Abstract
Hemoglobin-based oxygen carriers (HBOCs) are solutions of cell-free hemoglobin (Hb) that have been developed for replacement or augmentation of blood transfusion. It is important to monitor in vivo tissue hemoglobin content, total tissue hemoglobin [THb], oxy- and deoxy-hemoglobin concentrations ([OHb], [RHb]), and tissue oxygen saturation (StO2=[OHb]/[THb]×100%) to evaluate effectiveness of HBOC transfusion. We designed and constructed a broadband diffuse optical spectroscopy (DOS) prototype system to measure bulk tissue absorption and scattering spectra between 650 and 1000 nm capable of accurately determining these tissue hemoglobin component concentrations in vivo. Our purpose was to assess the feasibility of using DOS to optically monitor tissue [OHb], [RHb], StO2, and total tissue hemoglobin concentration ([THb]=[OHb]+[RHb]) during HBOC infusion using a rabbit hypovolemic shock model. The DOS prototype probe was placed on the shaved inner thigh muscle of the hind leg to assess concentrations of [OHb], [RHb], [THb], as well as StO2. Hemorrhagic shock was induced in intubated New Zealand white rabbits (N=6) by withdrawing blood via a femoral arterial line to 20% blood loss (10–15 cc/kg). Hemoglobin glutamer-200 (Hb-200) 1:1 volume resuscitation was administered following the hemorrhage. These values were compared against traditional invasive measurements, serum hemoglobin concentration (sHGB), systemic blood pressure, heart rate, and blood gases. DOS revealed increases of [THb], [OHb], and tissue hemoglobin oxygen saturation after Hb-200 infusion, while blood total hemoglobin values continued did not increase; we speculate, due to hyperosmolality induced hemodilution. DOS enables noninvasive in vivo monitoring of tissue hemoglobin and oxygenation parameters during shock and volume expansion with HBOC and potentially enables the assessment of efficacy of resuscitation efforts using artificial blood substitutes.
Keywords: hemorrhagic shock, blood substitute, diffuse optical spectroscopy
1 INTRODUCTION
Hemorrhage remains a leading cause of death in civilian and military trauma patients, and a major cause of morbidity and mortality in patients with medical illnesses, vascular events, and patients undergoing surgery. Limitations, availability, costs, infection risks, logistical issues, and complications of blood transfusions for resuscitation of hemorrhage have led to extensive efforts to develop safe and effective blood substitutes.1 A number of blood substitute approaches have been pursued, including development of an approved commercially available polymerized hemoglobin-based veterinary blood oxygen carrier (HBOC) product, hemoglobin glutamer-200 (Hb-200, Oxyglobin®, Biopure Corporation, Cambridge, Massachusetts).1–14
The goals of resuscitation from severe hemorrhage include restoration of hemodynamic stability and tissue oxygen delivery.15 Standard methods for assessment of adequacy of resuscitation during hemorrhage, including hematocrit, serum hemoglobin concentration, cardiac output, blood pressure, and pulse rate, are insensitive and nonspecific due to dynamic physiologic and reflex responses of the hemorrhaging patient.16–19 These difficulties in assessing adequacy of hemorrhage resuscitation are further compounded during blood substitute resuscitation due to interaction of complex factors, including differences between blood and cell-free blood substitutes.1,10,13 Some of the factors that may differentially affect tissue perfusion and oxygenation during hemoglobin-based blood substitute treatment include polymerized heme structures, heterogeneous polymer size distribution, NO scavenging, gas transfer from cell stromal free composition, and osmolality effects.1,6,12,13,20,21
Recently developed noninvasive diffuse optical spectroscopy (DOS) technology has been demonstrated to have the potential to noninvasively assess the physiologic changes associated with hemorrhage-induced reductions in tissue perfusion and the ability to indicate the degree of peripheral tissue hemoglobin oxygenation.15,22–27 DOS is a near-infrared (NIR) optical spectroscopic approach that can measure complex tissue-scattering induced light signal attenuation and use this information in the determination of broadband NIR absorption spectra in tissues, resulting in improved quantification due to its ability to measure absolute tissue chromophore concentrations compared to standard NIR methods, particularly in conditions where tissue scattering properties are changing dynamically, such as hemorrhage. This information can then be translated into quantitative measurements of the NIR absorbing components of the tissue including [OHb], [RHb], [THb], and tissue oxygen saturation (StO2= [OHb]/[THb] × 100%). Because absorption spectra of the oxygenated and deoxygenated Hb-200 are nearly identical to those of OHb and RHb,2–5 we proposed that noninvasive DOS should be capable of assessing the tissue response to hemorrhage and hemoglobin-based blood substitute resuscitation.
In this study, we investigate the ability of DOS to quantitatively detect changes in peripheral tissue perfusion occurring in vivo during hemorrhage and hemoglobin-based blood substitute resuscitation. We compare noninvasive DOS measures of [THb], StO2, [OHb], and [RHb] to standard hemorrhage assessment measures of heart rate, arterial pressures, and stroke volume during graded hemorrhage and blood substitute resuscitation to determine the ability of DOS to assess resuscitation response at the target tissue level. The major purpose of this study is to exemplarily demonstrate how DOS technology can assess tissue oxygenation restoration during HBOC infusion.
2 Materials and Methods
The study was approved by the Institutional Laboratory Animal Care and Use Committee, University of California, Irvine, and by the U.S. Air Force Animal Research programs, and performed in accordance with Federal, Department of Defense, and State Regulations.
The methods for animal preparation, anesthesia, monitoring, and DOS measurements have been previously described15,26,28 and are summarized below.
2.1 Anesthesia and Intubation
Male New Zealand white rabbits (N=6) (Western Oregon Rabbit Supply, Philomath, OR) weighing 3–5 kg (median 3.9 kg, mean 3.9 kg, standard error 0.166 and range 0.97 kg) were anesthetized with 2:1 ratio of ketamine HC1 (100 mg/mL) (Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, Michigan):Xylazine (20 mg/mL) (Anased, Lloyed Laboratories, Shenandoa, Iowa) at a dose of 0.75 mL/kg IM. After the IM injection a 22–24 gauge 1-in. catheter was placed in the animal’s marginal ear vein to administer intraverious (IV) anesthesia and secured with 1-in. standard porous adhesive tape. Maintenance anesthetic was dosed at 0.3 mL of 1:1 mixture of ketamine:xylazine IV (ketamine 100 mg/mL:xylazine 20 mg/mL) or continuous infusion of the 20 cc of the 1:1 solution diluted to 50 cc total in NS infused at 0.17 cc/min as needed. Depth of anesthesia was monitored according to established guidelines. Animals were intubated with a 3.0 endotracheal tube and mechanically ventilated (Harvard Apparatus dual phase control respirator, Holliston, Massachusetts) at respiration rate of 32/ min and a tidal volume of 50 cc and FiO2 of 100%. Pulse oximetry was accomplished with a probe placed on the tongue to measure SaO2 (Biox 3700 Pulse Oximeter, Ohmeda, Boulder, Colorado) and compared to arterial blood gas measurements.
2.2 Vascular Access, Blood Pressure, Blood Gasses
Left femoral arterial and femoral venous central lines (Cook 4.0 Fr, 18 Ga femoral artery catheter set, Cook, Inc., Bloomington, Indiana) were placed for arterial blood draws and systemic pressures. Pressures were obtained using a calibrated pressure transducer (TSD104A transducer and MP100 WSW System, Biopac Systems, Inc, Santa Barbara, California) and stored digitally. Mean, systolic, and diastolic pressures were determined from 5–10 s tracings. After each blood draw, lines were flushed with <0.5 cc of heparin (Elkins-Sinn, Inc., Cherry Hill, New Jersey) to prevent line thrombus occlusion. Arterial blood samples were measured with a blood gas analyzer calibrated to rabbit blood (IRMA Series 2000 Blood Analysis System, Diametrics Medical Inc., St Paul, Minnesota). Central venous blood samples were drawn from the femoral vein. Complete blood counts (CBC) were obtained from collected samples of venous blood and sent to an outside facility (Antech Diagnostics, Irvine, California) for analysis.
2.3 Noninvasive Measurements (Broadband Diffuse Optical Spectroscopy)
Detailed analysis of broadband DOS and the prototype system we constructed in our laboratory has been described previously.23,29–31 A multiwavelength, frequency domain (FD) photon migration instrument32,33 was combined with a steady-state (SS) NIR spectrometer for the noninvasive in vivo measurement of tissue chromophore concentration. A plastic probe containing the source and detector fibers was placed on the anterior medial surface of the right hind thigh for broad-band DOS measurement. The source and detector separation of 10 mm is used for both FD and SS acquisitions. The broad-band DOS prototype we used employs five laser diodes (661, 681, 783, 805, and 850 nm) and a fiber-coupled avalanche photodiode (APD) detector (Hamamatsu high-speed APD module C5658, Bridgewater, New Jersey). The APD detects the intensity-modulated diffuse reflectance signal at modulation frequencies between 50 and 300 MHz after propagating through the tissue. The absorption and reduced scattering coefficients are measured directly at each of the six laser diode wavelengths using the frequency-dependent phase and amplitude data. The reduced scattering coefficient is calculated throughout the NIR by fitting a power law to these five reduced scattering coefficients , where λ is wave-length, a is prefactor, and SP is scattering power).34–36 The acquired reduced scattering spectrum characterizes the scattering properties of tissue throughout the NIR. The SS acquisition is a broadband NIR reflectance measurement from 650 to 1000 nm that follows the FD measurements using a tungsten-halogen light source (Ocean Optics HL-2000, Dunedin, Florida) and a miniature spectrometer (BWTEK BTC611E, Newark, Delaware). It takes ~30 s to make a broadband DOS measurement. The intensity of the SS reflectance measurements are calibrated to the FD values of absorption and scattering to establish the absolute reflectance intensity. The absolute SS reflectance spectra are then analyzed to calculate tissue absorption coefficient (μa) spectra. Finally, the tissue concentrations of OHb, RHb, and H2O are calculated by a linear least-squares fit of the wavelength-dependent extinction coefficient spectra of each chromophore. The concentration calculation was conducted after each experiment using real-time data acquired during the study.
2.4 Experimental Procedures
A three-step, 20% hemorrhage model was used for these studies.15,26
After animal preparation, anesthesia, and intubation, baseline physiologic variable measurements were obtained, along with noninvasive DOS assessment of THb, OHb, and RHb. A 20% total hemorrhage goal was calculated (15 cc/kg). One-third of the calculated total hemorrhage volume was removed by withdrawal from the femoral arterial line over 30–60 s. Repeat physiologic and DOS measurements were obtained following the blood removal steps. The blood drawing and measurement process was repeated at 5 and 10 min until the 20% blood-loss goal was achieved after the third hemorrhage. Measurements took approximately 10–30 s to obtain.
After completion of the hemorrhage phase measurements, the animals were begun on a slow continuous infusion of Hb-200, Oxyglobin at a rate of 0.5 cc/min. Oxyglobin is a solution of ~13 g/dL of purified, polymerized bovine hemoglobin diluted in a modified Lactated Ringers solution. The Hb-200 infusion was continued until an infused volume equal to the total amount of blood that had been previously withdrawn was attained. Physiologic and DOS measurements were performed at one-third, two-thirds, and completion of the Oxyglobin infusion.
At completion of the experiment, each animal was euthanized with an intravenous injection of Eutha-6 (1.0–2.0 mL) through the marginal ear-vein catheter according to animal laboratory guidelines and the approved IACUC protocol.
2.4.1 Statistical analysis
One-way repeated measures analysis of variance (ANOVA) was used for comparison of outcome variables using SYSTAT ® version 10 (Systat Software, Inc., Point Richmond, CA). All data are presented as mean ± standard error (SE), and statistical significance were inferred when p<0.05.
3 Results
Table 1 lists the concentrations of broadband DOS OHb, RHb, THb, and StO2 as well as sHgb during each hemorrhage step and Oxyglobin resuscitation step.
Table 1.
Broadband DOS OHb, RHb, THb concentrations, and tissue oxygen saturation (StO2) and sHgb during hemorrhage and Oxyglobin resuscitation. Data are from six animals and are presented as mean±standard error.
| OHb (mM) | RHb (mM) | THb (mM) | StO2 (%) | sHgb (g/dL) | |
|---|---|---|---|---|---|
| Baseline | 35.3±6.5 | 18.9±2.6 | 54.2±8.5 | 63.4±4.2 | 14.8±0.4 |
| Hem 1 | 31.4±5.5 | 19.2±2.7 | 50.6±7.7 | 60.8±3.6 | 13.9±0.4 |
| Hem 2 | 28.4±5.4 | 20.0±2.7 | 48.4±7.4 | 56.9±4.4 | 13.0±0.4 |
| Hem 3 | 25.0±4.7 | 20.1±2.8 | 45.1±6.7 | 53.7±4.8 | 12.1±0.4 |
| Res 1 | 26.6±4.7 | 20.6±3.2 | 47.0±7.3 | 55.3±4.1 | 12.1±0.5 |
| Res 2 | 28.3±4.8 | 20.5±3.7 | 48.8±8.0 | 57.3±4.0 | 11.9±0.4 |
| Res 3 | ±29.0±4.8 | 21.4±3.9 | 50.4±8.1 | 56.9±4.0 | 11.9±0.5 |
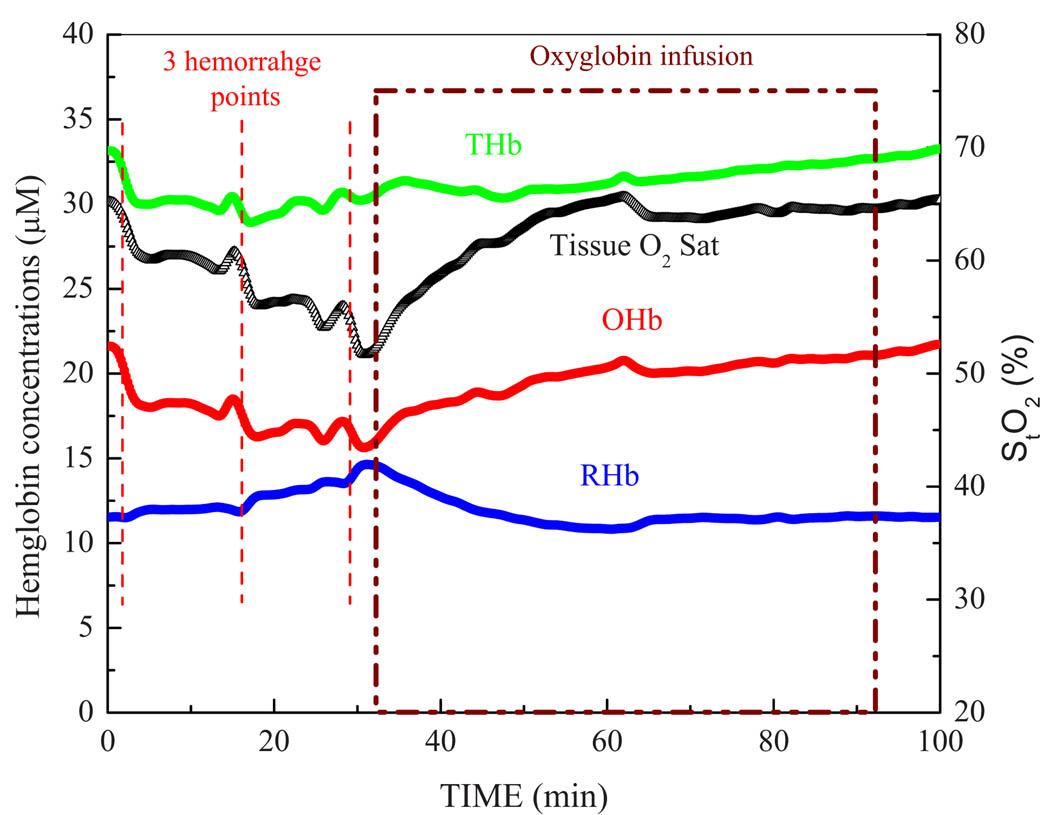
Figure 1 illustrates the hemoglobin concentrations ([OHb], [RHb], and [THb]=([OHb]+[RHb]) and StO2 during hemorrhage steps and continuous infusion of Oxyglobin as resuscitation fluid in a single animal. In this example, broadband DOS acquired the measurement every 10 s during the entire procedure.
Fig 1.
Continuous monitoring of tissue hemoglobin concentrations and StO2 using noninvasive DOS. DOS measurements were taken every 10 s. Data shown above are from a single rabbit.
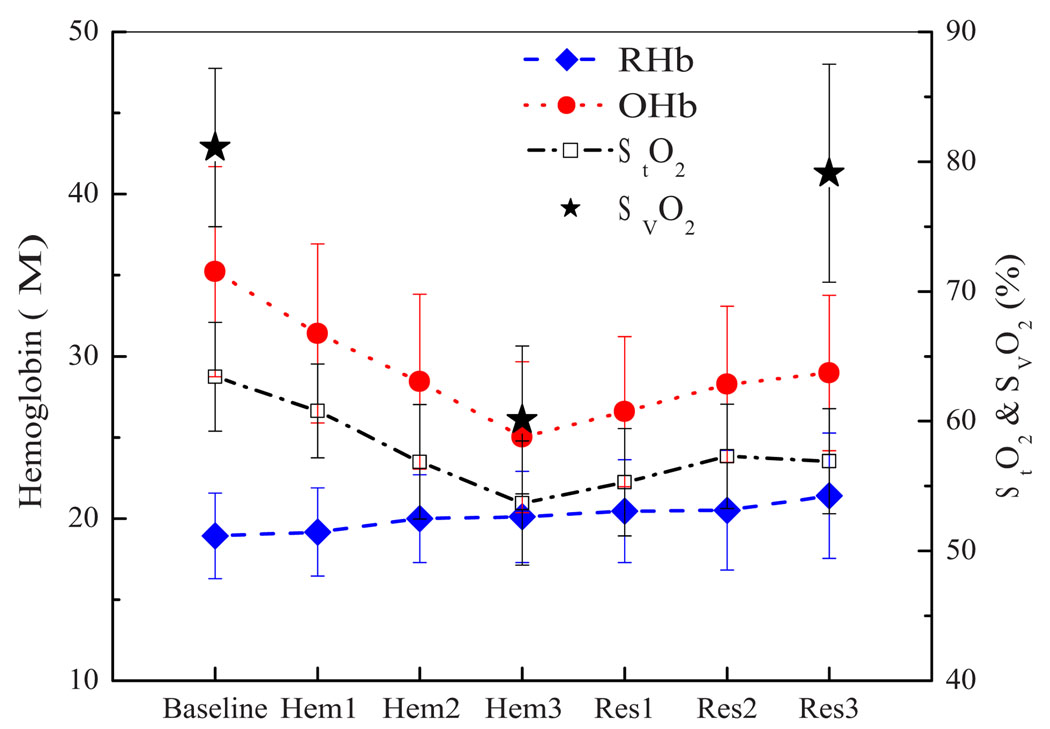
Figure 2 depicts the absolute concentrations of RHb, OHb, and tissue O2 saturation (StO2) during hemorrhage and resuscitation using Oxyglobin. [RHb] stayed relatively constant throughout hemorrhage and resuscitation phases (p=0.207, repeated measures ANOVA with all seven steps). [OHb] decreased significantly within subjects after each hemorrhage step (p<0.001) and increased during Oxyglobin infusion (p<0.001). StO2 decreased during the hemorrhage phase (p<0.001), but changes in StO2 were not statistically significant during the resuscitation (p=0.147), although the trend was similar to that of the increase in [OHb]. Venous oxygen saturation (SvO2) values from blood gas at baseline, the completion of hemorrhage and after Oxyglobin resuscitation are also plotted in Fig. 2, and they showed a similar trend. SvO2 significantly dropped during hemorrhage phase from the baseline value (−21%) and recovered back to the baseline after Oxyglobin infusion (p<0.05).
Fig 2.
DOS RHb and OHb concentrations and StO2 during hemorrhage and resuscitation using Oxyglobin. Venous oxygen saturation values at baseline, at the end of hemorrhage, and at the end of Oxyglobin resuscitation are also noted. Error bars represent standard error. Data are from six animals.
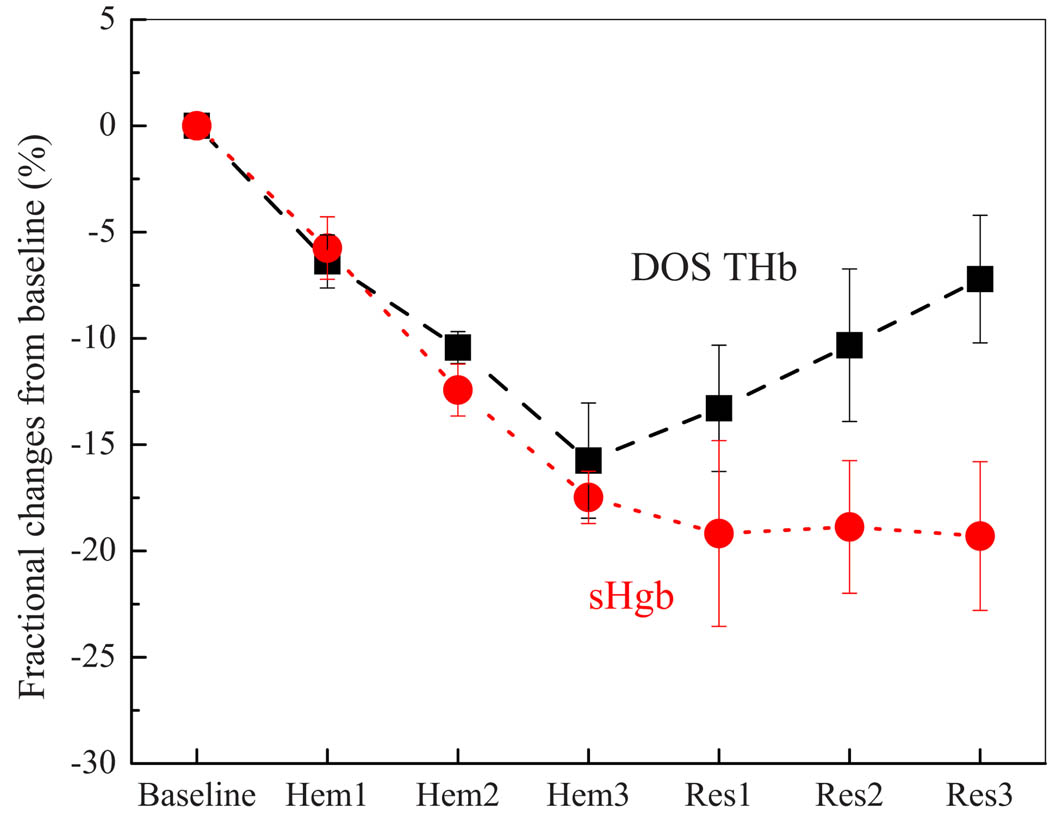
Figure 3 shows the normalized changes in DOS THb and sHgb during and hemorrhage steps and resuscitation with continuous infusion of Hb-200, Oxyglobin. Both DOS THb and sHgb were normalized by their respective baseline concentrations and fractional changes are plotted. Both DOS THb and sHgb decreased during hemorrhage phase (p<0.001). In order to determine the effect of Oxyglobin infusion on DOS THb and sHgb, repeated measures ANOVA were performed using the Hem3 time points (completion of hemorrhage) as the posthemorrhage baseline values. Changes in sHgb were insignificant (p=0.92), while DOS THb increased significantly after Oxyglobin infusion (p=0.002).
Fig 3.
Fractional changes of DOS THb and sHgb from baseline during hemorrhage and resuscitation using Oxyglobin. [RHb] stayed relatively constant throughout hemorrhage and resuscitation phases (p=0.207, repeated measures ANOVA with all seven steps). [OHb] decreased significantly within subjects after each hemorrhage step (p<0.001) and increased during Oxyglobin infusion (p<0.001). StO2 decreased during the hemorrhage phase (p<0.001), but changes in StO2 were not statistically significant during the resuscitation (p=0.147), although the trend was similar to that of the increase in [OHb]. Error bars represent standard error. Data are from six animals.
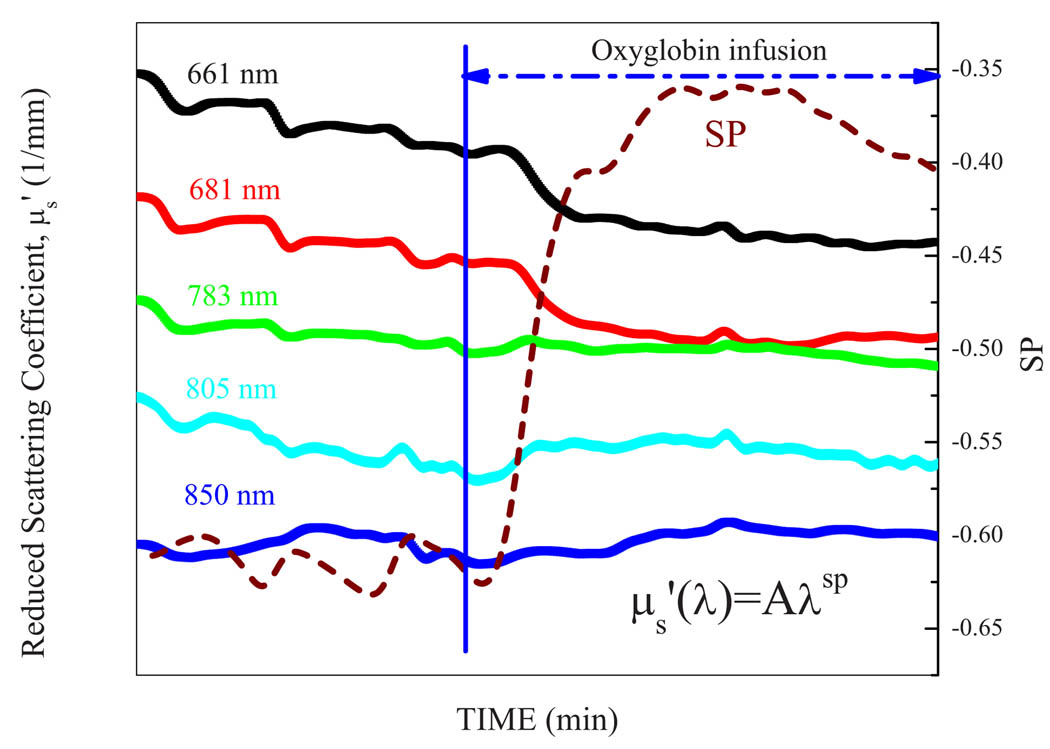
In Fig. 4 scattering changes were assessed during hemorrhage and Oxyglobin resuscitation. SP is determined by obtaining a power law fit of five individual at five laser diode wavelengths (, where λ is wavelength, a is prefactor). Scattering properties at the five discreet wavelengths sampled, as well as the power-fit SP showed demonstrated substantial changes during hemorrhage and blood substitute resuscitation.
Fig 4.
Example of changes in reduced scattering coefficient, (mm−1) during hemorrhage and Oxyglobin resuscitation. SP stands for scattering power of a power law fit of five individual at five laser diode wavelengths (, where λ is wavelength and a is a prefactor) in a representative rabbit. These data show the importance of accounting for the substantial scattering changes occurring with hemorrhage and blood substitute resuscitation when utilizing near-infrared spectroscopic methods.
4 Discussion
Improvements in diagnosis, monitoring, and optimization of treatment for patients with hemorrhage are essential for advances in medical, surgical, and trauma care. Advances in resuscitation products, including development of artificial blood substitutes and methods for assessing the adequacy of resuscitation at the target tissue level, are needed. Early changes that have been shown to occur with reduction in central blood volume during hemorrhage include reduction in cardiac output and decreased delivery of oxygen in the blood to peripheral tissues.18,19,37,38
In this study, as in previous studies, we have demonstrated the ability of noninvasive DOS to detect changes in tissue perfusion resulting from reduction in blood volume induced by progressive hemorrhage.15,26,27 The ability of DOS to quantitatively determine the extent of scattering-induced light attenuation provides for the ability to accurately obtain measurements of [THb], [OHb], [RHb], as well as tissue hemoglobin oxygen saturation under conditions of changing scattering properties as experienced during hemorrhage and resuscitation.15,23,24,26,27 Because DOS measures the average concentration of these components over the region of interrogation, the contributions of the arterioles, capillaries, and venules are included in the composite average tissue hemoglobin component concentration measurements.
As previously shown,15,26,27 during blood loss from hemorrhage, the [THb] measured by DOS decreases, likely due to a combination of vasoconstriction and decrease in intravascular hemoglobin (as a fraction of intravascular volume).15,27 During hemorrhage, the tissue [OHb] decreases, probably from a combination of physiologic factors including to vasoconstriction, decreased intravascular hemoglobin fraction, and increased conversion of OHb to RHb by the underperfused tissues.15 In contrast to decreasing [THb] and [OHb] during hemorrhage, tissue [RHb] levels do not change substantially during hemorrhage. This difference in response15 likely results in the decreased [RHb] from vasoconstriction, and decreased hematocrit are offset to varying degrees by increased [RHb] resulting from increased conversion of [OHb] to [RHb] at the tissue level.15 During resuscitation with whole blood, these hemorrhage-induced changes are reversed, as demonstrated in prior studies by our group15 using DOS and other groups using invasive methods.39,40
In the present study, a number of differences in DOS-measured responses are seen during blood substitute resuscitation compared to the previously demonstrated whole blood resuscitation effects. During the resuscitation phase, the total tissue hemoglobin measured by DOS increased toward baseline values with administration of hemoglobin-based blood substitute. This finding was expected due to the increased intravascular volume associated with blood substitute resuscitation, as well as the added hemoglobin content within the blood substitute.10,39 One might have predicted some blunting of this increase in total tissue hemoglobin concentration due to a possible component of NO scavenging-induced vasoconstriction reported with poly-heme-based blood substitutes, such as Oxyglobin, and other potential mechanisms leading to increased vascular resistance.1,20,21,39,41–45 However, such effects, if present, did not appear to substantially alter the increase in DOS-measured total tissue hemoglobin, suggesting that increased vascular volume predominates during blood substitute resuscitation as administered. These findings are consistent with findings from invasive hemodynamic monitoring studies in animals during HBOC resuscitation.40
In marked contrast to the DOS measured [THb], concurrently measured serum hemoglobin concentrations obtained from invasive blood samples did not rise during blood substitute resuscitation. This is not surprising given the fact that the HBOC, Oxyglobin, has a hemoglobin concentration of 13 g/dL, an osmolality of 300, and a colloid osmotic pressure of 43 mmHg.1 Administration of replacement fluid with this combination of hyper-osmolality and a 13 g/dL hemoglobin concentration, which is only slightly above that of the animals following acute hemorrhage (intravascular post hemorrhage hemoglobin concentration, averaged 12.1±0.4 g/dL compared to prehemorrhage baseline values of 14.8±0.4 g/dL), would be expected to draw fluid into the vascular space resulting in substantial increases in intravascular volume, but should cause only slight (if any) increases in intravascular hemoglobin concentration.6–8,20
These findings are consistent with prior studies where Oxyglobin infusion has been shown to restore hemodynamic parameters, including cardiac output and mesenteric arterial blood flow following hemorrhage, with increases in mean arterial blood pressure above baseline, but not to restore total intravascular hemoglobin and arterial blood oxygen content to baseline.20 The hyperoncotic properties of Oxyglobin result in rapid volume expansion with homogeneous microvascular perfusion and the ability to facilitate diffusive oxygen transfer, accelerating metabolic recovery.20 This would explain the discrepancy between DOS [THb] measurements (which include both intravascular hemoglobin concentration and tissue vascular volume) versus invasively obtained blood sample hemoglobin concentrations (which include only the intravascular hemoglobin concentration component) during blood substitute resuscitation. We propose that the DOS measurements therefore may reflect a more accurate measure of adequacy of tissue perfusion than serum hemoglobin concentrations alone10 under these conditions.
DOS measurements of tissue [OHb] demonstrate a reversal of the hemorrhage-induced tissue OHb reductions during resuscitation. These findings are paralleled by an improvement in measured venous blood oxygenation obtained by invasive blood gas analysis and suggest the ability to detect reversal of the increased oxygen extraction accompanied by increased conversion of OHb to RHb that had been induced during hypoperfusion. Using invasive muscle blood flow and muscle oxygen partial pressure measurements, Dreissen et al.39 and Cheung et al.40 had shown similar improvements in muscle tissue perfusion and oxygenation with Oxyglobin HBOC resuscitation following hemorrhage. The degree of reversal appears to be dependent on HBOC concentrations achieved during the resuscitation process.39,40
Substantial scattering changes occur in vivo during hemorrhage and resuscitation26 as a result of vascular changes, including vasoconstriction, vasodilation, and concentration of scatter-inducing red blood cells within the vasculature. During blood substitute resuscitation, the vasoactive effects of HBOCs, volume loading effects on the vasculature, as well as the absence of scattering cellular stroma all may possibly contribute to complex scattering alterations in bulk tissue during the blood substitute resuscitation phase. These scattering changes result in inaccuracies in standard near-infrared spectroscopy hemoglobin measurements that are overcome using DOS technology.
Stroma-free hemoglobin solutions have also been susceptible to methemoglobin (Met-Hgb) formation.20 Because of the marked spectral shifts in Met-Hgb, DOS is very sensitive in quantitatively detecting Met-Hgb levels in vivo,25 providing another potential advantage of DOS in monitoring blood substitute resuscitation. In the current studies, no significant increases in DOS-measured Met-Hgb levels were seen during short term follow-up of acute resuscitation with Oxyglobin HBOC.
There are a number of limitations in this study. DOS measures average tissue constituents to a depth of ~5 mm (at the source detector separation of 10 mm used in the studies). We have chosen to monitor peripheral muscle region as an indicator of central organ blood flow as suggested in previous studies.15,17,18,26 However, the DOS system that we employed uses a single-source detector separation that has the disadvantage of including superficial skin and fat regions in the interrogation field, which may behave differently during hemorrhage and resuscitation compared to the deeper muscle tissues.17,18,46
In addition, the current system measures at only one peripheral site. Regional variability, local traumatic injury, ischemia or hemorrhage at the site of measurement, or specific organ dysfunction would not be detected with the current design. Future planned designs of DOS device prototypes with collection in parallel from a number of sites and source detector separations could improve the quality and practicality of multisite data collection.
Within the DOS hemoglobin signals, there may be some component of interference by other NIR absorbers, such as tissue myoglobin. Although this may be of theoretical interest, from a practical standpoint it may not be necessary to fully separate these component contributions when examining tissue hemoglobin and hemoglobin oxygenation clinical effects of hemorrhage because they may be all be collective indicators of adequacy of perfusion.
We did not perform a specific set of control animal experiments for this particular study because previous studies from our laboratory have shown that Hb-O2, Hb-R, [THb] concentrations, and StO2 remain within 5% of baseline values at comparable time points. Likewise, sHgb, SaO2, SvO2, CO, and mean arterial pressure remained all within 6% of baseline values at the end of the previously reported study period, with the minimal changes seen being attributed to exposure to anesthesia effects and blood withdrawals for blood gas and sHgb analysis.26
5 Conclusions
Although there are a number of limitations of the current study, these promising initial findings suggest that noninvasive DOS measurements may be very useful for detection and optimization of the effects of blood substitute resuscitation on peripheral perfusion. Assessment of effectiveness of blood substitute resuscitation by conventional methods is currently limited because of the complexities of reflex responses to acute hemorrhage, discrepancies between volume of blood loss and changes in serum hemoglobin, as well as differences between stroma-free hemoglobin-based blood substitutes compared to red cell–based blood transfusions. This study demonstrates that DOS measurements may overcome some of these issues by providing information on the adequacy of restoration of tissue hemoglobin concentration and oxygenation. Future studies will be needed to determine the role of DOS in assessing the effects of hemorrhage and blood substitute resuscitation in humans.
Acknowledgments
This research was supported by funding from Grants No. AF-9550-04-1-0101, No. FOS-2004-0011A, and No. FOS-2004-0012A, and LAMMP No. 445474-30136. Blood substitute Hemoglobin glutamer-200 (Oxyglobin) was kindly supplied by Biopure, Inc, Cambridge, MA.
Contributor Information
Jangwoen Lee, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612.
Jae G. Kim, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612
Sari Mahon, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612.
Bruce J. Tromberg, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612
David Mukai, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612.
Kelly Kreuter, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612.
Darin Saltzman, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612.
Renee Patino, University of California, Irvine Department of Medicine Division of Pulmonary and Critical Care 200 South Manchester Boulevard, Suite 720 Orange, California 92868.
Robert Goldberg, University of California, Irvine Department of Medicine Division of Pulmonary and Critical Care 200 South Manchester Boulevard, Suite 720 Orange, California 92868.
Matthew Brenner, University of California, Irvine Beckman Laser Institute and Medical Clinic 1002 Health Sciences Road East Irvine, California 92612 and University of California, Irvine Department of Medicine Division of Pulmonary and Critical Care 200 South Manchester Boulevard, Suite 720 Orange, California 92868.
References
- 1.Driessen B, Jahr JS, Lurie F, Gunther RA. Inadequacy of low-volume resuscitation with hemoglobin-based oxygen carrier hemoglobin glutamer-200 (bovine) in canine hypovolemia. J. Vet. Pharmacol. Ther. 2001;24(1):61–71. doi: 10.1046/j.1365-2885.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 2.Jahr JS, Lurie F, Driessen B, Davis JA, Gosselin R, Gunther RA. The HemoCue, a point of care B-hemoglobin photometer, measures hemoglobin concentrations accurately when mixed in vitro with canine plasma and three hemoglobin-based oxygen carriers (HBOC) Can. J. Anaesth. 2002;49(3):243–248. doi: 10.1007/BF03020522. [DOI] [PubMed] [Google Scholar]
- 3.Jahr JS, Driessen B, Lurie F, Tang Z, Louie RF, Kost G. Oxygen saturation measurements in canine blood containing hemoglobin glutamer-200 (Bovine): in vitro validation of the NOVA CO-Oximeter. Vet. Clin. Pathol. 2001;30(1):39–45. doi: 10.1111/j.1939-165x.2001.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 4.Lurie F, Jahr JS, Driessen B. The novel HemoCu plasma/low hemoglobin system accurately measures small concentrations of three different hemoglobin-based oxygen carriers in plasma: hemoglobin glutamer-200 (bovine) (Oxyglobin), hemoglobin glutamer-250 (bovine) (Hemopure), and hemoglobin-Raffimer (Hemolink) Anesth. Analg. (Baltimore) 2002;95(4):870–873. doi: 10.1097/00000539-200210000-00014. TOC. [DOI] [PubMed] [Google Scholar]
- 5.Jahr JS, Lurie F, Driessen B, Tang Z, Louie RF, Kost G. Validation of oxygen saturation measurements in a canine model of hemoglobin-based oxygen carrier infusion. Am. J. Ther. 2003;10(1):21–28. doi: 10.1097/00045391-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Driessen B, Jahr JS, Lurie F, Golkaryeh MS, Gunther RA. Arterial oxygenation and oxygen delivery after hemoglobin-based oxygen carrier infusion in canine hypovolemic shock: a dose-response study. Crit. Care Med. 2003;31(6):1771–1779. doi: 10.1097/01.CCM.0000063476.79749.C1. [DOI] [PubMed] [Google Scholar]
- 7.Posner LP, Moon PF, Bliss SP, Gleed RD, Erb HN. Colloid osmotic pressure after hemorrhage and replenishment with Oxyglobin Solution, hetastarch, or whole blood in pregnant sheep. Vet. Anaesth. Analg. 2003;30(1):30–36. doi: 10.1046/j.1467-2995.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 8.Callan MB, Rentko VT. Clinical application of a hemoglobin-based oxygen-carrying solution. Vet. Clin. North Am. Small Anim. Pract. 2003;33(6):1277–1293. doi: 10.1016/s0195-5616(03)00119-0. vi. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenberger M. Transfusion medicine in exotic pets. Clin. Tech. Small Anim. Pract. 2004;19(2):88–95. doi: 10.1053/j.ctsap.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Cheung AT, Driessen B, Jahr JS, Duong PL, Ramanujam S, Chen PC, Gunther RA. Blood substitute resuscitation as a treatment modality for moderate hypovolemia. Artif. Cells Blood Substit Immobil Biotechnol. 2004;32(2):189–207. doi: 10.1081/bio-120037827. [DOI] [PubMed] [Google Scholar]
- 11.March H, Barger A, McCullough S, Schaeffer D, Macwilliams P. Use of the ADVIA 120 for differentiating extracellular and intracellular hemoglobin. Vet. Clin. Pathol. 2005;34(2):106–109. doi: 10.1111/j.1939-165x.2005.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 12.Buehler PW, Boykins RA, Jia Y, Norris S, Freedberg DI, Alayash AI. Structural and functional characterization of glutaraldehyde-polymerized bovine hemoglobin and its isolated fractions. Anal. Chem. 2005;77(11):3466–3478. doi: 10.1021/ac050064y. [DOI] [PubMed] [Google Scholar]
- 13.King DR, Cohn SM, Proctor KG. Resuscitation with a hemoglobin-based oxygen carrier after traumatic brain injury. Sitzungsber. K. Preuss. Akad. Wiss. 2005;59(3):553–560. 59(3), discussion 560-552. [PubMed] [Google Scholar]
- 14.Cheung AT, Duong PL, Driessen B, Chen PC, Jahr JS, Gunther RA. Systemic function, oxygenation and microvascular correlation during treatment of hemorrhagic shock with blood substitutes. Clin. Hemorheol Microcirc. 2006;34(1–2):325–334. [PubMed] [Google Scholar]
- 15.Lee J, Cerussi AE, Saltzman D, Waddington T, Tromberg BJ, Brenner M. Hemoglobin measurement patterns during noninvasive diffuse optical spectroscopy monitoring of hypovolemic shock and fluid replacement. J. Biomed. Opt. 2007;12(2):024001. doi: 10.1117/1.2715189. [DOI] [PubMed] [Google Scholar]
- 16.Wo CC, Shoemaker WC, Appel PL, Bishop MH, Kram HB, Hardin E. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit. Care Med. 1993;21(2):218–223. doi: 10.1097/00003246-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Soller BR, Yang Y, Soyemi OO, Ryan KL, Rickards CA, Walz JM, Heard SO, Convertino VA. Noninvasively determined muscle oxygen saturation is an early indicator of central hypovolemia in humans. J. Appl. Physiol. 2008;104(2):475–481. doi: 10.1152/japplphysiol.00600.2007. [DOI] [PubMed] [Google Scholar]
- 18.Soller BR, Ryan KL, Rickards CA, Cooke WH, Yang Y, Soyemi OO, Crookes BA, Heard SO, Convertino VA. Oxygen saturation determined from deep muscle, not thenar tissue, is an early indicator of central hypovolemia in humans. Crit. Care Med. 2008;36(1):176–182. doi: 10.1097/01.CCM.0000295586.83787.7E. [DOI] [PubMed] [Google Scholar]
- 19.Gosain A, Rabkin J, Reymond JP, Jensen JA, Hunt TK, Upton RA. Tissue oxygen tension and other indicators of blood loss or organ perfusion during graded hemorrhage. Surg. Today. 1991;109(4):523–532. [PubMed] [Google Scholar]
- 20.Driessen B, Jahr JS, Lurie F, Gunther RA. Effects of isovolemic resuscitation with hemoglobin-based oxygen carrier Hemoglobin glutamer-200 (bovine) on systemic and mesenteric perfusion and oxygenation in a canine model of hemorrhagic shock: a comparison with 6% hetastarch solution and shed blood. Vet. Anaesth. Analg. 2006;33(6):368–380. doi: 10.1111/j.1467-2995.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- 21.Kubulus D, Rensing H, Paxian M, Thierbach JT, Meisel T, Redl H, Bauer M, Bauer I. Influence of heme-based solutions on stress protein expression and organ failure after hemorrhagic shock. Crit. Care Med. 2005;33(3):629–637. doi: 10.1097/01.ccm.0000156295.48075.49. [DOI] [PubMed] [Google Scholar]
- 22.Tromberg BJ, Coquoz O, Fishkin JB, Anderson ER, Pham D, Brenner M, Svaasand LO. Frequency-domain photon migration (FDPM) measurements of normal and malignant cell and tissue optical properties. In: Sevick-Muraca E, Benaron D, editors. Biomedical Optical Spectroscopy and Diagnostics. 1996. pp. 111–116. [Google Scholar]
- 23.Pham TH, Coquoz O, Fishkin JB, Anderson E, Tromberg BJ. Broad bandwidth frequency domain instrument for quantitative tissue optical spectroscopy. Rev. Sci. Instrum. 2000;71(6):2500–2513. [Google Scholar]
- 24.Albert C, Richard Van W, Feizal W, Bruce T. Noninvasive monitoring of red blood cell transfusion in very low birthweight infants using diffuse optical spectroscopy. J. Biomed. Opt. 2005;10(5):051401. doi: 10.1117/1.2080102. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, El-Abaddi N, Duke A, Cerussi AE, Brenner M, Tromberg BJ. Noninvasive in vivo monitoring of methemoglobin formation and reduction with broadband diffuse optical spectroscopy. J. Appl. Physiol. 2006;100(2):615–622. doi: 10.1152/japplphysiol.00424.2004. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Saltzman DJ, Cerussi AE, Gelfand DV, Milliken J, Waddington T, Tromberg BJ, Brenner M. Broadband diffuse optical spectroscopy measurement of hemoglobin concentration during hypovolemia in rabbits. Physiol. Meas. 2006;27(8):757–767. doi: 10.1088/0967-3334/27/8/009. [DOI] [PubMed] [Google Scholar]
- 27.Pham TH, Hornung R, Ha HP, Burney T, Serna D, Powell L, Brenner M, Tromberg BJ. Noninvasive monitoring of hemodynamic stress using quantitative near-infrared frequency-domain photon migration spectroscopy. J. Biomed. Opt. 2002;7(1):34–44. doi: 10.1117/1.1427046. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Armstrong J, Kreuter K, Tromberg BJ, Brenner M. Non-invasive in vivo diffuse optical spectroscopy monitoring of cyanide poisoning in a rabbit model. Physiol. Meas. 2007;28(9):1057–1066. doi: 10.1088/0967-3334/28/9/007. [DOI] [PubMed] [Google Scholar]
- 29.Bevilacqua F, Berger AJ, Cerussi AE, Jakubowski D, Tromberg BJ. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl. Opt. 2000;39(34):6498–6507. doi: 10.1364/ao.39.006498. [DOI] [PubMed] [Google Scholar]
- 30.Jakubowski DJ. PhD thesis. Irvine: Beckman Laser Institute, University of California; 2002. Developement of broadband quantitative tissue optical spectroscopy for the non-invasive characterization of breast disease. [Google Scholar]
- 31.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasnad L, Butler J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Opt. Eng. 2000;2(1–2):26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fantini S, Franceschini M, Maier JS, Walker SA, Barbieri B, Gratton E. Frequency-domain multichannel optical detector for noninvasive tissue spectroscopy and oximetry. Opt. Eng. 1995;34:32–42. [Google Scholar]
- 33.Pogue BW, Patterson MS. Frequency-domain optical absorption spectroscopy of finite tissue volumes using diffusion theory. Phys. Med. Biol. 1994;39:1157–1180. doi: 10.1088/0031-9155/39/7/008. [DOI] [PubMed] [Google Scholar]
- 34.Graaff R, Aarnoose JG, Zijp JR, Sloot PMA, de Mul FFM, Greve J, Koelink MH. Reduced light-scattering properties for mixtures of spherical particles: a simple approximation derived from Mie calculation. Appl. Opt. 1992;31:1370–1376. doi: 10.1364/AO.31.001370. [DOI] [PubMed] [Google Scholar]
- 35.Mourant JR, Fuselier T, Boyer J, Johnson T, Bigio IJ. Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl. Opt. 1997;36:949–957. doi: 10.1364/ao.36.000949. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt JM, Kumar G. Optical scattering properties of soft tissue: a discrete particle model. Appl. Opt. 1998;37:2788–2797. doi: 10.1364/ao.37.002788. [DOI] [PubMed] [Google Scholar]
- 37.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J. Appl. Physiol. 2004;96(4):1249–1261. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- 38.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am. J. Physiol. 1998;275(6 Pt 2):R1909–R1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 39.Driessen B, Zarucco L, Gunther RA, Burns PM, Lamb SV, Vincent SE, Boston RA, Jahr JS, Cheung AT. Effects of low-volume hemoglobin glutamer-200 versus normal saline and arginine vasopressin resuscitation on systemic and skeletal muscle blood flow and oxygenation in a canine hemorrhagic shock model. Crit. Care Med. 2007;35(9):2101–2109. doi: 10.1097/01.CCM.0000277040.31978.3D. [DOI] [PubMed] [Google Scholar]
- 40.Cheung AT, To PL, Chan DM, Ramanujam S, Barbosa MA, Chen PC, Driessen B, Jahr JS, Gunther RA. Comparison of treatment modalities for hemorrhagic shock. Artif. Cells Blood Substit Immobil Biotechnol. 2007;35(2):173–190. doi: 10.1080/10731190601188257. [DOI] [PubMed] [Google Scholar]
- 41.Standl T. Haemoglobin-based erythrocyte transfusion substitutes. Expert Opin. Biol. Ther. 2001;1(5):831–843. doi: 10.1517/14712598.1.5.831. [DOI] [PubMed] [Google Scholar]
- 42.Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc. Res. 1996;32(4):632–643. [PubMed] [Google Scholar]
- 43.Spahn DR, Leone BJ, Reves JG, Pasch T. Cardiovascular and coronary physiology of acute isovolemic hemodilution: a review of nonoxygen-carrying and oxygen-carrying solutions. Anesth. Analg. (Baltimore) 1994;78(5):1000–1021. doi: 10.1213/00000539-199405000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Winslow RM. Current status of blood substitute research: towards a new paradigm. J. Intern Med. 2003;253(5):508–517. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 45.Winslow RM. Current status of oxygen carriers (‘blood substitutes’): 2006. Vox Sang. 2006;91(2):102–110. doi: 10.1111/j.1423-0410.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit. Care. 2004;8(5):373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]