank Montana Compton for her assistance as well as the patients who generously volunteered for these studies.
Footnotes
OCIS codes: 170.3880, 170.3890, 170.5280, 170.6510, 170.6935, 120.3890.
References
- 1.Chance B, Nioka S, Zhang J, Conant EF, Hwang E, Briest S, Orel SG, Schnall MD, Czerniecki BJ. Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: a six-year, two-site study. Acad. Radiol. 2005;12:925–933. doi: 10.1016/j.acra.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Durduran T, Choe R, Yu G, Zhou C, Tchou JC, Czerniecki BJ, Yodh AG. Diffuse optical measurement of blood flow in breast tumors. Opt. Lett. 2005;30:2915–2917. doi: 10.1364/ol.30.002915. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Q, Cronin EB, Currier AA, Vine HS, Huang M, Chen N, Xu C. Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction. Radiology (Oak Brook, Ill.) 2005;237:57–66. doi: 10.1148/radiol.2371041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerussi A, Shah N, Hsiang D, Durkin A, Butler J, Tromberg BJ. In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J. Biomed. Opt. 2006;11:044005. doi: 10.1117/1.2337546. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Choe R, Shah N, Durduran T, Yu G, Durkin A, Hsiang D, Mehta R, Butler J, Cerussi A, Tromberg BJ, Yodh AG. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J. Biomed. Opt. 2007;12:051903. doi: 10.1117/1.2798595. [DOI] [PubMed] [Google Scholar]
- 6.Xu RX, Young DC, Mao JJ, Povoski SP. A prospective pilot clinical trial evaluating the utility of a dynamic near-infrared imaging device for characterizing suspicious breast lesions. Breast Cancer Res. 2007;9:R88. doi: 10.1186/bcr1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S N, Cerussi AE, Gordon D, Durkin A, Hill B, Compton M, Wenzel L, Tromberg BJ. Biomedical Topical Meeting (BIO) Optical Society of America; 2006. Integration of diffuse optical technology into clinical settings for breast health applications; p. SC2. [Google Scholar]
- 8.MacKinnon NB, Cardeno M, Au S, MacAulay CE, Pikkula BM, Serachitopol D, Follen M, Park SY, Richards-Kortum R. Advanced Biomedical and Clinical Diagnostic Systems V. SPIE; 2007. Design of a multispectral digital colposcope. [Google Scholar]
- 9.Pham TH, Coquoz O, Fishkin JB, Anderson E, Tromberg BJ. Broad bandwidth frequency domain instrument for quantitative tissue optical spectroscopy. Rev. Sci. Instrum. 2000;71:2500–2513. [Google Scholar]
- 10.Bevilacqua F, Berger AJ, Cerussi AE, Jakubowski D, Tromberg BJ. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl. Opt. 2000;39:6498–6507. doi: 10.1364/ao.39.006498. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Saltzman DJ, Cerussi AE, Gelfand DV, Milliken J, Waddington T, Tromberg BJ, Brenner M. Broadband diffuse optical spectroscopy measurement of hemoglobin concentration during hypovolemia in rabbits. Physiol. Meas. 2006;27:757–767. doi: 10.1088/0967-3334/27/8/009. [DOI] [PubMed] [Google Scholar]
- 12.Cerussi A, Hsiang D, Shah N, Compton M, Mehta R, Durkin AF, Tromberg B. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4014–4019. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakubowski DB, Cerussi AE, Bevilacqua F, Shah N, Hsiang D, Butler J, Tromberg BJ. Monitoring neoadjuvant chemotherapy in breast cancer using quantitative diffuse optical spectroscopy: a case study. J. Biomed. Opt. 2004;9:230–238. doi: 10.1117/1.1629681. [DOI] [PubMed] [Google Scholar]
- 14.Tanamai W, Cerussi A, Hsiang D, Mehta R, Tromberg BJ. Assessing final pathological response to neoadjuvant chemotherapy using diffuse optical spectroscopy. Breast Cancer Research and Treatment. 2007:S218. [Google Scholar]
- 15.Kukreti S, Cerussi A, Tanamai V, Tromberg B, Rita M, David H, Gratton E. Intrinsic near-infrared spectroscopic biomarkers applied for evaluation of final pathological response to neaoadjuvant chemotherapy. Breast Cancer Research and Treatment. 2007:S250. [Google Scholar]
- 16.Klifa C, Li A, Hattangadi J, Shah N, Gibbs J, DeMicco E, Watkins M, Proctor E, Cerussi A, Tromberg B, Hylton N. Biomedical Topical Meeting (BIO) Optical Society of America; 2006. Study of breast tissue composition using magnetic resonance imaging and diffuse optical spectroscopy. paper SG4. [Google Scholar]
- 17.Zijlstra WG, Buursma A, Meeuwsen-van der Roest WP. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991;37:1633–1638. [PubMed] [Google Scholar]
- 18.Kou L, Labrie D, Chylek P. Refractive indices of water and ice in the 0.65–2:5 μm spectral range. Appl. Opt. 1993;32:3531–3540. doi: 10.1364/AO.32.003531. [DOI] [PubMed] [Google Scholar]
- 19.Eker C. Ph.D. thesis. Lund Institute of Technology; 1999. Optical characterization of tissue for medical diagnostics. [Google Scholar]
- 20.Cerussi AE, Jakubowski D, Shah N, Bevilacqua F, Lanning R, Berger AJ, Hsiang D, Butler J, Holcombe RF, Tromberg BJ. Spectroscopy enhances the information content of optical mammography. J. Biomed. Opt. 2002;7:60–71. doi: 10.1117/1.1427050. [DOI] [PubMed] [Google Scholar]
- 21.Cerussi A, Hsiang D, Shah N, Mehta R, Durkin A, Butler J, Tromberg BJ. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4014–4019. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finberg HJ. Whither (wither?) the ultrasound specialist? J. Ultrasound Med. 2004;23:1543–1547. doi: 10.7863/jum.2004.23.12.1543. [DOI] [PubMed] [Google Scholar]
- 23.Meeks SL, Buatti JM, Bouchet LG, Bova FJ, Ryken TC, Pennington EC, Anderson KM, Friedman WA. Ultrasound-guided extracranial radiosurgery: technique and application. Int. J. Radiat. Oncol. Biol. Phys. 2003;55:1092–1101. doi: 10.1016/s0360-3016(02)04406-1. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology (Oak Brook, Ill.) 2006;239:385–391. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 25.Nioka S, Wen S, Zhang J, Du J, Intes X, Zhao Z, Chance B. Simulation study of breast tissue hemodynamics during pressure perturbation. Adv. Exp. Med. Biol. 2005;566:17–22. doi: 10.1007/0-387-26206-7_3. [DOI] [PubMed] [Google Scholar]
- 26.Carp SA, Kauffman T, Fang Q, Rafferty E, Moore R, Kopans D, Boas D. Compression-induced changes in the physiological state of the breast as observed through frequency domain photon migration measurements. J. Biomed. Opt. 2006;11:064016. doi: 10.1117/1.2397572. [DOI] [PubMed] [Google Scholar]
- 27.Boverman G, Fang Q, Carp SA, Miller EL, Brooks DH, Selb J, Moore RH, Kopans DB, Boas DA. Spatiotemporal imaging of the hemoglobin in the compressed breast with diffuse optical tomography. Phys. Med. Biol. 2007;52:3619–3641. doi: 10.1088/0031-9155/52/12/018. [DOI] [PubMed] [Google Scholar]
- 28.Jiang S, Pogue BW, Paulsen KD, Kogel C, Poplack SP. In vivo near-infrared spectral detection of pressure-induced changes in breast tissue. Opt. Lett. 2003;28:1212–1214. doi: 10.1364/ol.28.001212. [DOI] [PubMed] [Google Scholar]