Abstract
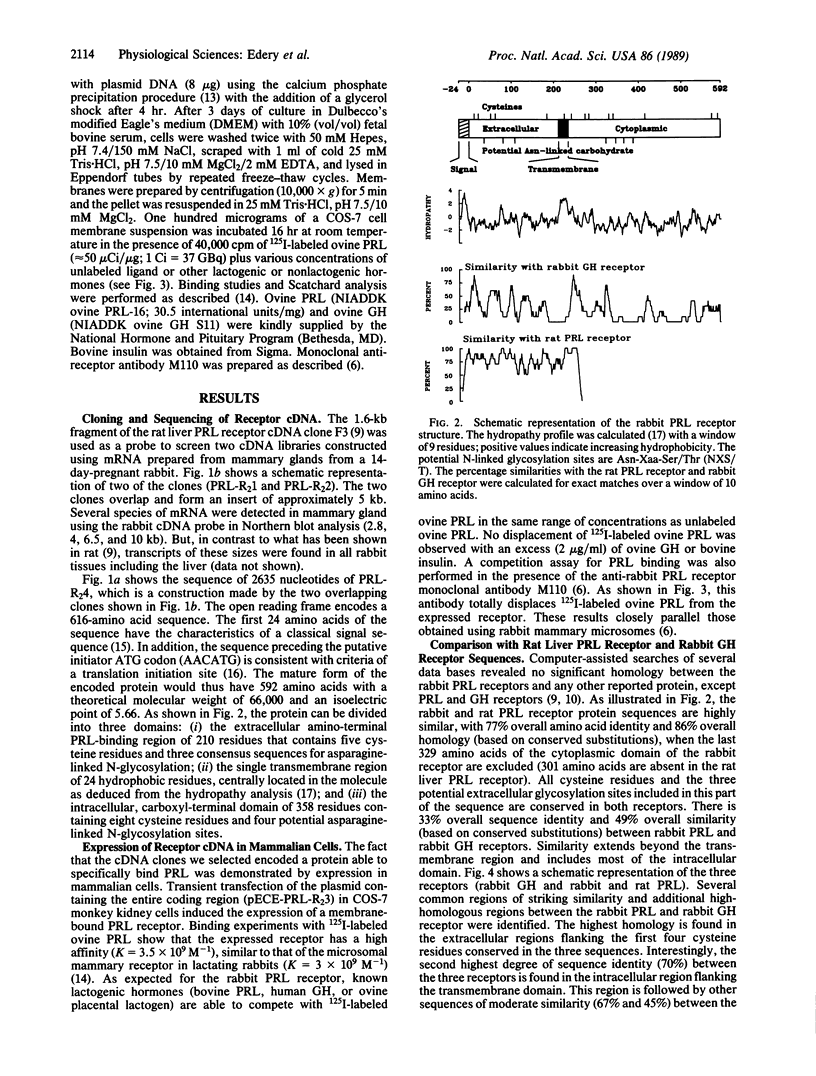
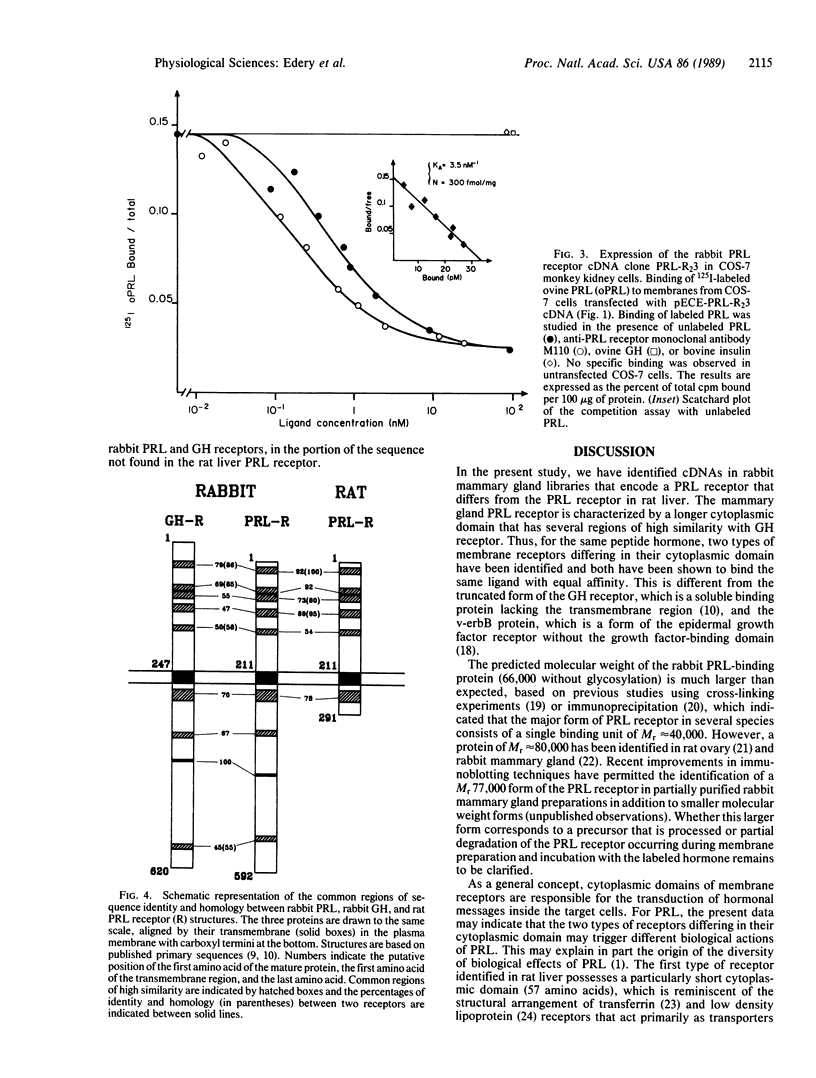
Two lambda gt11 clones containing fragments of cDNA encoding the prolactin receptor from rabbit mammary gland were isolated using a rat liver prolactin receptor cDNA probe. An 1848-base-pair open reading frame encodes a mature prolactin-binding protein of 592 amino acids that contains three domains: (i) the extracellular, amino-terminal, prolactin-binding region of 210 residues; (ii) the transmembrane region of 24 residues; and (iii) the intracellular, carboxyl-terminal domain of 358 residues. This latter domain is much longer than the cytoplasmic domain (57 residues) previously described for the rat liver prolactin receptor. In addition, the sequence identity of this form of prolactin receptor with the growth hormone receptor is extended in the cytoplasmic domain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonifacino J. S., Dufau M. L. Structure of the ovarian lactogen receptors. Analysis with bifunctional cross-linking reagents. J Biol Chem. 1984 Apr 10;259(7):4542–4549. [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P., Kelly P. A. Evolution of prolactin receptors in rabbit mammary gland during pregnancy and lactation. Endocrinology. 1977 May;100(5):1348–1356. doi: 10.1210/endo-100-5-1348. [DOI] [PubMed] [Google Scholar]
- Djiane J., Dusanter-Fourt I., Katoh M., Kelly P. A. Biological activities of binding site specific monoclonal antibodies to prolactin receptors of rabbit mammary gland. J Biol Chem. 1985 Sep 25;260(21):11430–11435. [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Kelly P. A., Djiane J. Immunological recognition of the prolactin receptor: identification of a single binding unit of molecular weight approximately 42,000. Biochimie. 1987 Jun-Jul;69(6-7):639–646. doi: 10.1016/0300-9084(87)90183-0. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Gascuel O., Danchin A. Protein export in prokaryotes and eukaryotes: indications of a difference in the mechanism of exportation. J Mol Evol. 1986;24(1-2):130–142. doi: 10.1007/BF02099961. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Aubert M. L., Djiane J., Kraehenbuhl J. P. Binding sites for lactogenic and somatogenic hormones from rabbit mammary gland and liver. J Biol Chem. 1983 Jan 10;258(1):305–314. [PubMed] [Google Scholar]
- Katoh M., Djiane J., Kelly P. A. Monoclonal antibodies against rabbit mammary prolactin receptors. Specific antibodies to the hormone binding domain. J Biol Chem. 1985 Sep 25;260(21):11422–11429. [PubMed] [Google Scholar]
- Katoh M., Djiane J., Kelly P. A. Prolactin-binding components in rabbit mammary gland: characterization by partial purification and affinity labeling. Endocrinology. 1985 Jun;116(6):2612–2620. doi: 10.1210/endo-116-6-2612. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin regulation of casein gene expression: possible mediators. Endocrinology. 1980 Jan;106(1):252–259. doi: 10.1210/endo-106-1-252. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Hogan M. L., Sauer R., Rosenblum I. Y., Greenwood F. C. Sequences of pituitary and placental lactogenic and growth hormones: evolution from a primordial peptide by gene reduplication. Proc Natl Acad Sci U S A. 1971 Apr;68(4):866–870. doi: 10.1073/pnas.68.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Shiu R. P., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974 Aug;95(2):521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- Sakai S., Ike F. Two separate receptors for prolactin in the rabbit mammary gland. Endocrinol Jpn. 1987 Dec;34(6):863–870. doi: 10.1507/endocrj1954.34.863. [DOI] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]


