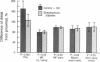Abstract
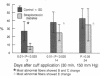
To determine the effect of diabetes on the development of axonal degeneration after acute nerve compression, the mobilized peroneal nerves of rats with streptozotocin-induced diabetes and of control rats were compressed at 150 mmHg (1 mmHg = 133 Pa) for 30 min by using specially devised cuffs. At three intervals after compression--3 days, rats diabetic for 31 wk; 14 days, diabetic for 6 wk; and 24 days, diabetic for 31 wk--groups of nerves were studied to assess numbers and sizes of fibers above, at, and below the cuff and to assess frequency of fiber degeneration in teased fibers from nerve distal to the cuff. Teased fibers with pathologic abnormalities were more frequent in nerves from controls than in nerves from diabetic rats in all three groups but the difference was statistically significant only at 3 and 14 days after compression. The lack of significant difference at 24 days may be explained by higher rates of disappearance of degenerating products and of fiber regeneration at 24 than at 3 and 14 days. This study provides evidence that in addition to delaying the reported functional deficit of vibratory detection threshold and conduction block during nerve compression, diabetes also may partially prevent axonal injury. Low nerve myo-inositol concentration did not predispose diabetic nerve to acute compression injury. If these results also apply to human diabetes and if repeated acute compression is involved in the genesis of fiber degeneration in entrapment, then a higher frequency of entrapment neuropathy among diabetics might be due to mechanisms other than increased susceptibility of fibers to acute compression--e.g., possibly to greater constriction of nerve due to pathologic alterations of the carpal ligament.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A., Nair C. P., Midgley R. Experimental progressive compression neuropathy in the rabbit. Histologic and electrophysiologic studies. Arch Neurol. 1971 Apr;24(4):358–364. doi: 10.1001/archneur.1971.00480340090010. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984 Oct;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Dyck P. J. Experimental hypertrophic neuropathy. Pathogenesis of onion-bulb formations produced by repeated tourniquet applications. Arch Neurol. 1969 Jul;21(1):73–95. doi: 10.1001/archneur.1969.00480130087010. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Zimmerman B. R., Vilen T. H., Minnerath S. R., Karnes J. L., Yao J. K., Poduslo J. F. Nerve glucose, fructose, sorbitol, myo-inositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med. 1988 Sep 1;319(9):542–548. doi: 10.1056/NEJM198809013190904. [DOI] [PubMed] [Google Scholar]
- Jakobsen J. Axonal dwindling in early experimental diabetes. I. A study of cross sectioned nerves. Diabetologia. 1976 Dec;12(6):539–546. doi: 10.1007/BF01220629. [DOI] [PubMed] [Google Scholar]
- Low P. A., Schmelzer J. D., Ward K. K., Yao J. K. Experimental chronic hypoxic neuropathy: relevance to diabetic neuropathy. Am J Physiol. 1986 Jan;250(1 Pt 1):E94–E99. doi: 10.1152/ajpendo.1986.250.1.E94. [DOI] [PubMed] [Google Scholar]
- Low P. A., Ward K., Schmelzer J. D., Brimijoin S. Ischemic conduction failure and energy metabolism in experimental diabetic neuropathy. Am J Physiol. 1985 Apr;248(4 Pt 1):E457–E462. doi: 10.1152/ajpendo.1985.248.4.E457. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Myers R., Powell H. Nerve compression injury and increased endoneurial fluid pressure: a "miniature compartment syndrome". J Neurol Neurosurg Psychiatry. 1983 Dec;46(12):1119–1124. doi: 10.1136/jnnp.46.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER D. W., LAMBERT E. H., BASTRON J. A., SPRAGUE R. G. The neuropathies associated with diabetes mellitus. A clinical and electromyographic study of 103 unselected diabetic patients. Neurology. 1961 Apr;11(4):275–284. doi: 10.1212/wnl.11.4.275. [DOI] [PubMed] [Google Scholar]
- Ochoa J., Fowler T. J., Gilliatt R. W. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat. 1972 Dec;113(Pt 3):433–455. [PMC free article] [PubMed] [Google Scholar]
- Phalen G. S. Reflections on 21 years' experience with the carpal-tunnel syndrome. JAMA. 1970 May 25;212(8):1365–1367. [PubMed] [Google Scholar]
- Richardson P. M., Thomas P. K. Percussive injury to peripheral nerve in rats. J Neurosurg. 1979 Aug;51(2):178–187. doi: 10.3171/jns.1979.51.2.0178. [DOI] [PubMed] [Google Scholar]
- STEINESS I. Influence of diabetic status on vibratory perception during ischaemia. Acta Med Scand. 1961 Sep;170:319–338. doi: 10.1111/j.0954-6820.1961.tb00245.x. [DOI] [PubMed] [Google Scholar]
- STEINESS I. Vibratory perception in diabetics during arrested blood flow to the limb. Acta Med Scand. 1959 Mar 4;163(3):195–205. doi: 10.1111/j.0954-6820.1959.tb10400.x. [DOI] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The effects of hypoxia on the excitability of the isolated peripheral nerves of alloxan-diabetic rats. J Neurol Neurosurg Psychiatry. 1969 Oct;32(5):462–469. doi: 10.1136/jnnp.32.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Windebank A. J., Natarajan V., Lambert E. H., Schmid H. H., Dyck P. J. Interstitial hyperosmolarity may cause axis cylinder shrinkage in streptozotocin diabetic nerve. J Neuropathol Exp Neurol. 1980 Nov;39(6):710–721. doi: 10.1097/00005072-198011000-00010. [DOI] [PubMed] [Google Scholar]