Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo
The Milan group identifies a new gene involved in the sorting and delivering of basally secreted proteins, like laminins, to the basement membrane in Drosophila. Scarface is a target of JNK signaling and Scarface mutant embryos show defects in germ-band retraction and dorsal closure, two morphogenetic processes that rely on the activity of integrins and laminins. Polarized localization of laminin A at the basement membrane is compromised in these embryos.
Keywords: JNK, morphogenesis, integrin, basement membrane
Abstract
Cell–matrix interactions brought about by the activity of integrins and laminins maintain the polarized architecture of epithelia and mediate morphogenetic interactions between apposing tissues. Although the polarized localization of laminins at the basement membrane is a crucial step in these processes, little is known about how this polarized distribution is achieved. Here, in Drosophila, we analyse the role of the secreted serine protease-like protein Scarface in germ-band retraction and dorsal closure—morphogenetic processes that rely on the activity of integrins and laminins. We present evidence that scarface is regulated by c-Jun amino-terminal kinase and that scarface mutant embryos show defects in these morphogenetic processes. Anomalous accumulation of laminin A on the apical surface of epithelial cells was observed in these embryos before a loss of epithelial polarity was induced. We propose that Scarface has a key role in regulating the polarized localization of laminin A in this developmental context.
Introduction
Integrins, which are heterodimeric transmembrane receptors, interact with laminins, which are extracellular matrix molecules, to mediate signalling, stable adhesion of differentiated epithelia to the basement membrane (BM) and transient adhesion during cell spreading or migration (Bokel & Brown, 2002). Considerable progress has been made in our understanding of the mechanisms involved in the polarized transport of integrins and other basolateral membrane proteins, but little is known about how laminins and other basally secreted proteins are sorted and delivered specifically to the BM (Rodriguez-Boulan et al, 2005). Although a pathway involved in the polarized transport of BM proteins was proposed many years ago (Caplan et al, 1987; Boll et al, 1991), the first protein regulating this process has only recently been characterized (Denef et al, 2008). Crag, a protein with domains involved in membrane transport and localized in early and recycling endosomes, has been shown to have a role in regulating the polarized secretion of BM proteins in Drosophila epithelial cells.
Here, in Drosophila, we characterize the embryonic role of scarface (scarf; Fig 1), a gene encoding for a putative secreted serine protease-like molecule without catalytic activity (Bonin & Mann, 2004). We present evidence that scarf expression is regulated by c-Jun amino-terminal kinase (JNK) signalling and scarf mutant embryos show defects in germ-band retraction (GBR) and dorsal closure (DC)—morphogenetic processes that rely on the activity of integrins and laminins (Leptin et al, 1989; Schock & Perrimon, 2003; Narasimha & Brown, 2004; Reed et al, 2004; Homsy et al, 2006). Our data indicate that Scarf has a key role in regulating the polarized localization of laminin A (LanA) in this developmental context.
Figure 1.
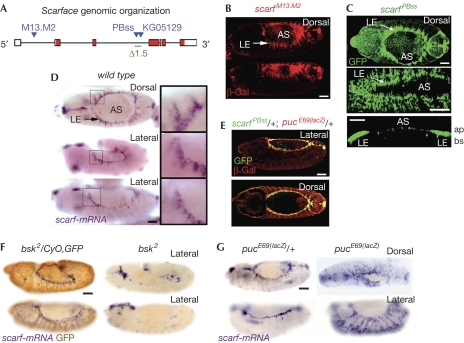
scarf is regulated by JNK in leading edge cells. (A) Genomic organization of the scarf locus. P element (KG05129) and piggyBac (PBss and M13.M2) insertions (blue triangles), the scarf Δ1.5 deletion (green), exons (white boxes) and the open reading frame (red boxes) are shown. (B–G) Embryos of stages 12–14 labelled to visualize β-gal, GFP or scarf mRNA expression. (C) The bottom panel shows a cross-sectional view. (D) Magnifications of the boxed tissues are shown. Scale bars, 25 μm. ap, apical; AS, aminoserosa; bs, basal; GFP, green fluorescent protein; JNK, c-Jun amino-terminal kinase; LE; leading edge; mRNA, messenger RNA.
Results And Discussion
During GBR, the tail end of the germ band, or embryo proper, interacts with the amnioserosa (AS), an epithelium of large flat cells that does not contribute to the larva, and moves to its final posterior position (Fig 2E; Schock & Perrimon, 2002). After GBR, the ectoderm has a gap on its dorsal side that is occupied by the AS. The dorsal-most cells of the ectoderm, on both sides of the embryo, are in contact with the AS and are called leading edge (LE) cells. During DC, LE cells direct the movement of the epidermal sheets migrating dorsally over the apical side of the AS until they meet and fuse at the dorsal midline (Fig 2E; Jacinto et al, 2002). Our attention was drawn to the function of scarf on the basis of its embryonic expression pattern. Two piggyBac insertions, scarf PBss(GFP) and scarf M13.M2(lacZ; Fig 1A), were expressed at a high level in LE cells during GBR and DC and at a low level in AS cells (Fig 1B,C). The expression of scarf was confirmed by in situ hybridization (Fig 1D). It was also expressed in the head and tail regions, and in the ventral ectoderm in a segmental pattern (Fig 1D and data not shown).
Figure 2.
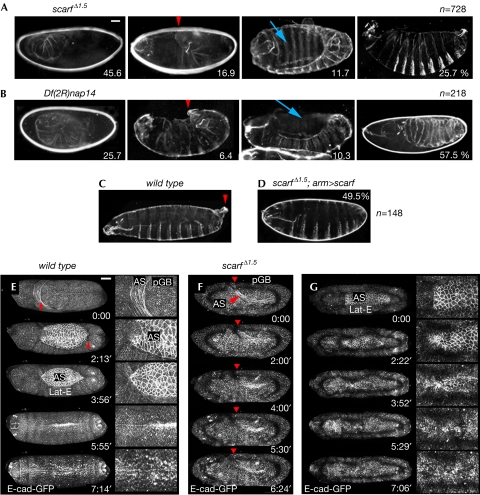
Germ-band retraction and dorsal closure defects in the absence of scarf. (A–D) Cuticle preparations of the indicated embryos. Red arrowheads point to the posterior tail and blue arrows to a hole in the dorsal part of the cuticle. Percentages of embryos with the corresponding phenotypes and the number of embryos scored (n) are shown. (E–G) Live image sequences of developing ubi-E-cad–GFP (E) and ubi-E-cad–GFP scarf Δ1.5 (F,G) embryos during GBR and DC. E-cad–GFP is shown in white. Red arrowheads point to the pGB. (E) In wild-type embryos, GBR and DC last around 3 h each. (F,G) Defects in GBR and DC are observed in the scarf Δ1.5 embryos. Movies are shown as supplementary information online. Scale bars, 25 μm. AS, aminoserosa; DC, dorsal closure; GBR, germ-band retraction; GFP, green fluorescent protein; pGB, posterior end of the germ band.
The JNK pathway is activated in LE cells and leads to the expression of the signalling molecule Dpp and the phosphatase puckered (Puc). The scarf gene was expressed in the same cells as puc and dpp at the LE (Fig 1E; supplementary Fig S1 online). Reduced JNK—basket (Bsk) in Drosophila—or expression of a dominant-negative version of Bsk or Puc, which mediates a feedback loop repressing JNK activity (Martin-Blanco et al, 1998), led to the loss of scarf expression in LE cells (Fig 1F; supplementary Fig S1 online). Loss of puc activity, which leads to increased levels of JNK activity, or expression of an activated version of JNK-activating kinase—Hemipterous in Drosophila—led to the expansion of the scarf expression domain throughout the lateral ectoderm (Fig 1G; supplementary Fig S1 online). Dorsal cells had a stronger response to increased JNK, suggesting that JNK requires the activity of another factor expressed in this region to induce scarf. As stated, the JNK cascade drives the expression of the secreted molecule Dpp. In embryos mutant for the Dpp receptor Thickveins, scarf expression at the LE was not lost and ectopic activation of the Dpp pathway did not induce the ectopic expression of scarf (supplementary Fig S1 online). These results indicate that scarf expression is induced by JNK in LE cells.
Homozygosis or trans-heterozygosis for the piggyBac insertions scarf PBss and scarf M13.M2 caused semi-lethality and survivors had scars on the head (data not shown; Bonin & Mann, 2004), phenotypes that were reverted by precise excision of the transposon elements (supplementary information online). As embryos showed no obvious cuticle phenotype (data not shown), we generated stronger alleles of scarf by imprecise excision of a P element located in the third intron of scarf (scarf KG05129; Fig 1A). We isolated one allele (scarf Δ1.5) that led to the loss of scarf messenger RNA expression (supplementary Fig S2 online). This is an embryonic lethal allele and trans-heterozygous combinations of scarf Δ1.5 or Df(2R)nap14, a deficiency that uncovers the scarf locus, with scarf PBss or scarf M13.M2 were semi-lethal and resulted in scars on the head (data not shown). Homozygous animals for scarf Δ1.5 or Df(2R)nap14 showed similar phenotypes (Fig 2A,B; supplementary Table S1 online). Their larval cuticles were either dorsally wrinkled or presented a dorsal hole or a U-shaped phenotype (Fig 2A–C), which is suggestive of failures in GBR and DC. A high proportion of embryos showed undifferentiated cuticles. Ubiquitous expression of scarf largely rescued the scarf Δ1.5 phenotype (Fig 2D; supplementary Table S1 online).
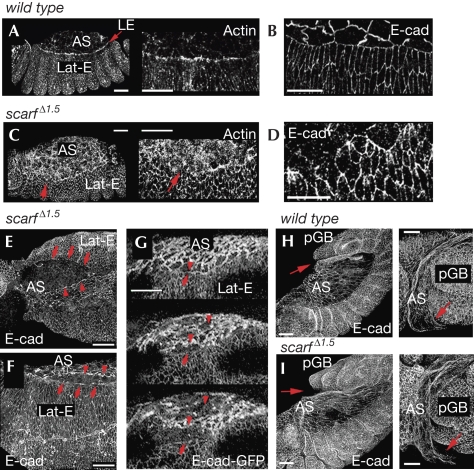
Time-lapse recordings indicated that these embryos were delayed in development (supplementary Table S2 online) and showed a failure in GBR (Fig 2E,F; supplementary Fig S3 online; supplementary Movies S1–S3 online) and DC (Fig 2E,G; supplementary Movies S4,S5 online). Frequently, the attachment of the AS to the tail end of the germ band was compromised (Fig 3H,I) and the germ band did not retract (Fig 2F; supplementary Movie S2 online) or retracted only partly (supplementary Fig S3 online; supplementary Movie S3 online). In embryos that were able to retract (Fig 2G), DC started and LE cells began to direct the movement of the epidermal sheets migrating dorsally over the apical side of the AS. However, either the AS detached from the neighbouring epidermal sheet (Fig 3E–G; supplementary Movie S5 online) or the epidermal sheets met and fused at the dorsal midline but the dorsal epidermis reopened (Fig 2G; supplementary Movie S4 online). We analysed the requirement of scarf in DC in greater detail in fixed staged embryos. During the initial steps of DC, lateral ectodermal cells start to elongate dorsally and an actin cable at the LE is formed, which helps to generate a linear fence at the interface between LE and AS cells (Fig 3A,B; Jacinto et al, 2002). In scarf Δ1.5 embryos, elongation of the lateral ectoderm was compromised frequently, the actin cable was not formed properly, the interface between LE and AS cells became irregular (Fig 3C,D), and eventually rips appeared at the AS–LE interface (Fig 3E–G). The elongation of lateral ectodermal cells and adhesion between AS and LE cells are mediated by the activity of Dpp (Affolter et al, 1994; Fernandez et al, 2007) and JNK activity controls the polymerization of actin into a cable at the LE (Jacinto et al, 2002). In embryos lacking scarf, the activity of the Dpp and JNK signalling pathways was not affected (supplementary Fig S4 online). Thus, Scarf has a role in DC without affecting the activity levels of these pathways.
Figure 3.
Dorsal closure and aminoserosa attachment defects in the absence of scarf. Wild-type (A,B,H) and scarf Δ1.5 (C–G,I) embryos labelled to visualize actin and E-cadherin (E-cad) protein expression or expression of an E-cad–GFP transgene. Red arrows in (A,C) point to the LE; those in (E–G) point to Lat-E cells; and those in (H,I) point to the attachment between AS cells and pGB. Red arrowheads in (E–G) point to AS cells. Scale bars, 25 μm. AS, amnioserosa; GFP, green fluorescent protein; Lat-E, lateral ectodermal; LE, leading edge; pGB, posterior end of the germ band.
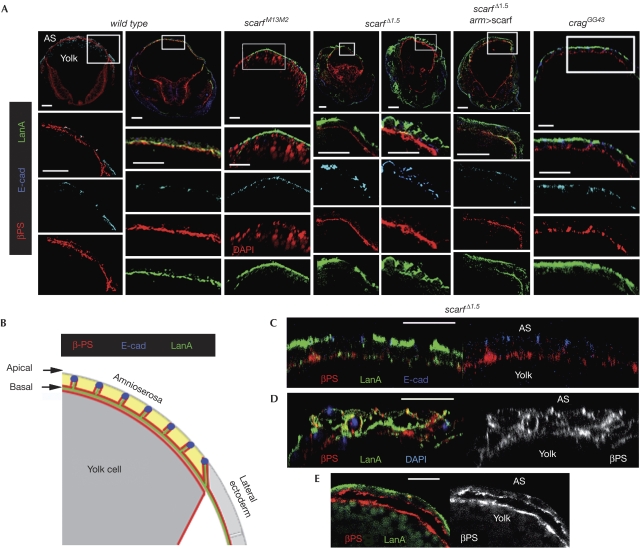
Integrins and laminins have a fundamental role in GBR and DC by mediating interactions between AS cells and neighbouring cell populations. As defects were observed in these processes on scarf depletion and a reduction in scarf levels increased the frequency of cuticle phenotypes caused by depletion of the β-integrin position-specific (βPS) subunit (supplementary Table S1 online), we examined the contribution of Scarf to the regulation of the expression levels and subcellular localization of these proteins. Cross-sectional views of properly staged wild-type and scarf Δ1.5 embryos stained for βPS integrin revealed similar levels and localization of integrins (Fig 4A,B). The JNK signalling regulates the expression of the βPS integrin subunit during DC (Homsy et al, 2006). Although ectopic activation of JNK induced increased expression of βPS integrin, ectopic expression of scarf did not exert this effect (supplementary Fig S5 online). We then analysed the expression of LanA, one of the two existing fly α-laminins. In wild-type embryos, high levels of LanA were localized at the BM of AS cells (Fig 4A,B). Interestingly, scarf embryos revealed strong defects in the polarized distribution of LanA. It was localized on the apical side of the AS epithelium and its protein levels were largely reduced in the BM (Fig 4A; supplementary Fig S6 online). Ubiquitous expression of scarf largely rescued the levels of LanA at the BM (Fig 4A). We observed similar defects in the polarized distribution of LanA in the lateral ectoderm of scarf mutant embryos (supplementary Fig S6 online), and these defects were also rescued largely by the ubiquitous expression of scarf (supplementary Fig S6 online). Apico-basal polarity of AS cells, visualized by the basal localization of βPS integrin and the localization of E-cadherin (E-cad) at the adherens junctions, was not affected in hypomorphic scarf PBss and scarf M13.M2 embryos (Fig 4A; supplementary Fig S6 online) and in some scarf Δ1.5 embryos (Fig 4C) even though LanA was localized on the apical side. However, scarf depletion was frequently found to cause defects in the apico-basal polarity of AS cells and this was accompanied by multilayering of the AS epithelium (Fig 4D; supplementary Fig S6 online). These observations suggest that before inducing a loss of epithelial integrity, the absence of Scarf caused aberrant localization of LanA on the apical side of the AS epithelium and reduced LanA in the BM, without affecting the distribution of apical and baso-lateral proteins. As integrins are involved in maintaining epithelial polarity (Schock & Perrimon, 2003; Fernandez-Minan et al, 2008), these results suggest that the observed loss of cell polarity is a consequence of reduced integrin-mediated adhesion to the BM. Consistent with this view, defects in the attachment of the AS cells to the underlying yolk cell were observed frequently (Fig 4E).
Figure 4.
Defects in the polarized distribution of laminin A in the absence of scarf. (A,C–E) Cross-sectional views of wild-type and mutant embryos at stages 12–14 labelled to visualize the subcellular localization of E-Cadherin (E-cad), DAPI or βPS integrin and laminin A (LanA). Lower panels in (A) show magnification of the boxed fields. (B) Schematic representation of a cross-sectional view of the wild-type embryo shown in (A). Scale bars, 25 μm. AS, aminoserosa; DAPI, 4′,6-diamidino-2-phenylindole; PS, position specific.
Although the defects observed in scarf Δ1.5 embryos resemble those caused by βPS integrin depletion, only defects in the attachment of the AS with the underlying yolk cells are found in lanA mutant embryos (Schock & Perrimon, 2003; Narasimha & Brown, 2004). Mutations in wing blister (wb), the only other fly α-laminin, cause defects in GBR and in the attachment between AS cells and the posterior germ band (Schock & Perrimon, 2003). These data suggest that LanA and Wb have a redundant function and that Scarf most probably promotes BM localization of not only LanA but also Wb. Consistent with the proposed redundancy, halving the dose of lanA or wb increased the frequency of the βPS integrin loss-of-function cuticle phenotype (supplementary Table S1 online). We were neither able to monitor the expression of Wb protein in AS cells using the available antibodies nor address the role of Scarf in the polarized distribution of other BM proteins, such as Perlecan or Collagen IV, as these two proteins were not expressed in AS cells (supplementary Fig S7 online).
These results indicate that Scarf depletion causes defects in the BM localization of LanA and in epithelial apico-basal polarity. The defects resemble those observed in follicle cells (that is, epithelial cells covering the fly oocyte) mutant for crag, a gene encoding for a protein localized in early and recycling endosomes (Denef et al, 2008) and proposed to regulate protein transport and membrane deposition of BM proteins. Zygotic removal of crag induced anomalous localization of LanA on the apical side of AS cells (Fig 4A; supplementary Fig S6 online). Zygotic or both maternal and zygotic removal of crag produced cuticles with a weak dorsally wrinkled phenotype (supplementary Table S1 online and data not shown), suggesting that Crag has a redundant function with another protein in the fly embryo and that the low BM levels of LanA observed in these embryos are sufficient to exert their function. Interestingly, halving the dose of scarf expression gave rise to a large proportion of crag cuticles with phenotypes similar to those caused by scarf depletion (supplementary Table S1 online).
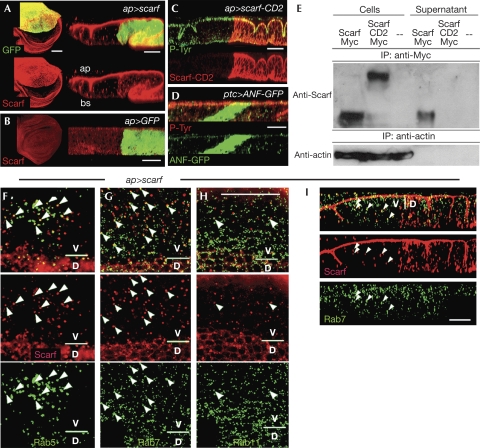
We next raised antibodies against Scarf to analyse its subcellular localization. As the antibodies did not work properly at embryonic stages and did not detect endogenous levels of Scarf protein, we used the wing imaginal disc, a monolayered epithelium, to analyse its subcellular localization. Scarf was not detected in wild-type wing cells (Fig 5B). When scarf and green fluorescent protein (GFP) were expressed in a restricted domain in the wing disc, Scarf was detected not only in the GFP-labelled scarf-producing cells, but also on the apical side of the epithelium at long distances from the source (Fig 5A). When atrial natriuretic factor–GFP—a fusion between the secreted rat atrial natriuretic peptide and GFP—was expressed, the protein was also observed at long distances from the source; however, it did not accumulate on the apical side of the epithelium (Fig 5D). Scarf was found to localize in small punctate structures throughout the cytoplasm of scarf non-producing cells (Fig 5F–I). These structures corresponded either to Rab5-positive early endosomes (Fig 5F; 38% of the Scarf vesicles (n=185) were labelled by Rab5–GFP), to Rab7-positive late endosomes (Fig 5G,I; 22%; n=353), or to Rab11-positive recycling endosomes (Fig 5H; 5%; n=140). Together, these results indicate that Scarf is secreted apically and is internalized through endocytosis by non-producing cells. To confirm that Scarf is a secreted protein, Drosophila S2 cells were transfected with either a scarf–myc-tagged transgene or a transgene driving the expression of a myc-tagged membrane-tethered form of Scarf (Scarf–CD2; Fig 5C; supplementary information online). Scarf was isolated not only from the protein extract of the scarf–myc-expressing cells but also from the supernatant (Fig 5E). Transfected Scarf–CD2 and endogenous actin were not observed in the supernatant (Fig 5E).
Figure 5.
Scarf is a secreted protein. (A–D) Z or XY confocal sections of (A) ap-Gal4>UAS-GFP,UAS-scarf (left panel), (B) ap-Gal4>UAS-GFP (left panel), (C) ap-Gal4>UAS-GFP,UAS-scarf-CD2 and (D) ptc-Gal4>UAS-ANF-GFP wing discs labelled to visualize GFP, Scarf and phospho-tyrosine protein expression. (E) Actin (lower immunoblot) and Myc-tagged versions of Scarf and Scarf-CD2 (upper immunoblot) from cell lysates and conditioned medium of S2 cells expressing Scarf–Myc, Scarf–CD2–Myc or an empty vector were immunoprecipitated using actin or Myc antibodies, respectively, and probed using actin and Scarf antibodies. (F–H) Sub-apical XY and (I) Z confocal sections of ap-Gal4>UAS-scarf wing discs labelled to visualize co-localization (white arrowheads) of Scarf protein and (F) Rab5–GFP, (G,I) Rab7–GFP and (H) Rab11–GFP. ap-Gal4 drives the expression of scarf in the dorsal compartment. Scale bars, 25 μm. ap, apical; bs, basal; D, dorsal compartment; GFP, green fluorescent protein; V, ventral compartment.
Here, we characterize the role of scarf, a JNK-regulated gene, in GBR and DC, two morphogenetic processes that rely on cell–matrix interactions between the AS epithelium and neighbouring cell populations. We present evidence that Scarf is a secreted protein expressed in LE cells and involved in promoting the localized accumulation of LanA in the BM of AS cells. Although low levels of Scarf were detected in AS cells (Fig 1B,C), the cuticle phenotype of scarf mutant embryos and the defects observed in BM localization of LanA and epithelial integrity of AS cells were rescued largely by driving expression of a scarf transgene only in ectodermal cells (supplementary Table S1 online; Fig 4; supplementary Fig S6 online), indicating that Scarf is acting as a secreted protein. Three alternative hypotheses might explain the role of Scarf and Crag in the polarized localization of LanA to the BM. As Crag and Scarf are localized specifically on the apical side of the epithelium (Fig 5A; Denef et al, 2008), they would have a repulsive role, inhibiting the targeting of LanA-containing vesicles with apical membranes. Alternatively, LanA might be secreted apically and basally and be stabilized preferentially on the basal side or degraded on the apical side. As Scarf encodes for a putative serine protease-like protein without catalytic activity, Scarf would function, in this scenario, to facilitate the effect of a cascade of serine proteases involved in the degradation of LanA in the apical domain of epithelial cells. Finally, in scarf mutant cells, LanA might be transported from the basal to the apical side through transcytosis, a mechanism responsible for the formation of a small apical BM cap over pre-invasive epithelial cells during Drosophila oogenesis (Medioni & Noselli, 2005).
Another secreted serine protease-like protein without catalytic activity, Masquerade, regulates cell–matrix interactions at the somatic-muscle attachment in the fly embryo, a process that also depends on integrin and laminin activity (Murugasu-Oei et al, 1995). We speculate that a number of non-functional serine protease-like proteins, such as Masquerade and Scarf, are regulated temporally and spatially to promote the appropriate localization of BM proteins in a context-dependent manner to facilitate cell–matrix interactions and to ensure the maintenance of epithelial integrity. Similarly, among the large class of vertebrate functional and non-functional serine protease-like proteins involved in remodelling the extracellular matrix (Lopez-Otin & Matrisian, 2007), some of them might exert a similar action.
Methods
Drosophila strains. scarf PBss (Bonin & Mann, 2004); scarf M13.M2, a piggyBac-lacZ insertion, and scarf KG05129, a P element insertion, are localized in the first and third intron, respectively, of the scarf locus. scarf Δ1.5 is an excision of the scarf KG05129 insertion lacking the 5′ end of the P element and containing an intronic deletion of 404 bp of the nearby 5′ genomic region (Fig 1A). Df(2R)nap14 is a deficiency, the breakpoints of which are 41B and 42B1. UAS-Scarf and other stocks are described in supplementary information online and in the FlyBase database.
Antibodies. Rat E-cad and mouse βPS antibodies were obtained from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, USA), rabbit anti-LanA was provided by S. Baumgartner, other antibodies are available commercially and antibodies against Scarf are described in supplementary information online. Embryo thick sections were performed as reported by Narasimha & Brown (2004).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank M. Averof, S. Baumgartner, D. Bohmann, N. Denef, N. Gorfinkiel, A. Jacinto, R. Mann, E. Martin-Blanco, G. Morata, M. Ringuette, T. Schupbach, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for reagents; N. Gorfinkiel, A. Jacinto and M. D. Martín-Bermudo for comments on the paper; T. Yates for help in preparing the paper; and the Institute for Research in Biomedicine Advanced Digital Microscopy Core Facility for help in the microscopy and image processing. M.M.'s laboratory is funded by the Ministerio de Ciencia e Innovación BFU2007-64127/BMC) and the European Molecular Biology Organization Young Investigator Programme.
Footnotes
The authors declare that they have no conflict of interest.
References
- Affolter M, Nellen D, Nussbaumer U, Basler K (1994) Multiple requirements for the receptor serine/threonine kinase thick veins reveal novel functions of TGFβ homologs during Drosophila embryogenesis. Development 120: 3105–3117 [DOI] [PubMed] [Google Scholar]
- Bokel C, Brown NH (2002) Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell 3: 311–321 [DOI] [PubMed] [Google Scholar]
- Boll W, Partin JS, Katz AI, Caplan MJ, Jamieson JD (1991) Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc Natl Acad Sci USA 88: 8592–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin CP, Mann RS (2004) A piggyBac transposon gene trap for the analysis of gene expression and function in Drosophila. Genetics 167: 1801–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD (1987) Dependence on pH of polarized sorting of secreted proteins. Nature 329: 632–635 [DOI] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schupbach T (2008) Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell 14: 354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, Jacinto A (2007) Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev 124: 884–897 [DOI] [PubMed] [Google Scholar]
- Fernandez-Minan A, Cobreros L, Gonzalez-Reyes A, Martin-Bermudo MD (2008) Integrins contribute to the establishment and maintenance of cell polarity in the follicular epithelium of the Drosophila ovary. Int J Dev Biol 52: 925–932 [DOI] [PubMed] [Google Scholar]
- Homsy JG, Jasper H, Peralta XG, Wu H, Kiehart DP, Bohmann D (2006) JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev Dyn 235: 427–434 [DOI] [PubMed] [Google Scholar]
- Jacinto A, Woolner S, Martin P (2002) Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev Cell 3: 9–19 [DOI] [PubMed] [Google Scholar]
- Leptin M, Bogaert T, Lehmann R, Wilcox M (1989) The function of PS integrins during Drosophila embryogenesis. Cell 56: 401–408 [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM (2007) Emerging roles of proteases in tumour suppression. Nat Rev Cancer 7: 800–808 [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A (1998) puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev 12: 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C, Noselli S (2005) Dynamics of the basement membrane in invasive epithelial clusters in Drosophila. Development 132: 3069–3077 [DOI] [PubMed] [Google Scholar]
- Murugasu-Oei B, Rodrigues V, Yang X, Chia W (1995) Masquerade: a novel secreted serine protease-like molecule is required for somatic muscle attachment in the Drosophila embryo. Genes Dev 9: 139–154 [DOI] [PubMed] [Google Scholar]
- Narasimha M, Brown NH (2004) Novel functions for integrins in epithelial morphogenesis. Curr Biol 14: 381–385 [DOI] [PubMed] [Google Scholar]
- Reed BH, Wilk R, Schock F, Lipshitz HD (2004) Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr Biol 14: 372–380 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A (2005) Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6: 233–247 [DOI] [PubMed] [Google Scholar]
- Schock F, Perrimon N (2002) Cellular processes associated with germ band retraction in Drosophila. Dev Biol 248: 29–39 [DOI] [PubMed] [Google Scholar]
- Schock F, Perrimon N (2003) Retraction of the Drosophila germ band requires cell–matrix interaction. Genes Dev 17: 597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.