Abstract
Human immunodeficiency virus type 1 (HIV-1) transcription relies on its transactivating Tat protein. Although devoid of a signal sequence, Tat is released by infected cells and secreted Tat can affect uninfected cells, thereby contributing to HIV-1 pathogenesis. The mechanism and the efficiency of Tat export remained to be documented. Here, we show that, in HIV-1-infected primary CD4+ T-cells that are the main targets of the virus, Tat accumulates at the plasma membrane because of its specific binding to phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2). This interaction is driven by a specific motif of the Tat basic domain that recognizes a single PI(4,5)P2 molecule and is stabilized by membrane insertion of Tat tryptophan side chain. This original recognition mechanism enables binding to membrane-embedded PI(4,5)P2 only, but with an unusually high affinity that allows Tat to perturb the PI(4,5)P2-mediated recruitment of cellular proteins. Tat–PI(4,5)P2 interaction is strictly required for Tat secretion, a process that is very efficient, as ∼2/3 of Tat are exported by HIV-1-infected cells during their lifespan. The function of extracellular Tat in HIV-1 infection might thus be more significant than earlier thought.
Keywords: HIV-1, PIP2, Tat, toxin, unconventional secretion
Introduction
Some cell proteins, generally small ones (<40 kDa), are secreted despite their lack of signal sequence. These proteins include homeodomain transcription factors, as well as numerous cytokines, including FGF-1, FGF-2 and IL-1β (Nickel and Rabouille, 2009). Their secretion pathways are collectively designed as nonclassical or unconventional export routes (Dupont et al, 2007; Nickel and Rabouille, 2009). These processes are intriguingly diverse and, so far, there seems to be as many pathways as proteins. Although the overall secretion route has been identified for most proteins that are secreted in an unconventional manner, mechanistic details on these pathways are lacking (Keller et al, 2008; Nickel and Rabouille, 2009).
The human immunodeficiency virus type 1 (HIV-1) transactivating protein Tat is a small (∼11 kDa) and basic protein that binds to the transactivation-response element (TAR) on nascent RNA and recruits cyclin T1, a component of the transcription elongation factor P-TEFb. As Tat promotes efficient transcription from the 5′ long-terminal repeat (LTR) of HIV-1, it has, therefore, a crucial function in virus multiplication within infected T-cells (Jeang et al, 1999). In agreement with this transcriptional function, Tat was found to accumulate within the nucleus or nucleolus of HIV-1-infected (Ranki et al, 1994) or Tat-transfected (Marasco et al, 1994) T-cell lines.
Although Tat is devoid of signal sequence, it is secreted by infected cells using an unconventional secretion pathway and in the absence of cell lysis (Ensoli et al, 1990). The fact that nanomolar Tat concentrations are present in the sera of HIV-1-infected patients (Xiao et al, 2000) indicates that this secretion process should be efficient and there is increasing evidence that circulating Tat is implicated in viral multiplication, AIDS development and HIV-1-associated dementia. Strikingly, secreted Tat acts as a viral toxin, inducing deleterious effects on numerous cell types. For instance, extracellular Tat can deregulate cytokine secretion by monocytes and lymphocytes, and directly induce lymphocyte and neuron death (Rubartelli et al, 1998; Gallo, 1999; Huigen et al, 2004). Nevertheless, both the efficiency and the mechanism of Tat secretion remain to be elucidated.
Phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) is a phospholipid that is specifically concentrated within the inner leaflet of the plasma membrane to which it can recruit proteins involved in several important cell activities such as endocytosis, phagocytosis, exocytosis and cell adhesion (De Matteis and Godi, 2004; Di Paolo and De Camilli, 2006). Here, we showed that Tat concentrates at the plasma membrane in primary T-cells. We identified PI(4,5)P2 as the cell component responsible for Tat recruitment at this level, and found that Tat–PI(4,5)P2 interaction enables Tat secretion. Intriguingly, Tat only binds PI(4,5)P2 when present in a membrane. This is due to the concomitant membrane insertion of Tat single Trp that is required to stabilize Tat–PI(4,5)P2 interaction. This binding is actually so tight that Tat can displace cell proteins from PI(4,5)P2. We also found that secreted Tat strongly affects HIV-1 multiplication in CD4+ primary T-cells.
Results
A cellular ELISA assay to monitor Tat secretion by T-cells
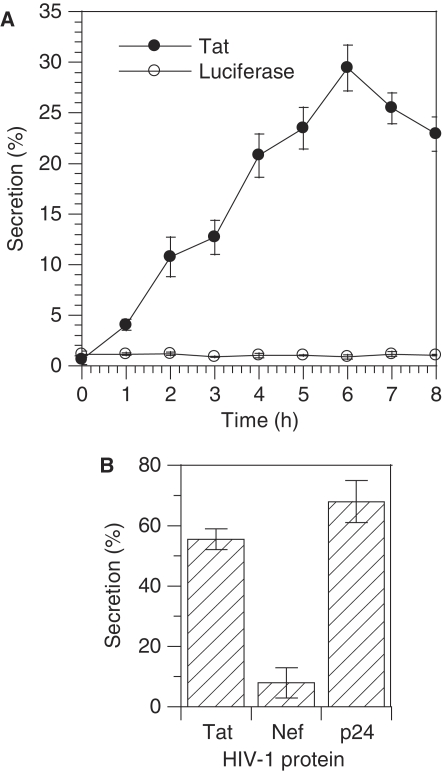
A first aim of this study was to set up a quantitative Tat secretion assay. A problem for Tat detection is the poor affinity of most anti-Tat antibodies. We tested 11 of them and found a couple that enabled reliable Tat detection by sandwich ELISA. Another problem was Tat susceptibility to oxidation. We observed that secreted Tat is readily oxidized by air oxygen and this makes it unreactive to antibodies (data not shown). To circumvent this difficulty, we modified a standard ELISA procedure. Jurkat cells, which are CD4+ T-lymphocytes similar to most HIV-1-infected cells (Stebbing et al, 2004), were first transiently transfected by plasmids coding for Tat and luciferase (used as a control for cell lysis) before plating onto 96-well plates earlier coated with anti-Tat monoclonal antibodies. Cells were then incubated for various times in degassed medium containing a low concentration of reductant (20 μM 2-mercaptoethanol) before assaying plate-bound Tat by ELISA. This ‘cellular ELISA' procedure enabled antibodies to capture secreted Tat before it becomes oxidized. Under these conditions, Tat secretion was linear up to 6 h. After this time, up to 30% of cellular Tat had been secreted by Jurkat cells (Figure 1A), whereas no release of cotransfected luciferase was observed. Hence, ∼5% of cellular Tat is secreted hourly by transfected Jurkat cells.
Figure 1.
Tat secretion is an efficient process. (A) Time dependence of Tat secretion by transfected Jurkat cells. Cells were transfected with Tat and luciferase expression vectors. After 48 h, they were plated onto ELISA plates precoated with anti-Tat monoclonal antibody. Secretion was allowed for the indicated time and was calculated as medium/(cell+medium) (%) for both Tat and luciferase. (B) Secretion of Tat, Gag p24 and Nef by HIV-1-infected primary CD4+ T-cells. Primary CD4+ T-cells were isolated, stimulated and infected with HIV-1. Three days after, cells were plated onto anti-Tat-coated ELISA plates for 15 h. Tat binding takes place under native conditions and this assay only detects free Tat in the medium, whereas the ELISA used to assay extracellular p24 and Nef involves the use of a detergent that releases any virion-incorporated protein.
Tat secretion by HIV-1-infected cells is an efficient process
We then examined the efficiency of Tat secretion in a biologically relevant system. We purified primary CD4+ T-cells, infected them with HIV-1 and assayed Tat secretion 3 days later, i.e. shortly after the beginning of infection to minimize cell lysis. Less than 10% of the cells were infected after this time, and we had to leave cells 12–18 h in the plate to capture enough Tat to allow reliable quantitation. After 15 h, ∼55% of total Tat (cells + medium) was found in the medium (Figure 1B) and extracellular concentration reached ∼0.25 nM. It should be noted that, because Tat capture by antibodies takes place under native conditions, the cellular ELISA assay only detects free Tat in the medium and not any Tat that could be incorporated into virions. Infected cells secrete Tat almost as efficiently as the viral capsid protein p24, whereas Nef was not significantly exported (Figure 1B). According to the average half-life of 2.2 days for an infected primary CD4+ T-cell (Perelson et al, 1996), that secretes 3.7±0.2% of cellular Tat hourly, it can be calculated that 2/3 of Tat is exported (i.e. 195% of the intracellular amount).
Tat is membrane bound in HIV-1-infected T-cells
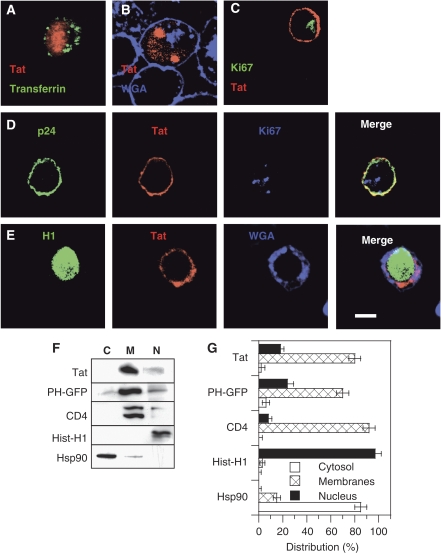
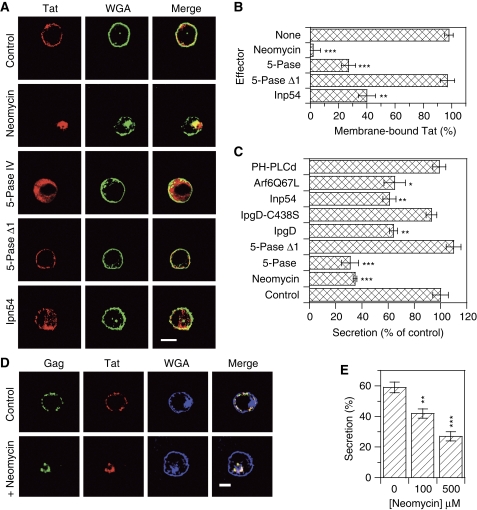
In the search for membrane compartments involved in Tat export, we examined Tat localization in CD4+ T-cells. We first used Tat-transfected cells. In agreement with earlier studies performed on cell lines (Stauber and Pavlakis, 1998; Marcello et al, 2001), in stably transfected Jurkat Tat-III cells (Figure 2A) or conventional Jurkat cells transiently transfected by Tat (Figure 2B), Tat concentrated within the nucleus and nucleoli. Nevertheless, in Tat-transfected primary CD4+ T-cells, Tat localized at the plasma membrane (Figure 2C). On HIV-1 infection, Tat concentrated at the plasma membrane of primary CD4+ T-cells (Figure 2D and E) together with Gag (p24). In some cells, especially at late stages of infection, Tat was also observed in the cytosol (Figure 2E; Supplementary Figure S1). The same localization was obtained using either a polyclonal or a monoclonal anti-Tat antibody, and controls were performed using antibodies against the nucleolar antigen Ki67 or histone H1 to check for the proper permeabilization of the nuclear envelope during immunofluorescence labelling of primary cells (Figure 2C–E; Supplementary Figure S1).
Figure 2.
Tat is membrane bound in HIV-1-infected primary CD4+ T-cells. (A–E) Immunofluorescence. Cells were fixed with paraformaldehyde and permeabilized with saponin. Tat, Gag p24, histone H1 or Ki67 were then revealed by immunofluorescence, as indicated. A fluorescent lectin (WGA) was used to localize the plasma membrane and the trans-Golgi network (TGN). (A) Jurkat-TatIII cells, which are stable Tat-transfected cells, were labelled with 100 nM fluorescent transferrin for 40 min to localize endosomes before fixation. (B) Jurkat cells were transiently transfected by Tat. (C) Tat-transfected-activated primary CD4+ T-cells. (D, E) HIV-1-infected primary CD4+ T-cells. Images are representative medial representative confocal sections. Bar, 5 μm. Wide field views are shown in Supplementary Figure S1. (F, G) Cell fractionation. Primary CD4+ T-cells were transfected with Tat or PHPLCδ-EGFP. One day later, cells were fractionated in three fractions, cytosolic (C), membranes (M) and nucleus (N). Proteins were precipitated and separated by tricine SDS–PAGE before western blots that were revealed using antibodies against the indicated proteins (F). An anti-GFP was used to detect PHPLCδ-EGFP. (G) The experiment was repeated three times and films were scanned for band quantification. The results are mean±s.e.m. (n=3).
As it was surprising to find a transcription factor such as Tat concentrated at the plasma membrane, we tried to confirm this result using cell fractionation. Primary CD4+ T-cells were thus transfected with Tat before cell fractionation in three different fractions containing cytosolic, membrane-associated and nuclear proteins. The efficiency of the fractionation procedure was examined using markers such as the cytosolic chaperone Hsp90 that was primarily observed in the cytosolic fraction, and histone H1 that was only found in the nuclear fraction (Figure 2F and G). The integral membrane protein CD4 mainly localized to the membrane fraction. For reasons that will become clearer later, a transfected EGFP chimera of PH domain of PLCδ (PHPLCδ), a well characterized specific probe for PI(4,5)P2 (De Matteis and Godi, 2004), was used as a marker for peripheral membrane proteins. This construct concentrated into the membrane fraction (80%), although it was present to some extent (24%) in the nuclear fraction. This distribution is consistent with PI(4,5)P2 intracellular localization (Watt et al, 2002). Tat was essentially observed (80%) in the membrane fraction, although 18% were present in the nucleus (Figure 2F and G). Altogether with immunofluorescence data, these results showed that Tat is concentrated at the plasma membrane in primary CD4+ T-cells and strongly suggested that Tat secretion could directly take place at this level.
Tat could be significantly detected in the nucleus by cell fractionation only, indicating that Tat concentration at this level is below the detection threshold of immunofluorescence, and that the nucleus is not a major destination for Tat in primary CD4+ T-cells. The fact that the virus replicates shows Tat accumulation within the nucleus is not required for efficient transactivation. To examine this point more directly using the same cellular background, we hooked the nuclear export signal (NES) of Rev to Tat C-terminus. As shown earlier in HeLa cells (Stauber and Pavlakis, 1998; Sivakumaran et al, 2009), the resulting Tat-NES was excluded from the nucleus of transiently transfected Jurkat cells and was exclusively observed in the cytosol or at the plasma membrane (Supplementary Figure S2A). We then compared the efficiency of transactivation by Tat-NES with that of native Tat using a dose-response curve. The efficiency of transactivation by Tat-NES was ∼70% of that of native Tat and this ratio remained constant whatever the plasmid doses, even for low Tat/LTR ratios (Supplementary Figure S2B). This result confirmed that efficient transactivation by Tat does not require Tat nuclear/nucleolar accumulation. This is consistent with the very high affinity of the cyclin T1–Tat–TAR ternary complex that assembles with subnanomolar Kd values (Zhang et al, 2000). We will come back to this point in the Discussion section.
Tat binds liposomal PI(4,5)P2 with a high affinity and specificity
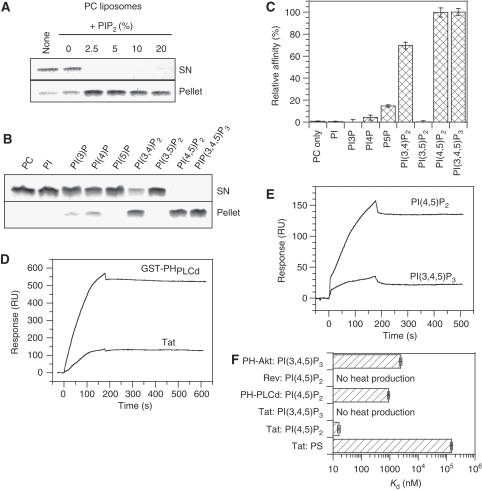
The next question was thus how Tat was recruited at the plasmalemma. We noticed that Tat had a putative-binding site for PI(4,5)P2 in its basic domain (49-RKKRRQRRR-57). We first examined whether Tat actually bound PI(4,5)P2 using a conventional liposome sedimentation assay (Barret et al, 2000). Tat was quantitatively pelleted with liposomes as soon as they contained 2.5% PI(4,5)P2 (Figure 3A), which is the approximate concentration of PI(4,5)P2 in the inner leaflet of the plasma membrane (Lemmon, 2003). If PI(4,5)P2 was involved in Tat recruitment at the plasma membrane, Tat should specifically bind to this phosphoinositide. We thus examined the specificity of Tat binding to different phosphoinositides. Tat bound, with roughly the same efficiency, to PI(4,5)P2 and PI(3,4,5)P3, to a lesser extent to PI(3,4)P2, and did not show any significant affinity to any other phosphoinositide (Figure 3B and C).
Figure 3.
Tat binds with high affinity to lipsomes containing PI(4,5)P2. (A) Effect of PI(4,5)P2 concentration on Tat binding to liposomes. Multilamellar PC liposomes were incubated with Tat before ultracentrifugation. The pellet and supernatant (SN) were analysed by SDS–PAGE, and gels were stained with Coomassie blue. (B) Specificity of Tat binding to phosphoinositides. Liposomes included 2.5% (mol/mol) of the indicated phosphoinositide. (C) Specificity of phosphoinositide recognition by Tat deduced from liposome sedimentation assays. The results are mean±s.e.m. (n=3). (D) SPR sensorgrams of Tat (200 nM) and GST–PHPLCδ (200 nM) binding to immobilized liposomes containing PC (75%), PE (20%) and PI(4,5)P2 (5%). (E) SPR sensorgrams showing the binding of Tat (200 nM) to liposomes containing 5% of either PI(4,5)P2 or PI(3,4,5)P3. (F) Affinities for PS, PI(4,5)P2 or PI(3,4,5)P3 of different phosphoinositide-binding proteins used in this study. Kd were determined using ITC. No heat production was detectable when mixing PI(3,4,5)P3 liposomes with Tat indicating that, according to ITC, Tat does not bind PI(3,4,5)P3.
It was also important to check whether Tat bound phosphatidylserine (PS), which is a negatively charged phospholipid present at high concentration (∼18%) within the inner leaflet of the plasma membrane (Corbin et al, 2004). We found that Tat quantitatively sedimented with PS-containing liposomes only when the PS concentration reached 20% (Supplementary Figure S3). Hence, Tat interacted with PS in this sedimentation assay.
To identify among PS, PI(4,5)P2 and PI(3,4,5)P3 the phospholipid(s) that was or were most likely to be responsible for Tat recruitment to the plasma membrane, we measured the Kd of their interaction with Tat. To this end, we first immobilized small unilamellar vesicles on chips for surface plasmon resonance (SPR) experiments (Honing et al, 2005). Tat strongly bound to chips coated with vesicles containing 5% PI(4,5)P2 (Figure 3D and E) and this binding was inhibited when anti-PI(4,5)P2 antibodies were applied before Tat (not shown). Regarding affinity, Tat dissociated very weakly from immobilized PI(4,5)P2 liposomes (Figure 3D and E), with a dissociation rate close to the sensitivity limit of the biosensor apparatus, and the Kd we obtained (Kd <0.3 nM) was overestimated.
To validate these findings, we used a GST-tagged version of the PH domain of PLCδ (PHPLCδ) that specifically recognizes PI(4,5)P2 (De Matteis and Godi, 2004). Immobilized PI(4,5)P2 liposomes bound GST–PHPLCδ with a Kd of 4 nM (Figure 3D). This value is very close to that (6.6 nM) obtained before using the same experimental system (Zimmermann et al, 2002). It should be noted that in these SPR experiments, Tat (∼11 kDa) always gave a smaller signal (140–160 RU) compared with GST–PHPLCδ (∼44 kDa) that gave ∼550 RU (Figure 3D). This is likely due to the difference in size between the two proteins. Indeed, the ratio of protein masses (4) is essentially the same as the RU ratio (3.7). We then checked Tat specificity for phosphoinositides in this experimental system. When PI(4,5)P2 was replaced by PI or PI4P, Tat did not bind anymore (Supplementary Figure S4). These results thus confirmed the specificity of Tat binding deduced from centrifugation assays (Figure 3B and C). SPR experiments also showed that Tat had at least a 10-fold higher affinity for PI(4,5)P2 as compared with PI(3,4,5)P3 (Figure 3E). The apparent discrepancy between these results and the equivalent binding of Tat to these lipids observed when using the cosedimentation assay (Figure 3B and C) is due to the fact that the latter assay is not very quantitative and does not discriminate between ligands unless they display dramatically different affinities (Blin et al, 2008).
SPR data were confirmed by isothermal tritration calorimetry (ITC), a technique that is quantitative and enables reliable measurements of affinities over a wide-concentration range (nM to μM). ITC results showed that Tat bound PI(4,5)P2 liposomes with a high affinity (Kd ∼ 16 nM; 1 PI(4,5)P2/Tat), whereas Tat poorly recognized PS (Kd>100 μM; 3 PS/Tat) and did not bind to PI(3,4,5)P3 in this system (Figure 3F). Altogether, SPR and ITC data showed that Tat is a specific PI(4,5)P2 ligand that displays a very high affinity for this lipid. Indeed, Tat avidity for PI(4,5)P2 (0.3 nM for SPR and 16 nM for ITC) is 20- to 50-fold higher than that of PHPLCδ (6.6 nM for SPR and 900 nM for ITC). As a negative control, we also used HIV-1 Rev that owns a basic domain (Truant and Cullen, 1999). Nevertheless, this does not endow Rev with PI(4,5)P2-binding properties (Figure 3F).
Tat is a strong and specific PI(4,5)P2 ligand intracellularly
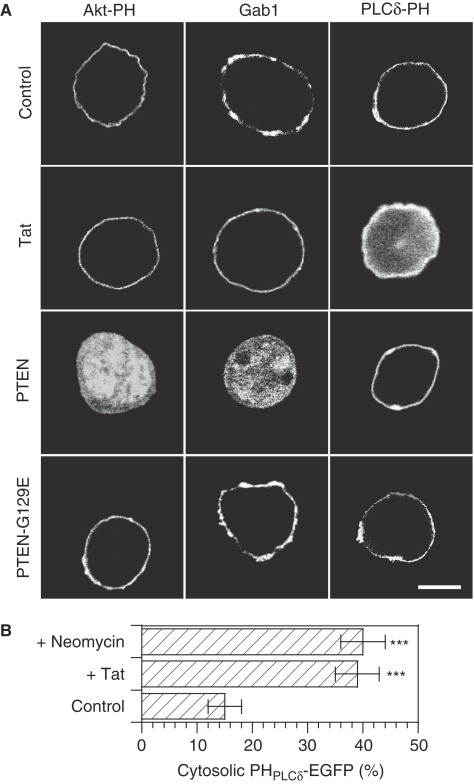
As a number of proteins containing basic motifs were found to bind both PI(4,5)P2 and PI(3,4,5)P3 (Heo et al, 2006), it was important to check whether Tat was a specific PI(4,5)P2 ligand intracellularly. It was also interesting to examine whether the superior affinity of Tat for PI(4,5)P2 compared with PHPLCδ was not only observed using SPR or ITC, but also on living cells. To examine these issues, we used PHPLCδ and PHAkt domains that are specific ligands of PI(4,5)P2 and PI(3,4,5)P3, respectively (Varnai and Balla, 1998; Astoul et al, 2001). When measured by ITC, we found the Kd of both interactions to be 0.9 μM for GST–PHPLCδ/PI(4,5)P2 interaction and 2.4 μM for GST–PHAkt/PI(3,4,5)P3 (Figure 3F), consistent with published data (Lemmon et al, 1995; Stephens et al, 1998). As these values lie within the same range, the ability of Tat to compete with these PH domains for their favourite phosphoinositide will, therefore, directly reflect Tat affinity for this lipid in the cellular context. For these competition experiments, we used Jurkat cells, as they lack phosphatases PTEN and SHIP that degrade PI(3,4,5)P3 into PI(4,5)P2 and PI(3,4)P2, respectively. These cells thus have a high basal level of PI(3,4,5)P3 (Astoul et al, 2001) and specific ligands for this lipid such as PHAkt domain (Astoul et al, 2001) and Gab1 (Maroun et al, 2003), therefore, localize at their plasma membrane (Figure 4A). Transfected Tat was able to displace an PHPLCδ-EGFP probe, but neither PHAkt-EGFP nor Gab1-EGFP from the membrane (Figure 4A). To have a more precise idea of the efficiency of PHPLCδ-EGFP displacement, we performed image quantification on n>30 cells. The results showed that, in control cells, 15% of PHPLCδ-EGFP is cytosolic. This fraction rose to 40% when Tat was cotransfected (Figure 4B). As a positive control for displacement of the PHPLCδ probe, we used neomycin. This cationic antibiotic binds tightly to the headgroup of phosphoinositides, thereby inhibiting protein binding to PI(4,5)P2 (Aharonovitz et al, 2000). In neomycin treated cells, 39% of PHPLCδ-EGFP was cytosolic, just as observed on Tat cotransfection (Figure 4B). Hence, Tat is able to efficiently displace PHPLCδ-EGFP from PI(4,5)P2. As a positive control for the displacement of PI(3,4,5)P3 probes to the cytosol, we cotransfected cells with PTEN that induced quantitative relocalization of both PI(3,4,5)P3 probes to the cytosol, whereas PHPLCδ-EGFP remained at the plasma membrane, as expected. The inactive (G129E) PTEN mutant had no effect (Figure 4A). Collectively, these data confirmed biochemical data (Figure 3F) and showed that Tat is a PI(4,5)P2 selective ligand that does not significantly interact with PI(3,4,5)P3 intracellularly. They also corroborated biochemical results indicating that Tat has a higher affinity for PI(4,5)P2 compared with PHPLCδ and indicated that, despite the fact that Tat is not easily visualized at the plasma membrane of Jurkat cells, it is nevertheless recruited at this level in a PI(4,5)P2-dependent manner. The efficiency of Tat nuclear import is such that it apparently perturbs the stability of Tat–PIP2 interaction, thereby decreasing Tat residence time at the plasma membrane. Nuclear accumulation, therefore, likely explains why it is difficult to visualize Tat at the plasmalemma in Jurkat cells.
Figure 4.
Tat is a high-affinity PI(4,5)P2 ligand intracellularly. (A) Tat selectively displaces PHPLCδ-EGFP from the plasma membrane. Jurkat cells were transfected as indicated using vectors coding for GAB1-EGFP or PHAkt-EGFP that both bind PI(3,4,5)P3, or of PHPLCδ-EGFP that is specific to PI(4,5)P2, together with an equivalent amount of PTEN plasmid (WT that transforms PI(3,4,5)P3 into PI(4,5)P2, or inactive mutant), Tat vector or empty plasmid (control). Cells were grown for 24 h, then fixed for confocal microscopic examination. Bar, 5 μm. (B) Quantification of the efficiency of PHPLCδ-EGFP displacement to the cytosol. Images from n>30 cells were recorded and the percentage of cell-fluorescence signal originating from the cytosol was determined. Control cells were transfected with PHPLCδ-EGFP and empty vector, cells + Tat were cotransfected with PHPLCδ-EGFP and Tat (ratio 1:1), cells + neomycin were treated with 2 mM neomycin for 1 h at 37°C before fixation. The significance of differences with control conditions was assessed using an unpaired, two-sided Student's t-test (***P<0.001).
PI(4,5)P2 is required for Tat targeting to the plasma membrane and for Tat secretion
To assess the function of this Tat–PI(4,5)P2 interaction in Tat secretion, we first used neomycin that masks PI(4,5)P2. This drug displaced Tat from the membrane of transfected primary CD4+ T-cells (Figure 5A and B), and strongly inhibited Tat secretion by Jurkat cells (−60%, Figure 5C). Cell transfection with a phosphoinositide 5-phosphatase IV (5-pase IV) that hydrolyses PI(4,5)P2 (Ono et al, 2004) strongly displaced Tat towards the cytosol (Figure 5A and B) and concomitantly inhibited Tat secretion by 65% (Figure 5C). Conversely, the 5-pase IV Δ1 inactive mutant affected neither Tat localization nor secretion (Figure 5A–C). The Ipn54 yeast 5-phosphatase (Pendaries et al, 2006) partly displaced Tat to the cytosol (Figure 5A and B) and inhibited Tat secretion by 40% (Figure 5C). A similar level of inhibition was achieved by the IpgD bacterial 4-phosphatase (Pendaries et al, 2006), whereas the IpgD C438S inactive mutant had no effect on Tat secretion by Jurkat cells (Figure 5C). A permanently activated mutant of Arf6 (Arf6-Q67L) that induces the formation of PI(4,5)P2-positive actin-coated endosomes that are no longer able to recycle back to the plasma membrane (Brown et al, 2001) reduced Tat secretion by 40% (Figure 5C). Both Arf6-Q67L and IpgD were toxic when expressed in primary T-cells. We also found that PHPLCδ, even when overexpressed, does not inhibit Tat secretion (Figure 5C), confirming that Tat is an intriguingly strong PI(4,5)P2 ligand. Altogether, the use of these various PI(4,5)P2 effectors that either masked, hydrolysed or displaced this lipid confirmed that Tat is bound to PI(4,5)P2 intracellularly. They further indicated that this binding is responsible for Tat recruitment to the plasma membrane that is a prerequisite for Tat secretion by Jurkat cells.
Figure 5.
Tat secretion is inhibited when PI(4,5)P2 is masked, hydrolysed or displaced from the plasma membrane. (A) Tat intracellular localization in activated CD4+ primary T-cells. Cells were cotransfected with 1 μg of pBi-Tat and 1 μg of the indicated effector. 5-pase is 5-phosphatase IV and 5-pase Δ1, an inactive mutant. After 1 day, and a 1 h treatment with 2 mM neomycin when indicated, cells were fixed for Tat staining by immunofluorescence. The plasma membrane and the TGN were revealed using WGA. Representative median confocal sections. Bar, 5 μm. (B) Quantification of the immunofluorescence results. Images from n>20 cells were recorded and the percentage of cell-fluorescence signal originating from the plasma membrane was determined. (C) Tat secretion by Jurkat cells. Tat, luciferase and the indicated effector plasmid were transfected into Jurkat cells before assaying Tat secretion 48 h later. When indicated, 10 mM neomycin was present during the secretion assay. Higher PHPLCδ doses were not more effective (data not shown). (D) Neomycin displaces Tat and Gag from the plasma membrane of infected cells. HIV-1-infected primary T-cells were treated with 2 mM neomycin for 1 h before fixation and localization of Tat and Gag p24 by immunofluorescence combined with WGA staining. Representative median confocal sections; bar, 5 μm. (E) Neomycin inhibits Tat secretion by HIV-1-infected primary T-cells in a dose-dependent manner. Cells were incubated in the ELISA plate for 15 h at 37°C in the presence of the indicated concentration of neomycin before processing. The significance of differences with control conditions was assessed using a two-sided Student's t-test (***P<0.001; **P<0.01; *P<0.02).
It was important to check whether this was also the case in a biologically relevant system. Treatment of HIV-1-infected primary CD4+ T-cells for 1 h with 2 mM neomycin resulted in quantitative displacement of Tat and Gag (p24) towards peri-Golgi compartments, that is around the main wheat germ agglutinin (WGA)-positive structure (Figure 5D). Neomycin toxicity limited the drug concentration that could be used during the overnight incubation required to assay Tat secretion by infected cells. Nevertheless, we found that Tat secretion by infected primary CD4+ T-cells was inhibited in a dose-dependent manner by neomycin (Figure 5E), confirming that Tat secretion by HIV-1-infected cells relies on PI(4,5)P2 binding.
Tat binds PIP2 using a specific motif of its basic domain
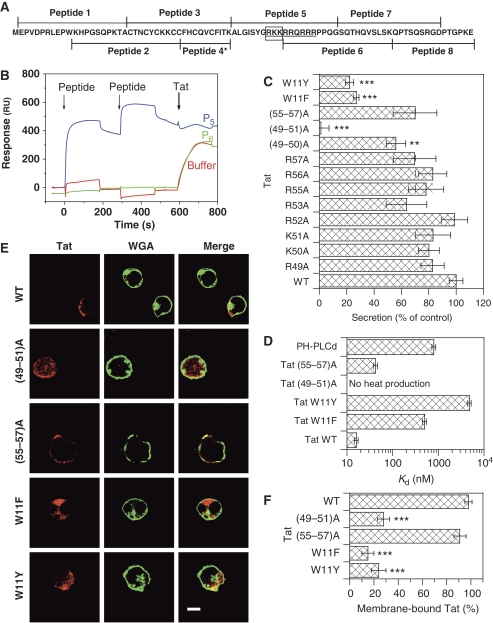
We then examined whether the Tat basic domain was indeed responsible for PI(4,5)P2 binding. To this end, we first used SPR and overlapping 20 mer peptides covering the Tat sequence (Figure 6A). Each peptide was injected twice to achieve saturation of binding sites as much as possible, before applying Tat to assess whether Tat fixation was affected by the peptide. Peptide 5 (residues 42–61), which covers the entire basic domain (residues 49–57) efficiently bound to PI(4,5)P2 albeit, contrary to Tat, it readily dissociated (Figure 6B). It should be noted, therefore, that the Tat basic domain, also known as the ‘protein transduction domain', that is used for protein vectorization into cells (Joliot and Prochiantz, 2004) is a weak PI(4,5)P2 ligand (Kd ∼600 nM for peptide 5 according to SPR). Peptide 5 nevertheless prevented Tat binding, whereas peptide 6 (residues 52–71), although encompassing most of the basic domain and containing five of its eight basic residues, did not bind PI(4,5)P2 and, therefore, did not prevent Tat binding (Figure 6B). The remaining peptides behaved as peptide 6 (not shown). SPR data, therefore, indicated that Tat binding to PI(4,5)P2 is mediated by its basic domain, and that at least one residue among the three basic residues 49–51 is involved in this interaction. We then performed an Ala scan on the basic residues of the domain to identify important residues. The secretion efficiency of these point mutants was essentially unaffected (Figure 6C). Nevertheless, when the first residues of the domain were progressively replaced by Ala, the secretion efficiency dropped from 82% (R49A) to 58% ((49–50)A) and 1% ((49–51)A). Equivalent mutations on the other end of the domain only weakly affected secretion (70% of secretion for (55–57)A). To monitor and compare the capacity of these mutants to bind PI(4,5)P2, we used ITC. We found (Figure 6D) that recombinant Tat(49–51)A did not significantly bind PI(4,5)P2, whereas Tat (55–57)A showed an affinity (Kd=42 nM) similar to that of native Tat (Kd=16 nM). Intracellular localization analysis by immunofluorescence confirmed biochemical data as Tat(55–57)A localized to the plasma membrane of transfected primary CD4+ T-cells, whereas Tat(49–51)A was essentially cytosolic (Figure 6E and F). Similar data were obtained using cell fractionation, except that in that case, Tat(49–51)A was observed in the nuclear and not in the cytosolic fraction (Supplementary Figure S5).
Figure 6.
Identification of Tat residues involved in PI(4,5)P2 binding. (A) Sequence of HIV-1 Tat BH10 and of the peptides used for this study. The residues of the basic domain are underlined, and those of the PIP2-binding site are boxed. Peptide 4* contains twice the indicated sequence. Cys-containing peptides were reduced with 10 mM DTT before use. (B) SPR sensorgrams illustrating the inhibition by Tat peptides of Tat binding to PI(4,5)P2 liposomes. Peptides were injected twice before applying Tat, as indicated by the arrows. The results of peptide 5 (P5) and 6 (P6) are shown. The other peptides behaved as P6 or buffer. The running buffer for SPR was 0.4 M NaCl, 10 mM HEPES, pH 7.4. (C) Secretion assays. Jurkat cells were transfected with luciferase and the indicated Tat mutant before assaying Tat secretion by ELISA 48 h later. (D) Affinity of Tat mutants and PHPLCδ for PIP2 liposomes as measured by ITC. Liposomes were sequentially injected into a microcalorimeter cuvette containing the protein. Heat production was monitored and enabled Kd determination. Tat(49–51)A did not produce any heat and, therefore, did not bind PIP2. (E) Intracellular localization of Tat mutants. Primary CD4+ T-cells were transfected with the indicated Tat mutant; 24 h later, cells were fixed and permeabilized for Tat immunofluorescence combined with WGA staining. Bar, 5 μm. (F) Quantification of the immunofluorescence results. Images from n>20 cells were recorded and the percentage of cell-fluorescence signal originating from the plasma membrane was determined. The significance of differences with WT Tat was assessed using a two-sided Student's t-test (***P<0.001; **P<0.01).
The fact that Tat (55–57)A preserved essentially native PI(4,5)P2 binding and secretion capacities shows that the loss of these activities by Tat(49–51)A is not merely due to the lack of three positive charges. These combined immunofluorescence, SPR, ITC and secretion data showed that Tat binds PI(4,5)P2 through residues 49–51 of its basic domain and confirmed that PI(4,5)P2 binding is a prerequisite for Tat secretion.
Tat requires a lipid membrane for insertion on PIP2 binding
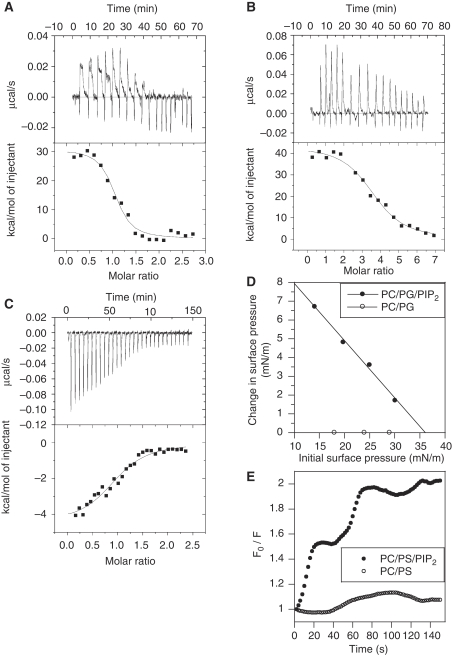
As three positive charges of Tat are involved in the binding of PI(4,5)P2 that has 3–5 negative charges (McLaughlin et al, 2002), the Tat–PI(4,5)P2 interaction should be stoichiometric. Titration of Tat with PI(4,5)P2–liposomes by ITC indeed revealed a stoichiometry of one PI(4,5)P2 per Tat molecule (Table 1). Surprisingly, this specificity was lost when using a water-soluble PI(4,5)P2 analogue, diC8-PI(4,5)P2, or the PI(4,5)P2 head group (Ins(1,4,5)P3) for which a 3–4 ligands/Tat interaction was observed, indicating that these soluble ligands bound to virtually all Tat-positive charges (n=17). In addition, when using liposomes, the ITC injection peaks were followed by a characteristic slow binding event (Figure 7A) that was not observed when using diC8-PI(4,5)P2 (data not shown), Ins(1,4,5)P3 (Figure 7B) or for the PHPLCδ–liposome interaction (Figure 7C). These three titration curves (Figure 7A-C) were strikingly different.
Table 1. Titration calorimetry of inositol phosphate binding to Tat and PHPLCδ.
| N (Tat:ligand) | Kd (nM) | ΔG (kJ/mol) | ΔH (kJ/mol) | TΔS (kJ/mol) | |
|---|---|---|---|---|---|
| PHPLCδ:PIP2 liposomes | 1:1.0 | 900 | −34.8 | −18.7 | +16.1 |
| Tat:Ins(1,4,5)P3 | 1:3.6 | 24 | −42.8 | +181.4 | +224.2 |
| Tat:PIP2 liposomes | 1:1.0 | 16 | −43.9 | +129.2 | +173.1 |
| N, stoichiometry; Kd, dissociation constant. Precisions on Kd are±10%. | |||||
| Proteins were present in the cell and PIP2 liposomes (PC/PG/PI(4,5)P2 75/20/5) or Ins(1,4,5)P3 were sequentially injected. | |||||
Figure 7.
Tat inserts into membranes on PI(4,5)P2 binding. Raw ITC data of Tat binding to (A) PI(4,5)P2 liposomes (PC/PG/PI(4,5)P2 75/20/5) and (B) Ins(1,4,5)P3. (C) Binding of GST–PHPLCδ to PI(4,5)P2 liposomes. Ins(1,4,5)P3 or PI(4,5)P2 liposomes were injected into the protein solution and heat production was monitored. The heat resulting from buffer injection was subtracted from the binding curves. Tat binding to diC8-PI(4,5)P2 showed narrow heat production peaks just as Ins(1,4,5)P3 (data not shown). (D) Tat inserts into phospholipid monolayers. Tat insertion into monolayers was monitored using Δπ as a function of π0. Monolayers were made of PC/PG (75/25) or PC/PG/PI(4,5)P2 (75/20/5) as indicated, and the subphase was 150 mM NaCl, 50 mM citrate, pH 7.0. For conversion, 1 mN/m=1 dyne/cm. Similar data were obtained using PS instead of PG (data not shown). (E) 10-DN quenching of Tat Trp fluorescence. Tat (2.5 μM) was quickly mixed with lipid vesicles composed of PC/PS (75/25) or PC/PS/PIP2 (75/20/5) containing or not 10% of the 10-DN quencher. Trp fluorescence was then recorded every 2 s. F0/F is the ratio of fluorescence intensity in samples without quencher relative to that with 10-DN (Caputo and London, 2003). The results of a representative experiment that was repeated three times.
Regarding the thermodynamics, PI(4,5)P2 recognition by PH domains is known to be an enthalpy-driven reaction (Lemmon et al, 1995) (Table 1), as expected for a binding event involving hydrogen bonding or ionic interactions with phosphate groups. Surprisingly, under the same experimental conditions, Tat interactions with PI(4,5)P2 liposomes were driven by a very favourable entropy that compensated for a large unfavourable enthalpy (Table 1). This unfavourable enthalpy was unexpected, and the association of Tat with PI(4,5)P2 liposomes clearly seems to be atypical in this respect. We also noted a significant reduction in favourable entropy for Tat titrations with liposomes as compared with those with Ins(1,4,5)P3 (Table 1) or diC8-PI(4,5)P2 (data not shown). Together with the slow binding event apparent for Tat–liposome interactions (Figure 7A), this could be indicative for Tat folding and/or membrane insertion.
These ITC results thus indicated that Tat requires a membrane to bind PI(4,5)P2, and suggested that the intrinsically unstructured Tat adopted a 3D-fold on interaction with PI(4,5)P2–liposomes and directly engaged interactions with the lipid membrane itself. This interpretation was consistent with the observations that Tat did not bind to PI(4,5)P2 spotted on a blot membrane or coated on an ELISA plate (data not shown). This was directly confirmed by the results of monolayer penetration experiments. In these studies, phospholipid monolayers of a chosen initial surface pressure (π0) were spread at constant area and the change in surface pressure (Δπ) was monitored after protein injection into the subphase. The extrapolation of the Δπ versus π0 plot yields the critical surface pressure (πc), which provides a π0 upper limit of a monolayer that a protein can insert into. The cell surface pressure of biological membranes has been estimated to be 30–35 mN/m (or dyne/cm), and any protein that presents a πc above this threshold can, therefore, spontaneously penetrate into biological membranes (Stahelin et al, 2003). Although Tat did not insert into PC/PG monolayers, in the presence of 5% PI(4,5)P2, Tat showed exceptionally high membrane penetrating power (πc∼36 mN/m; Figure 7D), that is higher than that of Epsin ENTH domain (πc∼31 mN/m; Stahelin et al, 2003) for instance. This result indicated that Tat can spontaneously insert into the inner leaflet of the plasma membrane.
Molecular basis for Tat-high affinity for PIP2
How does Tat insert into membranes on PI(4,5)P2 binding? According to the poor affinity of the transduction peptide for liposomal PI(4,5)P2 (Figure 6B), this interaction involves, in addition to the Tat basic domain, the participation of other parts of the molecule. Among hydrophobic residues that could be involved in membrane interaction, Tat owns a single and well-preserved Trp (Supplementary Figure S6; Pantano and Carloni, 2005) that enables Tat insertion into the endosome membrane during endocytosis of extracellular Tat (Yezid et al, 2009).
To examine whether Tat Trp indeed inserted into the membrane on Tat binding to PI(4,5)P2, we monitored the quenching of Tat-Trp fluorescence on binding to liposomes containing the hydrophobic quencher 10-doxylnonadecane (10-DN) (Caputo and London, 2003). Strong Trp-fluorescence quenching was observed when using PC/PS/PI(4,5)P2/10-DN vesicles, whereas fluorescence was not affected when using vesicles lacking PI(4,5)P2 (Figure 7E). This result confirmed that Tat inserts into the membrane on PI(4,5)P2 binding, and showed that Tat Trp is involved in this insertion process. Regarding the kinetics, insertion was essentially complete within 2 min. This is in agreement with the time scale of heat production detected during Tat–liposome interaction (Figure 7A).
We then made two conservative (W11F and W11Y) mutations on Trp11. We first monitored whether these mutations preserved native Tat biological activity, as monitored using the transactivation activity of the protein. This activity indeed requires Tat interaction with several partners (proteins and RNA) that recognize different domains of the protein (Jeang et al, 1999). The W11F mutation induced a drop of 50% in transactivation activity. This indicated a conformational problem in this mutant. Tat-W11Y showed native transactivation activity, suggesting that it preserved a normal conformation and reactivity of the molecule (Yezid et al, 2009).We then monitored the PIP2-binding capacity of these mutants by ITC. Replacement of Tat-Trp11 induced a drastic loss in the avidity for PI(4,5)P2 as Tat-W11F and Tat-W11Y bound PI(4,5)P2 30- and 300-fold less efficiently than WT Tat, respectively (Figure 6D). Keeping in mind the reservations regarding Tat-W11F conformation, it should be noted that the affinity of Trp11-mutant for PI(4,5)P2 is consistent with the respective ability of these residues to insert into membranes: Trp>>Phe >Tyr (White and Wimley, 1999).
To confirm the function of Tat-W11 in PI(4,5)P2 binding and Tat recruitment at the plasma membrane, we examined the intracellular localization of W11 mutants. They were both largely cytosolic (Figure 6E and F). This observation was confirmed by the results of secretion assays, as both W11F and W11Y mutations strongly inhibited Tat secretion by Jurkat cells (by ∼80%; Figure 6C). Collectively, these results showed that Tat Trp11 enables Tat membrane insertion that is strictly required for Tat binding to PI(4,5)P2, and thus for Tat secretion. This tight link and coupling between membrane penetration and PIP2 binding is the basis of the Tat requirement for a lipid membrane to bind PI(4,5)P2.
Secreted Tat is biologically active
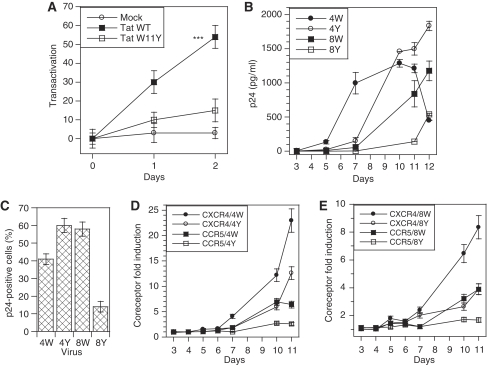
It was important to check the biological activity of secreted Tat in our experimental system. To this end, we first used a well-established transcellular transactivation assay (Helland et al, 1991; Marcuzzi et al, 1992; Ensoli et al, 1993). Donor cells were transfected with Tat (WT or W11Y) or EGFP as a control, and recipient cells with luciferase reporter plasmids. Donor and recipient cells were then mixed and collected for luciferase assays after various times of cocultivation. A time-dependent transcellular transactivation was observed in the case of WT-Tat (Figure 8A). Tat-W11Y that is poorly secreted (Figure 6C) and unable to reach the cytosol of target cells (Yezid et al, 2009) did not transactivate in this assay, although it showed native transactivation activity when coexpressed with luciferase vectors (Yezid et al, 2009), Hence, secreted Tat is capable to enter recipient cells to transactivate, and is thus biologically active.
Figure 8.
Secreted Tat is biologically active. (A) Transcellular transactivation by secreted Tat. Jurkat cells were transfected with WT Tat, Tat-W11Y or EGFP (mock); 18 h after transfection, cells were mixed (1/1) with Jurkats cells transfected with luciferase vectors, one encoding firefly luciferase under the control of HIV-1 LTR, and the other renilla luciferase under the control of a Tat-independent promoter. Cells were harvested for luciferase assays after the indicated coculture time, and transactivation was calculated using the increase in the firefly/renilla activity ratio (%). (B, C) Secreted Tat differentially affects virus replication according to tropism. Jurkat CD4-CCR5 T-cells that express both CXCR4 and CCR5 were infected with an X4 (pNL4-3) or an R5 (AD8) HIV-1 variant, bearing either WT Tat or Tat-W11Y as indicated by 4W, 4Y, 8W and 8Y. Infection was monitored using (B) p24 ELISA of culture supernatants and (C) intracellular p24 staining and FACS analysis using quadrant statistics. Data shown are representative of three different experiments. Similar results were obtained using PBMCs as target cells (data not shown). (D, E) Secreted Tat induces coreceptor expression. Coreceptor expression by Jurkat CD4-CCR5 T-cells infected with X4 (D) or R5 (E) variants was monitored by FACS analysis. Expression was quantified as the average fluorescence of the cell population, and the fold induction of CXCR4 and CCR5 was obtained by comparison with expression in control samples.
Secreted Tat strongly affects HIV-1 multiplication
Transcellular transactivation assays are not very sensitive (Ensoli et al, 1993), and we thought of using another effect of extracellular Tat to confirm that secreted Tat is active on target cells. Extracellular Tat was found to affect HIV-1 multiplication at the level of virus coreceptors (Huang et al, 1998; Weiss et al, 1999; Ghezzi et al, 2000; Xiao et al, 2000). As a Tat mutant that transactivates properly, but is not secreted, was not available, these studies used recombinant Tat and not Tat produced by infected cells. We took advantage of the Tat-W11Y mutant that retains native transactivation activity (Yezid et al, 2009), but is unable to cross membranes (Figure 6C; Yezid et al, 2009). We prepared recombinant viruses bearing the W11Y mutation either in an X4 or an R5 background, and used these viruses to infect CCR5-transfected Jurkat T-cells or PBMCs. These systems provided similar results. We monitored infection using extracellular- and intracellular-p24 assays and assessed coreceptor expression at the cell surface using FACS analysis. The results of extracellular p24 determinations (Figure 8B for Jurkat CD4-CCR5 cells) showed that all viruses were replicative. Regarding X4 (pNL4-3) viruses, the WT initially replicated more quickly, but a maximum was reached at day 10 and virus production decreased at later times. The highest virus titres were obtained with the mutant (Tat-W11Y) X4 virus (+40% compared with WT). Intracellular p24 assays by FACS (Figure 8C) or western blots (not shown) confirmed that X4-Tat-W11Y replicated more efficiently than the WT X4 viruses. Secreted WT Tat induced very strongly (up to 23-fold) CXCR4 expression (Figure 8D). CCR5 induction was lower (up to six-fold), but this weaker effect could be related to the specific genetic background of Jurkat CD4-CCR5 cells. The mutant (Tat-W11Y) X4 virus was much less efficient in increasing coreceptor levels (up to 12-fold for CXCR4 and three-fold for CCR5) and its effect likely represents the participation of infected cells.
When R5 (AD8) viruses were used, the mutant replicated less actively than the WT (Figure 8B and C). As noticed for X4 viruses, the WT R5 virus was at least two-fold more efficient than the mutant in increasing coreceptor expression (Figure 8E).
Altogether, these results are consistent with established Tat effects on HIV-1 coreceptors. The effect on CXCR4 is the most complex. Extracellular Tat at 2–200 nM is known to bind CXCR4, thereby inhibiting infection by X4 viruses (Ghezzi et al, 2000; Xiao et al, 2000). Nevertheless, as shown earlier using subnanomolar concentrations of exogenous Tat (Huang et al, 1998), Tat strongly induced CXCR4 expression (Figure 8D). As a result of these two opposite and concentration-dependent effects, the WT X4 virus started to multiply more efficiently than the mutant (Figure 8B). This is noticeable at day 7 in which WT X4 had already induced significant CXCR4 expression (Figure 8D). After day 10, when Tat accumulated in the medium, its inhibitory effect on the multiplication of WT X4 became dominant (Figure 8B and C). This concentration-dependent effect of Tat might modulate the spread of X4 viruses in patients by favouring infection in which infected cells are scarce (low Tat concentration) and inhibiting viral production in which infected cells are concentrated (high Tat concentration).
Regarding R5 viruses, our data confirmed those obtained using recombinant Tat and showing that extracellular Tat induced CCR5 (Huang et al, 1998; Weiss et al, 1999). Therefore, WT R5 viruses that trigger efficient Tat secretion have more CCR5 available for entry than W11Y R5 viruses (Figure 8E) and as a result multiply more quickly (Figure 8B). Secreted Tat thus regulates HIV-1 multiplication at the coreceptor level. For X4 viruses, this effect, positive when infection starts, becomes negative when Tat reach a threshold concentration in the nanomolar range, whereas Tat favours the multiplication of R5 viruses.
Discussion
Although HIV-1-infected cells were found to secrete Tat 19 years ago (Ensoli et al, 1990), this process remained poorly characterized (Chang et al, 1997). Meanwhile, a lot of studies showed that extracellular Tat was indeed a viral toxin that could affect the biological activity or the life span of different cell types, (Rubartelli et al, 1998; Gallo, 1999; Huigen et al, 2004). Nevertheless, Tat function was thought to be essentially restricted to the control of viral RNA production (Chiu et al, 2002). This general belief was largely due to the observations that transfected Tat was a nuclear or a nucleolar protein in most laboratory T-cell lines such as Jurkats (Figure 2A and B).
Tat intracellular localization
Here, we found that Tat accumulated at the plasma membrane in primary CD4+ T-cells. This was observed in both HIV-1-infected and Tat-transfected cells using immunofluorescence and cell fractionation (Figure 2). Hence, viral infection is not involved in Tat targeting to the plasmalemma in this cell type. As HIV-1 replicates in primary CD4+ T-cells, nuclear accumulation is not required for transactivation. This interpretation is consistent with the subnanomolar affinities involved in the assembly of the cyclin T1–Tat–TAR ternary complex (Zhang et al, 2000). As these interactions are at least 20-fold tighter than Tat–PI(4,5)P2 interaction, and as significant amounts of Tat are bound to PI(4,5)P2 in the infected cell (Figure 2D and E), all TAR should have bound a Tat molecule. Hence, in primary CD4+ T-cells, Tat transcriptional activity only requires and uses minute Tat amounts, allowing the infected cell to export most of the protein.
This interpretation was confirmed by the results obtained using Jurkat cells transfected with Tat-NES. The NES, when attached to Tat C-terminus, caused its relocalization to the cytosol and the plasma membrane, whereas transactivation activity was only moderately affected (Supplementary Figure S2). Hence, significant transactivation by Tat does not require Tat nuclear/nucleolar accumulation and Tat nuclear-concentration levels required to insure robust transactivation are below the immunofluorescence detection threshold.
Tat secretion is efficient and relies on PI(4,5)P2 binding
We found that, although unconventional, HIV-1 Tat secretion is an efficient process as 4–5% of cell-associated Tat is exported hourly. Interestingly, the extracellular Tat concentration observed after overnight incubation of infected CD4+ T-cells (5.106 cells/ml) reached 0.25 nM, a value that falls within the Tat-concentration range (0.18–3.6 nM) that was observed in the sera of HIV-1-infected patients (Xiao et al, 2000). This secretion efficiency also means that during the life span of the infected cell ∼2/3 of synthesized Tat will be exported, showing that the outside medium is a major destination for Tat.
Tat secretion relies on PI(4,5)P2 binding. Such a requirement was recently observed for the unconventional secretion of FGF-2, although compared with Tat, this cytokine showed lower specificity and affinity for PI(4,5)P2. Moreover, FGF-2 does not require a membrane to bind PI(4,5)P2 (Temmerman et al, 2008). Hence, although these proteins are similarly targeted to the plasma membrane, the mechanisms of PI(4,5)P2 recognition and membrane translocation are likely different for Tat and FGF-2.
Tat is not the only HIV-1 protein recruited by PI(4,5)P2 at the plasma membrane. This is also the case for Gag (Ono et al, 2004) and both proteins were indeed found to colocalize at the infected-cell plasmalemma (Figure 2D and E) and on peri-Golgi structures when they were displaced from PI(4,5)P2 by neomycin (Figure 5D). As HIV-1 virions were found to be enriched in this phosphoinositide (Chan et al, 2008), this raises the possibility that Tat could be included into virions, as discussed earlier (Chertova et al, 2006).
Molecular bases for Tat–PI(4,5)P2 high affinity
The bases for Tat–PI(4,5)P2 interaction are original in several respects. It was first surprising to find that, although the Tat basic domain contains eight basic residues out of a total of nine, only the first three were involved in PI(4,5)P2 recognition. This indicates that Tat, although refractory to crystallization and for which no precise 3D structure is currently available, nevertheless undergoes some kind of molecular organization, at least on ligand docking. This interpretation was confirmed by ITC data showing that liposomal PI(4,5)P2 induced Tat conformational modifications (Figure 7A).
The sequence of molecular remodelling triggered by PI(4,5)P2 binding and culminating with Tat membrane insertion and translocation remains to be identified. Nevertheless, as Tat Trp is buried within resting Tat (Bayer et al, 1995), whereas PI(4,5)P2 binding generated structural modifications that could be detected by ITC (Figure 7A), it seems likely that interaction with PI(4,5)P2 precedes and triggers Trp exposure.
Tat is an original PI(4,5)P2 ligand because of its high affinity and its strict requirement for a membrane to bind this lipid. These two specificities have the same molecular basis, Tat single Trp residue that inserts into membranes on PI(4,5)P2 binding, stabilizes the interaction and allows Tat secretion. These events are thus governed by residues 11 and 49–51 of Tat. Interestingly, despite HIV-1 high mutation rate (Walker and Burton, 2008), among the most conserved Tat residues from different viral isolates, residues 11 and 49–51 were indeed present, but not 55–57 (Supplementary Figure S6; Pantano and Carloni, 2005).
The high affinity of Tat for PI(4,5)P2 indicates that the function of this interaction is unlikely restricted to Tat secretion. Indeed, overexpression of PHPLCδ prevents the PI(4,5)P2-mediated recruitment at the plasma membrane of cell machineries that enable clathrin-mediated endocytosis (Jost et al, 1998), phagocytosis (Botelho et al, 2000) or exocytosis (Holz et al, 2000) and inhibits these processes. As Tat has a 50-fold better affinity for PI(4,5)P2 than PHPLCδ (Table 1) and can accordingly displace it from the cell membrane (Figure 4), it could, therefore, be reasonably concluded that Tat, even at minute concentrations, would produce similar or more pronounced effects on these cell activities, which are crucial for proper immune system function. As Tat is exported and can enter uninfected cells (Vendeville et al, 2004), this original and intriguing capacity of Tat to perturb PI(4,5)P2-mediated protein recruitment could be involved in immune system dysfunction during AIDS.
Conclusion
The main question raised by our study is why does HIV-1 trigger massive Tat export? The answer probably lies in the vast body of literature that described the pronounced effects of extracellular Tat on a variety of cell types (Rubartelli et al, 1998; Gallo, 1999; Huigen et al, 2004). The preservation among Tats of important residues involved in Tat secretion indeed indicates that the paracrine activity of Tat is critical for HIV-1 multiplication and could be an interesting target to develop future anti-HIV-1 therapeutics.
Materials and methods
Expression vectors
Tat mutants were generated from pBi-Tat or Pet11d-Tat (BH10 isolate, 86 residues) using Quickchange kits (Stratagene). Coding sequences were entirely sequenced (Cogenics, Meylan, France). Recombinant Tat or Tat mutants were purified from Escherichia coli as described (Vendeville et al, 2004). Expression vectors coding for PHPLCδ-EGFP and its inactive mutant (R40L) were kindly provided by Tamas Balla (NICHD, Bethesda); PTEN and its G129E mutant by Patrick Raynal (INSERM, Toulouse, France); 5-phosphatase IV and its Δ1 mutant by Akira Ono (NCI, Frederick); Arf6-Q67L by Julie Donaldson (NIH, Bethesda); IpgD, IpgD-C438S and Inp54 by Bernard Payrastre (INSERM, Toulouse); PHAkt-EGFP and GAB1-EGFP by Georges Bismuth (Institut Cochin, Paris, France) and GST–PHPLCδ by Alexander Gray (University of Dundee, UK).
Cells
Primary CD4+ T-cells were purified from human blood by density gradient centrifugation (Ficoll Hypaque) and immunomagnetic-positive selection (Dynal). The human CD4+ T-cell line Jurkat E6.1 was from the American Type Culture Collection. Cells were transfected using electroporation or infected by HIV-1 as indicated. Cell fractionation was performed using a subcellular protein fractionation kit (Thermo Scientific). Details are provided in Supplementary Data.
Secretion assays
Jurkat cells were electroporated (Vendeville et al, 2004) with pBi-Tat (20 μg), pBi-firefly luciferase (2 μg), pUHD (2 μg; required for expression from pBi vector) and effector plasmid (10 μg) when indicated. Two days after transfection, cells were washed and resuspended in medium prepared from degassed RPMI, 10% FCS and freshly diluted 20 μM 2-mercaptoethanol. They were then transferred (7.5 × 105 cells/well, in triplicate) to an ELISA FluoroNunc plate that had been coated overnight at 4°C with monoclonal anti-Tat antibody (1 μg/ml in 50 mM sodium carbonate pH 9.6) before saturation with 8% (w/v) skimmed milk in phosphate-buffered saline (PBS). Dilutions of recombinant Tat (19 pg—1.25 ng/well) were also added to the plate for calibration purposes. After 6 h at 37°C (except when otherwise stated), cells were transferred to microtubes, centrifuged and the supernatant was frozen for later luciferase assay. Cells were resuspended in passive lysis buffer prepared from degassed water. Part of the lysate was then used directly to assay intracellular Tat by ELISA using a fresh plate prepared as described above (including Tat standards), whereas the rest was frozen for the luciferase assay that was performed using a Promega kit. The end of the ELISA procedure involved washes with PBS then with PBS/0.05% Tween, incubation with rabbit anti-Tat antibodies for 1 h, washes with PBS/Tween, 1 h with peroxidase-labelled goat anti-rabbit antibodies, washes with PBS/Tween and revelation using 3,3,5,5-tetramethylbenzidine. Secretion was calculated using the Tat amounts present in the medium and intracellularly using the medium/(intracellular + medium) formula. The same protocol was used for infected cells. They were collected 3 days after infection (p24 from 48 to 88 ng/ml), before plating 106 cells/well of the ELISA plate and overnight incubation (12–18 h). HIV-1 p24 and Nef secretion were quantified using commercial sandwich ELISA kits (Ingen, France and ImmunoDiagnostics Inc., respectively).
Immunofluorescence
T-cells were allowed to adhere to alcian-blue-coated coverslips for 3 min at room temperature and were fixed in 3.7% paraformaldehyde before permeabilization with 0.1% saponin. Tat, Ki67 or Histone H1 were then revealed by indirect immunofluorescence (Vendeville et al, 2004). WGA-Cy5 was used to label glycoproteins on the plasma membrane and in the TGN. For quantification of Tat or PHPLCδ displacement from the plasma membrane, the ImageQuant software was used. Two circles (or more elaborated forms) were drawn on the cell-fluorescence image, one just outside the cell and one just beneath the membrane. The fluorescence signal present in the circles was then used to calculate the proportion of cell labelling coming from the cytosol and the plasma membrane. A minimum of 30 cells were scored for each condition.
Transcellular transactivation assays
Jurkat cells were transfected (Vendeville et al, 2004) with 10 μg of a vector expressing WT Tat, Tat-W11Y or EGFP; 18 h after transfection, cells were mixed (1/1) with Jurkats cells transfected with 7 μg of pGL3-LTR, which expresses firefly luciferase under control of the Tat-activated HIV-1-LTR promoter, and 1 μg of pRL-TK (Promega) that codes for renilla luciferase under the control of the herpes simplex virus thymidine kinase promoter. Cells were harvested for dual luciferase assays (Promega) after the indicated coculture time, and transactivation was calculated using the increase in the firefly/renilla activity ratio (%) (Vendeville et al, 2004).
Lipid techniques
Liposome sedimentation assays were performed as described (Barret et al, 2000). Small unilamellar vesicles (Méré et al, 2005) were used for SPR (Zimmermann et al, 2002) and ITC (Lemmon et al, 1995) analysis. Fluorescence and monolayer experiments were performed as detailed (Stahelin et al, 2003; Méré et al, 2005). Details are provided in Supplementary Data.
Supplementary Material
Acknowledgments
We thank all the people who, directly or indirectly, provided valuable material for this study. We are indebted to the AIDS Research and Reference Reagent Program, NIH, USA and the Programme EVA Centre for AIDS Reagents, NIBSC, UK for reagents. We are also grateful to Nicole Lautredou (IURC Montpellier RIO Imaging) for assistance with confocal analysis, to David Perrais, Laurent Chaloin and David Fenard for help in preliminary experiments, to Laurent Chiche for Tat sequence comparative analysis and to Catherine Lévèque for English editing. This work was supported by grants from the ANRS and Sidaction.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S (2000) Intracellular pH regulation by Na(+)/H(+) exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol 150: 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astoul E, Edmunds C, Cantrell DA, Ward SG (2001) PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol 22: 490–496 [DOI] [PubMed] [Google Scholar]
- Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V (2000) Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol 151: 1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer P, Kraft M, Ejchart A, Westendorp M, Frank R, Rosch P (1995) Structural studies of HIV-1 Tat protein. J Mol Biol 247: 529–535 [DOI] [PubMed] [Google Scholar]
- Blin G, Margeat E, Carvalho K, Royer CA, Roy C, Picart C (2008) Quantitative analysis of the binding of ezrin to large unilamellar vesicles containing phosphatidylinositol(4,5)-bisphosphate. Biophys J 94: 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S (2000) Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol 151: 1353–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG (2001) Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol 154: 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo GA, London E (2003) Using a novel dual fluorescence quenching assay for measurement of tryptophan depth within lipid bilayers to determine hydrophobic alpha-helix locations within membranes. Biochemistry 42: 3265–3274 [DOI] [PubMed] [Google Scholar]
- Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR (2008) Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol 82: 11228–11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11: 1421–1431 [DOI] [PubMed] [Google Scholar]
- Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW Jr, Sowder RC II, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE (2006) Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol 80: 9039–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YL, Ho CK, Saha N, Schwer B, Shuman S, Rana TM (2002) Tat stimulates cotranscriptional capping of HIV mRNA. Mol Cell 10: 585–597 [DOI] [PubMed] [Google Scholar]
- Corbin JA, Dirkx RA, Falke JJ (2004) GRP1 pleckstrin homology domain: activation parameters and novel search mechanism for rare target lipid. Biochemistry 43: 16161–16173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Godi A (2004) PI-loting membrane traffic. Nat Cell Biol 6: 487–492 [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Dupont E, Prochiantz A, Joliot A (2007) Identification of a signal peptide for unconventional secretion. J Biol Chem 282: 8994–9000 [DOI] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345: 84–86 [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol 67: 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RC (1999) Tat as one key to HIV-induced immune pathogenesis and Tat toxoid as an important component of a vaccine. Proc Natl Acad Sci USA 96: 8324–8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi S, Noonan DM, Aluigi MG, Vallanti G, Cota M, Benelli R, Morini M, Reeves JD, Vicenzi E, Poli G, Albini A (2000) Inhibition of CXCR4-dependent HIV-1 infection by extracellular HIV-1 Tat. Biochem Biophys Res Commun 270: 992–996 [DOI] [PubMed] [Google Scholar]
- Helland DE, Welles JL, Caputo A, Haseltine WA (1991) Transcellular transactivation by the human immunodeficiency virus type 1 tat protein. J Virol 65: 4547–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T (2006) PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314: 1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA (2000) A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J Biol Chem 275: 17878–17885 [DOI] [PubMed] [Google Scholar]
- Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ (2005) Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell 18: 519–531 [DOI] [PubMed] [Google Scholar]
- Huang L, Bosch I, Hofmann W, Sodroski J, Pardee AB (1998) Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J Virol 72: 8952–8960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigen MC, Kamp W, Nottet HS (2004) Multiple effects of HIV-1 trans-activator protein on the pathogenesis of HIV-1 infection. Eur J Clin Invest 34: 57–66 [DOI] [PubMed] [Google Scholar]
- Jeang KT, Xiao H, Rich EA (1999) Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem 274: 28837–28840 [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A (2004) Transduction peptides: from technology to physiology. Nat Cell Biol 6: 189–196 [DOI] [PubMed] [Google Scholar]
- Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL (1998) Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol 8: 1399–1402 [DOI] [PubMed] [Google Scholar]
- Keller M, Ruegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132: 818–831 [DOI] [PubMed] [Google Scholar]
- Lemmon MA (2003) Phosphoinositide recognition domains. Traffic 4: 201–213 [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, O'Brien R, Sigler PB, Schlessinger J (1995) Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA 92: 10472–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco WA, Szilvay AM, Kalland KH, Helland DG, Reyes HM, Walter RJ (1994) Spatial association of HIV-1 tat protein and the nucleolar transport protein B23 in stably transfected Jurkat T-cells. Arch Virol 139: 133–154 [DOI] [PubMed] [Google Scholar]
- Marcello A, Cinelli RA, Ferrari A, Signorelli A, Tyagi M, Pellegrini V, Beltram F, Giacca M (2001) Visualization of in vivo direct interaction between HIV-1 TAT and human cyclin T1 in specific subcellular compartments by fluorescence resonance energy transfer. J Biol Chem 276: 39220–39225 [DOI] [PubMed] [Google Scholar]
- Marcuzzi A, Weinberger J, Weinberger OK (1992) Transcellular activation of the human immunodeficiency virus type 1 long terminal repeat in cocultured lymphocytes. J Virol 66: 4228–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun CR, Naujokas MA, Park M (2003) Membrane targeting of Grb2-associated binder-1 (Gab1) scaffolding protein through Src myristoylation sequence substitutes for Gab1 pleckstrin homology domain and switches an epidermal growth factor response to an invasive morphogenic program. Mol Biol Cell 14: 1691–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D (2002) PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31: 151–175 [DOI] [PubMed] [Google Scholar]
- Méré J, Morlon-Guyot J, Bonhoure A, Chiche L, Beaumelle B (2005) Acid-triggered membrane insertion of pseudomonas exotoxin A involves an original mechanism based on pH-regulated tryptophan exposure. J Biol Chem 280: 21194–21201 [DOI] [PubMed] [Google Scholar]
- Nickel W, Rabouille C (2009) Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10: 148–155 [DOI] [PubMed] [Google Scholar]
- Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO (2004) Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci USA 101: 14889–14894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano S, Carloni P (2005) Comparative analysis of HIV-1 Tat variants. Proteins 58: 638–643 [DOI] [PubMed] [Google Scholar]
- Pendaries C, Tronchere H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B (2006) PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J 25: 1024–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD (1996) HIV-1 dynamics in vivo: virion clearance rate, infected cell life span, and viral generation time. Science 271: 1582–1586 [DOI] [PubMed] [Google Scholar]
- Ranki A, Lagerstedt A, Ovod V, Aavik E, Krohn KJ (1994) Expression kinetics and subcellular localization of HIV-1 regulatory proteins Nef, Tat and Rev in acutely and chronically infected lymphoid cell lines. Arch Virol 139: 365–378 [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Poggi A, Sitia R, Zocchi MR (1998) HIV-I Tat: a polypeptide for all seasons. Immunol Today 19: 543–545 [DOI] [PubMed] [Google Scholar]
- Sivakumaran H, van der Horst A, Fulcher AJ, Apolloni A, Lin MH, Jans DA, Harrich D (2009) Arginine methylation increases the stability of human immunodeficiency virus type 1 Tat. J Virol 83: 11694–11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin RV, Long F, Peter BJ, Murray D, De Camilli P, McMahon HT, Cho W (2003) Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and epsin N-terminal homology (ENTH) domains. J Biol Chem 278: 28993–28999 [DOI] [PubMed] [Google Scholar]
- Stauber RH, Pavlakis GN (1998) Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology 252: 126–136 [DOI] [PubMed] [Google Scholar]
- Stebbing J, Gazzard B, Douek DC (2004) Where does HIV live? N Engl J Med 350: 1872–1880 [DOI] [PubMed] [Google Scholar]
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279: 710–714 [DOI] [PubMed] [Google Scholar]
- Temmerman K, Ebert AD, Muller HM, Sinning I, Tews I, Nickel W (2008) A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic 9: 1204–1217 [DOI] [PubMed] [Google Scholar]
- Truant R, Cullen BR (1999) The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol 19: 1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Balla T (1998) Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol 143: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B (2004) HIV-1 Tat enters T-cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell 15: 2347–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BD, Burton DR (2008) Toward an AIDS vaccine. Science 320: 760–764 [DOI] [PubMed] [Google Scholar]
- Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM (2002) Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J 363: 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Nath A, Major EO, Berman JW (1999) HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol 163: 2953–2959 [PubMed] [Google Scholar]
- White SH, Wimley WC (1999) Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct 28: 319–365 [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT (2000) Selective CXCR4 antagonism by tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA 97: 11466–11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezid H, Konate K, Debaisieux S, Bonhoure A, Beaumelle B (2009) Mechanism for HIV-1 TAT insertion into the endosome membrane. J Biol Chem 284: 22736–22746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tamilarasu N, Hwang S, Garber ME, Huq I, Jones KA, Rana TM (2000) HIV-1 TAR RNA enhances the interaction between Tat cyclin T1. J Biol Chem 275: 34314–34319 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Meerschaert K, Reekmans G, Leenaerts I, Small JV, Vandekerckhove J, David G, Gettemans J (2002) PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol Cell 9: 1215–1225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.