Abstract
Epithelial tissues are essential for the function of almost all major organs and altering their polarized architecture leads to a variety of diseases. The directed deposition of basement membrane proteins is a crucial but poorly understood process, important to maintain epithelial structure. In this issue of EMBO reports, the Drosophila gene Scarface is shown to regulate it.
EMBO Rep (2010) advance online publication. doi: 10.1038/embor.2010.43
Epithelial tissues perform essential barrier and transport functions in almost all major organs, and the inability to properly form or maintain their polarized architecture has been implicated in various diseases, including cancer. In this issue of EMBO reports, the Milan group implicates the Drosophila gene scarface (scarf) in the polarized deposition of basement membrane (BM) proteins (Sorrosal et al, 2010), a process which is crucial for maintaining epithelial structure.
Mammalian epithelial cells have a polarized structure maintained by intercellular tight junctions, which separate distinct apical and basolateral plasma membrane (PM) domains. Many proteins have been found to be targeted specifically to either the apical or basolateral PM, where they are unable to diffuse across the tight junctional barrier (Fig 1; Tanos & Rodriguez-Boulan, 2008). Another defining characteristic of epithelia is the existence of a complex network of secreted extracellular matrix proteins—such as laminin, collagens and heparan sulphate proteoglycans (HSPGs)—at the basal surface of epithelial cells, which are collectively known as the BM (Yurchenco et al, 2004). Basolateral-specific cell-surface receptors, such as integrins and dystroglycan, transduce signals from the BM and promote the adhesion of epithelial cells to the substratum. The BM is also crucial for specifying positional cues during the establishment and maintenance of epithelial cell polarity and, consequently, the proper formation of epithelial tissues.
Figure 1.
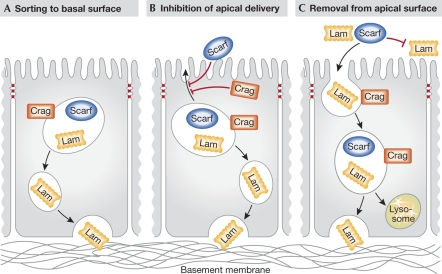
How could Scarf and Crag regulate the proper localization of basement membrane proteins to the epithelial basal surface? (A) By actively sorting BM cargo—such as laminin (Lam)—into vesicles bound for delivery to the basal surface. (B) Through the inhibition of the delivery or fusion of BM-containing vesicles to the apical domain. (C) By removing BM from the apical surface through endocytosis and/or proteolysis. BM, basement membrane; Scarf, Scarface.
The vesicular trafficking pathways that are required for the transport of newly synthesized and recycled PM proteins specifically to the apical or basolateral domains of epithelial cells have been the topic of intense investigation (Tanos & Rodriguez-Boulan, 2008). By contrast, the pathways governing the secretion of extracellular matrix proteins that are exclusive to the apical or basolateral surfaces have been much less studied. Do the same pathways control the delivery of both PM and secreted proteins to the correct cell-surface domain? This seems not to be the case, as several groups have shown that the delivery of basolateral-specific PM proteins and secreted BM proteins can be uncoupled (Boll et al, 1991; Caplan et al, 1987; Cohen et al, 2001; de Almeida & Stow, 1991). Since these initial reports, few details have been elucidated regarding the molecular components of these two distinct pathways. However, two recent studies have taken significant steps to shed light on this important process (Denef et al, 2008; Sorrosal et al, 2010).
…the pathways governing the secretion of extracellular matrix proteins that are exclusive to the apical or basolateral surfaces have been much less studied
By using the power of a genetic mosaic screen, Denef and colleagues isolated mutations in the Drosophila gene crag that are defective in the polarity and organization of the follicular epithelial (FE) cells (Denef et al, 2008). Notably, before the loss of polarity in crag mutant FE cells, the BM proteins perlecan—which is an HSPG—laminin and collagen IV were found not only on the basal surface but also mislocalized to the apical side of the cells. Importantly, the normal localization of several apical and basolateral PM proteins was unaffected in these cells, indicating that Crag does not regulate their polarized transport. This study also showed that mislocalized apical perlecan did not originate through transcytosis from the basal surface. Thus, crag seems to be required specifically for proper polarized secretion of newly synthesized BM components, a function that is probably essential to the general role of crag in maintaining epithelial architecture. crag encodes a protein containing multiple DENN domains and a functional calmodulin-binding domain. The precise function of the DENN domains is unknown; however, they are found in several proteins involved in protein transport, such as Rab interactors. Consistent with a role in protein transport, Crag is localized to Rab5- and Rab11-positive endosomes, and to the apical and lateral cortex of FE cells. Surprisingly, Crag protein has not been detected at the basal surface. The presence of crag homologues in vertebrates suggests a potentially conserved role for this gene in epithelial BM protein deposition.
The identification of the first putative molecular components that regulate polarized BM deposition is a significant advance in the field of epithelial biology
In this issue of EMBO reports, Sorrosal and colleagues examine the function of the Drosophila gene scarf (Sorrosal et al, 2010), which they show is expressed in the leading edge (LE) cells of the dorsal ectoderm and the amnioserosa (AS) epithelial cells of the embryo. Both LE and AS cells have important roles during embryonic dorsal closure, and a scarf loss-of-function allele induced defects in this process, as well as other phenotypes indicative of compromised AS epithelial cell polarity and adhesion. Similar phenotypes were also observed in mutants of crag, laminin A and the integrin βPS subunit, and a reduction of scarf levels acted synergistically to enhance some of the defects associated with crag and integrin βPS mutations. Although mutation of scarf led frequently to the loss of AS epithelial cell architecture and polarity, an analysis of the embryos that retained epithelial cell morphology revealed mislocalization of laminin to the apical surface. Unlike laminin, integrins were still localized properly to the basolateral domain. This defect is essentially identical to that observed for crag mutant FE and AS epithelial cells, indicating that—similar to Crag—Scarf seems to specifically regulate the proper polarized deposition of BM proteins, and that defects in this process might lead subsequently to disorganized epithelial architecture.
Although the authors were unable to examine endogenous Scarf protein in AS cells, they expressed Scarf in the wing imaginal disc epithelium and analysed its subcellular localization. Similar to Crag, Scarf localization was observed at the apical and lateral surfaces of cells but was absent from the basal surface. Additionally, Scarf protein could be found in Rab5-, Rab7- and Rab11-positive endosomes of cells that do not express Scarf, indicating that Scarf is a secreted protein that can be taken up by neighbouring cells. These observations are in agreement with the prediction that Scarf is a homologue of vertebrate secreted trypsin-like serine proteases that lack catalytic activity.
The identification of the first putative molecular components that regulate polarized BM deposition is a significant advance in the field of epithelial biology. Although no detailed mechanistic information has emerged from these studies, the analysis of Scarf and Crag function reveals several possible general models of how polarized BM protein localization might be achieved (Fig 1). The localization of both Scarf and Crag to endosomes is consistent with a potential role of these proteins in the vesicular transport of BM components. Scarf and Crag could act to properly sort newly synthesized and recycled BM components into vesicles destined for the basal surface. They could also act after the sorting of cargo to regulate the delivery or fusion of these vesicles at the basal surface. If Scarf or Crag regulate BM deposition at one of these later steps, they might act as inhibitory factors in vesicle transport or fusion with the apical membrane, as both are detected only at the apical and lateral domains and not at the basal surface. This would explain why BM protein exocytosis is misdirected to the apical surface in the absence of Scarf or Crag. Although apical BM deposition in crag mutants is derived from newly synthesized protein and not transcytosis of BM proteins, this does not rule out a role for Scarf in this process. Another possibility is the involvement of Crag and Scarf in removing BM proteins from the apical surface through endocytosis and/or degradation. The predominant view is that exocytosis of BM components is polarized towards the basolateral domain (Boll et al, 1991; Caplan et al, 1987; Natori et al, 1992; Unemori et al, 1990). However, if BM is exocytosed at both the apical and basal domains or accumulates at the apical surface non-cell-autonomously after secretion from nearby tissues, BM proteins would subsequently need to be stabilized only at the basal surface or actively removed from the apical surface. The putative molecular identity of Scarf as a serine protease that is secreted apically makes the latter model particularly attractive. Although Scarf lacks a crucial catalytic residue for protease activity, it could plausibly still function to regulate this process. Further insight into the mechanism of action of these two proteins, as well as the identification of additional factors will be required to distinguish between the various models of BM deposition and determine if the pathway is conserved in mammals.
…analysis of Scarf and Crag function reveals several possible general models of how polarized BM protein localization might be achieved
References
- Boll W et al. (1991) PNAS 88: 8592–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MJ et al. (1987) Nature 329: 632–635 [DOI] [PubMed] [Google Scholar]
- Cohen D et al. (2001) Traffic 2: 556–564 [DOI] [PubMed] [Google Scholar]
- de Almeida JB, Stow JL (1991) Am J Physiol 261: C691–C700 [DOI] [PubMed] [Google Scholar]
- Denef N et al. (2008) Dev Cell 14: 354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori Y et al. (1992) Am J Physiol 262: F131–F137 [DOI] [PubMed] [Google Scholar]
- Sorrosal G et al. (2010) EMBO Rep [Epub 9 Apr 2010] doi:10.1038/embor.2010.43 [Google Scholar]
- Tanos B, Rodriguez-Boulan E (2008) Oncogene 27: 6939–6957 [DOI] [PubMed] [Google Scholar]
- Unemori EN et al. (1990) J Biol Chem 265: 445–451 [PubMed] [Google Scholar]
- Yurchenco PD et al. (2004) Matrix Biol 22: 521–538 [DOI] [PubMed] [Google Scholar]