Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O
The pathogenic bacterium Streptococcus pyogenes utilizes a pore-forming protein, streptolysin O (SLO) in a process called cytolysin-mediated translocation (CMT) to inject NAD+-glycohydrolase toxin (SPN) into the host cell cytosol. Caparon and colleagues probe the importance of pore formation using SLO mutants that are unable to form pores. They find that pore formation is not required for CMT, but that it is essential for the cytotoxicity of S. pyogenes.
Keywords: Streptococcus pyogenes, streptolysin O, NAD+ glycohydrolase, cytolysin-mediated translocation, cholesterol-dependent cytolysin
Abstract
Bacterial toxin injection into the host cell is required for the virulence of numerous pathogenic bacteria. Cytolysin-mediated translocation (CMT) of Streptococcus pyogenes uses streptolysin O (SLO) to translocate the S. pyogenes nicotinamide adenine dinucleotide-glycohydrolase (SPN) into the host cell cytosol, resulting in the death of the host cell. Although SLO is a pore-forming protein, previous studies have shown that pore formation alone is not sufficient for CMT to occur. Thus, the role and requirement of the SLO pore remains unclear. In this study, we constructed various S. pyogenes strains expressing altered forms of SLO to assess the importance of pore formation. We observed that SLO mutants that are unable to form pores retain the ability to translocate SPN. In addition, SPN translocation occurs after inhibition of actin polymerization, suggesting that CMT occurs independently of clathrin-mediated endocytosis. Moreover, despite the ability of mutants to translocate SPN, their cytotoxic effect requires SLO pore formation.
Introduction
Secretion and trafficking of bacterial toxins into host cells is essential for the virulence of nearly all pathogenic bacteria (Odenbreit et al, 2000; Alto et al, 2006). Cytolysin-mediated translocation (CMT) in Streptococcus pyogenes—an organism that causes a wide range of complications, including mild diseases (such as pharyngitis and impetigo), life-threatening invasive diseases (such as necrotizing fasciitis) and post-infectious sequelae (such as glomerulonephritis and rheumatic fever)—uses the multimeric pore-forming cytolysin streptolysin O (SLO) to translocate the S. pyogenes nicotinamide adenine dinucleotide (NAD+)-glycohydrolase (SPN) across the host cell membrane (Madden et al, 2001; Bricker et al, 2002). S. pyogenes NAD+-glycohydrolase cleaves β-NAD+ to produce nicotinamide and ADP-ribose, and it can also perform cyclase and ADP-ribosyltransferase reactions (Karasawa et al, 1995; Stevens et al, 2000). Although the mechanism of translocation is unknown, deletion of either slo or spn leads to both decreased cytotoxicity and virulence, suggesting that CMT is important for pathogenesis (Madden et al, 2001; Bricker et al, 2002, 2005).
A simple model predicts that translocation of SPN into the host cell occurs by diffusion through the 30 nm SLO pore. However, several observations suggest that the process is more complex, including that co-infection with isogenic slo and spn mutants is unable to restore CMT (Madden et al, 2001), that the related cytolysin perfringolysin O (PFO) cannot complement an SLO− mutant (Meehl & Caparon, 2004), and that CMT can discriminate between SPN and other streptococcal proteins (Ghosh & Caparon, 2006). Furthermore, deletion of specific amino-acid residues in SPN and SLO renders each of them incompetent for CMT, without altering their other functions (NADase activity and pore formation, respectively; Meehl & Caparon, 2004; Ghosh & Caparon, 2006). Altogether, data indicate that both SPN and SLO have an active role in CMT and that SPN does not diffuse passively through the SLO pore.
The ability to uncouple SLO pore formation from CMT indicates that the presence of pores is not sufficient for SPN translocation. This raises the question as to whether pore formation is even necessary for CMT. The SLO and PFO proteins are members of the cholesterol-dependent cytolysin (CDC) family, found in several pathogenic Gram-positive bacterial species. The three-dimensional structure of PFO and several other CDCs reveals that these proteins have an analogous domain structure and use a similar mechanism to form pores (Tweten, 2005). The bacterium secretes soluble cytolysin monomers and pore formation begins when the monomers bind to the host cell membrane through domain 4 of the protein. The monomers then associate to form an oligomeric structure, known as the prepore complex, which is bound to the cell surface but does not insert into the host cell membrane. Finally, conformational changes in domain 3 of each monomer convert three pairs of α-helical bundles into two transmembrane β-hairpins. This transformation leads to the insertion of the prepore complex into the membrane to form a functional pore with a lumen ranging from 30 to 50 nm in diameter (Tweten, 2005). The mechanism of pore formation has been studied extensively in PFO. Specific mutations in domain 3 lock the protein at different stages of pore formation (Tweten, 2005). In this study, we generated an analogous set of locked SLO mutants and used these to assess the necessity of the SLO pore in CMT.
Results
SLO mutants locked at various stages of oligomerization
On the basis of studies using PFO, the substitution of two conserved adjacent glycine residues located in domain 3 of SLO (Table 1, monomer-locked), with two valine residues, was predicted to inhibit the interactions that lead to the oligomerization of subunits (Tweten, 2005). The substitution of a conserved domain 3 tyrosine residue with alanine (Table 1, prepore-locked) was predicted to allow monomers to bind to and form an oligomeric structure on the host cell membrane but prevent the insertion of the pore (Tweten, 2005).
Table 1. Bacterial stains used in this study.
| Strain | Relevant genotype/descriptiona | Comments | Reference |
|---|---|---|---|
| JRS4 | wild type/wt | Scott et al (1986) | |
| SLO6 | sloΔ113−447/SLO− | SLO-deficient mutant | Ruiz et al (1998) |
| SPN1 | spnΔ177−319/SPN− | SPN-deficient mutant | Madden et al (2001) |
| NGM1 | sloY255A/prepore-locked | Expresses prepore-locked SLO | This study |
| NGM2 | sloG398V/G399V/monomer-locked | Expresses monomer-locked SLO | This study |
| SLOΔ37−102 | sloΔ37−102/SLOΔNT | Expresses SLO lacking an amino-terminal translocation domain | Meehl & Caparon (2004) |
| NGM3 | sloΔ37−102,Y255A/preporeΔNT | Expresses prepore-locked SLO lacking an amino-terminal translocation domain | This study |
| SLO, streptolysin O; SPN, Streptococcus pyogenes nicotinamide adenine dinucleotide-glycohydrolase; NT, amino terminus; wt, wild type. | |||
| aAllelic replacement was used to substitute the endogenous allele in wild-type JRS4 with the mutant alleles. | |||
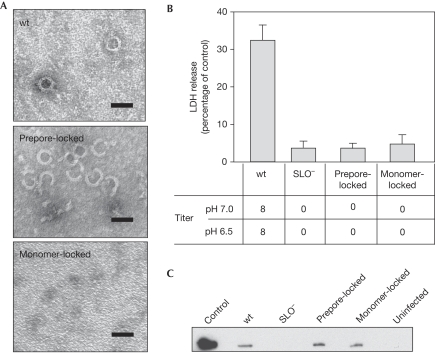
To verify oligomerization phenotypes, the mutant SLO proteins were expressed with a 6 × histidine linker at their carboxy-termini and purified from Escherichia coli (supplementary Fig S1 online). The soluble purified proteins were incubated in the presence of a cholesterol and phospholipid mixture mimicking biological membranes and examined by negative-stain electron microscopy. As expected, wild-type SLO formed oligomeric ring structures in the presence of the membrane constituents (Fig 1A). The prepore-locked SLO was able to form ring structures similar to wild type, but these structures were not observed when the monomer-locked protein was incubated with the lipid mixture (Fig 1A).
Figure 1.
Prepore- and monomer-locked mutants of streptolysin O localize to host cell membranes but cannot form pores. (A) Negative-stain electron microscopy was used to visualize the ability of the purified SLO mutant proteins to oligomerize in the presence of cholesterol and phospholipids. The images shown are representative of data from three independent experiments. Scale bars, 50 nm. (B) The pore-forming ability of the indicated SLO proteins was assessed by haemolytic activity at pH 7.0 and 6.5 (titres shown below) and by the release of LDH from A549 cells (compared with total LDH from lysed cells control, shown above). A ‘0' indicates that no lysis was detected with undiluted supernatant. Data represent mean±s.e.m. values derived from three independent experiments. (C) After infection for 3.75 h, A549 cells were subjected to fractionation with Triton X-100. A western blot analysis of the insoluble fraction compared with a precipitate prepared from an overnight culture supernatant (control) is shown. The image shown is representative of data obtained from three independent experiments. LDH, lactate dehydrogenase; SLO, streptolysin O; wt, wild type.
To confirm functional phenotypes, western blot analysis showed that the wild-type, prepore-locked and monomer-locked SLO proteins were stable and expressed at equivalent levels (supplementary Fig S2 online). As expected, treatment of A549 lung carcinoma cells with cell-free overnight-culture supernatants from a strain expressing wild-type SLO resulted in the release of lactate dehydrogenase (LDH). By contrast, treatment with supernatants from strains expressing prepore- and monomer-locked SLO promoted minimal LDH release, which was indistinguishable from that produced by an SLO− mutant (Fig 1B). Furthermore, unlike wild-type SLO, prepore- and monomer-locked SLO could not promote detectable lysis of erythrocytes at either neutral or acidic pH (Fig 1B). These data confirm that the mutant SLO proteins are not capable of forming a functional pore.
SLO mutants are associated with cell membranes
The process of oligomerization and pore formation requires cytolysins to bind to the host cell membrane. After an infection with the wild-type and mutant strains, A549 cells were fractionated with Triton X-100. Western blot analysis illustrated that wild-type SLO localized to the Triton-X-100-insoluble membrane fraction (Fig 1C). Prepore-locked and monomer-locked SLO proteins also localized to the A549 cell membrane fraction and were present in amounts similar to wild-type SLO (Fig 1C). None of these proteins were detected in the Triton X-100-soluble fraction (data not shown). These results indicate that even though mutant SLO proteins were unable to form pores, both bound to the membrane as efficiently as wild-type SLO.
Pore formation is not required for CMT
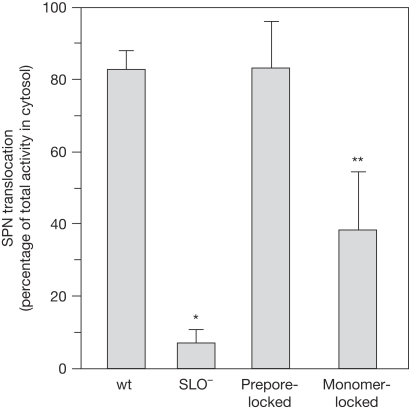
To assess the requirement for pore formation in CMT, the prepore- and monomer-locked strains were used to infect A549 cells. The efficiency of SPN translocation was evaluated according to the percentage of the total SPN expressed, which was translocated into the A549 cell cytosol. Consistent with the requirement for SLO, the wild-type strain was CMT-competent with most (typically more than 70%) of the total SPN expressed in the A549 cell cytosolic fraction, whereas the SLO− mutant was markedly deficient for CMT (typically less than 10%; Fig 2). By contrast, even though prepore-locked SLO was unable to form pores, the mutant strain translocated SPN at levels indistinguishable from wild type (Fig 2). Although reduced from that of the prepore-locked strain, the monomer-locked SLO strain was also CMT-competent and translocated SPN at levels significantly higher than the SLO− mutant (Fig 2; P<0.005). These data indicate that pore formation was not required for CMT and that, although not essential, SLO oligomerization did improve the efficiency of CMT.
Figure 2.
Cytolysin-mediated translocation is not dependent on pore formation but is enhanced by oligomerization. The ability of the SLO pore-forming mutants (Table 1) to translocate SPN into the A549 cell was analysed. The bars show the percentage of the total SPN expressed that was translocated into the A549 cytosolic fraction, after a 3.75 h infection. An ‘*' indicates that CMT is significantly lower than wild type (P<0.0001) and ‘**' indicates CMT is at levels higher than SLO− (P<0.005). The data represent the mean±s.e.m. values derived from at least three independent experiments. CMT, cytolysin-mediated translocation; SLO, streptolysin O; SPN, Streptococcus pyogenes nicotinamide adenine dinucleotide-glycohydrolase; wt, wild type.
Mutants use a similar CMT pathway
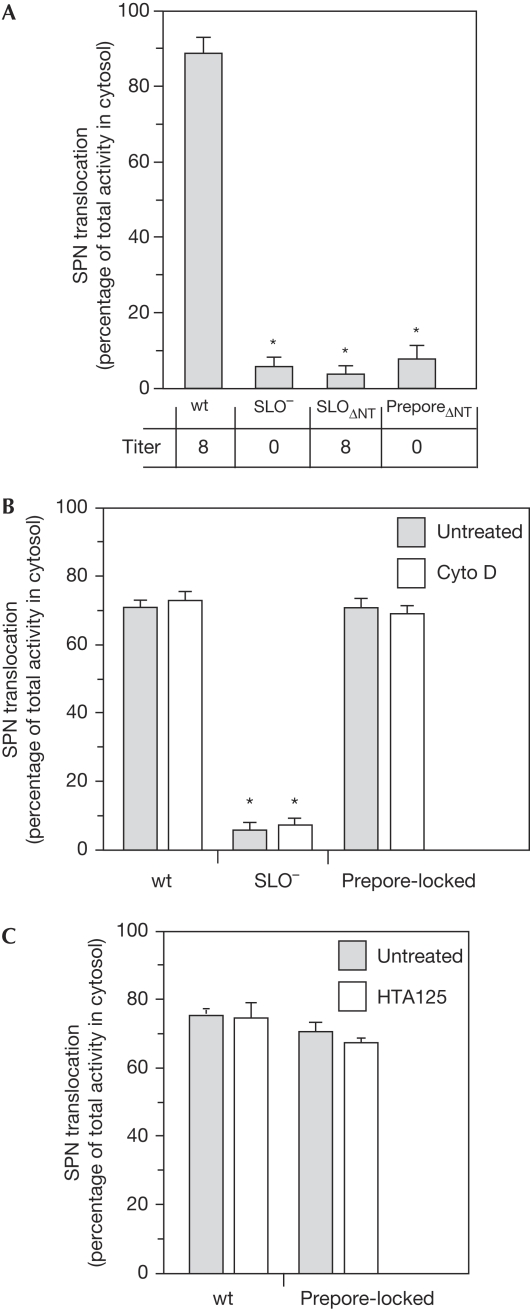
To ascertain whether the same SPN translocation pathway was used by the wild-type and prepore-locked strains, we examined the importance of the SLO amino terminus and sensitivity to cytochalasin D. A previous study demonstrated that deletion of 66 residues at the N terminus of SLO (SLOΔNT) prevented SPN translocation but did not affect the ability of the mutant protein to form pores (Meehl & Caparon, 2004). In addition, inhibitors of actin polymerization, known to inhibit the host cell clathrin-mediated endocytic pathway (supplementary Fig S4 online), did not prevent wild-type SLO from conducting CMT (Madden et al, 2001; Bricker et al, 2002). A strain was constructed that expressed a prepore-locked SLO that also lacked the N-terminal translocation domain (Table 1, preporeΔNT). As expected, this strain lost the ability to lyse erythrocytes, a characteristic of both wild type and the strain expressing SLO with only the N-terminal deletion (Table 1, SLOΔNT; Fig 3A). However, preporeΔNT also lost the competence of wild type for CMT (P<0.0001) and was as defective as SLOΔNT (Fig 3A), demonstrating that the ability of the prepore-locked strain to conduct CMT is also dependent on the N-terminal domain. Furthermore, similar to wild type, the presence of cytochalasin D did not alter the ability of the prepore-locked strain to conduct CMT (Fig 3B). Previous studies indicate that at least one CDC has the ability to bind to and signal through the Toll-like receptor 4 (TLR4; Malley et al, 2003; Srivastava et al, 2005). However, a specific TLR4 antagonist (HTA125) had no capacity to inhibit CMT caused by wild type or the prepore-locked strain (Fig 3C). These data show that the CMT pathway promoted by prepore-locked SLO is indistinguishable from that of wild-type SLO.
Figure 3.
The cytolysin-mediated translocation pathway used by the prepore-locked and wild-type SLO are indistinguishable. (A) CMT efficiencies of the indicated strains (top) and haemolytic titres (bottom) were assessed and presented as described for Fig 2. CMT efficiency by the indicated strains (Table 1) after treatment of A549 cells with (B) cytochalasin D or (C) the TLR4 antagonist HTA125 are shown. Data represent mean±s.e.m. values and an ‘*' indicates that CMT is significantly lower than wild type (P<0.0001). CMT, cytolysin-mediated translocation; SLO, streptolysin O; SPN, Streptococcus pyogenes nicotinamide adenine dinucleotide-glycohydrolase; TLR4, Toll-like receptor 4; wt, wild type.
Pore formation is required for cytotoxicity
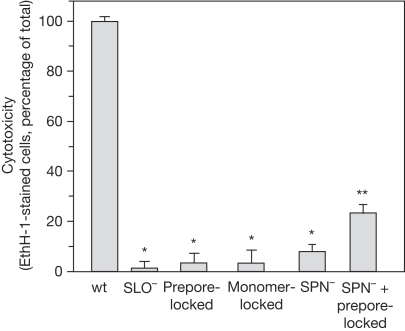
To determine whether cytotoxicity requires both SLO pore formation and SPN translocation, A549 cells were infected with the wild-type and mutant strains, and membrane integrity was assessed by staining with the membrane-impermeable fluorescent probe ethidium homodimer 1 (EthH-1; Madden et al, 2001). After 5 h, most wild-type-infected A549 could not exclude EthH-1, indicating that their membranes were compromised (Fig 4). By contrast, infection with the prepore- and monomer-locked strains resulted in a more than 90% reduction in the number of EthH-1-staining cells compared with wild type (P<0.0001). These reduced levels were similar to those due to infection by SLO− and SPN− strains (Fig 4). This reduction in cytotoxicity by the locked mutants occurred despite the presence of cytosolic SPN. Although reduced from wild type, co-infection by SPN−—which produces SLO—and prepore-locked strains produced a significantly higher number of cells with damaged membranes compared with SLO− mutant alone (Fig 4, P<0.0001). These data indicate that although SPN translocation occurred in the absence of an SLO pore, the synergistic SLO/SPN cytotoxic response requires both the translocation and pore-forming activities of SLO.
Figure 4.
Cytotoxicity requires both streptolysin O pore formation and SPN translocation. The percentage of A549 cells with cytosolic EthH-1 staining after infection by the indicated strains is shown (Table 1). An ‘*' indicates that EthH-1 staining of A549 cells is significantly lower than wild type (P<0.0001) and ‘**' indicates that EthH-1 staining is at levels higher than SLO− (P<0.0001). Data represent mean±s.e.m. values derived from at least three independent experiments. EthH-1, ethidium homodimer 1; SLO, streptolysin O; SPN, Streptococcus pyogenes nicotinamide adenine dinucleotide-glycohydrolase.
Discussion
Cytolysin-mediated translocation has been compared previously with specialized secretion systems, such as the Gram-negative type III secretion system, which inject effector proteins into the host cell cytosol (Gauthier & Finlay, 2001). It has been established that SPN is translocated through CMT and that SLO is necessary for this process. According to an early model of CMT, SLO actively translocated SPN into the host cell cytosol through the lumen of its pore. However, data presented here show that the SLO pore is not required for SPN translocation.
Although these data do not reveal the pathway for SPN uptake, they do contribute to a growing appreciation that CDCs possess alternative activities that do not rely on pore formation. For example, after binding to the membrane, listeriolysin O and pneumolysin initiate signalling events that result in histone dephosphorylation and deacetylation in the absence of pores, causing alterations in the host cell gene expression profile (Hamon et al, 2007). Similarly, pneumolysin can stimulate cytokine secretion and caspase I activation independently of pore formation (Baba et al, 2002; Shoma et al, 2008). Overall, these reports suggest that cytolysins with dual functionality might be prevalent and that pore formation might be a secondary activity in certain circumstances.
Pore formation was necessary for cytotoxicity, but the mechanism underlying this synergistic effect is also unclear. However, it is known that cells have the capacity to heal SLO pores (Walev et al, 2001; Idone et al, 2008) and it is possible that SPN could interfere with this process. Cellular wound healing is also a localized process (Idone et al, 2008), which could explain why a mixed infection only partly restored cytotoxicity, as the site of SPN delivery and that of pore formation would not be coupled efficiently. However, it cannot be ruled out that the prepore-locked protein exhibited a dominant-negative effect though the formation of mixed oligomers that resulted in an overall reduction in pore formation.
Given that pore formation is not required for CMT, data presented here suggest a more active role for the host cell. Streptolysin O is known to bind to cholesterol through domain 4, thus implicating clathrin-independent endocytosis through lipid rafts (Kirkham & Parton, 2005; Mayor & Pagano, 2007), followed by the release of SPN into the host cell cytosol as a possible CMT mechanism. Several CDCs, including listeriolysin O and PFO, have been shown to localize to cholesterol-enriched domains of membranes (Ohno-Iwashita et al, 2004; Gekara et al, 2005), suggesting that SLO also might be present in similar membrane domains. A recent report argues against this pathway and indicates that the initial binding of PFO is preferentially to free cholesterol at the membrane surface, rather than to cholesterol associated with lipid rafts (Flanagan et al, 2009).
It is also becoming apparent that CDCs can exhibit marked differences in how they bind to the membrane, ranging from those that recognize a specific protein to those, such as PFO, that bind exclusively to cholesterol (Tweten, 2005). Interestingly, SLO demonstrates much less dependence on binding to cholesterol than does PFO (Giddings et al, 2003), suggesting that SLO might recognize an additional membrane receptor. Thus, a rigorous biophysical investigation of how SLO binds to the membrane, including determination of whether CMT is affected by ‘reprogramming' binding specificity by swapping domain 4 of SLO with that of other CDCs, probably holds the key to understanding the CMT uptake pathway.
Methods
Analysis of CMT. The S. pyogenes strains used in this study are listed in Table 1 and were constructed by standard methods as described in the supplementary information online. Analysis of CMT by using A549 cells was conducted as described previously (Meehl & Caparon, 2004; Ghosh & Caparon, 2006). Where indicated, cytochalasin D (2 μg/ml, cat. #C2618, Sigma, St Louis, MO, USA) or the TLR4 antagonist HTRA125 (10 μg/ml, cat. #HM2068, Cell Sciences) were added to the medium for 30 and 60 min, respectively, before infection by streptococcal strains. This cytochalasin D treatment was shown to inhibit endocytosis (supplementary Fig S4 online), but not CMT (Madden et al, 2001). This HTA125 treatment inhibited lipopolysaccharide-induced induction of interleukin-6 (supplementary Fig S3 online), similar to that observed elsewhere (Xie et al, 2009). Data presented here represent mean±s.e.m. values derived from at least three independent experiments.
Analysis of cytotoxicity. The integrity of A549 cell membranes after infection was assessed by the ability to exclude the membrane-impermeable fluorescent probe EthH-1 by using a commercial reagent (Live/Dead, cat. #L3224, Invitrogen, Carlsbad, CA, USA) as described previously (Madden et al, 2001). The ability to lyse erythrocytes and to promote LDH release was analysed as described previously (Ruiz et al, 1998; Madden et al, 2001; Meehl & Caparon, 2004). The data presented here represent mean±s.e.m. values derived from at least three independent experiments.
Statistical analysis. Any differences in the mean values of CMT translocation efficiencies or A549 cell cytotoxicity compared between various mutant and wild-type strains were tested for significance by the unpaired t-test (Glantz, 2002) and P-values less than 0.05 were considered significant.
A549 cell fractionation. After infection, A549 cells were lysed using Tris-buffered saline containing 1% Triton X-100 (cat. #T8787, Sigma), collected by scraping, and fractions were prepared by ultracentrifugation as described previously (Madden et al, 2001). Triton-X-100-insoluble and -soluble fractions were subjected to sodium dodecyl sulphate–polyacrylamidegel electrophoresis (SDS–PAGE) using an 8% gel and analysed by western blot analysis using SLO antiserum (Meehl & Caparon, 2004).
Purification of SLO. Mutant and wild-type slo alleles were expressed in E. coli by using the pBAD/gIII vector (cat. #V45001, Invitrogen) and were amplified by using the primers listed in supplementary Table S1 online. Expression constructs contained a C-terminal 6 × histidine tag (supplementary Table S1 online) and were expressed and purified by metal ion affinity chromatography using standard methods (see supplementary information online). Purity was assessed by SDS–PAGE and staining with Coomassie brilliant blue R250 (supplementary Fig S1 online).
Analysis of SLO oligomerization. Purified SLO proteins (0.65 μg) were incubated for 1 h at 25°C in the presence of a lipid mixture (0.5 mg/ml) containing about 50 mol% cholesterol (cat. #700000, Avanti) and about 50 mol% 1,2 dioleoyl-sn-glycero-3-phosphocholine (cat. #850375, Avanti, Alabaster, AL, USA) as described previously (Dang et al, 2005). Samples were allowed to absorb onto formvar/carbon-coated copper grids for 10 min. Grids were washed in dH2O and stained with 1% aqueous uranyl acetate (Ted Pella, Redding, CA, USA) for 1 min. Excess liquid was wicked off gently and grids were allowed to air-dry. Samples were viewed on a JEOL 1200EX transmission electron microscope ( JEOL USA, Peabody, MA, USA) at an accelerating voltage of 80 kV.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank W. Beatty for assistance with electron microscopy. This study was supported by Public Health Service Grant AI064721 from the National Institutes of Health (to M.G.C.) and partly by the Infectious Disease/Basic Microbial Pathogenic Mechanisms Training Grant 5T32AI007172 (to N.M.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Alto NM et al. (2006) Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124: 133–145 [DOI] [PubMed] [Google Scholar]
- Baba H, Kawamura I, Kohda C, Nomura T, Ito Y, Kimoto T, Watanabe I, Ichiyama S, Mitsuyama M (2002) Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity. Infect Immun 70: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker AL, Cywes C, Ashbaugh CD, Wessels MR (2002) NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol Microbiol 44: 257–269 [DOI] [PubMed] [Google Scholar]
- Bricker AL, Carey VJ, Wessels MR (2005) Role of NADase in virulence in experimental invasive group A streptococcal infection. Infect Immun 73: 6562–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang TX, Hotze EM, Rouiller I, Tweten RK, Wilson-Kubalek EM (2005) Prepore to pore transition of a cholesterol-dependent cytolysin visualized by electron microscopy. J Struct Biol 150: 100–108 [DOI] [PubMed] [Google Scholar]
- Flanagan JJ, Tweten RK, Johnson AE, Heuck AP (2009) Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry 48: 3977–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A, Finlay BB (2001) Bacterial pathogenesis: the answer to virulence is in the pore. Curr Biol 11: R264–R267 [DOI] [PubMed] [Google Scholar]
- Gekara NO, Jacobs T, Chakraborty T, Weiss S (2005) The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol 7: 1345–1356 [DOI] [PubMed] [Google Scholar]
- Ghosh J, Caparon MG (2006) Specificity of Streptococcus pyogenes NAD+ glycohydrolase in cytolysin-mediated translocation. Mol Microbiol 62: 1203–1214 [DOI] [PubMed] [Google Scholar]
- Giddings KS, Johnson AE, Tweten RK (2003) Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci USA 100: 11315–11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz S (2002) Primer of Biostatistics. New York, NY, USA: McGraw Hill [Google Scholar]
- Hamon MA, Batsche E, Regnault B, Tham TN, Seveau S, Muchardt C, Cossart P (2007) Histone modifications induced by a family of bacterial toxins. Proc Natl Acad Sci USA 104: 13467–13472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW (2008) Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Takasawa S, Yamakawa K, Yonekura H, Okamoto H, Nakamura S (1995) NAD+-glycohydrolase from Streptococcus pyogenes shows cyclic ADP-ribose forming activity. FEMS Microbiol Lett 130: 201–204 [DOI] [PubMed] [Google Scholar]
- Kirkham M, Parton RG (2005) Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 1746: 349–363 [DOI] [PubMed] [Google Scholar]
- Madden JC, Ruiz N, Caparon M (2001) Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in Gram-positive bacteria. Cell 104: 143–152 [DOI] [PubMed] [Google Scholar]
- Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT (2003) Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA 100: 1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8: 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl MA, Caparon MG (2004) Specificity of streptolysin O in cytolysin-mediated translocation. Mol Microbiol 52: 1665–1676 [DOI] [PubMed] [Google Scholar]
- Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R (2000) Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287: 1497–1500 [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y, Shimada Y, Waheed AA, Hayashi M, Inomata M, Nakamura M, Maruya M, Iwashita S (2004) Perfringolysin O, a cholesterol-binding cytolysin, as a probe for lipid rafts. Anaerobe 10: 125–134 [DOI] [PubMed] [Google Scholar]
- Ruiz N, Wang B, Pentland A, Caparon M (1998) Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol Microbiol 27: 337–346 [DOI] [PubMed] [Google Scholar]
- Scott JR, Guenthner PC, Malone LM, Fischetti VA (1986) Conversion of an M group A Streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med 164: 1641–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoma S, Tsuchiya K, Kawamura I, Nomura T, Hara H, Uchiyama R, Daim S, Mitsuyama M (2008) Critical involvement of pneumolysin in production of interleukin-1α and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase 1 activation. Infect Immun 76: 1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R (2005) The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun 73: 6479–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DL, Salmi DB, McIndoo ER, Bryant AE (2000) Molecular epidemiology of nga and NAD glycohydrolase/ADP-ribosyltransferase activity among Streptococcus pyogenes causing streptococcal toxic shock syndrome. J Infect Dis 182: 1117–1128 [DOI] [PubMed] [Google Scholar]
- Tweten RK (2005) Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun 73: 6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, Bhakdi S (2001) Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA 98: 3185–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XH, Law HK, Wang LJ, Li X, Yang XQ, Liu EM (2009) Lipopolysaccharide induces IL-6 production in respiratory syncytial virus-infected airway epithelial cells through the toll-like receptor 4 signaling pathway. Pediatr Res 65: 156–162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.