EMBO J 29 8, 1318–1330 (2010); published online March042010 The pathways by which neurotransmitter-filled presynaptic vesicles (SVs) are generated and recycled have been debated for a long time. Glyvuk et al (2010) in this issue of The EMBO Journal describe an unanticipated role for the clathrin adaptor AP-1 and in particular its σ1B subunit in SV recycling. SV reformation is defective in σ1B-deficient mice, which instead accumulate large endosome-like vacuoles. These defects are paired with reduced motor coordination and long-term spatial memory. This work thus not only provides novel insights into the role of clathrin/AP-1 coats in SV recycling from endosomes, but also unravels a molecular mechanism that may contribute to some forms of X-linked mental retardation.
Synaptic transmission involves the fusion of neurotransmitter-filled synaptic vesicles (SVs) with the presynaptic plasma membrane at the active zone. To sustain neurotransmission and to prevent expansion of the plasma membrane, SVs undergo a local cycle of reformation, whereby SV proteins and lipids are re-internalized and SVs are regenerated (Murthy and De Camilli, 2003; Ryan, 2006). SVs display a specific protein and lipid composition that enables them to store and release the neurotransmitter, to be targeted to or cluster near release sites, and to undergo multiple rounds of exo-endocytosis. Defective vesicle cycling or exo-endocytic coupling has been linked to deficits in short-term synaptic plasticity, memory, and cognitive performance, including mental retardation and schizophrenia in humans (Murthy and De Camilli, 2003). The question which mechanisms contribute to SV recycling dates back to the early days of neurophysiology and the discovery by Katz that the neurotransmitter is released in predefined quanta. Are all SVs created equal? Do all SVs undergo full fusion with the plasmalemma? Are SVs solely regenerated from the plasma membrane proper or are endosomal intermediates involved? These fundamental questions (Ryan, 2006) have been difficult to answer, but a combination of genetic and optical tools seem well-suited to tackle them.
This is precisely the path that Glyvuk et al (2010) have followed. The authors generated knockout (KO) mice lacking expression of AP-1/σ1B. X-chromosome-encoded σ1B is one out of three isogenes (termed A–C) for the tiny σ subunit of AP-1 (comprising γ1, β1, μ1 and σ1 adaptins) localized to the trans-Golgi network (TGN) and endosomes. In contrast to mice deficient in γ1 or μ1A adaptins, which suffer from early embryonic lethality, σ1B-KO mice survive to adulthood and develop normally, suggesting that σ1A and σ1C adaptins can partially compensate for σ1B function. However, σ1B-KO animals are hypoactive and show behavioral abnormalities such as balancing problems and impaired spatial learning. These defects are reminiscent of those seen in patients suffering from X-linked metal retardation due to premature STOP codons present in their σ1B gene (Tarpey et al, 2006). How can these phenotypes be explained at the cellular and molecular level?
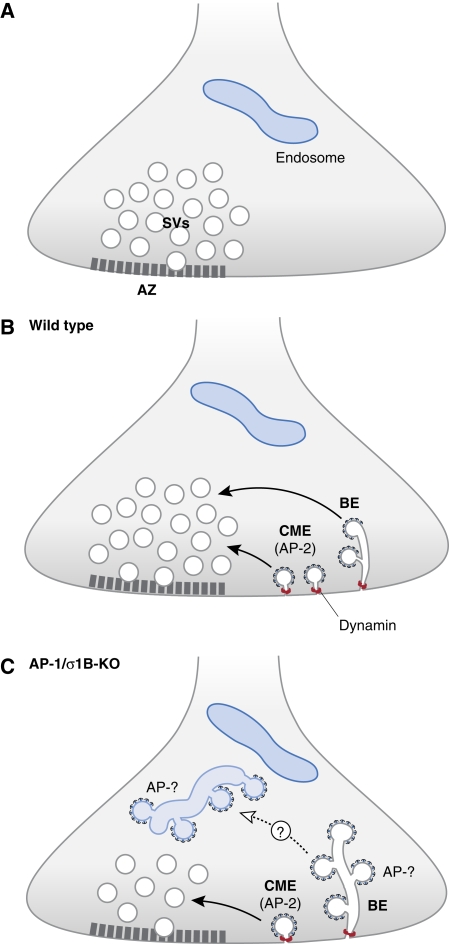
Surprisingly, Glyvuk et al (2010) found that presynaptic function, in particular recycling of SVs, was perturbed in σ1B-deficient animals. KO mice showed a severe reduction in the total number of SVs at hippocampal boutons at rest and upon activity. Instead, nerve terminals from σ1B-KO mice accumulated enlarged vacuoles, reminiscent of endosomes in non-neuronal cells. Frequently, these endosome-like structures contained clathrin-coated buds of undefined molecular identity, which did not appear to be connected to the plasmalemma (Figure 1). Most strikingly, replenishment of release-competent SVs after full depletion of the total recycling SV pool was significantly slower than in wild-type littermates. Hence, the authors propose an unanticipated function for AP-1/σ1B-containing clathrin coats in SV reformation from endosomes. Moreover, the new study lends further support to the idea that bulk endocytosis (BE) and subsequent consumption of endosome-like vacuoles but not kiss-and-run (Zhang et al, 2009) represents the major alternative route for SV recycling under conditions in which clathrin-mediated endocytosis (CME) becomes limiting.
Figure 1.
Hypothetical model for the role of different clathrin/AP complexes in SV recycling. (A) SVs are clustered near the active zone (AZ). (B) In wild type SVs are recycled mainly by CME. Sustained high-level activity induces BE involving formation of vacuoles. Fission likely involves dynamin (red circles). SV reformation from vacuoles occurs, at least in part, by clathrin-dependent budding. (C) Nerve terminals of AP-1/σ1B-KO mice display reduced numbers of SVs. Clathrin-coated buds accumulate on vacuoles that might have originated from BE. Buds may contain AP-1/σ1A, AP-2, or AP-3.
Glyvuk et al (2010) argue that the CME of SV membranes represents a kinetic bottleneck of the recycling pathway. Under conditions of sustained activity BE provides a compensatory mechanism to balance high exocytic load with matching endocytic activity. Vacuolar membrane invaginations are then consumed by undefined budding events that chop these membranes into small vesicles that may re-enter the SV cycle (Figure 1A). It is this consumption step that the authors envision to depend on AP-1/σ1B (Figure 1B). The experimental evidence for this model at present remains indirect. AP-1, as its relative AP-2, is one of the major recruitment factors for clathrin and loss of either protein complex results in depletion of clathrin-coated pits from TGN/endosomes or the plasmalemma, respectively. Why then do AP-1/σ1B-KO mice accumulate clathrin-coated pits on endosome-like vacuoles? One possibility is that other σ1 isoproteins such as σ1A do a poor job in functionally replacing σ1B on endosomes. It is also possible that clathrin coats are formed by AP complexes other than AP-1 (Kim and Ryan, 2009). This question requires further study. Moreover, based on the observed SV depletion from resting terminals of σ1B-deficient neurons, an additional role for AP-1/σ1B in SV biogenesis at the TGN cannot be ruled out.
An equally important open point is the origin and identity of the endosomal vacuoles found in σ1B-deficient presynaptic terminals. The data suggest that they are derived from previously fused SV membranes and/or the plasma membrane. Similar vacuoles were seen at Drosophila neuromuscular synapses following acute inactivation of clathrin (Heerssen et al, 2008; Kasprowicz et al, 2008), a condition causing impaired neurotransmission and defective recycling of SVs upon intense stimulation. Compensatory upregulation of BE then causes massive accumulation of vacuolar membranes inside stimulated boutons, which, however, lack morphologically recognizable coats, in contrast to σ1B mutants (Glyvuk et al, 2010). One might thus assume that the vacuoles observed in σ1B-KO mice result from BE. Whether such invaginations have fused with bona fide endosomes or solely represent a patch of internalized plasmalemma needs to be determined.
The enigma remains: why do neurons require multiple routes for recycling of functional SVs? Neurons have to respond to a broad spectrum of stimulus frequencies, yet, remain neurotransmission-competent over extended periods of time. Although the crucial role of CME in the maintenance of SV pools is undisputed, it is clear that this pathway operates with limited kinetics and sorting capacity that would limit exo-endocytic vesicle cycling during intense stimulation. Non-specific bulk uptake of membrane in the vicinity of the release site may provide a kinetic relief from this bottleneck and allow neurons to maintain neurotransmission during high-level activity. However, BE may not come without a price. Bulk internalization of large membrane chunks will not suffice to maintain the morphological and biochemical characteristics of SVs. Thus, additional sorting steps downstream of the actual internalization event are needed and this may be precisely where AP-1/σ1B and, perhaps also AP-3, kick in. A potential path for future studies thus pertains to the question of whether σ1B-KO mice show defects in sorting of specific SV components and how this may relate to the phenotype in mice (Glyvuk et al, 2010) and by extension in human patients suffering from this form of X-linked mental retardation (Tarpey et al, 2006).
Acknowledgments
Work in the authors' laboratory is supported by grants from DFG (SFB765/B4; SFB740/HA2686/4-1; Exc 257-Neurocure), the Schram Foundation, and the European Science Foundation (Euromembranes-SYNAPSE, HA2686/6-1).
Footnotes
The authors declare that they have no conflict of interest.
References
- Glyvuk N, Tsytsyura Y, Geumann C, D'Hooge R, Hüve J, Kratzke M, Baltes J, Böning D, Klingauf J, Schu P (2010) AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J 29: 1318–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen H, Fetter RD, Davis GW (2008) Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol 18: 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz J, Kuenen S, Miskiewicz K, Habets RL, Smitz L, Verstreken P (2008) Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J Cell Biol 182: 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA (2009) Synaptic vesicle recycling at CNS snapses without AP-2. J Neurosci 29: 3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P (2003) Cell biology of the presynaptic terminal. Annu Rev Neurosci 26: 701–728 [DOI] [PubMed] [Google Scholar]
- Ryan TA (2006) A pre-synaptic to-do list for coupling exocytosis to endocytosis. Curr Opin Cell Biol 18: 416–421 [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Stevens C, Teague J, Edkins S, O'Meara S, Avis T, Barthorpe S, Buck G, Butler A, Cole J, Dicks E, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jones D, Menzies A, Mironenko T, Perry J et al. (2006) Mutations in the gene encoding the sigma 2 subunit of the adaptor protein complex 1, AP1S2, cause X-linked mental retardation. Am J Hum Genet 79: 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW (2009) The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 323: 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]