Abstract
Objectives:
To review the evidence for efficacy and metabolic effects of atypical antipsychotics (AAPs), and to propose a metabolic monitoring protocol for AAP use in children and adolescents.
Methods:
A PubMed search was performed to obtain all studies related to efficacy, metabolic side-effects, and monitoring in those less than 18 years of age.
Results:
There are no approved indications for AAP use in children and adolescents in Canada. Based on US Food and Drug Administration approvals and a review of randomized controlled trials, we identified 7 indications for AAP use that target specific symptoms in youth including schizophrenia, bipolar I disorder, autism, pervasive developmental disorder, disruptive behaviour disorders (including conduct disorder and ADHD), developmental disabilities and Tourette Syndrome. A wide range of metabolic effects including weight gain, increased waist circumference, dysglycemia, dyslipidemia, hypertension, elevated hepatic transaminases and prolactin levels have been reported. We have developed a proposal for metabolic monitoring that includes anthropometric measurements and laboratory testing at baseline and appropriate intervals thereafter.
Conclusion:
There is an urgent need for national clinical practice guidelines that provide, not only appropriate treatment algorithms for AAP-use based on evidence, but also address metabolic monitoring and subsequent management of complications in this vulnerable population.
Keywords: atypical antipsychotics, children, adolescents, efficacy, metabolic, monitoring
Introduction
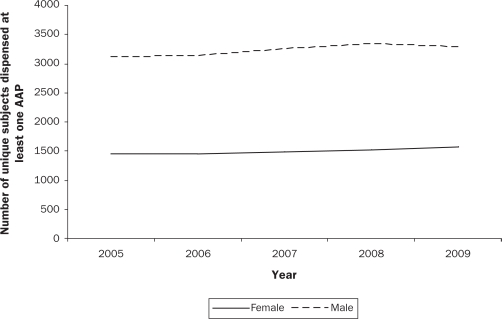
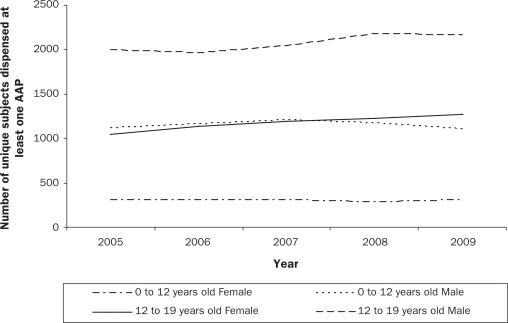
Second-generation or atypical antipsychotics (AAPs) have steadily replaced first-generation or typical antipsychotics since their introduction in the late 1980’s. In parallel with rising adult prescription rates (Hermann et al., 2002; Mond, Morice, Owen, & Korten, 2003), there is a growing body of literature demonstrating that prescriptions of AAPs to children and adolescents have increased substantially over the last decade. In the United States (US), the number of office-based visits by youth that included an AAP prescription increased 6-fold from 201,000 in 1993 to 1,224,000 in 2002 (Olfson, Blanco, Liu, Moreno, & Laje, 2006). A more recent study (Domino & Swartz, 2008) reported that the average age of AAP users declined from 49 years of age to 43 years of age in the US, as a result of a shift in prescriptions from the elderly towards youth, who accounted for 15% of all AAP prescriptions in 2005, doubled from 7% in 1996. While similar national data are not available for Canada, prescriptions of AAPs for children under 14 years old increased 10-fold between 1997 and 2007 in British Columbia (Therapeutics initiative, 2009). Consistent with US data (Cooper et al., 2006; Olfson et al., 2006), BC Pharmacare data (Pharmacare, 2009) indicate that prescriptions of AAPs are more common in boys than in girls (Figure 1a), with a substantial number of children under age 12 receiving these medications (Figure 1b). In a recent Canadian survey of child psychiatrists and developmental pediatricians (Doey, Handelman, Seabrook, & Steele, 2007), 12% of all AAP prescriptions were for children 8 years of age and under, which is of particular concern.
Figure 1a.
Sex distribution of atypical antipsychotic prescriptions in youth ≤ 19 years
Figure 1b.
Distribution of atypical antipsychotic prescriptions by age group and sex
Reasons that may have contributed to the increased prescription rates of AAPs in youth include the following: increasing evidence that illnesses such as bipolar disorder (Geller, Tillman, & Bolhofner, 2007) and schizophrenia (Addington & Rapoport, 2009) may present in childhood; arguable overgeneralization of clinical trial data from efficacy trials in adults and sub-populations of children and youth; and, most importantly, a perception of an improved side-effect profile particularly with respect to extrapyramidal symptoms (EPS) including tardive dyskinesia (Correll, Leucht, & Kane, 2004; Kane, 2001) than the first-generation or typical antipsychotic medications. However, in adults, there has been a clear link between AAPs and metabolic adverse effects for some time. Many studies in adults confirm that AAPs can precipitate weight gain (Tandon & Halbreich, 2003), hyperlipidemia (Meyer & Koro, 2004) and insulin resistance (Nasrallah & Newcomer, 2004; Wu et al., 2006), and that their use is associated with development of metabolic syndrome (Meyer, Koro, & L’Italien, 2005; Shirzadi & Ghaemi, 2006) and type 2 diabetes (Cohen, 2004; Henderson, 2002; Melkersson & Dahl, 2004; Newcomer, 2005).
There have been recent reports (Correll, 2009; Panagiotopoulos, Ronsley, & Davidson, 2009) of similar metabolic effects in children and adolescents. Of concern, there are data (Doey et al., 2007) indicating that a substantial percentage of physicians prescribe these medications for symptoms and diagnoses that have not been previously studied [e.g., poor frustration tolerance (75%), impulsivity (65%), obsessive-compulsive disorder (52%), insomnia (36%), post traumatic stress disorder (34%), depression (30%), and eating disorders (26%)]. These observations, combined with the increase in AAP prescription rates, indicate that there is an urgent need to systematically review both the existing evidence for use of AAPs, and their metabolic effects.
Therefore, our objectives with regard to children and adolescents under 18 years of age (collectively referred to as youth) are to summarize the evidence for efficacy of AAPs, review the existing literature assessing the metabolic effects of AAPs, and propose a protocol for metabolic monitoring in youth prescribed AAPs.
Methods
Efficacy
A PubMed search was performed to obtain all efficacy studies using the following key words: children or adolescents or youth or pediatric; atypical or second generation antipsychotics; aripiprazole or clozapine or olanzapine or paliperidone or quetiapine or risperidone or ziprasidone; efficacy; safety; indications. Only randomized controlled trials (RCTs) with a primary outcome of efficacy of one or more AAP conducted in individuals under 18 years of age were included. Case reports, review articles and open label trials assessing indications for AAP efficacy were not included due to space limitations. Information about current US Food and Drug Administration (FDA) approvals for children was obtained from the FDA website (Food and Drug Administration, 2010). Information about current Health Canada approvals was obtained from the Health Canada website (Health Canada, 2009).
Metabolic effects
A PubMed search was performed to obtain all studies assessing metabolic side effects using the following key words: children or adolescents or youth or pediatric; atypical or second generation antipsychotics; aripiprazole or clozapine or olanzapine or paliperidone or quetiapine or risperidone or ziprasidone; weight gain, obesity, dyslipidemia, hypercholesterolemia, hypertriglyceridemia, hyperglycemia, diabetes, insulin resistance, hypertension or blood pressure, hepatic transaminases, metabolic side effects, monitoring. Given that rare side effects may not be identified from randomized clinical trials and that regulatory agencies have relied on case series of serious adverse events (Gitlin, Julie, Spurr, Lim, & Juarbe, 1998; Kohlroser, Mathai, Reichheld, Banner, & Bonkovsky, 2000; Malik, Prasad, Saboorian, Thiele, & Malet, 2000) as a basis for removing a drug from the market to protect patient safety, all studies with a primary or secondary outcome measure of the above metabolic side effects conducted in individuals under 18 years of age were included. Studies were also included for review if they contained guidelines for monitoring of metabolic side effects in AAP-treated youth or adults. Additional articles were obtained from references and poster publications. Studies pertaining to cardiovascular disease (with the exception of hypertension/blood pressure), thyroid, parathyroid, or gonadal effects are beyond the scope of this review.
Classification
Articles were classified into level of evidence categories based on the Centre for Evidence Based Medicine criteria (Cache Limited, 2010).
Results
Efficacy
A total of 42 RCTs assessing efficacy of AAPs in children and adolescents were identified. Of these, 41 RCTs demonstrated a positive outcome, and one double-blind, placebo controlled RCT of quetiapine (DelBello et al., 2009) for symptoms of depression in adolescents 12–18 years of age with bipolar I disorder demonstrated no improvement.
While there are currently no Health Canada approved indications, there are several FDA approved indications for AAP use in children and adolescents. Table 1 summarizes the target symptoms, and mental health conditions, including the specific age ranges, for which there is either FDA approval or RCT evidence for use in this population.
Table 1.
Evidence supporting atypical antipsychotic use for specific indications
| Antipsychotic | Indication | Target symptoms | Age range (years) | Evidence |
|---|---|---|---|---|
| Aripiprazole | Autism§ | Irritability | 6–17 | Owen et al., 2009, Marcus et al., 2009. |
| Bipolar I Disorder§ | Manic or mixed episodes | 8–17 (10–17)§ | Findling et al., 2009, Tramontina et al., 2009. | |
| Schizophrenia§ | Positive and negative symptoms | 13–17 | Findling et al., 2008. | |
| Clozapine | Schizophrenia | Psychosis | 8–18 | Sporn et al., 2007. |
| Olanzapine | Bipolar I Disorder§ | Manic or mixed episodes (acute and maintenance treatment) | 13–17 | Tohen et al., 2007 |
| PDD | Aggression | 6–14 | Hollander et al., 2006. | |
| Schizophrenia§ | Positive and negative symptoms | 13–17 | Kryzhanovskaya et al., 2009. | |
| Quetiapine | Schizophrenia§ | Positive and negative symptoms | 13–17 | www.clinicaltrials.gov |
| Bipolar I Disorder§ | Manic episodes | 12–18 (10–17)§ | DelBello et al., 2006, Del Bello et al., 2002. | |
| Conduct disorder | Aggression | 12–17 | Connor et al., 2008. | |
| Risperidone | Autism§ | Irritability, Aggression, Communication, Hyperactivity, affect regulation | 2–18 (5–16)§ | Aman et al., 2008, Aman et al., 2005, McCracken et al., 2005, McCracken et al., 2002, McDougle et al., 2005, Miral et al., 2008, Nagaraj et al., 2006, Pandina et al., 2007, Shea et al., 2004. |
| Bipolar I Disorder§ | Manic or mixed episodes | 10–17 | www.clinicaltrials.gov, www.clinicaltrials.gov (Johnson et al., 2008), DelBello M et al., 2005, Wilson et al., 2009. | |
| Developmental Disabilities, Subaverage IQ | Aggression, SIB | 6–18 | Buitelaar et al., 2001, Zarcone et al., 2001. | |
| Disruptive Behaviour Disorders (including ADHD) | Conduct problems, Irritability, Hyperactivity, Aggression | 4–14 | Aman et al., 2009, Aman et al., 2002, Armenteros et al., 2007, Biederman et al., 2006, Correia Filho et al., 2005, Findling et al., 2004, Reyes et al., 2006, Snyder et al., 2002. | |
| Schizophrenia§ | Positive and negative symptoms | 13–17 | Haas et al., 2009. | |
| Tourette Syndrome | Tics | 7–17 | Bruggeman et al., 2001, Gaffney et al., 2002, Gilbert et al., 2004, Scahill et al., 2003. | |
| Ziprasidone | Bipolar I Disorder | Mania | 10–17 | DelBello et al., 2008. |
| Tourette Syndrome | Tics | 7–17 | Salle et al., 2000. |
- FDA Approved Indication
Metabolic Effects
A total of 24 studies were identified using our search criteria, and are presented in Table 2.
Table 2.
Summary of studies assessing metabolic effects of antipsychotics as a primary outcome
| Type of Study (Level of evidence) | AP | Length of study (weeks) | Study sample (N, %male, mean age [years]) | Metabolic Outcomes | Reference |
|---|---|---|---|---|---|
| Randomized controlled trial (1a) | R | 8 | N=101, 81% | prolactin | Anderson, et al., 2007. |
| 8.8 | |||||
| Prospective open label (1b) | A, O, Q, R | 12 | N=272, 57%; | weight, weight%, BMI, zBMI, fat mass, waist circumference, lipids, triglycerides, glucose, insulin | Correll, et al., 2009. |
| 13.9 | |||||
| Prospective open label (1b) | A, C, O, Q, R, Z | 12 | N=153; 77.8%; | body composition parameters, lipids, glucose, insulin, metabolic syndrome | Penzner, et al., 2009. |
| 11.3 | |||||
| Prospective open label (1b) | R | 4 | N=120, 76% | alanine aminostransferase, aspartate aminotransferase, gamma glutamyltransferase, alkaline phosphatase | Erdogan, et al., 2008. |
| 8.56 | |||||
| Open label or double blind (1b) | C, H, O | 6–8 | N=57, 60% | prolactin | Alfaro, et al., 2002. |
| C: 14.2, H: 13.8, O: 14.5 | |||||
| Cross sectional open label (2b) | Q, R | N/A | N=194, 77% | zBMI, BMI, waist circumference, glucose, insulin, lipids, metabolic syndrome | Weiss, et al., 2009. |
| 11.6 | |||||
| Naturalistic longitudinal (2b) | O, Q, R | 24 | N=66, 66.6% | zBMI, lipids, glucose, thyroid stimulating hormone | Fraguas, et al., 2008. |
| 15.2 | |||||
| Prospective open label (2b) | C, O, R | 6 | N=45, 69% | weight, BMI, zBMI | Fleischhaker, et al., 2007. |
| C: 17.4, O: 15.7, R: 15.2 | |||||
| Prospective open label (2b) | R | 24 | N=63, 78% | weight, leptin | Martin, et al., 2004. |
| 8.6 | |||||
| Prospective open label (2b) | H, O, R | 12 | N=50, 62% | weight, BMI | Ratzoni, et al., 2002. |
| H: 17.3, O: 17.0, R: 17.1 | |||||
| Prospective open label (2b) | R | 10 | N=25, 88% | prolactin | Masi, et al., 2001. |
| 4.10 | |||||
| Prospective open label (2b) | C, H, O | 6 | N=35, 63% | prolactin | Wudarsky, et al., 1999. |
| 14.1 | |||||
| Retrospective chart review (2b) | C, O, Q, R | N/A | N=432, 62% | zBMI, glucose, lipids, blood pressure, metabolic syndrome | Panagiotopoulos, et al., 2009. |
| treated: 13.7, control: 13.9 | |||||
| Retrospective chart review (2b) | R | 151 | N=99, 88% | weight, BMI, zBMI, blood pressure, waist circumference, lipids, glucose, insulin | Calarge, et al., 2009. |
| normal BMI:11.9, high BMI:11.3 | |||||
| Retrospective cohort design (2b) | A, O, Q, R, Z | N/A | N=4140, 68% | BMI, type 2 diabetes, dyslipidemia, hypertension, cardiovascular disease, cerebrovascular/orthostatic hypotension adverse events | McIntyre, et al., 2008. |
| 10.4 | |||||
| Retrospective chart review (2b) | A, C, O, Q, R, Z | 4 | N=95, 57% | BMI, lipids | Patel, et al., 2007. |
| 14 | |||||
| Cross sectional study (2b) | O, Q, R | 1–127 | N=126, 61.9% | glucose, lipids, BMI, prolactin, thyroid stimulating hormone, glycosylated hemoglobin A1c, blood pressure | Laita, et al., 2007. |
| 15.62 | |||||
| Retrospective chart review (2b) | O, Q | 4–5 | N=103, 48% | weight, BMI | Patel, et al., 2004. |
| O: 14.2, Q: 14.4 | |||||
| Retrospective chart review (2b) | R | 34 | N=22, 86% | weight, triglycerides, total cholesterol | Martin, et al., 2002. |
| 12.8 | |||||
| Retrospective chart review (2b) | R | 24 | N=37, 76% | weight, BMI, tanner staging, zweight | Martin, et al., 2000. |
| 12.5 | |||||
| Retrospective chart review (2b) | R | 106 | N=38, 84% | aspartate aminotransferase, alanine aminotransferase, total bilirubin, weight | Szigethy, et al., 1999. |
| 10.6 | |||||
| Case series (3a) | R | N/A | N=3, 66% | prolactin | Madhusoodanan, et al., 2006. |
| cases aged 15, 17, 18 | |||||
| Letter to editor (3b) | R | N/A | N=12, 58% | diabetes mellitus | Koller, et al., 2004. |
| 13.5 | |||||
| Letter to editor (3b) | O, C | N/A | 20, 55% | hyperglycemia | Koller, et al., 2001. |
| cases age range: 13–18 |
Legend: A: Aripiprazole; AP: Antipsychotic; C: Clozapine; H: Haloperidol; N/A: not available; O: Olanzapine; Q: Quetiapine; R: Risperidone; Z: Ziprasidone
Lipids: total cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol
R: Risperidone; Z: Ziprasidone; zBMI: body mass index standardized for age and sex; zweight: weight standardized for age and sex
Absolute Weight Gain
Compared to adults, AAPs have been found to produce significantly greater weight gain in children and do so within a very short time frame (Correll & Carlson, 2006; Fleischhaker et al., 2007; Penzner et al., 2009; Ratzoni et al., 2002). The magnitude of absolute weight gain varies between AAPs, with olanzapine producing the greatest weight gain (Correll et al., 2009; Fleischhaker et al., 2007; Fraguas et al., 2008; Patel, Kistler, James, & Crismon, 2004). Correll reported significant weight gain within 4 weeks of AAP initiation (average weight gain: olanzapine 4.52 kg; quetiapine 2.87 kg; risperidone 2.72 kg; aripiprazole 1.61 kg), that remained significantly higher than that seen in untreated youth after 12 weeks of treatment (olanzapine 8.5 kg; quetiapine 6.1 kg; risperidone 5.34 kg; aripiprazole 4.4 kg). Due to insufficient enrollment, the ziprasidone group was not analyzed. Extreme weight gain (defined as an increase of ≥ 7% from initial baseline weight) was observed in 84% of olanzapine-treated, 56% of quetiapine-treated, 64% of risperidone-treated and 58% of aripiprazole-treated patients (Correll et al., 2009; Ratzoni et al., 2002). Of note, one study (Fleischhaker et al., 2007) demonstrated that olanzapine and risperidone, but not clozapine, caused a disproportionately higher weight gain in children and adolescents compared to adults. There are limited long-term studies of AAPs in which weight gain is a primary or secondary outcome measure. However, an RCT (Arango et al., 2009) comparing olanzapine and quetiapine in adolescent patients with a first psychotic episode, reported the mean incremental weight gain to be 15.5 kg and 5.4 kg respectively during the 6 month study. Although it has been argued that youth may be more vulnerable to AAP-induced weight gain than adults, recent work (Correll et al., 2009) challenges this hypothesis by suggesting that the greater weight gain in youth is related to less frequent AAP exposure compared with most adult samples.
Body mass index (BMI)
Because childhood and adolescence involves continuing increases in height and weight as part of normal growth, body mass index (BMI: weight (kg)/height (m)2) normalized for age and sex (BMI z-score) provides a more accurate method with which to assess the appropriateness of absolute weight gain in the context of linear growth. For children, overweight is defined as a BMI ≥ 85th percentile and obese as ≥ 95th percentile for age and sex (Center for Disease Control and Prevention, 2007) (corresponding to BMI z-scores of 1.04 and 1.64, respectively). An absolute increase in BMI z-score of ≥ 0.5 has been proposed as significant because this degree of growth-adjusted weight gain was found to increase the risk for metabolic syndrome by more than 50% (Correll & Carlson, 2006; Weiss et al., 2004). Within 12 weeks of treatment, Correll reported (Correll et al., 2009) an increase in BMI z-score of > 0.5 in 62% of olanzapine-treated, 36% of quetiapine-treated, 47% of risperidone-treated, and 22% of aripiprazole-treated youth. Overall, 17% of these patients had become overweight or obese. In the short-stay, child and adolescent psychiatric emergency (CAPE) unit at BC Children’s Hospital (BCCH), cross-sectional prevalence rates of obesity/overweight were more than double in AAP-treated youth (57%) compared with AAP-naïve youth (23%) (Panagiotopoulos et al., 2009). This finding is consistent with Patel (Patel et al., 2007), who reported an overall prevalence of 68% obesity/overweight in their inpatient sample which was 2.2 times greater than a sample from the general population (31%).
Waist Circumference
Waist circumference has been used as a surrogate measure of central adiposity, and has been shown to be a strong predictor of other components of the metabolic syndrome (Bitsori, Linardakis, Tabakaki, & Kafatos, 2009; Zimmet et al., 2007). There is a growing body of evidence that AAPs can result in a rapid increase in waist circumference. In the CAPE unit at BCCH, 49% of AAP-treated youth had a waist circumference ≥ 90th percentile compared to only 18% of AAP-naïve youth (Panagiotopoulos, Davidson, Weiss, 2009). In a subset of children (n=27) treated with both risperidone and a psychostimulant for ADHD, and enrolled in a protocol for metabolic monitoring at BCCH, increased waist circumference was documented in 68% (Weiss et al., 2009). Correll (Correll et al., 2009) reported significant mean increases in waist circumference after 12 weeks of therapy with olanzapine (8.6 cm), aripiprazole (5.4 cm), quetiapine (5.3 cm), and risperidone (5.1 cm) compared to untreated controls. Similar to increases in body weight, increases in waist circumference were noted within 4 weeks of treatment.
Glucose and Insulin
In the US FDA MedWatch Drug Surveillance System, there are several case series (Koller, Malozowski, & Doraiswamy, 2001; Koller, Cross, & Schneider, 2004; Koller, Weber, Doraiswamy, & Schneider, 2004) reporting the occurrence of diabetes and diabetic ketoacidosis in children and adolescents following treatment with clozapine, olanzapine, risperidone and quetiapine. Although most of these cases occurred within 6 months of drug initiation, some occurred as late as 12–24 months of therapy. Of 46 cases of quetiapine-related diabetes (Koller, Weber et al., 2004), 11 deaths were reported (age range 12–47 years). Significant elevations in fasting glucose (without overt diabetes symptoms) have been shown to occur following 12 weeks of olanzapine treatment (Correll et al., 2009; Sikich et al., 2008). The cross-sectional prevalence of impaired fasting glucose and/or type 2 diabetes from the CAPE unit at BCCH was 21.5% in AAP-treated youth, almost three times that seen in AAP-naïve youth (7.5%) (Panagiotopoulos et al., 2009), likely due to a longer duration of AAP treatment. While the exact pathophysiology of AAP-induced diabetes/hyperglycemia remains unclear, there is evidence (Calarge, Acion, Kuperman, Tansey, & Schlechte, 2009; Correll et al., 2009; Weiss et al., 2009) that AAPs promote insulin resistance with compensatory fasting hyperinsulinemia. Although rapid weight gain and central adiposity likely contribute to insulin resistance, emerging evidence from animal models (Chintoh et al., 2008; Savoy et al., 2008) suggest that AAPs may also exert direct effects on beta-cell function.
Lipids
Significant increases in total cholesterol have been documented following olanzapine and quetiapine treatment (Fraguas et al., 2008; Sikich et al., 2008), in triglycerides following olanzapine, quetiapine and risperidone treatment, and in LDL-cholesterol following aripiprazole and olanzapine treatment (Correll et al., 2009). Using administrative datasets, McIntyre and Jerrell (McIntyre & Jerrell, 2008) previously reported that the odds of incident dyslipidemia are higher for those exposed to multiple antipsychotics (OR 5.26; 95% confidence interval (CI): 1.64–16.82). The risk of patients with preexisting obesity and hypertension developing type 2 diabetes or dyslipidemia was 4.5 times higher following antipsychotic exposure than those without these pre-existing conditions. One study (Martin et al., 2004), found that almost 25% of the variance in triglyceride levels could be explained by weight gain alone, and another study (Patel et al., 2007) reported that BMI z-scores were positively associated with total cholesterol, triglycerides and LDL and negatively correlated with HDL.
Blood Pressure
The odds of developing incident hypertension have been reported to be significantly higher for antipsychotic-treated adolescents 13 years of age or older (OR 2.78; 95% CI: 1.69–4.55), but are not correlated to which antipsychotic they received (McIntyre & Jerrell, 2008). Consistent with these data, the cross-sectional prevalence of elevated blood pressure (defined as systolic or diastolic ≥ 90th percentile for age, sex, and height percentile) within the CAPE unit at BCCH was 54% in AAP-treated youth, three times that seen in AAP-naïve youth (18%) (Panagiotopoulos, Davidson, Weiss, 2009). However, two other studies in AAP-treated children and adolescents (Fraguas et al., 2008; Laita et al., 2007) have not found significant changes in blood pressure even when assessing long-term treatment.
Metabolic syndrome
The ‘metabolic syndrome’ refers to a condition in which there is central obesity and at least 2 additional criteria (high blood pressure, high triglyceride level, low HDL-cholesterol level, high fasting glucose) (Zimmet et al., 2007). Metabolic syndrome is a predictor of cardiovascular disease risk, particularly of atherosclerosis and stroke in adults (Li et al., 2003; Raitakari et al., 2003). The definition of metabolic syndrome is derived from adult studies and is not universally accepted in pediatrics. There is ongoing debate about the relative importance of the diagnosis compared to the individual abnormalities. The current literature indicates that the atherosclerotic process begins in childhood and that indices of metabolic syndrome track from childhood to adulthood (Berenson et al., 1998).
Studies assessing the impact of AAPs on metabolic syndrome in children are limited. Following short-term treatment (mean follow-up 10.8 weeks) with AAPs, the mean prevalence of metabolic syndrome was 1.6% with the highest rate documented in the quetiapine sub-group (6.5%) (Correll et al., 2009). In contrast, in the CAPE unit at BCCH, where the mean duration of AAP treatment at the time of assessment was 12 months, the prevalence of metabolic syndrome was 9 times higher in AAP-treated (27%) compared to AAP-naïve (2.9%) children and adolescents (p<0.001) with an increased prevalence of the following components, respectively: elevated waist circumference (49.1 vs. 17.9%; p<0.0001); hypertriglyceridemia (42.6 vs. 22.4%; p=0.015); impaired fasting glucose (16.1 vs. 2.6%; p=0.005); and hypertension (54 vs. 18%; p<0.0001). In addition, low HDL-cholesterol was seen in 16% of AAP-treated youth compared to 11% of AAP-naïve youth; however, this result was not statistically significant (Panagiotopoulos, Davidson, Weiss, 2009).
Hepatic Transaminases
Studies in children and adolescents regarding effects on hepatic transaminases during AAP treatment are limited. Woods (Woods, Martin, Spector, & McGlashan, 2002) reviewed the FDA MedWatch Drug Surveillance System regarding olanzapine and noted significantly higher risk ratios for hepatic transaminase abnormalities in children [under 10 years of age] (RR 3.4, 95% CI: 1.9–6.1) and adolescents [10–19 years of age] (RR 1.9, 95% CI: 1.5–2.4) compared to adults. These findings are consistent with other clinical trials (Gonzalez-Heydrich, Raches, Wilens, Leichtner, & Mezzacappa, 2003; Sikich et al., 2008). However, in risperidone-treated youth, data are conflicting. One case series (Kumra, Herion, Jacobsen, Briguglia, & Grothe, 1997) identified steatohepatitis in 2 of 13 children treated with risperidone, while other prospective studies (Erdogan et al., 2008; Szigethy, Wiznitzer, Branicky, Maxwell, & Findling, 1999) have reported low rates of clinically significant hepatic transaminase elevation. More research is needed to evaluate these abnormalities and the potential for steatohepatitis in this population.
Prolactin
There is accumulating evidence (Anderson et al., 2007) that children treated with risperidone often exhibit modest to marked elevations in prolactin. In a post-hoc analysis of 5 clinical trials (Findling et al., 2003) in children between 5–15 years of age with disruptive behaviour disorders who were treated with risperidone, there was a rapid rise of serum prolactin peaking at 1–2 months following initiation of therapy and returning to normal levels after 3–5 months. However, in a different study (Anderson et al., 2007) of children with autism between 5–17 years of age, the risperidone-induced increase in prolactin reached a 4-fold increase within the first 2 months, and remained significantly increased at 6 months (3-fold) and at 22 months of treatment (2-fold). While data (Alfaro et al., 2002) do not suggest any correlation between prolactin levels and risperidone dosage, a reduction in dosage has been associated with significantly decreased levels (Masi, Cosenza, & Mucci, 2001). While increases in prolactin levels are especially noted with risperidone, they can also occur with other AAPs, including olanzapine and clozapine (Alfaro et al., 2002; Dittmann et al., 2008; Laita et al., 2007; Wudarsky et al., 1999). These elevations appear to be particularly concerning in adolescents (Woods et al., 2002). In contrast, aripiprazole appears to decrease prolactin levels (Findling et al., 2008). While the majority of patients remain asymptomatic with mild prolactin elevation, more marked elevation has been reported to lead to gynecomastia in males, and galactor-rhea and amenorrhea in females (Madhusoodanan & Moise, 2006). Although there is evidence that AAP-induced increases in prolactin tend to diminish over time, the impact of chronic prolactin increases on gonadal hormones, growth and pubertal development and bone mineral density are unknown (Anderson et al., 2007).
Metabolic monitoring
Prompted by the growing body of evidence highlighting significant metabolic effects associated with AAP treatment in adults, the American Diabetes Association with the American Psychiatric Association published a consensus statement (American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, & North American Association for the Study of Obesity, 2004) in 2004 that specified a monitoring protocol including baseline assessments of personal/family history, body mass index, waist circumference, blood pressure, as well as fasting plasma glucose and lipid levels. In spite of these recommendations, and in conjunction with FDA warnings about metabolic adverse effects of AAPs, adherence to these monitoring recommendations has been low in adults (Haupt et al., 2009; Morrato et al., 2009).
In our retrospective chart review of 432 youth admitted to the CAPE unit at BCCH during a 2.5-year period, we found that only 39% of patients had their height and weight measured, 34% had their fasting glucose measured, and 32% had a lipid profile measured (Panagiotopoulos et al., 2009). Similarly, Haupt reported (Haupt et al., 2009) that baseline and 12-week testing rates for lipids and glucose in children were the lowest of all age groups. A survey of Australian child psychiatrists (Walter et al., 2008) revealed the following monitoring rates following AAP-initiation: height (50%); waist circumference (28%); blood pressure (46%); fasting glucose (52%); lipids (48%); prolactin (32%); hepatic transaminases (53%).
Based on the current literature regarding the time course of onset of metabolic complications, and other national consensus guidelines (American Diabetes Association et al., 2004; Woo, Harris, Houlden, 2005), we have created a metabolic monitoring protocol. Table 3 summarizes the proposed history, anthropometric and laboratory measures with suggested time intervals in the first year of treatment. Although beyond the scope of this review, reports of thyroid stimulating hormone and amylase abnormalities following quetiapine treatment (Feret & Caley, 2000; Gropper & Jackson, 2004; Kelly & Conley, 2005) support inclusion of these parameters in our metabolic monitoring protocol. More frequent assessments of metabolic parameters may be required based on clinical circumstances. This monitoring protocol is currently being piloted at BC Children’s Hospital and Vancouver Coastal Child & Youth Mental Health Teams.
Table 3.
Proposed metabolic monitoring protocol
| Assessment | Baseline | 1 month | 2 months | 3 months | 6 months | 9 months | 12 months† |
|---|---|---|---|---|---|---|---|
| Family History (diabetes,high cholesterol, cardiovascular disease, psychiatric history) | x | ||||||
| Risk Factors (smoking, physical activity, screen time, sugar-sweetened beverages) | x | ||||||
| Height | x | x | x | x | x | x | x |
| Weight | x | x | x | x | x | x | x |
| Waist Circumference | x | x | x | x | x | x | x |
| Blood Pressure | x | x | x | x | x | x | x |
| Fasting Plasma Glucose | x | x | x | x | |||
| Fasting Insulin | x | x | x | x | |||
| Fasting Total Cholesterol | x | x | x | x | |||
| Fasting LDL-Cholesterol | x | x | x | x | |||
| Fasting HDL-Cholesterol | x | x | x | x | |||
| Fasting Triglycerides | x | x | x | x | |||
| Aspartate Aminotransferase | x | x | x | x | |||
| Alanine Aminotransferase | x | x | x | x | |||
| Gamma Glutamyltransferase | x | x | x | x | |||
| Prolactin | x | x* | x* | x* | |||
| Amylase‡ | x | x | x | x | |||
| Thyroid Stimulating Hormone‡ | x | x | x |
and yearly thereafter or sooner if clinically indicated
as clinically indicated [ie. abnormalities in menstruation, galactorrhea/nipple discharge, sexual dysfunction, delays in pubertal development; signs of osteoporosis (fractures)]
quetiapine-treatment only
Discussion
There are no approved indications for AAP use in children and adolescents in Canada. In this paper, we have summarized the limited number of indications and target symptoms within specific age groups by AAP for which there is evidence to support efficacy in this population. Our review confirms that there is no evidence to support the use of these medications for insomnia or major depression. While naturalistic studies assessing clinical effectiveness would be helpful to guide clinical practice, these studies are limited to non-existent for these published indications. In view of the fact that AAPs are being prescribed for a wide range of symptoms and mental health conditions in children and adolescents (Doey et al., 2007; Harrison-Woolrych, Garcia-Quiroga, Ashton, & Herbison, 2007; Olfson et al., 2006) that are not supported by our comprehensive review of the literature, we strongly encourage health care professionals to review their prescribing practices in this vulnerable population. Furthermore, based on recent FDA warnings (US Food and Drug Administration, 2010) and our literature review, it is clear that olanzapine should not be used as a first-line treatment in youth. Given that a growing number of physicians without specialty training are also prescribing these medications, practice guidelines that provide an evidence-based algorithm for treatment (including alternatives to AAP use) are vital for ensuring that all children and adolescents with mental health conditions receive the best care.
In view of the substantial body of literature suggesting that AAPs have a wide range of metabolic effects on individuals who are still growing and developing, consistent metabolic monitoring including anthropometric measurements and laboratory testing at baseline and at appropriate intervals thereafter is necessary. As part of the routine psychiatric history, baseline documentation of familial and lifestyle risk factors for obesity, type 2 diabetes, dyslipidemia and cardiovascular disease should be performed to assist the prescriber in assessing and providing anticipatory guidance regarding potential metabolic risks. We propose that national consensus guidelines for monitoring and management of metabolic complications of AAPs are urgently required and will serve as a starting point for improving awareness and monitoring uptake in Canada. As a first step, following synthesis of the current evidence, we have prepared a metabolic monitoring protocol which is currently being piloted at BC Children’s Hospital and local community mental health teams.
Conclusion
Prescription of AAPs in children and adolescents should be guided by the available evidence in this age group, and not extrapolated from adult studies. It is incumbent on physicians to ensure that the benefits of AAP treatment outweigh the risks. There is an urgent need for clinical practice guidelines in Canada that provide, not only appropriate treatment algorithms for AAP-use based on indications, but also address metabolic monitoring and subsequent management of complications in this vulnerable population. In preparation for the development of such guidelines, the authors invite readers’ comments and feedback.
Acknowledgements/Conflict of Interest
Dr. Panagiotopoulos is supported by the Child & Family Research Institute and Canadian Diabetes Association Clinician Scientist Awards. This work is also funded by operating grants from The Lawson Foundation and the Canadian Diabetes Association.
References
- Addington AM, Rapoport JL. The genetics of childhood-onset schizophrenia: When madness strikes the pre-pubescent. Current Psychiatry Reports. 2009;11(2):156–161. doi: 10.1007/s11920-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro CL, Wudarsky M, Nicolson R, Gochman P, Sporn A, Lenane M, et al. Correlation of antipsychotic and prolactin concentrations in children and adolescents acutely treated with haloperidol, clozapine, or olanzapine. Journal of Child and Adolescent Psychopharmacology. 2002;12(2):83–91. doi: 10.1089/104454602760219126. [DOI] [PubMed] [Google Scholar]
- Aman MG, Hollway JA, Leone S, Masty J, Lindsay R, Nash P, et al. Effects of Risperidone on cognitive-motor performance and motor movements in chronically medicated children. Research in Developmental Disabilities. 2009;30:386–396. doi: 10.1016/j.ridd.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Aman MG, Hollway JA, McDougle CJ, Scahill L, Tierney E, McCracken JT, et al. Cognitive effects of risperidone in children with autism and irritable behavior. Journal of Child and Adolescent Psychopharmacology. 2008;18(3):227–236. doi: 10.1089/cap.2007.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Arnold LE, McDougle CJ, Vitiello B, Scahill L, Davies M, et al. Acute and long-term safety and tolerability of risperidone in children with autism. Journal of Child and Adolescent Psychopharmacology. 2005;15(6):869–884. doi: 10.1089/cap.2005.15.869. [DOI] [PubMed] [Google Scholar]
- Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorders, and subaverage IQ. Journal of Child and Adolescent Psychopharmacology. 2004;14(2):243–254. doi: 10.1089/1044546041649020. [DOI] [PubMed] [Google Scholar]
- Aman MG, De Smedt G, Derivan A, Lyons B, Findling RL, Risperidone Disruptive Behavior Study Group Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. The American Journal of Psychiatry. 2002;159(8):1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. American Psychiatric Association. American Association of Clinical Endocrinologists. North American Association for the Study of Obesity Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biological Psychiatry. 2007;61(4):545–550. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Arango C, Robles O, Parellada M, Fraguas D, Ruiz-Sancho A, Medina O, et al. Olanzapine compared to quetiapine in adolescents with a first psychotic episode. European Child & Adolescent Psychiatry. 2009;18(7):418–428. doi: 10.1007/s00787-009-0749-5. [DOI] [PubMed] [Google Scholar]
- Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/hyperactivity disorder: A placebo-controlled pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(5):558–565. doi: 10.1097/chi.0b013e3180323354. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. the bogalusa heart study. The New England Journal of Medicine. 1998;338(23):1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Wozniak J, Spencer T, Pandina G. Risperidone for the treatment of affective symptoms in children with disruptive behavior disorder: A post hoc analysis of data from a 6-week, multicenter, randomized, double-blind, parallel-arm study. Clinical Therapeutics. 2006;28(5):794–800. doi: 10.1016/s0149-2918(06)00132-9. [DOI] [PubMed] [Google Scholar]
- Bitsori M, Linardakis M, Tabakaki M, Kafatos A. Waist circumference as a screening tool for the identification of adolescents with the metabolic syndrome phenotype. International Journal of Pediatric Obesity: IJPO: An Official Journal of the International Association for the Study of Obesity. 2009;4(4):325–331. doi: 10.3109/17477160902914597. [DOI] [PubMed] [Google Scholar]
- Bruggeman R, van der Linden C, Buitelaar JK, Gericke GS, Hawkridge SM, Temlett JA. Risperidone versus pimozide in tourette’s disorder: A comparative double-blind parallel-group study. The Journal of Clinical Psychiatry. 2001;62(1):50–56. doi: 10.4088/jcp.v62n0111. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. Journal of Clinical Psychiatry. 2001;62(4):239–248. doi: 10.4088/jcp.v62n0405. [DOI] [PubMed] [Google Scholar]
- Cache Limited 2010Oxford centre for evidence-based medicine - levels of evidence Retrieved 11/23, 2009, from http://www.cebm.net/index.aspx?o=1025
- Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2009;19(2):101–109. doi: 10.1089/cap.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention CDC growth charts United States 2000. 2007.
- Chintoh AF, Mann SW, Lam L, Lam C, Cohn TA, Fletcher PJ, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. Journal of Clinical Psychopharmacology. 2008;28(5):494–499. doi: 10.1097/JCP.0b013e318184b4c5. [DOI] [PubMed] [Google Scholar]
- Cohen D. Atypical antipsychotics and new onset diabetes mellitus. An overview of the literature. Pharmacopsychiatry. 2004;37(1):1–11. doi: 10.1055/s-2004-815468. [DOI] [PubMed] [Google Scholar]
- Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18(2):140–156. doi: 10.1089/cap.2006.0007. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, Ray WA. Trends in prescribing of antipsychotic medications for US children. Ambulatory Pediatrics : The Official Journal of the Ambulatory Pediatric Association. 2006;6(2):79–83. doi: 10.1016/j.ambp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Correia Filho AG, Bodanese R, Silva TL, Alvares JP, Aman M, Rohde LA. Comparison of risperidone and methylphenidate for reducing ADHD symptoms in children and adolescents with moderate mental retardation. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(8):748–755. doi: 10.1097/01.chi.0000166986.30592.67. [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. The Journal of the American Medical Association. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU. Multiple antipsychotic use associated with metabolic and cardiovascular adverse events in children and adolescents. Evidence-Based Mental Health. 2009;12(3):93. doi: 10.1136/ebmh.12.3.93. [DOI] [PubMed] [Google Scholar]
- Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(7):771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: A systematic review of 1-year studies. The American Journal of Psychiatry. 2004;161(3):414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Chang K, Welge JA, Adler CM, Rana M, Howe M, et al. A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disorders. 2009;11(5):483–493. doi: 10.1111/j.1399-5618.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- Delbello M, Findling R, Wang P, Gundapeneni B, Versavel M.2008Poster: Efficacy and safety of ziprasidone in pediatric bipolar disorder Unpublished manuscript.
- DelBello MP, Kowatch RA, Adler CM, Stanford KE, Welge JA, Barzman DH, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(3):305–313. doi: 10.1097/01.chi.0000194567.63289.97. [DOI] [PubMed] [Google Scholar]
- Delbello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1216–1223. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Meyer E, Freisleder FJ, Remschmidt H, Mehler-Wex C, Junghanss J, et al. Effectiveness and tolerability of olanzapine in the treatment of adolescents with schizophrenia and related psychotic disorders: Results from a large, prospective, open-label study. Journal of Child and Adolescent Psychopharmacology. 2008;18(1):54–69. doi: 10.1089/cap.2006.0137. [DOI] [PubMed] [Google Scholar]
- Doey T, Handelman K, Seabrook JA, Steele M. Survey of atypical antipsychotic prescribing by Canadian child psychiatrists and developmental pediatricians for patients under 18 years of age. Canadian Journal of Psychiatry. 2007;52(6):363–368. doi: 10.1177/070674370705200605. [DOI] [PubMed] [Google Scholar]
- Domino ME, Swartz MS. Who are the new users of antipsychotic medications? Psychiatric Services. 2008;59(5):507–514. doi: 10.1176/appi.ps.59.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan A, Atasoy N, Akkurt H, Ozturk D, Karaahmet E, Yalug I, et al. Risperidone and liver function tests in children and adolescents: A short-term prospective study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(3):849–857. doi: 10.1016/j.pnpbp.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Feret BM, Caley CF. Possible hypothyroidism associated with quetiapine. The Annals of Pharmacotherapy. 2000;34(4):483–486. doi: 10.1345/aph.19186. [DOI] [PubMed] [Google Scholar]
- Findling RL, Nyilas M, Forbes RA, McQuade RD, Jin N, Iwamoto T, et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: A randomized, double-blind, placebo-controlled study. The Journal of Clinical Psychiatry. 2009;70(10):1441–1451. doi: 10.4088/JCP.09m05164yel. [DOI] [PubMed] [Google Scholar]
- Findling RL, Robb A, Nyilas M, Forbes RA, Jin N, Ivanova S, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. The American Journal of Psychiatry. 2008;165(11):1432–1441. doi: 10.1176/appi.ajp.2008.07061035. [DOI] [PubMed] [Google Scholar]
- Findling RL, Aman MG, Eerdekens M, Derivan A, Lyons B, Risperidone Disruptive Behavior Study Group Long-term, open-label study of risperidone in children with severe disruptive behaviors and below-average IQ. The American Journal of Psychiatry. 2004;161(4):677–684. doi: 10.1176/appi.ajp.161.4.677. [DOI] [PubMed] [Google Scholar]
- Findling RL, Kusumakar V, Daneman D, Moshang T, De Smedt G, Binder C. Prolactin levels during long-term risperidone treatment in children and adolescents. The Journal of Clinical Psychiatry. 2003;64(11):1362–1369. doi: 10.4088/jcp.v64n1113. [DOI] [PubMed] [Google Scholar]
- Fleischhaker C, Heiser P, Hennighausen K, Herpertz-Dahlmann B, Holtkamp K, Mehler-Wex C, et al. Weight gain associated with clozapine, olanzapine and risperidone in children and adolescents. Journal of Neural Transmission. 2007;114(2):273–280. doi: 10.1007/s00702-006-0602-7. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration 2010Atypical antipsychotic drugs information Retrieved 11/23, 2009, from http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm094303.htm
- Fraguas D, Merchan-Naranjo J, Laita P, Parellada M, Moreno D, Ruiz-Sancho A, et al. Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. The Journal of Clinical Psychiatry. 2008;69(7):1166–1175. doi: 10.4088/jcp.v69n0717. [DOI] [PubMed] [Google Scholar]
- Gaffney GR, Perry PJ, Lund BC, Bever-Stille KA, Arndt S, Kuperman S. Risperidone versus clonidine in the treatment of children and adolescents with tourette’s syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(3):330–336. doi: 10.1097/00004583-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers, school-aged children, adolescents, and adults. Journal of Child and Adolescent Psychopharmacology. 2007;17(2):217–222. doi: 10.1089/cap.2007.0017. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Batterson JR, Sethuraman G, Sallee FR. Tic reduction with risperidone versus pimozide in a randomized, double-blind, crossover trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(2):206–214. doi: 10.1097/00004583-200402000-00017. [DOI] [PubMed] [Google Scholar]
- Gitlin N, Julie NL, Spurr CL, Lim KN, Juarbe HM. Two cases of severe clinical and histologic hepatotoxicity associated with troglitazone. Annals of Internal Medicine. 1998;129(1):36–38. doi: 10.7326/0003-4819-129-1-199807010-00008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Heydrich J, Raches D, Wilens TE, Leichtner A, Mezzacappa E. Retrospective study of hepatic enzyme elevations in children treated with olanzapine, divalproex, and their combination. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(10):1227–1233. doi: 10.1097/00004583-200310000-00014. [DOI] [PubMed] [Google Scholar]
- Gropper D, Jackson CW. Pancreatitis associated with quetiapine use. Journal of Clinical Psychopharmacology. 2004;24(3):343–345. doi: 10.1097/01.jcp.0000126667.90691.88. [DOI] [PubMed] [Google Scholar]
- Haas M, Eerdekens M, Kushner S, Singer J, Augustyns I, Quiroz J, et al. Efficacy, safety and tolerability of two dosing regimens in adolescent schizophrenia: Double-blind study. The British Journal of Psychiatry: The Journal of Mental Science. 2009;194(2):158–164. doi: 10.1192/bjp.bp.107.046177. [DOI] [PubMed] [Google Scholar]
- Harrison-Woolrych M, Garcia-Quiroga J, Ashton J, Herbison P. Safety and usage of atypical antipsychotic medicines in children: A nationwide prospective cohort study. Drug Safety: An International Journal of Medical Toxicology and Drug Experience. 2007;30(7):569–579. doi: 10.2165/00002018-200730070-00002. [DOI] [PubMed] [Google Scholar]
- Haupt DW, Rosenblatt LC, Kim E, Baker RA, Whitehead R, Newcomer JW. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. The American Journal of Psychiatry. 2009;166(3):345–353. doi: 10.1176/appi.ajp.2008.08030383. [DOI] [PubMed] [Google Scholar]
- Health Canada 2009Drug product search Retrieved 02/02, 2010, from http://webprod.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp
- Henderson DC. Atypical antipsychotic-induced diabetes mellitus: How strong is the evidence? CNS Drugs. 2002;16(2):77–89. doi: 10.2165/00023210-200216020-00001. [DOI] [PubMed] [Google Scholar]
- Hermann RC, Yang D, Ettner SL, Marcus SC, Yoon C, Abraham M. Prescription of antipsychotic drugs by office-based physicians in the United States, 1989–1997. Psychiatric Services. 2002;53(4):425–430. doi: 10.1176/appi.ps.53.4.425. [DOI] [PubMed] [Google Scholar]
- Hollander E, Wasserman S, Swanson EN, Chaplin W, Schapiro ML, Zagursky K, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. Journal of Child and Adolescent Psychopharmacology. 2006;16(5):541–548. doi: 10.1089/cap.2006.16.541. [DOI] [PubMed] [Google Scholar]
- Kane JM. Extrapyramidal side effects are unacceptable. European Neuropsychopharmacology. 2001;11(Suppl 4):S397–403. doi: 10.1016/s0924-977x(01)00109-2. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR. Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. The Journal of Clinical Psychiatry. 2005;66(1):80–84. doi: 10.4088/jcp.v66n0111. [DOI] [PubMed] [Google Scholar]
- Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: Report of two cases and review of adverse events reported to the United States food and drug administration. The American Journal of Gastroenterology. 2000;95(1):272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Koller EA, Weber J, Doraiswamy PM, Schneider BS. A survey of reports of quetiapine-associated hyperglycemia and diabetes mellitus. The Journal of Clinical Psychiatry. 2004;65(6):857–863. doi: 10.4088/jcp.v65n0619. [DOI] [PubMed] [Google Scholar]
- Koller EA, Cross JT, Schneider B.2004Risperidone-associated diabetes mellitus in children Pediatrics 1132421–2.; author reply 421–2. [DOI] [PubMed] [Google Scholar]
- Koller E, Malozowski S, Doraiswamy PM. Atypical antipsychotic drugs and hyperglycemia in adolescents. The Journal of the American Medical Association. 2001;286(20):2547–2548. doi: 10.1001/jama.286.20.2547. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya L, Schulz SC, McDougle C, Frazier J, Dittmann R, Robertson-Plouch C, et al. Olanzapine versus placebo in adolescents with schizophrenia: A 6-week, randomized, double-blind, placebo-controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(1):60–70. doi: 10.1097/CHI.0b013e3181900404. [DOI] [PubMed] [Google Scholar]
- Kumra S, Herion D, Jacobsen LK, Briguglia C, Grothe D. Case study: Risperidone-induced hepatotoxicity in pediatric patients. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(5):701–705. doi: 10.1097/00004583-199705000-00022. [DOI] [PubMed] [Google Scholar]
- Laita P, Cifuentes A, Doll A, Llorente C, Cortes I, Parellada M, et al. Antipsychotic-related abnormal involuntary movements and metabolic and endocrine side effects in children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2007;17(4):487–502. doi: 10.1089/cap.2006.0039. [DOI] [PubMed] [Google Scholar]
- Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The bogalusa heart study. The Journal of the American Medical Association. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- Madhusoodanan S, Moise D. Risperidone-induced hyperprolactinemia in adolescents: A case series. The Journal of Clinical Psychiatry. 2006;67(7):1110–1113. doi: 10.4088/jcp.v67n0714. [DOI] [PubMed] [Google Scholar]
- Malik AH, Prasad P, Saboorian MH, Thiele DL, Malet PF. Hepatic injury due to troglitazone. Digestive Diseases and Sciences. 2000;45(1):210–214. doi: 10.1023/a:1005450519498. [DOI] [PubMed] [Google Scholar]
- Marcus RN, Owen R, Kamen L, Manos G, McQuade RD, Carson WH, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(11):1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- Martin A, Scahill L, Anderson GM, Aman M, Arnold LE, McCracken J, et al. Weight and leptin changes among risperidone-treated youths with autism: 6-month prospective data. The American Journal of Psychiatry. 2004;161(6):1125–1127. doi: 10.1176/appi.ajp.161.6.1125. [DOI] [PubMed] [Google Scholar]
- Martin A, L’Ecuyer S. Triglyceride, cholesterol and weight changes among risperidone-treated youths. A retrospective study. European Child & Adolescent Psychiatry. 2002;11(3):129–133. doi: 10.1007/s00787-002-0255-5. [DOI] [PubMed] [Google Scholar]
- Martin A, Landau J, Leebens P, Ulizio K, Cicchetti D, Scahill L, et al. Risperidone-associated weight gain in children and adolescents: A retrospective chart review. Journal of Child and Adolescent Psychopharmacology. 2000;10(4):259–268. doi: 10.1089/cap.2000.10.259. [DOI] [PubMed] [Google Scholar]
- Masi G, Cosenza A, Mucci M. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. Journal of Child and Adolescent Psychopharmacology. 2001;11(4):389–394. doi: 10.1089/104454601317261564. [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. The New England Journal of Medicine. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, et al. Risperidone for the core symptom domains of autism: Results from the study by the autism network of the research units on pediatric psychopharmacology. The American Journal of Psychiatry. 2005;162(6):1142–1148. doi: 10.1176/appi.ajp.162.6.1142. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Archives of Pediatrics & Adolescent Medicine. 2008;162(10):929–935. doi: 10.1001/archpedi.162.10.929. [DOI] [PubMed] [Google Scholar]
- Melkersson K, Dahl ML. Adverse metabolic effects associated with atypical antipsychotics: Literature review and clinical implications. Drugs. 2004;64(7):701–723. doi: 10.2165/00003495-200464070-00003. [DOI] [PubMed] [Google Scholar]
- Meyer J, Koro CE, L’Italien GJ. The metabolic syndrome and schizophrenia: A review. International Review of Psychiatry. 2005;17(3):173–180. doi: 10.1080/09540260500071798. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: A comprehensive review. Schizophrenia Research. 2004;70(1):1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD : A randomized, controlled, double-blind trial. European Child & Adolescent Psychiatry. 2008;17(1):1–8. doi: 10.1007/s00787-007-0620-5. [DOI] [PubMed] [Google Scholar]
- Mond J, Morice R, Owen C, Korten A. Use of antipsychotic medications in Australia between July 1995 and December 2001. The Australian and New Zealand Journal of Psychiatry. 2003;37(1):55–61. doi: 10.1046/j.1440-1614.2003.01110.x. [DOI] [PubMed] [Google Scholar]
- Morrato EH, Newcomer JW, Kamat S, Baser O, Harnett J, Cuffel B. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 2009;32(6):1037–1042. doi: 10.2337/dc08-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: Randomized, placebo-controlled, double-blind study. Journal of Child Neurology. 2006;21(6):450–455. doi: 10.1177/08830738060210060801. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Newcomer JW. Atypical antipsychotics and metabolic dysregulation: Evaluating the risk/benefit equation and improving the standard of care. Journal of Clinical Psychopharmacology. 2004;24(5 Suppl 1):S7–14. doi: 10.1097/01.jcp.0000142282.62336.e9. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: A comprehensive literature review. CNS Drugs. 2005;19:S1–S93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Archives of General Psychiatry. 2006;63(6):679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- Panagiotopoulos C, Davidson J, Weiss M. Treatment with atypical antipsychotics increases the risk for metabolic syndrome in youth with mental illness. Poster Presentation at the Annual Meeting of the American Academy of Child & Adolescent Psychiatry (AACAP); October 27–November 1 2009.2009. [Google Scholar]
- Panagiotopoulos C, Ronsley R, Davidson J. Increased prevalence of obesity and glucose intolerance in youth treated with second-generation antipsychotic medications. Canadian Journal of Psychiatry. 2009;54(11):743–749. doi: 10.1177/070674370905401104. [DOI] [PubMed] [Google Scholar]
- Pandina GJ, Bossie CA, Youssef E, Zhu Y, Dunbar F. Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. Journal of Autism and Developmental Disorders. 2007;37(2):367–373. doi: 10.1007/s10803-006-0234-7. [DOI] [PubMed] [Google Scholar]
- Patel NC, Hariparsad M, Matias-Akthar M, Sorter MT, Barzman DH, Morrison JA, et al. Body mass indexes and lipid profiles in hospitalized children and adolescents exposed to atypical antipsychotics. Journal of Child and Adolescent Psychopharmacology. 2007;17(3):303–311. doi: 10.1089/cap.2006.0037. [DOI] [PubMed] [Google Scholar]
- Patel NC, Kistler JS, James EB, Crismon ML. A retrospective analysis of the short-term effects of olanzapine and quetiapine on weight and body mass index in children and adolescents. Pharmacotherapy. 2004;24(7):824–830. doi: 10.1592/phco.24.9.824.36091. [DOI] [PubMed] [Google Scholar]
- Penzner JB, Dudas M, Saito E, Olshanskiy V, Parikh UH, Kapoor S, et al. Lack of effect of stimulant combination with second-generation antipsychotics on weight gain, metabolic changes, prolactin levels, and sedation in youth with clinically relevant aggression or oppositionality. Journal of Child and Adolescent Psychopharmacology. 2009;19(5):563–573. doi: 10.1089/cap.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmacare 2009Policy, outcomes evaluation and research, Pharmaceutical Services Division, Ministry of Health Services Unpublished manuscript.
- Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intimamedia thickness in adulthood: The cardiovascular risk in young Finns study. The Journal of the American Medical Association. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- Ratzoni G, Gothelf D, Brand-Gothelf A, Reidman J, Kikinzon L, Gal G, et al. Weight gain associated with olanzapine and risperidone in adolescent patients: A comparative prospective study. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(3):337–343. doi: 10.1097/00004583-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Reyes M, Buitelaar J, Toren P, Augustyns I, Eerdekens M. A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. American Journal of Psychiatry. 2006;163(3):402–410. doi: 10.1176/appi.ajp.163.3.402. [DOI] [PubMed] [Google Scholar]
- Salle F, Kurlan R, Goetz C, Singer H, Scahill L, Law G, et al. 2000Ziprasidone treatment of children and adolescents with tourette’s syndrome: A pilot study Journal of the American Academy of Child and Adolescent Psychiatry 393, pages. [DOI] [PubMed] [Google Scholar]
- Savoy YE, Ashton MA, Miller MW, Nedza FM, Spracklin DK, Hawthorn MH, et al. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: Evidence for the involvement of sympathetic regulation. Schizophrenia Bulletin. 2008;36(2):410–418. doi: 10.1093/schbul/sbn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Leckman JF, Schultz RT, Katsovich L, Peterson BS. A placebo-controlled trial of risperidone in tourette syndrome. Neurology. 2003;60(7):1130–1135. doi: 10.1212/01.wnl.0000055434.39968.67. [DOI] [PubMed] [Google Scholar]
- Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634–41. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- Shirzadi AA, Ghaemi SN. Side effects of atypical antipsychotics: Extrapyramidal symptoms and the metabolic syndrome. Harvard Review of Psychiatry. 2006;14(3):152–164. doi: 10.1080/10673220600748486. [DOI] [PubMed] [Google Scholar]
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. The American Journal of Psychiatry. 2008;165(11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PubMed] [Google Scholar]
- Snyder R, Turgay A, Aman M, Binder C, Fisman S, Carroll A, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(9):1026–1036. doi: 10.1097/00004583-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Vermani A, Greenstein DK, Bobb AJ, Spencer EP, Clasen LS, et al. Clozapine treatment of childhood-onset schizophrenia: Evaluation of effectiveness, adverse effects, and long-term outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(10):1349–1356. doi: 10.1097/chi.0b013e31812eed10. [DOI] [PubMed] [Google Scholar]
- Szigethy E, Wiznitzer M, Branicky LA, Maxwell K, Findling RL. Risperidone-induced hepatotoxicity in children and adolescents? A chart review study. Journal of Child and Adolescent Psychopharmacology. 1999;9(2):93–98. doi: 10.1089/cap.1999.9.93. [DOI] [PubMed] [Google Scholar]
- Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: Similar improved efficacy but different neuroendocrine side effects. Psychoneuroendocrinology. 2003;28(suppl 1):1–7. doi: 10.1016/s0306-4530(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Therapeutics initiative Increasing use of newer antipsychotics in children: A cause for concern? Therapeutics Initiative: Evidence Based Drug Therapy, April–June 2009. 2009.
- Tohen M, Kryzhanovskaya L, Carlson G, Delbello M, Wozniak J, Kowatch R, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. The American Journal of Psychiatry. 2007;164(10):1547–1556. doi: 10.1176/appi.ajp.2007.06111932. [DOI] [PubMed] [Google Scholar]
- Tramontina S, Zeni CP, Ketzer CR, Pheula GF, Narvaez J, Rohde LA. Aripiprazole in children and adolescents with bipolar disorder comorbid with attention-deficit/hyperactivity disorder: A pilot randomized clinical trial. The Journal of Clinical Psychiatry. 2009;70(5):756–764. doi: 10.4088/JCP.08m04726. [DOI] [PubMed] [Google Scholar]
- Turgay A, Binder C, Snyder R, Fisman S. Long-term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics. 2002;110(3):e34. doi: 10.1542/peds.110.3.e34. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration 2010Zyprexa (olanzapine): Use in adolescents Retrieved 02/02, 2010, from http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm198402.htm
- Walter G, DeLaroche A, Soh N, Hunt G, Cleary M, Malhi G, et al. Side effects of second-generation antipsychotics: The experiences, views and monitoring practices of Australian child psychiatrists. Australasian Psychiatry. 2008;16(4):253–262. doi: 10.1080/10398560801958549. [DOI] [PubMed] [Google Scholar]
- Weiss M, Panagiotopoulos C, Giles L, Gibbins C, Kuzeljevic B, Davidson J, et al. A naturalistic study of predictors and risks of atypical antipsychotic use in an attention-deficit/hyperactivity disorder clinic. Journal of Child and Adolescent Psychopharmacology. 2009;19(5):575–582. doi: 10.1089/cap.2009.0050. [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. The New England Journal of Medicine. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Woo V, Harris S, Houlden R. Canadian diabetes association position paper: Antipsychotic medications and associated risks of weight gain and diabetes. Canadian Journal of Diabetes. 2005;29(2):111. [Google Scholar]
- Woods SW, Martin A, Spector SG, McGlashan TH. Effects of development on olanzapine-associated adverse events. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1439–1446. doi: 10.1097/00004583-200212000-00015. [DOI] [PubMed] [Google Scholar]
- Wu RR, Zhao JP, Liu ZN, Zhai JG, Guo XF, Guo WB, et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology. 2006;186(4):572–578. doi: 10.1007/s00213-006-0384-5. [DOI] [PubMed] [Google Scholar]
- Wudarsky M, Nicolson R, Hamburger SD, Spechler L, Gochman P, Bedwell J, et al. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. Journal of Child and Adolescent Psychopharmacology. 1999;9(4):239–245. doi: 10.1089/cap.1999.9.239. [DOI] [PubMed] [Google Scholar]
- Zarcone JR, Hellings JA, Crandall K, Reese RM, Marquis J, Fleming K, et al. Effects of risperidone on aberrant behavior of persons with developmental disabilities: I. A double-blind crossover study using multiple measures. American Journal of Mental Retardation. 2001;106(6):525–538. doi: 10.1352/0895-8017(2001)106<0525:EOROAB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatric Diabetes. 2007;8(5):299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]