New Cdc2 Tyr 4 phosphorylation by dsRNA-activated protein kinase triggers Cdc2 polyubiquitination and G2 arrest under genotoxic stresses
Cdc2 is a subunit of M-phase kinase, which plays a key role in G2/M transition. Bae and colleagues investigate the regulation of Cdc2 under genotoxic stress. They find that PKR kinase mediates Cdc2 Tyr4 phosphorylation and that this plays a crucial role in Cdc2 downregulation and G2 arrest in cells treated with anti-cancer drugs.
Keywords: Cdc2, polyubiquitination, PKR, Tyr 4 phosphorylation, G2/M transition
Abstract
Cell division cycle 2 (Cdc2) protein is an essential subunit of M-phase kinase (MPK), which has a key role in G2/M transition. Even though the control of MPK activity has been well established with regard to the phosphorylation of Cdc2 at Thr 14 and/or Tyr 15 and Thr 161, little is known about the proteolytic control of Cdc2. In this study, we observed that Cdc2 was downregulated under genotoxic stresses and that double-stranded RNA-activated protein kinase (PKR) was involved in the process. The PKR-mediated Tyr4 phosphorylation triggered Cdc2 ubiquitination. Phospho-mimic mutations at the Tyr 4 residue (Y4D or Y4E) caused significant ubiquitination of Cdc2 even in the absence of PKR. Our findings demonstrate that (i) PKR, Ser/Thr kinase, phosphorylates its new substrate Cdc2 at the Tyr 4 residue, (ii) PKR-mediated Tyr 4-phosphorylation facilitates Cdc2 ubiquitination and proteosomal degradation, (iii) unphosphorylated Tyr 4 prevents Cdc2 ubiquitination, and (iv) downstream from p53, PKR has a crucial role in G2 arrest and triggers Cdc2 downregulation under genotoxic conditions.
Introduction
The G2/M transition in eukaryotes depends on the activity of the cell division cycle 2 (Cdc2) protein–cyclin B complex, known as the M-phase kinase (MPK; Labbe et al, 1988). Although the control of MPK activity has been well established with regard to Cdc2 phosphorylation and dephosphorylation at Thr 14, Tyr 15 and Thr 161 (Gautier et al, 1991; Mueller et al, 1995), little is known about the proteolytic control of Cdc2.
Double-stranded RNA (dsRNA)-activated protein kinase (PKR) was first identified as a cellular antiviral component induced by type I interferon (IFN-α/β; Kerr et al, 1977). Recently, however, several different roles have been suggested for PKR as a negative regulator of cell growth when induced under stress conditions or overexpressed (Alisi et al, 2005; Lee et al, 2007). We showed that PKR is a new p53 target gene and has a significant role in the tumour suppressor function of p53, such as G2 arrest and cell apoptosis, under genotoxic conditions (Yoon et al, 2009). In the study, we also observed Cdc2 downregulation downstream from the p53/PKR pathway under genotoxic conditions. It has been suggested that genotoxin-induced G2 arrest is probably associated with the inhibition of Cdc2 expression (Taylor & Stark, 2001; Le Gac et al, 2006). A previous study reported that PKR downregulated Cdc2 and cyclin B1 when overexpressed ectopically in Chinese hamster ovary cells (Dagon et al, 2001). However, to the best of our knowledge, no study has systematically examined the detailed mechanisms underlying PKR-mediated Cdc2 downregulation and associated G2 arrest under genotoxic conditions.
Results
PKR is involved in Cdc2 downregulation
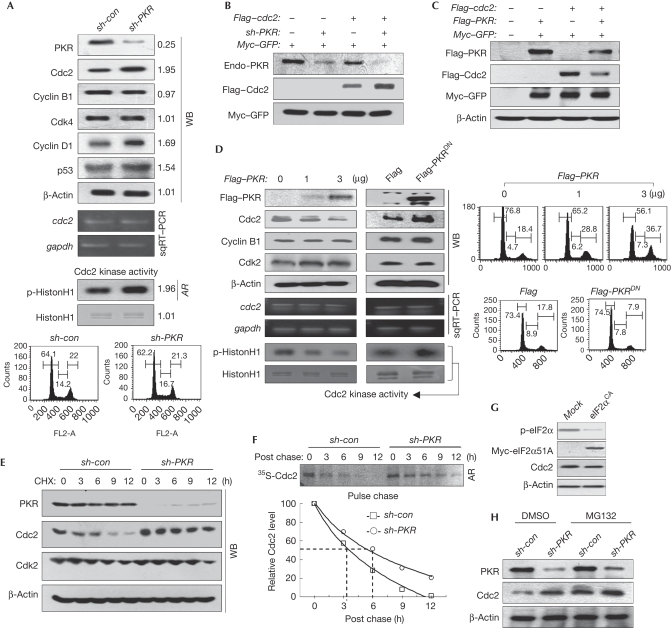
Endogenous Cdc2 levels and Cdc2-associated kinase activity were increased markedly, together with cyclin D1 and p53 as reported (Baltzis et al, 2007; Raven et al, 2008), in the PKR-knockdown (PKRKD) cells without any changes in the levels of cdc2 messenger RNA (mRNA) and other associated proteins at the same cell-cycle stage (Fig 1A). Furthermore, PKRKD cells had a high level of ectopic Cdc2 (Flag–cdc2) relative to the PKR-normal cells (Fig 1B). Ectopic expression of PKR reduced the level of ectopic Cdc2 (Fig 1C). Ectopic expression of PKR downregulated endogenous Cdc2 in a dose-dependent manner, which was accompanied by G2 arrest, whereas the levels of cdc2 mRNA and other proteins, such as Cdk2 or cyclin B1, were not affected by PKR expression (Fig 1D). In addition, the level of Cdc2 in cells transfected with a catalytically dead form of PKR, K296R (dominant-negative mutant; PKRDN), increased without affecting cdc2 mRNA levels and cell cycle (Fig 1D). This indicates that the catalytically active PKR downregulates the Cdc2 levels after transcription. The level of cyclin B1 is defined further under Discussion. The bands for Cdc2 disappeared rapidly in PKR-normal cells but not in PKRKD cells under the same cyclohexamide conditions (Fig 1E), and the half-life of Cdc2 was increased significantly by PKR-knockdown when assessed by pulse-chase experiments (Fig 1F), indicating that PKR downregulates Cdc2 by destabilizing Cdc2 after translation. We could not detect any differences in the levels of Cdc2 expression between the normal and eukaryotic initiation factor 2α (eIF2α) 51A constitutively active (eIF2αCA) cells (Fig 1G), suggesting that PKR-mediated Cdc2 downregulation is not due to translation inhibition. The Cdc2 downregulation was not observed in MG132-treated PKR-normal cells (Fig 1H), which suggests that PKR-mediated Cdc2 downregulation is probably associated with proteasomal degradation.
Figure 1.
PKR downregulates Cdc2. (A) Cell extracts of PKR-normal (sh-con) or PKRKD (sh-PKR) HEK293 cells were subjected to WB analysis (top). The mRNA level was assessed by sqRT–PCR (upper middle). Cdc2-associated kinase activity was assessed as described in the supplementary information online (lower middle). Relative band intensities were assessed through MCID analysis software (www.mcid.co.uk). sh-con or sh-PKR HEK293 cells were subjected to cell cycle analysis after PI staining (bottom). (B) PKR-normal (sh-PKR−) or PKRKD (sh-PKR+) HEK293 cells were transfected with Flag–cdc2 plasmid, together with Myc–GFP control, and were subjected to WB analysis. (C) Cdc2 levels in HEK293 cells, co-transfected with the plasmid combinations, were assessed. (D) HEK293 cells transfected with an increasing amount of Flag–PKR (left) or 3 μg of Flag–PKR/K296R (PKRDN, right) plasmids were subjected to WB analysis (top), sqRT–PCR (middle), Cdc2-associated kinase assay (bottom) and flow cytometry after PI staining (right). (E) The stability of Cdc2 was assessed in sh-con and sh-PKR HEK293 cells cultured in the presence of 75 μM CHX. (F) The stability of Cdc2 was assessed by pulse chase experiments as described in the supplementary information online (top). Relative band intensity was quantified with ImageJ software (http://rsbweb.nih.gov/ij; bottom). (G,H) Cdc2 levels were assessed in eIF2αCA HEK293 cells (G) and in sh-con and sh-PKR HEK293 cells that were treated with or without MG132 for 5 h before collection (H). AR, autoradiogram; Cdc2, cell division cycle 2; CHX, cycloheximide; DMSO, dimethyl sulphoxide; eIF2αCA, eukaryotic initiation factor 2α constitutively active; GFP, green fluorescent protein; HEK, human embryonic kidney; mRNA, messenger RNA; PI, propidium iodide; PKR(KD), double-stranded RNA-activated protein kinase (knockdown); sqRT–PCR, semi-quantitative reverse transcriptase PCR; WB, western blot.
PKR is involved in the polyubiquitination of Cdc2
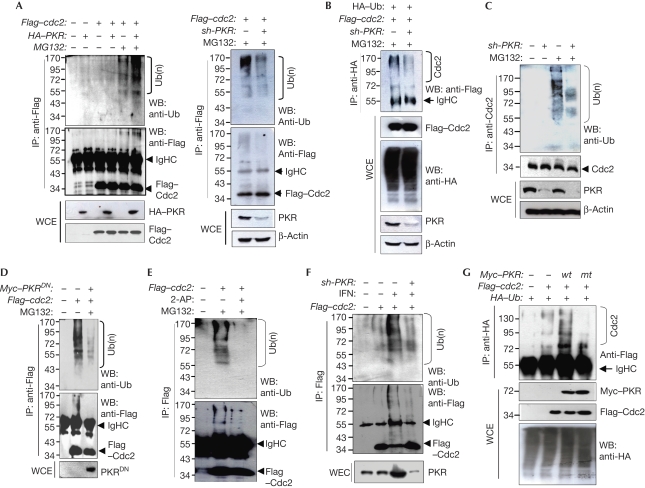
We then examined whether PKR is involved in Cdc2 ubiquitination or not. In cells co-transfected with Cdc2- and PKR-expressing plasmids, ectopically expressed Cdc2 (Flag–Cdc2 or Cdc2–Flag) was significantly polyubiquitinated in the presence of MG132 (Fig 2A; supplementary Fig S4A online). However, even in the presence of MG132, ectopic Cdc2 was ubiquitinated weakly in the PKRKD cells as compared with that in PKR-normal cells (Fig 2B). In the immunoprecipitation experiments, endogenous Cdc2 was ubiquitinated significantly in PKR-normal cells, but not in PKRKD cells when pretreated with MG132 (Fig 2C). The PKRDN form inhibited PKR-mediated Cdc2 ubiquitination, suggesting that catalytically active PKR is necessary for Cdc2 ubiquitination (Fig 2D). It was further evidenced by the results that Cdc2 ubiquitination was reduced significantly by treatment with 2-aminopurin (2-AP), a PKR-specific inhibitor (Fig 2E). Ectopically expressed Cdc2 was ubiquitinated strongly in the cells pretreated with type I IFN, whereas the band intensity was reduced markedly by PKR knockdown (Fig 2F), indicating that PKR is involved mainly in the IFN-mediated Cdc2 ubiquitination. This was confirmed further in the ectopic ubiquitin (Ub)-expressing haemagglutinin (HA) cells. In the immunoprecipitants of HA, ubiquitinated Cdc2 bands were detected clearly only in the wild-type PKR-expressing cells, not in the cells expressing mutant (mt) PKR (PKRDN; Fig 2G). Together, our findings suggest that PKR catalyses Cdc2 ubiquitination, resulting in the proteasomal degradation of Cdc2.
Figure 2.
PKR is involved in the polyubiquitination of Cdc2. (A) HEK293 cells co-transfected with Flag–cdc2 and/or HA–PKR plasmids were treated with MG132 as described in Methods. Cdc2 ubiquitination was assessed by WB analysis using anti-Ub after IP with anti-Flag. (B) PKR-normal (−) and PKRKD (+) cells were transfected with Flag–cdc2 plasmids. Cdc2 ubiquitinations were assessed after IP with anti-Flag (left) and anti-HA (right). (C) Cdc2 ubiquitinations were assessed in PKR-normal (−) and PKRKD (+) HEK293 cells. (D) Cdc2 ubiquitinations were assessed in HEK293 cells co-transfected with Flag–cdc2 and Myc–PKR/K296R (PKRDN) plasmids. (E) HEK293 cells, transfected with Flag–cdc2, were treated with 2 mM 2-AP for 24 h. Cdc2 ubiquitinations were assessed. (F) PKR-normal (−) and PKRKD (+) HEK293 cells were transfected with the Flag–cdc2 plasmid and then treated with 1,000 units of IFN-α for 24 h. Cdc2 ubiquitinations were assessed. (G) HEK293 cells were co-transfected with the Myc–PKR (wt or K296R mt), Flag–cdc2 and/or HA–Ub plasmids. Cdc2 band shifts were assessed. Cells were treated with MG132 before collection (F,G). All ubiquitination analyses were performed after normalization with Cdc2 in WCE or IP. 2-AP, 2-aminopurin; Cdc2, cell division cycle 2; HA, haemagglutinin; HEK, human embryonic kidney; IFN, interferon; IP, immunoprecipitation; mt, mutant; PKR(KD), double-stranded RNA-activated protein kinase (knockdown); Ub, ubiquitin; WB, western blot; WCE, whole cell extract; wt, wild type.
PKR phosphorylates amino-terminal Tyr 4 of Cdc2
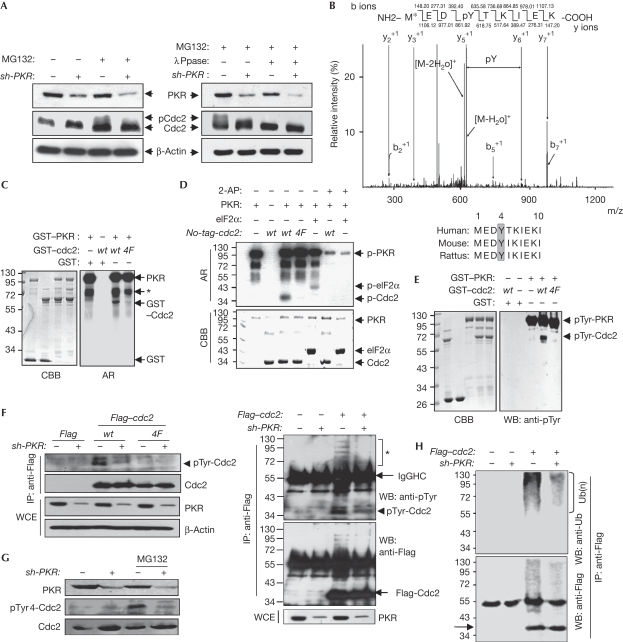
We next investigated whether PKR-mediated Cdc2 ubiquitination is associated with PKR-mediated Cdc2 phosphorylation. As shown in Fig 3A, Cdc2 bands were partly shifted in PKR-normal MG132-treated cells, and the shifted bands of Cdc2 disappeared by phosphatase (λPpase). However, these Cdc2 band shifts and the λPpase-mediated band disappearance were not observed in PKRKD cells (Fig 3A). These results suggest that PKR phosphorylates Cdc2, which probably leads to Cdc2 ubiquitination. In a tandem mass spectrometry analysis, Cdc2 was found to be phosphorylated at Tyr 4 on the N-terminal conserved sequence among species (Fig 3B). The PKR-mediated Cdc2 phosphorylation was abrogated by Y4F substitution or by 2-AP treatment in the in vitro kinase assays accompanied by autoradiography (Fig 3C,D) and western blot analysis (Fig 3E) regardless of the N-terminal glutathione-S-transferase tag (Fig 3D). Ectopic wild-type Cdc2 was also phosphorylated in PKR-normal but not in PKRKD cells, whereas mt Cdc2 (Y4F) was not phosphorylated in either cell type (Fig 3F). The phospho-Cdc2 band was detected clearly in the cells co-transfected with wild-type PKR and wild-type Cdc2, but not in cells co-transfected with either mt Cdc2 (Y4F) or with PKRDN (K296R; supplementary Fig S1 online). Furthermore, endogenous phospho-Cdc2 was detected in PKR-normal MG132-treated cells, but not detected clearly in PKRKD cells in the western blot analysis with phospho-Tyr 4-Cdc2 peptide-derived antiserum (Fig 3G; supplementary Fig S2 online). Together, our findings demonstrate that PKR phosphorylates the Cdc2 at Tyr 4. Phospho-Cdc2 was observed clearly by highly shifted multibands in the PKR-normal cells, but not in PKRKD cells (Fig 3H, left). When the same samples were analysed with the Ub antibody, highly shifted smeared band patterns were detected in the same lane (Fig 3H, right). These results suggest that PKR-mediated ubiquitination of Cdc2 seems to be associated with the PKR-mediated Cdc2 phosphorylation at Tyr 4. However, PKR-mediated Cdc2-Tyr 4 phosphorylation did not affect the inhibitory phosphorylation of Cdc2 at Thr 14 and Tyr 15 (supplementary Fig S3 online).
Figure 3.
PKR phoshorylates the Cdc2 at Tyr 4. (A) PKR-normal (−) and PKRKD (+) HEK293 cell extracts were treated with protein phosphatase (λPpase) at 30°C for 1 h. Protein levels and phosphorylation-mediated Cdc2 band shift were assessed. (B) ESI-MS/MS spectrum of Cdc2 tryptic peptides after the in vitro kinase reaction with PKR. The bottom panel shows the amino-terminal conserved sequence of Cdc2 among species. (C–E) Wild-type and mt (Y4F) Cdc2, GST-tagged (C,E) or untagged Cdc2 and 6His–eIF2α (D) recombinant proteins were incubated with preactivated PKR in the presence or absence of 5 mM 2-AP, and Cdc2 phosphorylations were assessed by autoradiogram (C,D) and WB analysis (E). The asterisk indicates PKR fragments. (F) PKR-normal (−) and PKRKD (+) HEK293 cells were transfected with wild-type or mt (Y4F) Flag–cdc2 plasmids. Cdc2 phosphorylations were assessed with pTyr mAb. (G) Endogeneous phospho-Cdc2 levels in PKR-normal (−) and PKRKD (+) HEK293 cells were assessed with rabbit pTyr-Cdc2 peptide-derived antiserum, prepared as described in supplementary information online. (H) PKR-normal (−) and PKRKD (+) HEK293 cells were transfected with Flag–cdc2 plasmid. Anti-Flag IP samples were assessed by WB analysis with pTyr (upper left) and Ub (upper right) mAbs after Cdc2 normalization. Both membranes were washed and restained with Flag mAb (lower). The asterisk indicates a shifted multiband detected by pTyr mAb. 2-AP, 2-aminopurin; AR, autoradiogram; CBB, Coomassie brilliant blue; Cdc2, cell division cycle 2; eIF2α, eukaryotic initiation factor 2α; GST, glutathione-S-transferase; HEK, human embryonic kidney; IP, immunoprecipitation; mAB; monoclonal antibody; mt, mutant; PKR(KD), double-stranded RNA-activated protein kinase (knockdown); Ub, ubiquitin; WB, western blot; WCE, whole cell extract; wt, wild type.
Tyr 4 phosphorylation facilitates Cdc2 ubiquitination
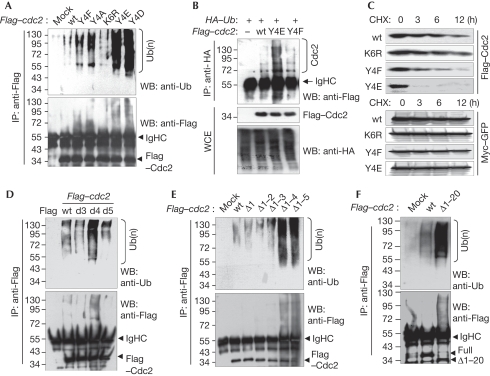
We next examined whether the PKR-mediated Tyr 4-phosphorylation of Cdc2 is inevitable for the polyubiquitination of Cdc2. As shown in Fig 4A, phosphor-mimicking Cdc2 mutants (Y4D and Y4E) were ubiquitinated strongly compared with wild-type Cdc2 (Tyr 4) and other mutants. Unexpectedly, Y4A and Y4F Cdc2 mutants were also ubiquitinated slightly higher than wild-type Cdc2 (Fig 4A). These results are delineated further under Discussion. In the immunoprecipitants of ectopic ubiquitin (HA–Ub), ubiquitinated Cdc2 bands were detected more clearly in Y4E mt Cdc2-expressing cells than in wild-type or Y4F mt-expressing cells (Fig 4B). We next investigated the stability of Cdc2 mutants. As shown in Fig 4C, the Y4E phosphor-mimicking mutant was degraded most rapidly (within 3 h) in the wild-type and mt Cdc2s. The Y4F mutant was also unstable compared with the wild-type or K6R mt (Fig 4C). The PKR-mediated ubiquitination and the stability of ectopic wild-type/mt Cdc2 were not impaired by N/carboxy-terminal Flag tag (supplementary Fig S4 online). We next examined several amino-acid deletion mutants to determine the role of unmodified Tyr 4 in Cdc2. Interestingly, the Tyr 4-deletion mutant was ubiquitinated most potently (Fig 4D) and the most unstable (supplementary Fig S5 online) among the three deletion mutants. The ubiquitination and associated stability of wild-type Cdc2 were changed significantly by PKR overexpression or knockdown, whereas those of Tyr 4 substitution or Tyr 4 deletion mutants of Cdc2 were not changed by the presence or absence of PKR (supplementary Fig S6 online). In the N-terminal serial deletion analysis, Cdc2 ubiquitination and the associated instability were not enhanced significantly until the first four amino-acid deletions (Fig 4E; supplementary Fig S7 online). In addition, the N-terminal 20 amino-acid deletion mutant of Cdc2 was ubiquitinated strongly compared with the wild-type Cdc2 (Fig 4F). These results suggest that (i) the Tyr 4 residue of Cdc2 has an important role in protecting Cdc2 from ubiquitination-mediated proteasomal degradation, and (ii) either Tyr 4 phosphorylation or genetic mutations at this site facilitate Cdc2 polyubiquitination.
Figure 4.
Phosphorylation or mutation of Cdc2 at Y4 facilitates the polyubiquitination of Cdc2. (A) HEK293 cells were transfected with Flag-tagged wild-type or mt cdc2 plasmids. Cdc2 ubiquitinations were assessed after IP. (B) HEK293 cells were transfected with wild-type or mt Flag–cdc2 plasmids together with HA–Ub plasmid. Cdc2 ubiquitinations were assessed. (C) HEK293 cells, co-transfected with wild-type and mt Flag–cdc2 and Myc–GFP plasmids, were treated with 75 μM CHX for the indicated time periods, and Cdc2 stabilities were assessed. (D–F) HEK293 cells were transfected with wild-type or (D) single amino-acid deletion mt, (E) amino-terminal serial deletion mutants or (F) N-terminal first 20 amino-acid deletion (Δ1–20) mt of Flag–cdc2. Cdc2 ubiquitinations were assessed. The stability of each mt and wild-type Cdc2 was assessed by CHX chase assay (bottom, D,E). All ubiquitination assays were performed after normalization with Cdc2. Cdc2, cell division cycle 2; CHX, cycloheximide; GFP, green fluorescent protein; HA, haemagglutinin; HEK, human embryonic kidney; IP, immunoprecipitation; mt, mutant; PKR, double-stranded RNA-activated protein kinase; Ub, ubiquitin; WB, western blot; wt, wild type.
G2 arrest and PKR-mediated Cdc2 degradation
We next examined whether genotoxin-mediated G2 arrest is associated with PKR-mediated Cdc2 degradation. In this study, we observed that the Cdc2 level and Cdc2 kinase activity were enhanced significantly in PKRKD cells compared with that in PKR-normal cells under several genotoxic conditions (supplementary Fig S8A online). Cdc2 levels were downregulated by doxorubicin (Dox) in a dose-dependent manner, but this downregulation was obliterated by PKR knockdown under the same conditions (supplementary Fig S8B online). Even under the genotoxic conditions, the Cdc2-enhancing patterns were detected in the PKRKD p53+/+ cells, and the patterns were similarly observed in the isogenic p53−/− cells (supplementary Fig S8C online). In response to Dox, Cdc2 was ubiquitinated significantly in the PKR-normal cells, whereas the ubiquitination was clearly diminished in the PKRKD or 2-AP-treated cells (supplementary Fig S8D online). These results suggest that Cdc2 downregulation by anti-cancer drugs is attributable largely to PKR-mediated Cdc2 ubiquitination, downstream from p53. As expected, Dox/etoposide-mediated G2 arrest was attenuated by PKR knockdown in human colorectal tumour cell line (HCT) 116 p53+/+ cells, whereas this attenuation by PKR knockdown was not observed in isogenic HCT116 p53−/− cells (supplementary Fig S9A online), suggesting that PKR has an important role in genotoxin-mediated G2 arrest downstream from p53. In addition, the G2 arrest by Dox was enhanced significantly by Cdc2 knockdown (si-cdc2) in both PKR-normal and PKRKD cells (supplementary Fig S9B online). The PKR-mediated G2 arrest was restored by Cdc2 overexpression (supplementary Fig S10 online). The PKRKD cells grew faster than normal cells and were resistant to Dox, but the effects of PKR knockdown were almost obliterated in the Cdc2 knockdown cells (supplementary Fig S11A online). These data imply that genotoxin-mediated G2 arrest and the inhibition of cell proliferation were associated with PKR-mediated Cdc2 downregulation. The PKRKD tumours also grew faster than PKR-normal tumours (supplementary Fig S11B online) and proved to be resistant to anti-cancer drugs (supplementary Fig S11C,D online). Together, our findings indicate that G2 arrest and tumour suppression by anti-cancer drugs can be attributed largely to PKR-mediated Cdc2 downregulation.
Discussion
A previous study showed that ectopic expression of PKR downregulates not only Cdc2 but also cyclin B1 in Chinese hamster ovary cells (Dagon et al, 2001). However, we could not detect any changes in the level of cyclin B1 in both PKRKD and PKR-overexpressing cells compared with PKR-normal cells (Fig 1A,D). The difference might be due to the treatment with a synthetic hormone (ecdysone) analogue, muristerone A, in the previous study.
In the mass spectrometry analysis and follow-up mutation study, we found that PKR phosphorylates the Tyr 4 residue of Cdc2 (Fig 3). The kinase, PKR, has been known as a serine/threonine protein kinase. A recent study, however, showed that PKR autophosphorylates its own Tyr residues (at Tyr 101, Tyr 162 and Tyr 293) for its full-scale catalytic activity (Su et al, 2006). Nevertheless, there have been no reports showing that PKR phosphorylates the Tyr residue of its counter substrate. It is worth noting that PKR phosphorylates the Tyr residue on its new substrate Cdc2.
The PKR-mediated Cdc2 phosphorylation at Tyr 4 seemed to be the best condition for Cdc2 ubiquitination (Fig 4). However, a series of mutation studies suggested that Cdc2 ubiquitination was not dependent exclusively on PKR-mediated Cdc2 phosphorylation. Rather, Tyr 4 phosphorylation may cause certain interactions or disruptions that result in some type of conformational changes in Cdc2, which may allow the Cdc2 E3 ubiquitin ligase to access the ubiquitination sites on Cdc2 as represented in supplementary Fig S12 online. In other words, as opposed to the bindings of Skp-Cullin-F-box protein/β-transduction repeat-containing protein (SCFβ-TrCP) E3 ligase (Watanabe et al, 2004) or steroid receptor coactivator 3 (SRC3)-specific E3 ligase (Wu et al, 2007), the binding of Cdc2 E3 ligase to the target region on Cdc2 seems unlikely to be dependent on phosphorylation. This was shown further by the serial deletion assays shown in Fig 4D–F. These results imply that unmodified Tyr 4 is essential for the maintaining Cdc2 stability and the G2/M transition under normal conditions.
In this study, we found that PKR-mediated Cdc2 Tyr 4 phosphorylation has a key role in Cdc2 downregulation and G2 arrest as a molecular switch in the cells treated with anti-cancer drugs. The Cdc2-specific E3 ubiquitin ligase remains to be identified.
Methods
Cells. The HCT116 p53+/+ and p53−/− cells were provided by B. Vogelstein (Johns Hopkins Oncology Center, Baltimore, MA, USA). The PKR knockdown (sh-PKR transformed) human embryonic kidney 293 and HCT116 cells, and the eIF2αCA cells were prepared as described previously (Lee et al, 2007; Yoon et al, 2009). Cells were transiently knocked down by transfection with 100 nM specific small interfering (si)RNAs or non-targeting siRNA as a control (Stealth nontarget siRNA, GC medium composition) by using the Oligofectamine reagent (Invitrogen, San Diego, CA, USA). The sequence of Cdc2 siRNA: si-Cdc2; 5′-UGUACCAGAGUGUUACUACCUCAUG-3′.
Ubiquitination assay. Cells were treated with 20 μM MG132 for 5 h before collection. As described previously (Lee et al, 2009), Flag-tagged, HA–Ub-attached, or endogenous Cdc2 were immunoprecipitated with Flag, HA or Cdc2 monoclonal antibodies in immunoprecipitation (IP) buffer (50 mM Tris–HCl (pH 7.5), 50 mM NaCl, 0.5% NP40, 10 mM NaF and 10 mM Na3VO4) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN, USA) at 4°C for 4 h, and the immunoprecipitants were then washed five times with IP buffer. After normalization with immunoprecipitated Cdc2, Cdc2 ubiquitination was analysed as described previously (Wu et al, 2007) by using a horseradish peroxidase-conjugated Ub monoclonal antibody P4D1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This study was supported by the Specific Basement grant from the Korea National Research Foundation (2009-0084683), and the Bio New Drug grants (A085033) from the Korean Ministry of Health and Welfare. This study was also partly supported by 63 Research Fund of Sungkyunkwan University.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alisi A, Mele R, Spaziani A, Tavolaro S, Palescandolo E, Balsano C (2005) Thr 446 phosphorylation of PKR by HCV core protein deregulates G2/M phase in HCC cells. J Cell Physiol 205: 25–31 [DOI] [PubMed] [Google Scholar]
- Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE (2007) The eIF2α kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 282: 31675–31687 [DOI] [PubMed] [Google Scholar]
- Dagon Y, Dovrat S, Vilchik S, Hacohen D, Shlomo G, Sredni B, Salzberg S, Nir U (2001) Double-stranded RNA-dependent protein kinase, PKR, down-regulates CDC2/cyclin B1 and induces apoptosis in non-transformed but not in v-mos transformed cells. Oncogene 20: 8045–8056 [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW (1991) cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67: 197–211 [DOI] [PubMed] [Google Scholar]
- Kerr IM, Brown RE, Hovanessian AG (1977) Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature 268: 540–542 [DOI] [PubMed] [Google Scholar]
- Labbe JC, Lee MG, Nurse P, Picard A, Doree M (1988) Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cdc2+. Nature 335: 251–254 [DOI] [PubMed] [Google Scholar]
- Le Gac G, Esteve PO, Ferec C, Pradhan S (2006) DNA damage-induced down-regulation of human Cdc25C and Cdc2 is mediated by cooperation between p53 and maintenance DNA (cytosine-5) methyltransferase 1. J Biol Chem 281: 24161–24170 [DOI] [PubMed] [Google Scholar]
- Lee ES, Yoon CH, Kim YS, Bae YS (2007) The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis. FEBS Lett 581: 4325–4332 [DOI] [PubMed] [Google Scholar]
- Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, Ha NC, Lane DP, Song J (2009) Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J 28: 2100–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG (1995) Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270: 86–90 [DOI] [PubMed] [Google Scholar]
- Raven JF, Baltzis D, Wang S, Mounir Z, Papadakis AI, Gao HQ, Koromilas AE (2008) PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2α phosphorylation. J Biol Chem 283: 3097–3108 [DOI] [PubMed] [Google Scholar]
- Su Q, Wang S, Baltzis D, Qu LK, Wong AH, Koromilas AE (2006) Tyrosine phosphorylation acts as a molecular switch to full-scale activation of the eIF2α RNA-dependent protein kinase. Proc Natl Acad Sci USA 103: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815 [DOI] [PubMed] [Google Scholar]
- Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, Osada H (2004) M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc Natl Acad Sci USA 101: 4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RC, Feng Q, Lonard DM, O'Malley BW (2007) SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129: 1125–1140 [DOI] [PubMed] [Google Scholar]
- Yoon CH, Lee ES, Lim DS, Bae YS (2009) PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Natl Acad Sci USA 106: 7852–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.