microRNA/Argonaute 2 regulates nonsense-mediated messenger RNA decay
Kim and colleagues provide evidence that Ago2 targets CBP80/20- and EJC-bound mRNAs and inhibits their nonsense-mediated mRNA decay (NMD). They also show that a subset of transcripts expected to be targeted for NMD are stabilized by miRNA-mediated gene silencing. The crosstalk between NMD and miRNA-mediated gene silencing sheds light on a new post-transcriptional regulation mechanism in mammalian cells.
Keywords: Ago2, microRNA, CBP80/20, eIF4E, NMD
Abstract
Imperfect base-pairing between microRNA (miRNA) and the 3′-untranslated region of target messenger RNA (mRNA) triggers translational repression of the target mRNA. Here, we provide evidence that human Argonaute 2 targets cap-binding protein (CBP)80/20-bound mRNAs and exon junction complex-bound mRNAs and inhibits nonsense-mediated mRNA decay (NMD), which is restricted tightly to CBP80/20-bound mRNAs. Furthermore, microarray analyses reveal that a subset of cellular transcripts, which are expected to be targeted for NMD, is stabilized by miRNA-mediated gene silencing. The regulation of NMD by miRNAs will shed light on a new post-transcriptional regulation mechanism of gene expression in mammalian cells.
Introduction
Immediately after transcription initiation, the cap structure at the 5′-end of pre-messenger RNA (pre-mRNA) is recognized by the nuclear cap-binding protein (CBP) complex, a heterodimer of CBP80 and CBP20 (Chang et al, 2007; Isken & Maquat, 2007). During the export of properly spliced mRNA through the nuclear pore complex (NPC), CBP80/20 recruits a ribosome to initiate translation, which is referred to as ‘the pioneer (or first) round of translation' or ‘CBP80/20-dependent translation' (Ishigaki et al, 2001; Chiu et al, 2004; Lejeune et al, 2004; Isken & Maquat, 2007; Kim et al, 2009). After the CBP80/20-dependent translation, CBP80/20 is replaced by the cytoplasmic CBP, eukaryotic translation initiation factor 4E (eIF4E). The cap-bound eIF4E recruits the ribosomes to trigger multiple rounds of translation, which is referred to as ‘steady-state translation' or ‘eIF4E-dependent translation'.
The CBP80/20-dependent translation occurs on mRNAs that harbour exon junction complex (EJC), which is deposited on mRNAs as a consequence of splicing. However, eIF4E-dependent translation occurs on EJC-free mRNAs. The CBP80/20-dependent translation preferentially requires the newly identified middle domain of eukaryotic initiation factor 4G (MIF4G) domain-containing protein, CBP80/20-dependent translation initiation factor (CTIF; Kim et al, 2009). The CTIF protein associates with CBP80 and eIF3, which is a crucial protein complex for the recruitment of the 40S ribosome. Analogously, eIF4E-dependent translation requires interactions of eIF4E–eIF4GI/II–eIF3 to recruit the 40S ribosome into mRNA. Even though CBP80 binds to CTIF more preferentially than eIF4GI/II (Kim et al, 2009), CBP80 can interact with eIF4GI (Ishigaki et al, 2001; Chiu et al, 2004; Lejeune et al, 2004), suggesting that CBP80–eIF4GI/II–eIF3 interactions might also be involved in CBP80/20-dependent translation.
The CBP80/20-dependent translation is coupled tightly to mammalian nonsense-mediated mRNA decay (NMD; Behm-Ansmant et al, 2007; Chang et al, 2007; Isken & Maquat, 2007; Neu-Yilik & Kulozik, 2008; Silva & Romao, 2009). NMD is a cellular mechanism by which aberrant mRNAs that harbour premature termination codons (PTCs) can be discriminated from normal mRNAs. Eventually, these faulty mRNAs will be eliminated, thus preventing the expression of truncated proteins. NMD is also believed to function as a post-transcriptional regulation mechanism for gene expression, as it targets a subset of normal cellular transcripts (Mendell et al, 2004; Wittmann et al, 2006).
MicroRNAs (miRNAs) are small endogenous non-coding RNAs that post-transcriptionally regulate gene expression by base-pairing with target mRNAs (reviewed in Eulalio et al, 2008). The mature miRNA assembles with Argonaute (Ago) family proteins, forming the miRNA-induced silencing complex (miRISC). The miRISC complex has been shown to inhibit gene expression of target mRNA in various ways. In particular, several steps of translation can be targeted for miRISC-mediated gene repression (Eulalio et al, 2008).
In this study, we show that human Ago2 is loaded onto CBP80-bound mRNAs and eIF4E-bound mRNAs. In addition, we observed that a subset of cellular transcripts, which are expected to be targeted for NMD, is stabilized by miRNA-mediated gene silencing. Accordingly, the abundance of NMD reporter mRNA, which contains a PTC and miR-122 target sequences at the 3′-untranslated region (3′UTR), decreases on treatment with 2′-O-methyl anti-miR-122. Our observations suggest crosstalk between NMD and miRNA-mediated gene silencing.
Results And Discussion
Human Ago2 is loaded onto CBP80/20-bound mRNAs
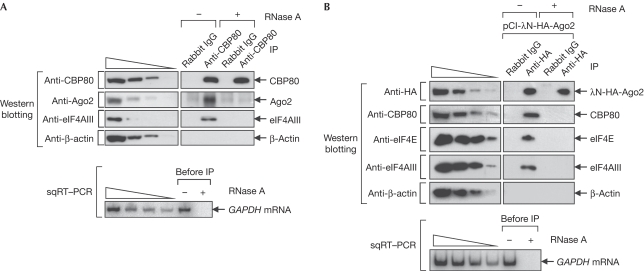
On the basis of the previous report that human Ago2 silences eIF4E-dependent translation (Eulalio et al, 2008), we reasoned that Ago2 might also silence CBP80/20-dependent translation. We performed immunoprecipitations to test whether human Ago2 is loaded onto CBP80/20-bound mRNAs (Fig 1). The results showed that endogenous Ago2 and eIF4AIII, which is a component of EJC, co-immunopurified with endogenous CBP80 in an RNase A-sensitive manner (Fig 1A). Furthermore, endogenous CBP80, eIF4E and eIF4AIII co-immunopurified with λN-haemagglutinin (HA)-Ago2 in an RNase A-sensitive manner (Fig 1B). Consistent with our findings, an RNA-mediated interaction between Ago2 and eIF4E has been reported previously (Chu & Rana, 2006). In addition, endogenous Ago2 was enriched in the immunoprecipitation of either Flag–CBP20 or Flag–eIF4E from total extracts of Cos-7 cells exposed to ultraviolet radiation, excluding the possibility that the RNA-mediated interaction between CBP80/20 and Ago2 occurs after cell lysis (supplementary Fig S1 online). As expected, endogenous eIF4AIII was enriched in the immunoprecipitation of Flag–CBP20, but not the immunoprecipitation of Flag–eIF4E (supplementary Fig S1 online). All immunoprecipitation results suggest that Ago2 is loaded efficiently onto CBP80/20- and EJC-bound mRNAs, and onto eIF4E-bound and EJC-free mRNAs, with CBP80/20 or eIF4E binding to the cap structure of the mRNA and with Ago2 binding to the 3′UTR of the mRNA.
Figure 1.
Human Ago2 is loaded onto CBP80/20- and eIF4E-bound mRNAs. (A) IPs of endogenous CBP80. IPs were performed using HeLa cell extracts and either CBP80 antibody or non-specific control antibody rabbit IgG. Upper panel: samples obtained before or after IP were analysed by western blotting by using the indicated antibodies. The four leftmost lanes, in which threefold serial dilutions of sample before IP were loaded, demonstrate that the western blotting was semi-quantitative. Lower panel: GAPDH mRNA was analysed by sqRT–PCR to demonstrate that the RNase A digestion was complete. The four leftmost lanes, in which twofold serial dilutions of cellular RNAs were analysed, demonstrate that the conditions used for RT–PCR were semi-quantitative. (B) IP of λN-HA-Ago2. The samples were analysed by western blotting (upper panel) or sqRT–PCR (lower panel) either before or after IP with HA antibody or non-specific control rat IgG. CBP, cap-binding protein; eIF, eukaryotic translation initiation factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HA, haemagglutinin; IP, immunoprecipitation; mRNA, messenger RNA; IgG, immunoglobulin G; sqRT–PCR, semi-quantitative reverse transcriptase PCR.
Loading of Ago2/miRISC onto 3′UTR abolishes NMD
Efficient loading of Ago2 onto CBP80/20-bound mRNAs led us to test whether Ago2 affects the events that occur on CBP80/20-bound mRNAs. As mammalian NMD is restricted only to CBP80/20-bound mRNAs, but not eIF4E-bound mRNAs (Ishigaki et al, 2001; Chiu et al, 2004; Chang et al, 2007; Isken & Maquat, 2007), we tested the effect of Ago2 on NMD.
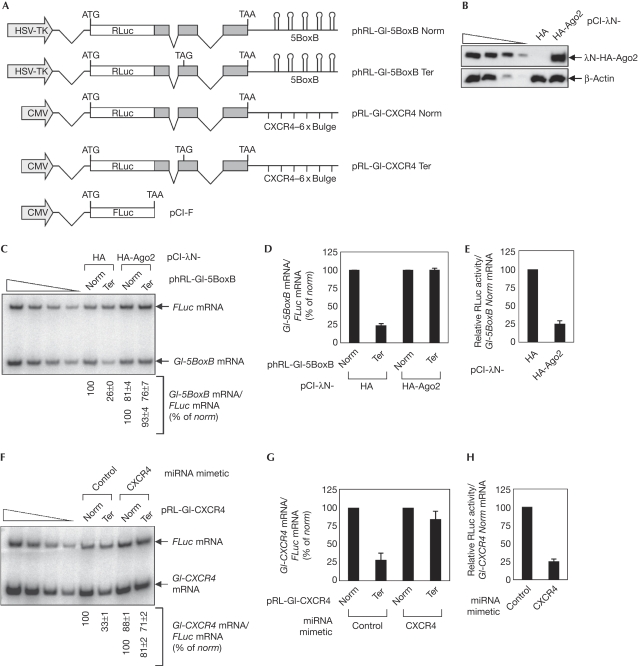
Two approaches were used: (i) artificial tethering of Ago2 to 3′UTR and (ii) loading of endogenous Ago2 or miRISC onto 3′UTR using CXCR4 miRNA mimetic. First, HA-Ago2 was tethered to the 3′UTR of NMD reporter Gl-5BoxB Norm and Ter mRNAs (Fig 2A) with the λN/boxB system (Kiriakidou et al, 2007). These tethering NMD reporter mRNAs were targeted efficiently for NMD, as demonstrated by the abrogation of NMD on downregulation of eIF4AIII, Upf1 or Y14 (supplementary Fig S2A–D online). The tethering results revealed that tethering of λN-HA-Ago2 inhibited NMD of Gl-5BoxB Ter mRNA by 3.6-fold (Fig 2C). Consistent with a previous report (Kiriakidou et al, 2007), tethering of λN-HA-Ago2 repressed overall translation of Gl-5BoxB Norm mRNA to 25% compared with that obtained from tethering of λN-HA (Fig 2E), marginally changing the mRNA level (Fig 2C). These results suggest that silencing of overall translation by tethered Ago2 was efficient under these conditions. By contrast, tethering of a negative control protein λN-HA-Ago2F2V2, in which two phenylalanines in the MC domain were substituted by valines (Kiriakidou et al, 2007), failed to inhibit NMD of Gl-5BoxB mRNA and did not significantly affect the abundance and overall translation of Gl-5BoxB Norm mRNA (data not shown). The semi-quantitative reverse transcriptase PCR (sqRT–PCR) results, shown in Fig 2C, were confirmed further by quantitative real-time PCR (Fig 2D).
Figure 2.
Artificial tethering of human Ago2 at the 3′UTR or loading of endogenous miRISC onto 3′UTR abrogates nonsense-mediated mRNA decay. (A) Schematic representations of the NMD reporter constructs. (B–E) Cos-7 cells were transiently transfected with (i) 1 μg of either pCI-λN-HA or pCI-λN-HA-Ago2, (ii) 0.1 μg of either phRL-Gl-5BoxB Norm or Ter, and (iii) 0.1 μg of pCI-F. (B) Western blotting of λN-HA-Ago2 using HA antibody. (C) sqRT–PCR of Gl-5BoxB mRNAs and FLuc mRNAs. The levels of Gl-5BoxB mRNAs were normalized to the levels of FLuc mRNAs. The normalized level of Gl-5BoxB Norm mRNA in the presence of λN-HA was set to 100% (upper numbers). Alternatively, the normalized levels of Gl-5BoxB Norm mRNA in the presence of each effector were set to 100% (lower numbers). (D) Quantitative real-time PCR of Gl-5BoxB mRNAs and FLuc mRNAs. The levels of Gl-5BoxB mRNAs were normalized to the levels of FLuc mRNAs. The normalized level of Gl-5BoxB Norm mRNA in the presence of λN-HA was set to 100%. (E) Translational efficiency of Gl-5BoxB Norm mRNAs. The relative RLuc activity (RLuc activity/FLuc activity) was normalized to the relative amount of Gl-5BoxB Norm mRNA (Gl-5BoxB Norm mRNA/FLuc mRNA). The normalized translation efficiency of Gl-5BoxB Norm mRNA in the presence of λN-HA was set to 100%. (F–H) HeLa cells (2 × 106) were transiently co-transfected with (i) miRNA-targeted NMD reporter plasmid, either pRL-Gl-CXCR4 Norm or Ter, (ii) the pCI-F, and (iii) either CXCR4 miRNA mimetic or non-specific control siRNA. (F,G) The abundance of Gl-CXCR4 Norm or Ter mRNAs and (H) translational efficiency of Gl-CXCR4 Norm mRNAs were analysed by (F) sqRT–PCR, (G) quantitative real-time PCR and (H) dual luciferase assay, respectively. 3′UTR, 3′-untranslated region; Ago2, Argonaute 2; HA, haemagglutinin; miRISC, microRNA-induced silencing complex; mRNA, messenger RNA; NMD, nonsense-mediated mRNA decay; siRNA, small interfering RNA; sqRT–PCR, semi-quantitative reverse transcriptase PCR.
Second, CXCR4 miRNA mimetic, which base-pairs imperfectly with CXCR4 miRNA target sites, was used to trigger the loading of endogenous Ago2 or miRISC onto the 3′UTR of NMD reporter Gl-CXCR4 Norm and Ter mRNAs, which contain six repeats of the CXCR4 miRNA-binding site (CXCR4–6 × Bulge) at the 3′UTR (Fig 2A) and were confirmed targets for NMD (supplementary Fig S2E,F online). The CXCR4 miRNA mimetic significantly abrogated the NMD of Gl-CXCR4 Ter mRNA, marginally affecting the abundance of Gl-CXCR4 Norm mRNA (Fig 2F). In addition, the overall translational efficiency of Gl-CXCR4 Norm mRNA decreased fourfold on treatment with CXCR4 miRNA mimetic (Fig 2H), indicating that CXCR4 miRNA mimetic-mediated translational repression was efficient under these conditions. The sqRT–PCR results, shown in Fig 2F, were confirmed further by quantitative real-time PCR (Fig 2G). These results suggest that tethering or loading of Ago2 (or miRISC) onto the 3′UTR of PTC-containing mRNAs abrogates NMD, possibly by targeting CBP80/20-dependent translation.
miRISC inhibits CBP80/20-dependent translation
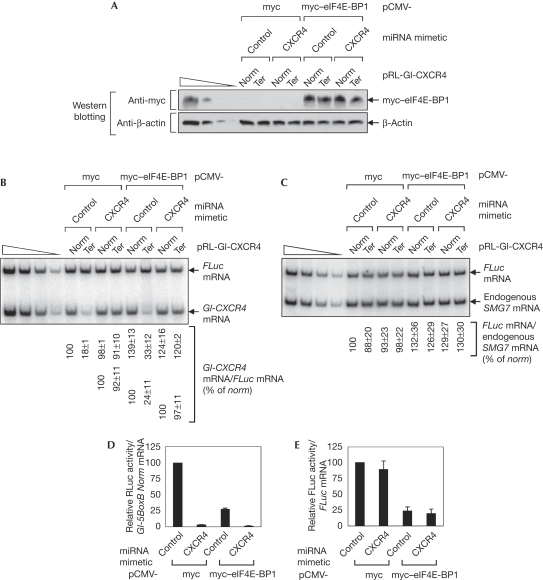
Several steps of eIF4E-dependent translation have been shown to be targeted for miRISC-mediated gene repression (reviewed in Eulalio et al, 2008). To determine clearly which step of NMD is targeted by Ago2-mediated silencing and to exclude the possibility that inhibition of eIF4E-dependent translation mediated by Ago2 indirectly affects the efficiency of NMD, we used eIF4E-binding protein 1 (eIF4E-BP1; Fig 3), the overexpression of which inhibits eIF4E-dependent translation, but not CBP80/20-dependent translation, owing to its competition with eIF4G for binding to eIF4E (Chiu et al, 2004). The results revealed that overexpression of myc–eIF4E-BP1 reduced the efficiency of overall translation to 25% (Fig 3D,E). Interestingly, the NMD of Gl-CXCR4 mRNA caused by the loading of endogenous miRISC onto the 3′UTR was not significantly affected by overexpression of myc–eIF4E-BP1 under these conditions (Fig 3B). Given that (i) Ago2 or miRISC inhibits the translation of target mRNAs (Eulalio et al, 2008), (ii) NMD is coupled tightly to CBP80/20-dependent translation (Ishigaki et al, 2001), (iii) tethering of Ago2 or loading of endogenous Ago2 at the 3′UTR abrogates NMD (Fig 2), and (iv) the selective inhibition of eIF4E-dependent translation by overexpression of eIF4E-BP1 does not influence the inhibition of NMD by loading of endogenous miRISC onto the 3′UTR (Fig 3), it is most likely that the abrogation of NMD is due to the inhibition of CBP80/20-dependent translation by Ago2 or miRISC.
Figure 3.
Overexpression of eIF4E-BP1 has no significant effect on abrogation of nonsense-mediated mRNA decay by loading of endogenous miRISC onto the 3′UTR. (A–E) Cos-7 cells were transiently transfected with (i) 4 μg of either pCMV-myc or pCMV-myc–eIF4E-BP1, (ii) 0.1 μg of either pRL-Gl-CXCR4 Norm or Ter, (iii) 0.1 μg of pCI-F, and (iv) 100 nM of either CXCR4 miRNA mimetic or non-specific control miRNA mimetic. (A) Western blotting of myc–eIF4E-BP1. (B) sqRT–PCR of Gl-CXCR4 mRNAs and FLuc mRNAs. The levels of Gl-CXCR4 mRNAs were normalized to the levels of FLuc mRNAs. The normalized level of Gl-CXCR4 Norm mRNA obtained from cells transfected with pCMV-myc was set to 100% (upper numbers). Alternatively, the normalized level of each Gl-CXCR4 Norm mRNA was set to 100% (lower numbers). (C) sqRT–PCR of FLuc mRNAs and endogenous SMG7 mRNAs. The normalized level of FLuc mRNA (FLuc mRNA/endogenous SMG7 mRNA) obtained from cells transfected with pCMV-myc was set to 100%. (D) Translational efficiency of Gl-CXCR4 Norm mRNAs. The RLuc activity was normalized to the relative amount of Gl-CXCR4 Norm mRNA. The normalized translation efficiency of Gl-CXCR4 Norm mRNA obtained from cells transfected with pCMV-myc and pRL-CXCR4 Norm was set to 100%. (E) Translational efficiency of FLuc mRNAs. FLuc activity was normalized to the relative amount of FLuc mRNA. 3′UTR, 3′ untranslated region; BP1, binding protein 1; miRISC, microRNA-induced silencing complex; mRNA, messenger RNA; NMD, nonsense-mediated mRNA decay; sqRT–PCR, semi-quantitative reverse transcriptase PCR.
Cellular NMD substrates regulated by Ago2/miRNAs
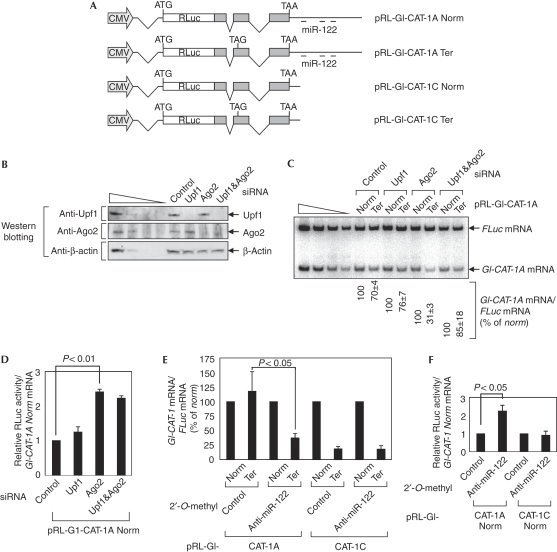
If a certain endogenous miRNA is loaded onto the 3′UTR of cellular NMD substrates and if its abundance is sufficient to silence NMD, the cellular NMD substrates would escape NMD. To test this, we assessed the effect of downregulation of the endogenous miRNA, miR-122, which represses translation of cationic amino-acid transporter 1 (CAT-1) mRNA (Bhattacharyya et al, 2006), on NMD in Huh-7 cells. The NMD reporter plasmids pRL-Gl-CAT-1 Norm and Ter, which contain CAT-1 3′UTR harbouring either miR-122-binding sites (pRL-Gl-CAT-1A) or no binding site (pRL-Gl-CAT-1C), were constructed (Fig 4A). As expected, Gl-CAT-1C Ter mRNA was efficiently targeted for NMD, whereas NMD of Gl-CAT-1A Ter mRNA was inhibited by 2.7-fold (supplementary Fig S3A online) and its translation was repressed by threefold (supplementary Fig S3B online). Interestingly, NMD of Gl-CAT-1A mRNA increased by 2.5-fold on downregulation of Ago2 (Fig 4C). The increase of NMD by downregulation of Ago2 was alleviated by downregulation of both Ago2 and Upf1 (Fig 4C). Notably, downregulation of Ago2 enhanced translation of Gl-CAT-1A Norm mRNA by 2.3-fold (Fig 4D), indicating that translational repression by Ago2 worked efficiently under these conditions. These results suggest that endogenous miRNA, miR-122, silences CBP80/20-dependent translation and hence NMD of Gl-CAT-1A Ter mRNA.
Figure 4.
Gl-CAT-1A Ter mRNA escapes nonsense-mediated decay in a manner that depends on Ago2 and miR-122. (A) Schematic representations of miR-122-targeted NMD reporter constructs. (B–D) Huh-7 cells were transiently transfected with 100 nM siRNA as indicated and, 2 days later, retransfected with 0.05 μg of pRL-Gl-CAT-1A harbouring Norm or Ter, and 0.05 μg of reference plasmid pCI-F. (B) Western blotting to demonstrate the specific downregulation by the indicated siRNAs. (C) sqRT–PCR of Gl-CAT-1A mRNAs and Gl-CAT-1C mRNAs. (D) Translational efficiencies of Gl-CAT-1A Norm mRNAs. (E, F) Huh-7 cells were transiently co-transfected with pRL-Gl-CAT-1 Norm or Ter, pCI-F, and either 2′-O-methyl anti-miR-122 oligonucleotide or non-specific 2′-O-methyl control oligonucleotide. (E) The abundance of Gl-CAT-1 Norm or Ter mRNAs and (F) the translational efficiency of Gl-CAT-1 Norm mRNAs were analysed by (E) quantitative real-time RT–PCR and (F) dual luciferase assay, respectively. P-values are indicated above the bar. CAT-1, cationic amino-acid transporter 1; mRNA, messenger RNA; NMD, nonsense-mediated messenger RNA decay; RT–PCR, reverse trascriptase–PCR; siRNA, small interfering RNA.
The inhibition of NMD of Gl-CAT-1A mRNA by endogenous miR-122 was confirmed more directly by using 2′-O-methyl antisense oligonucleotides. Treatment with 2′-O-methyl anti-miR-122, but not with the non-specific 2′-O-methyl control oligonucleotide, triggered the NMD of Gl-CAT-1A mRNA by 3.1-fold (Fig 4E). Under the same conditions, translation of Gl-CAT-1A Norm mRNAs, but not of Gl-CAT-1C Norm, was increased by 2.3-fold on treatment with 2′-O-methyl anti-miR-122 (Fig 4F), suggesting that silencing of overall translation by miR-122 works efficiently under these conditions. These results suggest that endogenous miR-122-containing miRISC loaded onto the 3′UTR abrogates NMD of Gl-CAT-1A mRNA.
Next, we performed microarray analysis using HeLa cell transcripts to search for miRNA-targeted cellular NMD substrates. We observed 54 transcripts (0.23% of the probe sets on the microarray) that were downregulated by at least 1.8-fold on Ago2 downregulation (supplementary Table S1A online). Only nine transcripts were upregulated on Ago2 downregulation (supplementary Table S1B online). Among the downregulated transcripts, the abundance of nine transcripts was restored by Ago2 or Upf1 downregulation (supplementary Table S1A online). Intriguingly, eight of those transcripts (except HIST1H2BD ) contained upstream open reading frame, which is one of the NMD-inducing features (Mendell et al, 2004). The levels of these transcripts were confirmed further by using sqRT–PCR (data not shown). These results suggest that a subset of cellular NMD substrates escape NMD in an Ago2- or miRNA-dependent manner.
Here, we provide evidence that Ago2 or miRISC targets CBP80/20-bound mRNAs, consequently inhibiting NMD. Ago2 or miRISC might target a specific factor involved in CBP80/20-dependent translation but not eIF4E-dependent translation. The observations obtained using overexpressed eIF4E-BP1 (Fig 3) suggest that eIF4G and other translation initiation factors recruited after eIF4G could be excluded as Ago2 or miRISC targets. However, the plausible candidates include CTIF and CBP80/20, which are preferentially involved in CBP80/20-dependent translation (Kim et al, 2009). These questions should be addressed in future studies.
Methods
Plasmid construction. Details are provided in the supplementary information online.
Cell culture and transfection. HeLa, Cos-7 and Huh-7 cells were grown in Dulbecco's modified Eagle's medium (Lonza, Walkersville, MD, USA) containing 10% fetal bovine serum (Lonza). Cells were transiently transfected with the indicated plasmids and 100 nM in vitro-synthesized small interfering RNA (Invitrogen, Carlsbad, CA, USA) by using Lipofectamine 2000 (Invitrogen) and Oligofectamine (Invitrogen), respectively, as described previously (Cho et al, 2009; Kim et al, 2009). The CXCR4 miRNA mimetic and non-specific control miRNA sequences were 5′-r(GUUUUCACUCCAGCUAACA)d(TT)-3′ and 5′-r(ACAAUCCUGAUCAGAAACC)d(TT)-3′, respectively, in which underlined sequences indicate a bulge generated by imperfect base-pairing between miRNA and target 3′UTR. At 2 days after transfection, cells were collected and total proteins and RNAs were purified as described previously (Cho et al, 2009; Kim et al, 2009).
sqRT–PCR and quantitative real-time PCR. sqRT–PCR and quantitative real-time PCR were performed as described previously (Cho et al, 2009; Kim et al, 2009).
Quantitative real-time PCR analyses using the LightCycler system (Roche Diagnostics, Mannheim, Germany) were performed with single-stranded complementary DNA and gene-specific oligonucleotides by using the Lightcycler 480 SYBR Green I Master Mix (Roche Diagnostics GmbH, Mannheim, Germany). The mRNA expression levels are the means of three independent experiments. Melting curves of PCR products were assessed for quality control.
Gene-specific oligonucleotides used in the study are as follows: 5′-CACTGGGCAGGTGTCCACTC-3′ (sense) and 5′-GTTCTGGATCATAAACTTTC-3′ (antisense) for the amplification of Gl-5BoxB, Gl-CXCR4 and Gl-CAT-1 mRNAs; 5′-CCAAAGGAGACCATCTGACC-3′ (sense) and 5′-CCTCATCTCGGCTTTCC-3′ (antisense) for SMG7 mRNA; 5′-TGGCAAATTCCATGGCACC-3′ (sense) and 5′-AGAGATGATGACCCTTTTG-3′ (antisense) for GAPDH mRNA; and 5′-CACTGGGCAGGTGTCCACTC-3′ (sense) and 5′-CTCTTCATAGCCTTATGCAG-3′ (antisense) for FLuc mRNA transcribed from pCI-F.
Immunoprecipitation and western blotting. Immunoprecipitation was performed as described previously (Cho et al, 2009; Kim et al, 2009). Cell extracts or immunopurified proteins were electrophoresed in sodium dodecyl sulphate–polyacrylamide (6–12%) and transferred to HyBond ECL nitrocellulose (Amersham, Little Chalfont, UK). The following antibodies were used: anti-Flag (Sigma, St Louis, MO, USA), anti-HA (Roche, Nutley, NJ, USA), anti-myc (Calbiochem, Darmstadt, Germany), anti-eIF4E (BD biosciences, San Jose, CA, USA), anti-eIF4AIII (a gift from R. Reed), anti-Upf1 (a gift from L.E. Maquat), anti-Ago2 (Upstate, Billerica, MA, USA), anti-glyceraldehyde 3-phosphate dehydrogenase (Calbiochem) and anti-β-actin (Sigma). Antibody against human CBP80 was raised in rabbits using the synthetic peptide GKELYEKKDAEMDRC (Ab Frontier, Seoul, Korea).
Microarray analysis. Total RNA was extracted from cells by using TRIzol Reagent (Invitrogen) and purified by using RNeasy columns (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Microarray analysis was carried out by Macrogen (Seoul, Korea). In brief, biotin-labelled complementary RNAs were generated by using the Illumina RNA amplification kit (Ambion) according to the manufacturer's instructions. The labelled complementary RNA samples were hybridized to each human-8 expression bead array (24,000 human gene chips) according to the manufacturer's instructions (Illumina, San Diego, CA, USA). The array signal was detected by using Amersham fluorolink streptavidin–Cy3 (GE Healthcare Bio-Sciences, Little Chalfont, UK) as described in the bead array manual. Hybridized chips were scanned in an Illumina bead array Reader confocal scanner (Illumina), and the scanned images were analysed with Illumina BeadStudio v3.1.3 (Gene Expression Module v3.3.8). Transcripts that were commonly downregulated or upregulated, by at least 1.8-fold, in two independent microarray analyses were considered differentially expressed. The microarray data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus web-based data repository (series ID: GSE16170).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank L.E. Maquat for providing Upf1 antibody, R. Reed for eIF4AIII antibody, W. Filipowicz and Z. Mourelatos for Ago2-tethering plasmids, and S.K. Jang for pcDNA3.1-FLuc-CXCR4–6 × Bulge plasmid. This study was supported by a grant from the National Research and Development Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0820090); a grant (PJ007050) from BioGreen 21 Program, Rural Development Administration, Republic of Korea; and the Korea Science and Engineering Foundation (KOSEF) Grant funded by the Korea government (MEST; No. 2009-0078061). J.C. was supported, in part, by a Seoul Science Fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Behm-Ansmant I, Kashima I, Rehwinkel J, Sauliere J, Wittkopp N, Izaurralde E (2007) mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett 581: 2845–2853 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W (2006) Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125: 1111–1124 [DOI] [PubMed] [Google Scholar]
- Chang YF, Imam JS, Wilkinson MF (2007) The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76: 51–74 [DOI] [PubMed] [Google Scholar]
- Chiu SY, Lejeune F, Ranganathan AC, Maquat LE (2004) The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes Dev 18: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Kim KM, Kim YK (2009) Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Mol Cell 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Chu CY, Rana TM (2006) Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol 4: e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Izaurralde E (2008) Getting to the root of miRNA-mediated gene silencing. Cell 132: 9–14 [DOI] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE (2001) Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 106: 607–617 [DOI] [PubMed] [Google Scholar]
- Isken O, Maquat LE (2007) Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21: 1833–1856 [DOI] [PubMed] [Google Scholar]
- Kim KM, Cho H, Choi K, Kim J, Kim BW, Ko YG, Jang SK, Kim YK (2009) A new MIF4G domain-containing protein, CTIF, directs nuclear cap-binding protein CBP80/20-dependent translation. Genes Dev 23: 2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidou M, Tan GS, Lamprinaki S, de Planell-Saguer M, Nelson PT, Mourelatos Z (2007) An mRNA m7G cap-binding-like motif within human Ago2 represses translation. Cell 129: 1141–1151 [DOI] [PubMed] [Google Scholar]
- Lejeune F, Ranganathan AC, Maquat LE (2004) eIF4G is required for the pioneer round of translation in mammalian cells. Nat Struct Mol Biol 11: 992–1000 [DOI] [PubMed] [Google Scholar]
- Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC (2004) Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet 36: 1073–1078 [DOI] [PubMed] [Google Scholar]
- Neu-Yilik G, Kulozik AE (2008) NMD: multitasking between mRNA surveillance and modulation of gene expression. Adv Genet 62: 185–243 [DOI] [PubMed] [Google Scholar]
- Silva AL, Romao L (2009) The mammalian nonsense-mediated mRNA decay pathway: to decay or not to decay! Which players make the decision? FEBS Lett 583: 499–505 [DOI] [PubMed] [Google Scholar]
- Wittmann J, Hol EM, Jack HM (2006) hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol Cell Biol 26: 1272–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.