Abstract
The solubilization of biological membranes by detergents has been used as a major method for the isolation and purification of membrane proteins and other constituents. Considerable interest in this field has resulted from the finding that different components can be solubilized selectively. Certain membrane constituents are incorporated into small micelles, whereas others remain in the so-called detergent-resistant membrane domains that are large enough to be separated by centrifugation. The detergent resistant fractions contain an elevated percentage of cholesterol, and thus its interaction with specific lipids and proteins may be key for membrane organization and regulation of cellular signaling events.
This report focuses on the solubilization process induced by the sucrose monoester of myristic acid, β-D-Fructofuranosyl-6-O-myristyl-α-D-glucopyranoside (MMS), a nonionic detergent. We studied the effect of the head group and the cholesterol content on the process. 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and Dioctadecyl dimethylammonium chloride (DODAC) vesicles were used, and the solubilization process was followed using Laurdan (6-Dodecanoyl-2-dimethylaminonaphthalene) Generalized Polarization (GP) measurements, carried out in the cuvette and in the 2-photon microscope.
Our results indicate that: (i) Localization of the MMS moieties in the lipid bilayer depend on the characteristics of the lipid polar head group and influence the solubilization process. (ii) Insertion of cholesterol molecules into the lipid bilayer protects it from solubilizaton and (iii) the microscopic mechanism of solubilization by MMS implies the decrease in size of the individual liposomes.
Keywords: POPC, DODAC, vesicles, liposomes, sucrose ester, solubilization, cholesterol
1. Introduction
Solubilization of membrane components from synthetic and biological membranes by detergents has long been used to understand the nature and properties of different bilayers. The use of micelle-forming surfactants is one of the preferred methods for isolation of specific membrane constituents (Kragh-Hansen et al. 1998; Yakushi et al. 2004), and several studies on lipid-detergent interactions have been published which use either liposomes mimicking biological systems or actual biological systems (Lichtenberg 1985; Inoue et al. 1988; de la Maza and Parra 1993a; Kragh-Hansen et al. 1998; Lopez et al. 2000a; Lopez et al. 2000b; Lopez et al. 2001a; Cocera et al. 2002; Lopez et al. 2002; Deo et al. 2005; Lichtenberg et al. 2005).
Detergents such as Triton X-100 are used to isolate membrane structures called DRMs (detergent resistant membranes), mainly constituted by sphingomyelin and cholesterol. Since the DRMs were isolated from natural membranes, researchers have looked for these structures in vivo. This putative cholesterol-rich domains (rafts) are conceived as small (10– 200 nm), heterogeneous and highly dynamic structures (Pike 2006). Cell membranes may contain several distinct raft sub-types with distinct morphology, size, density and molecular composition. Rafts properties, existence and relationship with the isolated structures DMRs are still controversial issues and have been reviewed in several articles (Zidovetzki and Levitan 2007; Pike 2005; Munro 2003). Lipid plasma membrane composition and organization may be involved in several cellular processes including complex cell signaling pathways (Brown and Rose 1992; Lichtenberg et al. 2005) and changes in cholesterol content have been proposed as a possible tuning key in this mechanisms, therefore as a membrane stabilizer (Chern et al. 2006). Detailed studies on the interaction of detergents with membranes containing cholesterol are needed to understand all the issues previously described.
Sucrose esters correspond to a class of nonionic detergents that are non-toxic, skin compatible, non-polluting and biodegradable. Also, their synthesis is not dependent on the petrochemical industry, and they can be prepared from renewable sources. In addition, the surface activity described for commercial mixtures of sucrose esters is similar to that of alkyl glucosides, a family of detergents currently employed in membrane solubilization studies (Jackson et al. 1982; Lopez et al. 2000c; Lopez et al. 2001; Granizo et al. 2004). Consequently, sucrose esters may be valuable candidates to replace and/or improve the use of alkyl glucosides in membranes studies.
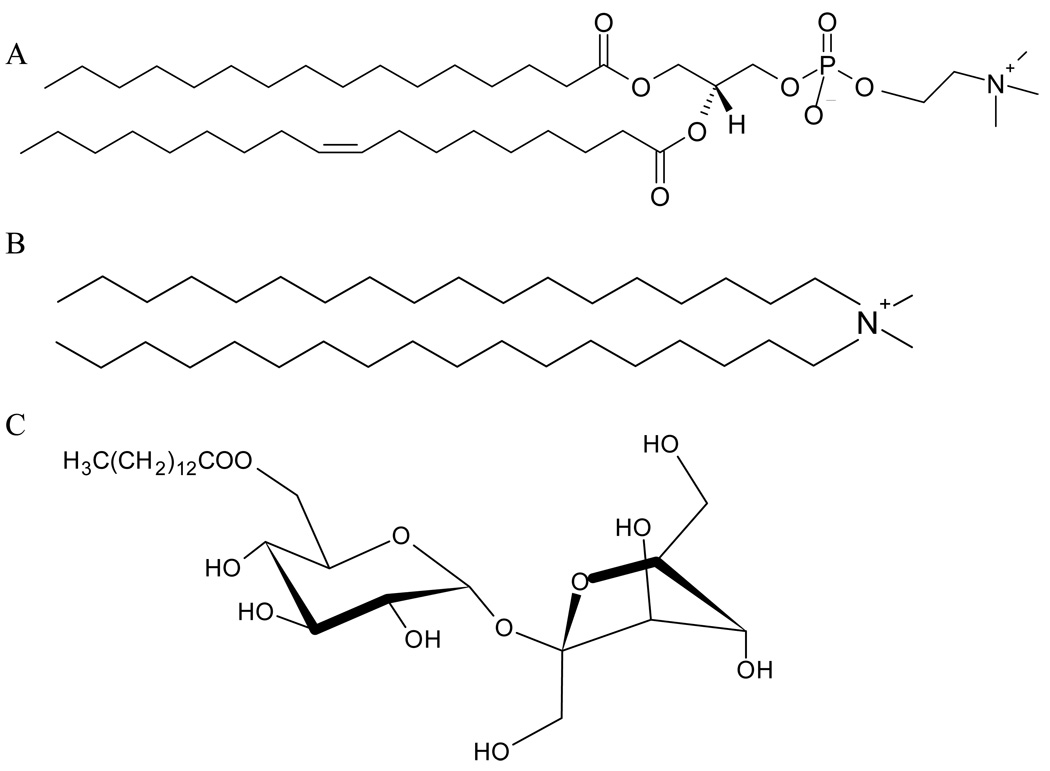
The primary objective of this work was to study the solubilization process induced by the nonionic detergent β-D-Fructofuranosyl-6-O-myristyl-α-D-glucopyranoside (MMS). We studied the effect of the head group of the lipid forming the bilayer being solubilized, and the effect of inserting cholesterol in the bilayer. Two lipids were used: Palmitoyl-2-oleoyl-sn-glycero-3- phosphocholine (POPC) and Dioctadecyl-dimethyl-ammonium chloride (DODAC). POPC is a well known phospholipid able to form fluid zwitterionic vesicles at ambient temperature (Tm = − 2.5°C) (Koynova and Caffrey 1998). DODAC, a synthetic surfactant, able to form cationic vesicles possesses a higher transition temperature (Tm = 39°C) and a small dimethyl ammonium head (Carmona-Ribeiro and Chaimovich 1986; Lissi et al. 1992; Nascimento et al. 1998; Lemp et al. 2003). Figure 1 shows the molecular structures of the compounds employed in this study, POPC, DODAC and MMS. For the cholesterol studies, different ratios of cholesterol:lipid (POPC or DODAC) were used to fabricate either SUVs (small unilamellar vesicles) or GUVs (giant unilamellar vesicles) for spectroscopic or microscopic studies respectively. Fluorescence spectroscopy (emission spectra) and two-photon fluorescence microscopy were used with Laurdan as a fluorescent probe. Laurdan, is currently used to determine changes in water content in the bilayer as a measurement of the membrane’s fluidity, which can then be extrapolated to determine the polarity of the membrane’s microenvironments (Parasassi et al. 1998; Harris et al. 2002).
Figure 1.
Molecular structure of POPC (A), DODAC (B) and MMS (C).
Our data show that the insertion of the MMS molecules into POPC or DODAC bilayers is different, and that the solubilization process is affected accordingly. The location of cholesterol in the bilayer depends on the nature of the lipid forming the bilayer, and consequently the solubilization process is also affected by cholesterol. Our data also give information about the microscopic events underlying the solubilization process.
2. Materials and methods
2.1. Materials
The sucrose monoester, β-D-Fructofuranosyl-6-O-myristyl-α-D-glucopyranoside (MMS), was synthesized by a modification of the Osipow-Snell method (Osipow et al. 1956; York et al.1956) that yields a relatively complex mixture of mono- (mainly 6-O and presumably 1-O), di and tri-esters. To isolate the monoester, a chromatographic procedure previously described was employed (Becerra et al. 2006) The NMR spectra, obtained on a Bruker ADX 300 spectrometer, with DMSOd-6 containing 5% of CH3OD to avoid micellization, are in good agreement with previously reported spectra of monoesters (Vlahov et al. 1997). 1-Palmitoyl-2- oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol (Chol) from Avanti Polar Lipids (Alabaster, AL) and Laurdan, from Molecular Probes Invitrogen (Carlsbad, CA) were employed without further purification. Dioctadecyl-dimethyl-ammonium chloride (DODAC), from HergaInd. (Brazil) was purified as described elsewhere (Encinas et al. 1989). The clear solution was filtered through a 0.45 µm nylon membrane, stored at 5°C and used within 1–2 weeks. All solvents employed were HPLC quality from Merck. Water was treated with Milli-Q equipment from Waters Corporation (Milford, MA).
2.2. Liposome preparation
Small unilamellar liposomes (SUVs) were obtained by ultrasonication of 10mM POPC or DODAC suspensions in buffer or Milli-Q water, respectively, with a Cole Parmer Ultrasonic Homogenizer. SUVs with different cholesterol concentrations were obtained by mixing POPC or DODAC with cholesterol in chloroform solution, removing organic solvent under a N2 stream, and, after buffer (or water) addition, sonicating the sample until the dispersion became clear. The required sucrose ester concentration was achieved by adding small aliquots of concentrated MMS solutions in DMSO. As previously reported, mixtures did not show spectral differences after equilibrating for 24–48 hours (Becerra et al. 2006). For giant unilamellar liposomes (GUVs), phospholipid stock solutions were prepared in chloroform at concentrations of 0.2 mg/ml. GUVs with POPC (or DODAC) alone were formed as well as GUVs made form binary mixtures POPC/Chol (or DODAC/Chol) at several molar ratios. The electroformation method developed by Angelova and Dimitrov (Angelova and Dimitrov 1986), with further modifications as described elsewhere (Sanchez et al. 2002), was used to prepare the giant vesicles. After GUVs were formed the temperature was adjusted to the desired value. The temperature was measured inside the vesicle formation chamber at theplatinum wires, using a digital thermometer (model 400B, Omega, Stamford, CT) with a precision of 0.1°C. Experiments were carried out in the same chamber with an inverted fluorescence microscope (Axiovert 35, Zeiss, Thornwood, NY). Using this method, vesicles remained attached to the platinum wires, which allowed us to perform the particular experiments on single GUV without vesicle drifting. A charge-coupled device video camera (CCD-Iris, Sony, Tokyo, Japan) was used to follow GUV formation and to select the target vesicle. The fluorescent probe was added to the system once the GUVs were formed.
2.3. Fluorescence spectroscopy
Steady state fluorescence measurements of Laurdan were accomplished in a Fluorolog Tau-2 spectrofluorometer (SPEX, Jobin Ybon) at 25.0 ± 0.5°C. The values of Generalized Polarization of Laurdan (3 µM, λex = 364nm, λem = 440nm and 490nm), were determined by using the expression of Parasassi et al.(Parasassi et al. 1991; Parasassi et al. 1998), Eq (1):
| (1) |
2.4. Two-photon intensity images
Images were collected on a scanning two-photon fluorescence microscope designed in the Laboratory for Fluorescence Dynamics (So et al. 1995). A LD-Achroplan 20× long working distance air objective with a N.A. of 0.4 (Zeiss, Holmdale, NJ) was used. A mode-locked titanium-sapphire laser (Mira 900, Coherent, Palo Alto, CA) pumped by a frequency-doubled Nd:vanadate laser (Verdi, Coherent) set to 780 nm, was used as the two-photon excitation light source. A galvanometer-driven x-y scanner (Cambridge Technology, Watertown, MA) was positioned in the excitation path to achieve beam scanning in both the x and y directions. The sample received from 5 to 9 mW of 780 nm excitation light, and a frame rate of 9s/frame was used to acquire the 256 × 256 pixel images. The fluorescence emission was observed through a broad band-pass filter from 350 nm to 600 nm (BG39 filter, Chroma Technology, Brattleboro, VT). A miniature photomultiplier (R5600-P, Hamamatsu, Bridgewater, NJ) was used for light detection in the photon counting mode. A two-channel detection system was attached for generalized polarization (GP, defined above) image collection.
2.5. Determination of the effective molar ratio of detergent to lipid in the bilayers (Re) and the partitioning coefficient (K)
The equilibrium partition model (Lichtenberg 1985; Almog et al. 1990) predicts a coefficient (K) for the distribution of a surfactant between the lipid bilayer and the aqueous media. In a vesicular solution this parameter is defined as:
| (2) |
where CB and CW are the millimolar surfactant concentrations in the lipid and aqueous pseudo phases, respectively and L is the lipid concentration (DODAC or POPC in our experiments).
When the parameter Re is defined as the effective molar ratio of surfactant to lipid in the bilayers (CB/L), Eq. 2 can be rewritten as:
| (3) |
The process of liposome solubilization can be characterized by two critical parameters, Csat and Csol. The first, Csat, is the surfactant concentration necessary to saturate the liposome bilayer, and Csol is the concentration needed to achieve a complete solubilization of the bilayer into mixed micelles. According to previous studies (Paternostre et al. 1988; Almog et al. 1990; de la Maza and Parra 1993b; Lopez et al. 2001b) both critical concentrations exhibit a linear dependence on the phospholipid concentration, as stated by Eq. 4.
| (4) |
where, Ct is the total surfactant concentration at each breakpoint, and the superindexes indicate the different observed critical points for POPC and DODAC vesicles. This relation allows determination of the effective surfactant to lipid molar ratios for surfactant-saturated vesicles (Re sat) and for lipid saturated mixed-micelles (Re sol) as well as the concentration of aqueous monomeric surfactant (Cw) coexisting with the aggregates.
3. Results
3.1. Effect of the radius of curvature of liposome on the bilayer properties
The radius of curvature of a liposome may affect properties of the membrane such as its permeability and molecular order. In this study we used two types of lipid systems: small unilamellar vesicles (SUVs) and giant unilamellar vesicles (GUVs). Since the two systems show a large difference in size (around 50 nm and 10–100 µm respectively) and therefore in radius of curvature, we designed the experiments to show that these systems can indeed be compared in our measurements. GP values for SUVs are measured by classical (cuvette) fluorescence spectroscopy while GUVs are analyzed on the two-photon excitation fluorescence microscope. GP values of SUVs and GUVs made of POPC containing different amounts of cholesterol were also compared. From 0 to 30% cholesterol no significant differences were found for the GP value in both systems (when normalized against the GP of Laurdan in DMSO). Instead, over 30% cholesterol we detected differences that could be attributed to changes in the cholesterol distribution among the membrane, as previously reported for phospholipid artificial and biological bilayers (Nemeczt and Schroeder 1988; Thewalt and Bloom 1992; Williams et al. 2000). In order to avoid misinterpretations, in this study we restrict our experiments to cholesterol concentrations below 30%.
3.2. Solubilization of vesicles by MMS
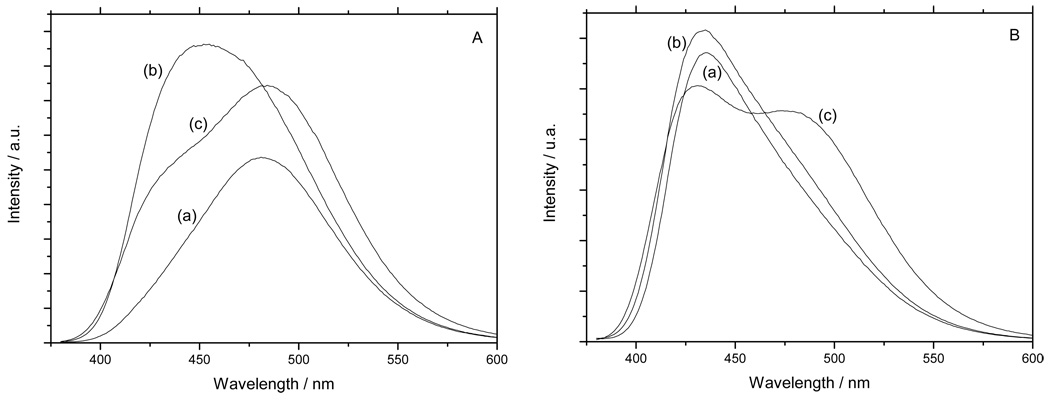
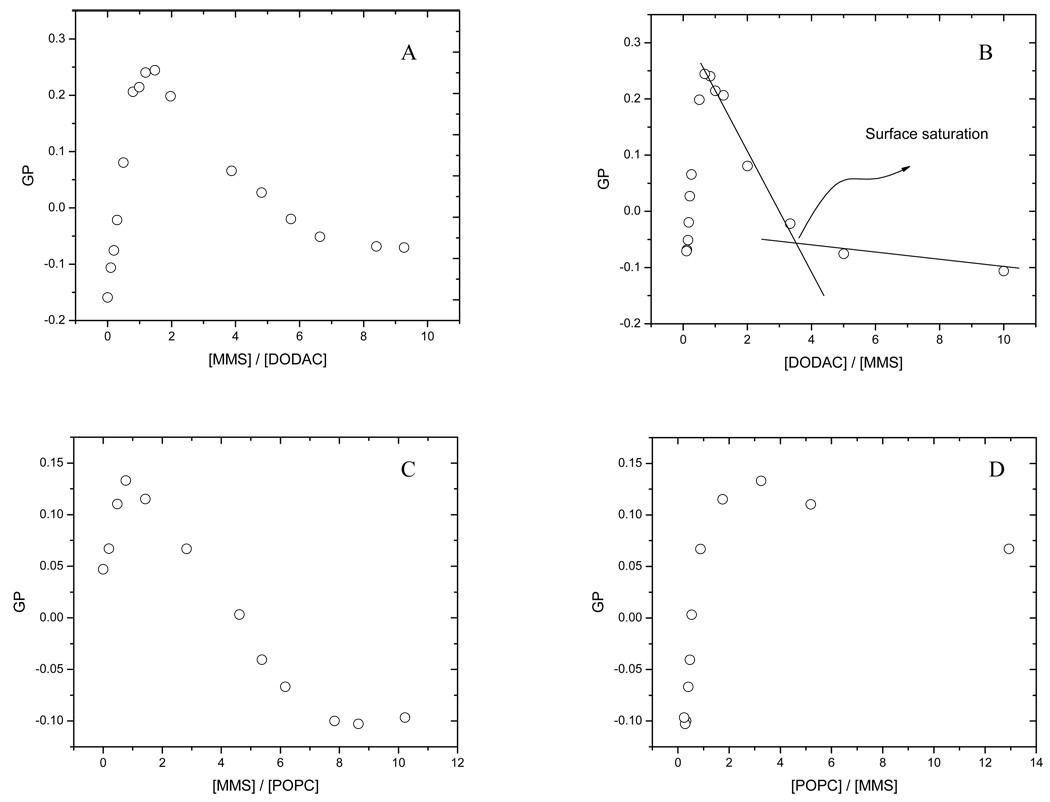
We followed the effect of the addition of MMS on DODAC and POPC vesicles by measuring Laurdan general polarization (GP). The GP parameter measures the shift of the Laurdan emission spectra due to changes in the water content of the membrane and has been previously used to follow solubilization of different liposomes by detergents (Heerklotz et al. 1994; Paternostre et al. 1995; Becerra et al. 2006). Figure 2 shows the spectral shift observed on the Laurdan emission when MMs is incorporated into the DODAC and POPC bilayers. The plot of the GP values versus MMS concentration corresponds to the solubilization profile for the system, and the one for DODAC vesicles is shown in. Figure 3A. At low MMS concentrations an increase in GP is observed, until a maximum value of 0.25 at a ratio detergent/lipid of 1.2. We defined this initial GP change as ΔGPsat and it has an average value near 0.4 GP units in this case. After this maximum, the GP value decreased until reaching a stable value of 0.1 GP units at a ratio detergent/lipid equals 9.5. In Figure 3B the same data from Figure 3A have been plotted but the abscissa is the reciprocal (corresponding to the inverse of surfactant concentration). The presence of a breakpoint at DODAC/MMS ratio of 3.5 indicates the existence of an additional critical point before bilayer saturation is reached. This point corresponds to the parameter previously defined by us, as surface saturation (Becerra et al. 2006). The solubilization profile for POPC liposomes with MMS is shown in Figure 3C. The general shape of the solubilization profile remains similar to the one observed for DODAC, with and smaller ΔGPsat (in average 0.07 GP units). However, the reciprocal plot (Figure 3D) for POPC does not show the breakpoint corresponding to a surface saturation, suggesting a different position of surfactant in the bilayer surface (Figure 3D).
Figure 2.
Emission profile of laurdan from DODAC-15%Chol (A) and POPC- 20%Chol (B): pure vesicles (a), vesicles saturated with MMS (b) and vesicles near the critical point of solubilization with MMS (c).
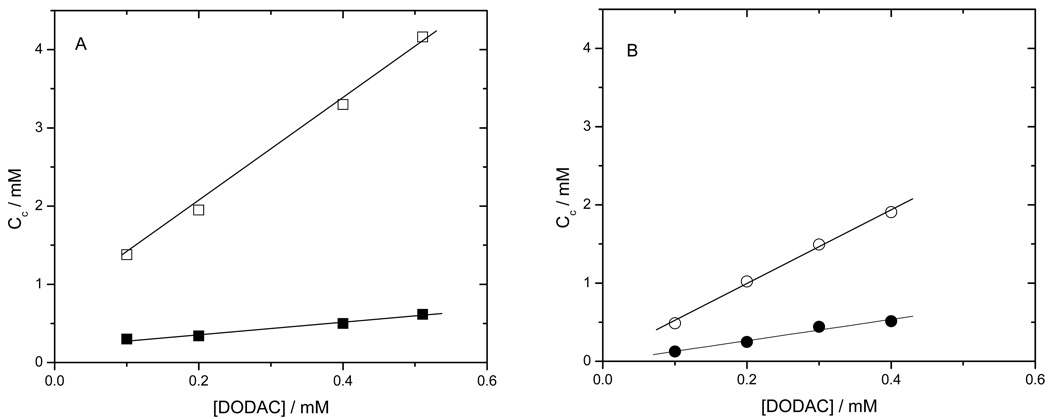
Figure 3.
Solubilization profiles of: DODAC 0.4mM vesicles plotted against MMS DODAC ratio (A) and against its reciprocal (DODAC vs MMS) (B), in this plot the surface saturation point can be observed. And POPC 0.4mM liposomes plotted against MMS concentration (C) and against its reciprocal (D).
3.3. Effect of Cholesterol on the solubilization parameters
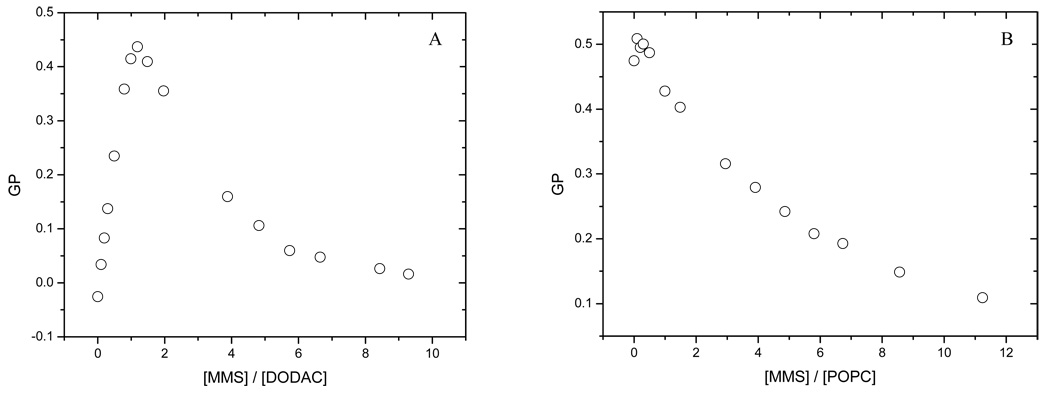
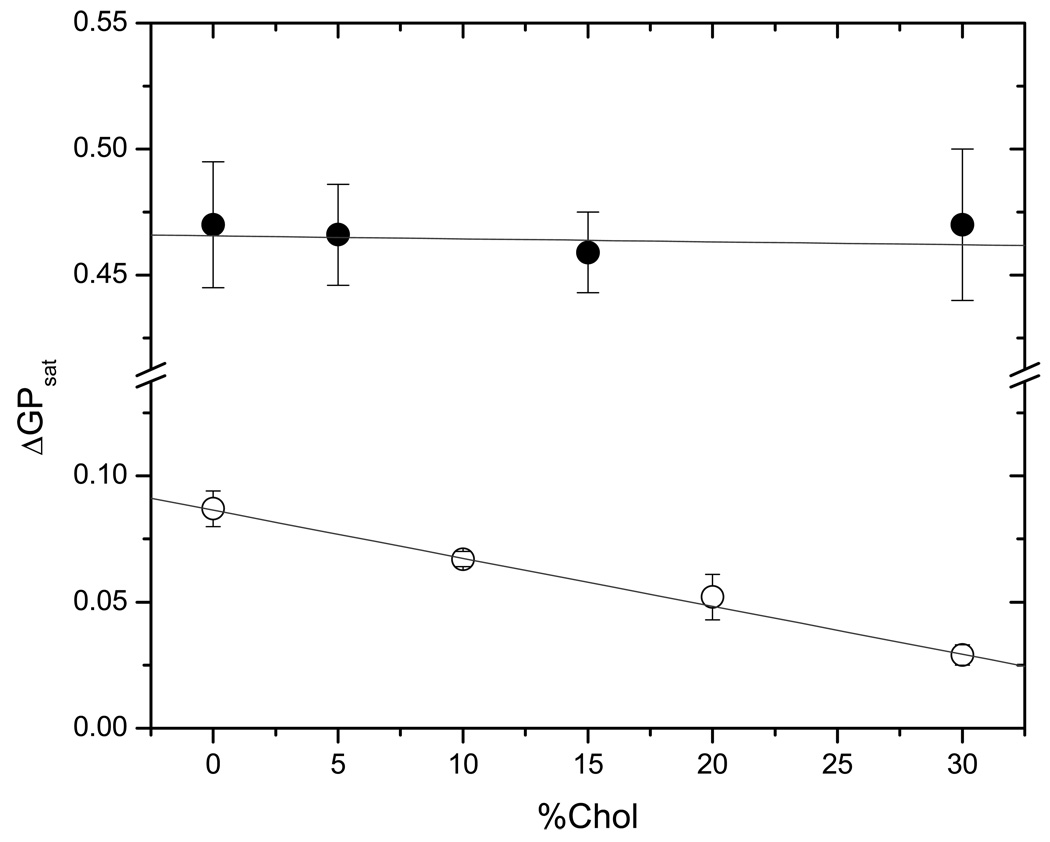
The effect of cholesterol on the solubilization process of POPC and DODAC by MMS was determined by varying the ratio POPC (DODAC):cholesterol in the mixtures. Figure 4 shows the solubilization profile for the two systems containing 30% cholesterol (maximum percentage used in this work). For DODAC containing 30% cholesterol (Figure 4A), the shape of the solubilization profile does not change significantly compared to the profile in absence of it (Figure 3A) with an average value for ΔGPsat = 0.45 GP units. However, for POPC, even if the general shape of the solubilization profile does not change with respect to the profile for pure POPC (Figure 3B), the ΔGPsat parameter has a much smaller value, 0.03 GP units (Figure 4B). The shape of the solubilization profiles for DODAC and POPC at intermediate cholesterol concentrations remains similar to the one shown in Figures 3 and 4, indicating that these systems also follow the model of the three stages (Helenius and Simons 1975; Lichtenberg 1985). The effect of increasing cholesterol concentration on the ΔGPsat for POPC and DODAC is shown in Figure 5. While ΔGPsat is nearly independent of the amount of cholesterol present in DODAC SUVs, there is a small, but consistent decrease on the value of ΔGPsat as cholesterol increases in POPC vesicles. Tables 1 and 2 show the effect of cholesterol on the critical parameters for the solubilization process of DODAC (Table 1) and POPC (Table 2), by MMS. The critical parameters were determined using Eq. 4, and the linear fit of experimental data can be seen in Figure 6. These critical parameters correspond to the minimum molar concentration of surfactant needed to saturate (Resat) or solubilize (Resol) one mol of lipid forming vesicles and are useful to establish the effect of the presence of cholesterol on the capability of the surfactant to incorporate into the bilayer. Low values for Resol are explained in terms of the formation of mixed micelles, poor in surfactant but with a lipid high content, characteristic of a very efficient solubilizing agent. On the other hand, less efficient solubilizing agents will show high Resol. The K constant is related to the affinity of the surfactant for the aqueous and lipid phases. This constant can be calculated from the previously determined Re values (using Eq. 3). The values obtained for the partition coefficients show the same trend discussed for the effective detergent to lipid molar ratio.
Figure 4.
Solubilization profile of DODAC (A) and POPC (B) small unilamellar vesicles loaded with 30% of Cholesterol.
Figure 5.
Dependence of ΔGPsat, (defined in the main text), against percentage of cholesterol present in the bilayer, for POPC (○) and DODAC (●) vesicles. Intersections correspond to Ctss.
Table 1.
Parameters obtained for the solubilization of DODAC SUVs at 25°C with MMS. The values were determined at each critical point of the process and in the presence of increasing amounts of cholesterol. (n.d. not determined)
|
~% mol cholesterol |
Surface Saturation | Saturation | Solubilization | |||
|---|---|---|---|---|---|---|
| Re | K (mM−1) | Re | K (mM−1) | Re | K (mM−1) | |
| 0 | 0.22 ± 0.05 | 4.5 | 1 12 ± 0.07 | 13 | 6.68 ± 0.50 | 22 |
| 5 | 0.29 ± 0.06 | 5.6 | 0.92 ± 0.2 | 11 | 6.93 ± 1.10 | 22 |
| 15 | 0.45 ± 0.03 | 7.8 | 1 36 ± 0.25 | 14 | 4.74 ± 0.31 | 21 |
| 30 | 0.31 ± 105 | 5.9 | 0.93 ± 0.04 | 12 | n.d. | n.d. |
Table 2.
Parameters obtained for the solubilization of POPC SUVs at 25°C with MMS. The values were determined at each critical point of the process and in the presence of increasing amounts of cholesterol. (n.d. not determined)
|
~% mol cholesterol |
Saturation | Solubilization | ||
|---|---|---|---|---|
| Re | K (mM−1) | Re | K (mM−1) | |
| 0 | 0.46 ± 0.09 | 79 | 6.48 ± 0.68 | 22 |
| 10 | 0.54 ± 0.10 | 88 | 8.14 ± 0.77 | 22 |
| 20 | 0.65 ± 0.11 | 98 | 4.44 ± 0.37 | 20 |
| 30 | n.d. | n.d. | 6.04 ± 0.45 | 21 |
Figure 6.
Plot of solubilization and saturation critical concentrations (Ct sol (○,□) and Ct sat (●,□)) against DODAC concentration with MMS employed as surfactant. For (A) pure DODAC vesicles and (B) DODAC-15%Chol vesicles.
Some of the critical parameters are not reported in Tables 1 and 2 for technical problems: for POPC plus 30% cholesterol, the small ΔGPsat observed made the determination of the corresponding critical parameter inaccurate. For DODAC plus 30% cholesterol, the critical parameters were not determined because the concentrations of MMS for complete solubilization of bilayers could not be reached.
3.4. Microscopic observation of vesicles solubilization process
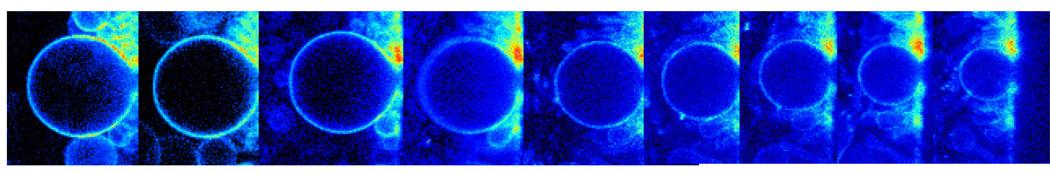
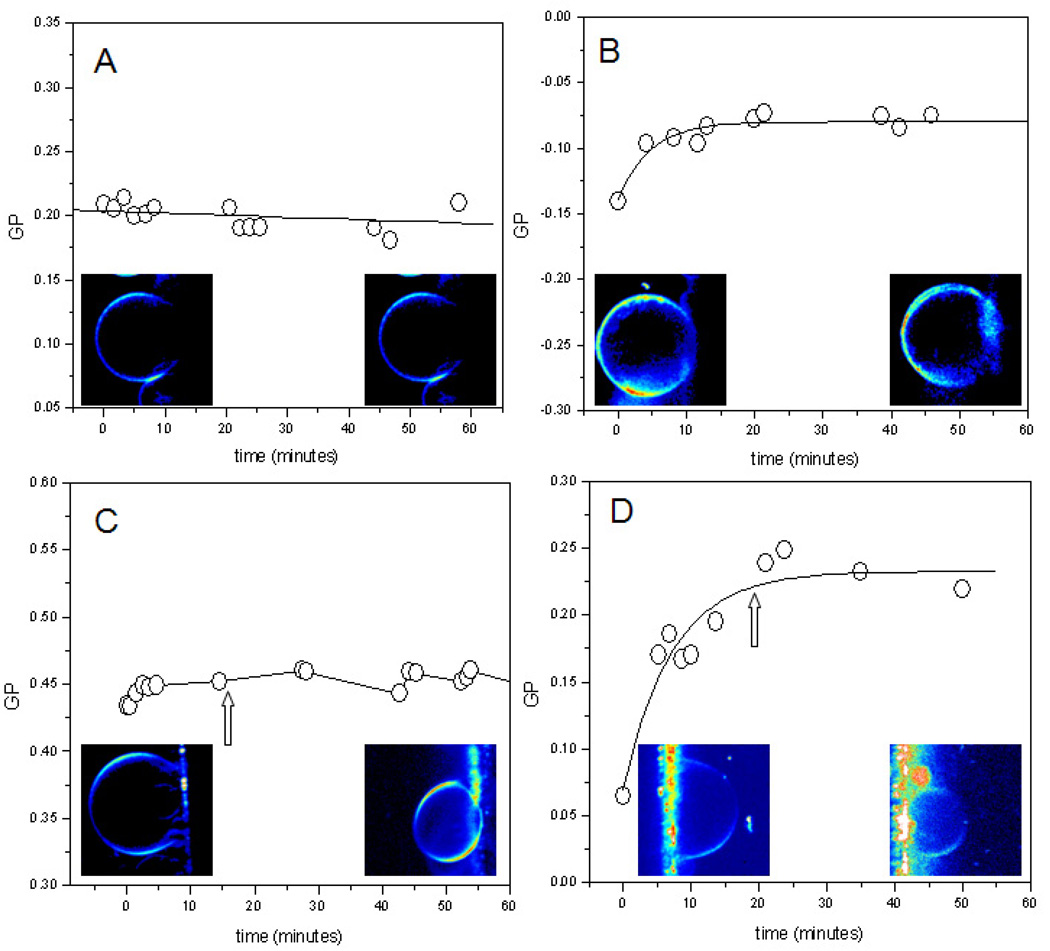
The solubilization process of POPC and DODAC by MMS, using GUVs and Laurdan GP measurements in the 2-photon excitation microscope was also studied. This approach enabled us to obtain information about the changes occurring on individual liposomes during the process of solubilization. The spatial resolution of the microscopy technique allow differentiation of the GP changes coming from the GUV that is being solubilized, from those coming from the mixed micelles produced during the solubilization process, information that is not possible to obtain from cuvette measurements, where an average GP value from all the labeled structures is registered. As a general observation, the solubilization by MMS of the two systems (POPC and DODAC) with or without cholesterol produces a decrease in the overall volume of the GUV and as the solubilization proceeds in time. Figure 7 shows the changes in volume produced on a POPC GUV after the addition of 1µM MMS. The first image (time = 0) shows the equatorial view of a GUV (target GUV) of approximately 40 µm attached to the wire. After the addition of MMS, one can observe a continuous decrease in size of the target vesicle that almost disappears after 40 minutes. Simultaneously, the material in the background of the image increases as long as Laurdan is solubilized together with the POPC. This material in the image background corresponds to the small micelles leaving the target GUV and other GUVs in the experimental chamber. We added MMS to GUVs made of pure lipids (POPC and DODAC) and containing cholesterol. The concentration of MMS required to obtain a similar change in volume is smaller for GUVs made of pure compounds than that needed for GUVs containing cholesterol (data not shown). For GUVs of pure POPC (Figure 8A) and DODAC (Figure 8B), a concentration of0.6 mg/ml of MMS was used. The size of the GUVs does not change, and the GP values after the addition of MMS give information about the bilayer saturation process. In agreement with cuvette experiments, bilayer saturation of POPC GUVs induce insignificant change in the GP value of the target vesicle (Figure 8A), instead saturation of the DODAC bilayer induces, in all the cases studied, an increase of the GP value (Figure 8B). This observation shows that the insertion of the MMS molecules into the two studied bilayers is different. For GUVs containing cholesterol, higher concentrations of MMS were used (12.0 mg/mL). Figures 8C and 8D show the changes in the GP value for POPC and DODAC vesicles containing 30% and 26% cholesterol respectively. In both cases a decrease in the overall size was observed after the addition of MMS (arrow in Figures 8C and 8D shows the beginning of size decrease), indicating that the solubilization process has begun. Before solubilization started, the observed behavior was similar to that observed at low concentration of MMS: a minor change (decrease/increase) in the GP value for POPC (Figure 8C), and an increase in GP value for DODAC.
Figure 7.
Intensity image of POPC GUV after addition of MMS (1µM final concentration), showing the change in size during solubilization process.
Figure 8.
Solubilization process of: A POPC GUVs with 0.6 µg/mL of MMS; B DODAC GUVs with 0.6 µg/mL of MMS; C POPC GUVs with 30% of Cholesterol and 12.0 µg/mL of MMS and D DODAC GUVs with 26% of Cholesterol and 12.0 µg/mL of MMS
4. Discussion
This manuscript address several interrelated issues: (i) the effect of the head group of liposomal lipids (comparing DODAC to POPC) on the solubilization of the liposomes by the surfactant MMS, (ii) the effect of cholesterol on the solubilization process by MMS (i.e. micellization of the phospholipids and cholesterol) and (iii) the mechanism of solubilization of GUVs, as observed by time-dependent two-photon excitation microsocopy.
In this study the fluorescent dye Laurdan, was used as a reporter of the water content in the lipid bilayer, and two different methodologies were utilized: spectroscopic measurements of SUVs in cuvette and microscopic measurements of GUVs in a two-photon microscope. The first approach allows us to study the physicochemical aspects of the process and the second gives information about the liposome solubilization process at the single liposome level. The results obtained with both methodologies are in full agreement reporting the saturation stage (both report the GP values when there are no changes in size) and they are complementary for the solubilization stage (spectroscopy measurements report the GP values from the liposome and micelles and microscopy can separate the two contributions). Thus our results indicate that the combination of these two methodologies can be used in solubilization studies and give valuable information about the process.
Effect of the head group of liposomal lipids on the solubilization by the surfactant MMS
The solubilization profile of DODAC vesicles with MMS (Figure 3A) is in agreement with the general interpretation reported before (Becerra et al. 2006) and with the three stage model proposed to describe the solubilization of liposomes in the presence of amphiphillic compounds (Helenius and Simons 1975; Jackson et al. 1982; Lichtenberg 1985): the initial increase in GP (ΔGPsat) originates from the incorporation of surfactant into the bilayer with the concomitant increase in packing density, and the presence of the MMS sucrose groups on the surface of the bilayer blocks the water access to the areas surrounding the LAURDAN molecules. A limit is reached when the membrane is saturated by the detergent. The decrease in GP values after the maximum is attained is attributed to the rupture of bilayers and mixed micelles formation, and finally, the plateau value corresponds to complete solubilization of the bilayers into mixed micelles (solubilization point Resol). In the case of pure DODAC vesicles, ΔGPsat of 0.4 GP units upon MMS incorporation (Figure 3A) reflects an important blocking of water access into the bilayer. The change in slope observed in Figure 3B and reported as the surface saturation point, Ress, in Table 1, has been attributed to the reorientation of sucrose moieties (from a parallel to a perpendicular position relative to the vesicle surface) on the vesicle surface before bilayer saturation (Becerra et al. 2006), A similar change in slope before saturation was reported for egg phosphatidylcholine using octyl glucoside, and was ascribed to the opening of the vesicles (Ollivon et al. 1988). For our system, the microscopy experiments show that during saturation the vesicles remain intact and when solubilization starts the vesicles decrease in size and mixed micelles are released until the liposome disappears without being bilayer opening a main mechanism in the whole process (Figure 7). When the behavior just described is compared with the solubilization profile for pure POPC with MMS (Figure 3C), clearly two differences can be observed (comparison of Figure 3A and Figure 3C): (i) ΔGPsat for POPC (0.07 GP units) is smaller than ΔGPsat for DODAC (0.45 GP units) and (ii) the change of slope before the saturation observed in the case DODAC (Figure 3B) is not observed in POPC (Figure 3D). Both differences could be indicative of a different location of surfactant in the surface of vesicle. Comparing the solubilization parameters for the pure compounds (Table 1 for DODAC and 2 for POPC) it is clear that more MMS is needed to saturate the DODAC bilayer (Resat comparison DODAC = 1.12 and POPC = 0.46) but the detergent is equally effective to solubilize both of them (Resol comparison (DODAC = 6.68 and POPC = 6.48). These results indicate that the insertion of the surfactant in the two lipid bilayers may be different.
Effect of cholesterol on the solubilization process by MMS
For DODAC vesicles the presence of cholesterol does not affect the values for the different effective molar ratios (Table 1) with the exception of vesicles with 15% cholesterol, where it seems to be a ‘local’ discontinuity and all the critical parameters show a different behavior. For POPC liposomes the results obtained in the presence of cholesterol are different. The saturation parameter (Re sat) shows a continuous increase as the content of cholesterol increases, indicating that in order to achieve membrane saturation in the presence of cholesterol, higher concentrations of MMS are needed. This behavior has been previously described as a cholesterol protecting effect (Chern et al. 2006). On the other hand, Resol shows a singularity at 10 and 20% cholesterol (Table 2), indicating a less favorable (Resol=8.14) but easier (Resol =4.44) solubilization process in the presence of 10 and 20 % cholesterol respectively. Dilatometric studies performed in DPPC-cholesterol bilayer show singularities at compositions ranging between 17.5 and 20.0% in mol of cholesterol (Melchior et al. 1980) that may be related with the behavior reported here for POPC-cholesterol.
Mechanism of solubilization of GUVs: time-dependent two-photon excitation microscopy
The results obtained using bi-photonic microscopy GP imaging show the solubilization process of individual GUVs. During the solubilization process the individual liposomes decrease in size (Figure 7 and 8) as the mixed micelles (detergent-lipid) are beingreleased, in agreement with the postulated mechanisms of mixed micelles formation. El Kiratand Morandat (El Kirat and Morandat 2007), using AFM interpreted the formation of holes on the supported bilayer after the addition of Triton X-100 to the removal of lipids from some areas of the bilayer. Our microscopy data using MMS also show that during the solubilization process, lipids are being removed from the bilayer. In our case, namely the unsupported GUVS systems, the removal of lipids (lipid-detergent micelles) from the bilayer is manifestedas a decrease in the GUV size.
The effect of cholesterol on the susceptibility of the membranes to detergents is a complex process dependent on several factors such as temperature, lipid composicion, lipid phase among others (Ahmed et al. 1997; Sot et al. 2002). Reports in the literature suggest a protecting role of cholesterol against solubilization by detergents (Deo and Somasundaran 2003; Schnitzer et al. 2005; El Kirat and Morandat 2007) and our data add information along the same lines. We observed that the concentration of MMS needed to solubilize (decrease in size) GUVs made of the pure compounds (POPC and DODAC) is smaller than the concentration needed to solubilize GUVs containing cholesterol. This last observation suggests that isolation of structures such as DRMs from membranes may occur via extraction of the solubilizable components by the detergent.
Our microscopy data indicate that GP of the target GUV does not change while solubilizing and the spectral data in cuvette show a decrease in GP during the solubilization stage. This apparent contradiction can be explained by considering the fact that in the images the GP values only come from the bilayer of a single GUV, however, in the cuvette the GP values correspond to mean values including a population of target vesicles and any other labeled structures (remaining bilayers and leaving mixed micelles). Thus, results obtained with both methodologies (Laurdan emission spectra and Laurdan two-photon imaging) are in full agreement reporting the saturation stage, and complementary for the solubilization stage.
5. Concluding remarks
Our data can be explained with the postulate that in the solubilization process by MMS, the size and topology of lipid heads have a determinant effect on the location of the sucrose moiety of the monoester during the saturation stage and further solubilization. DODAC molecules with a small cationic head (dimethyl ammonium group with two hydrocarbonated tails of 18 carbons) can form bilayers with an interface relatively permeable to water molecules, according to the GP values observed for DODAC vesicles. On these bilayers MMS will insert its tail between the DODAC molecules but the sucrose head will remains outside, covering the interface and therefore blocking water access. The presence of cholesterol seems to have a small influence on the incorporation of surfactant previous to the saturation and on the subsequent solubilization. Additionally the dependence of GPsat with cholesterol, Figure 5 indicates that the presence of cholesterol does not affect the access of water to the probe location. For POPC liposomes, the size of the polar head (phosphocholine) forces the sucrose moiety to locate in a different position in the bilayer. The cis unsaturations present in one of the acyl chains introduce kinks that force a looser packing and lower phase transition temperatures. When the MMS molecules insert in the POPC bilayer, they can now penetrate deeper and the sucrose moiety can be located in the plane of the phospholipid polar heads and not “over” the liposome surface. When cholesterol is present in the bilayer, the POPC kinks will limit the reported ability of cholesterol to order, to mix homogeneously, to reduce interfacial elasticity and to decrease in-plane elasticity (Needham et al. 1988; Rawicz et al.2000). Cholesterol will thus locate more superficially than in bilayers formed by lipids with saturated acyl chains such as DPPC or in this case by DODAC molecules (having also saturated hydrocarbon chains). Therefore, the presence of cholesterol will block the access of water to the probe location (decrease in the ΔGPsat values, Figure 5) and will interfere in the insertion of the MMS molecules in the case of POPC, where both, surfactant and cholesterol will compete for a similar location in the bilayer. However, the effect of the presence of sucrose moieties below the surface, between heads of phospholipidic bilayers, may not be so important. The microscopic view of the solubilization process by MMS revealed common issues on the process: the solubilization process by MMS implies shrinking of the liposome while the mixed lipid-detergent micelles are being released, and when cholesterol is present in the bilayer, the concentration of MMS required for solubilization is larger that when pure compounds form the bilayer.
Acknowledgements
This work was supported by funds from Fondecyt 1040573 and 1080412. Also GG thanks DI (University of Chile). SS and EG acknowledge the Division of Research Resources of the National Institutes of Health (Grant PHS 5 P41 RR-03155).
References
- Ahmed SN, Brown DA, London E. On the Origin of Sphingolipid/Cholesterol Rich Detergent-Insoluble Cell Membranes: Physiological Concentrations of Cholesterol and Sphingolipid Induce Formation of a Detergent-Insoluble, Liquid-Ordered Lipid Phase in Model Membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- Almog S, Litman BJ, Wimley W, Cohen J, Wachtel EJ, Barenholz Y, Benshaul A, Lichtenberg D. States of Aggregation and Phase-Transformations in Mixtures of Phosphatidylcholine and Octyl Glucoside. Biochemistry. 1990;29:4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Angelova MI, Dimitrov DS. Liposome Electroformation. Faraday Discussion. Chem. Soc. 1986;81:303–311. [Google Scholar]
- Becerra N, de la Nuez LR, Zanocco AL, Lemp E, Gunther G. Solubilization of dodac small unilamellar vesicles by sucrose esters - A fluorescence study. Colloid Surface A. 2006;272:2–7. [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell-surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Carmona-Ribeiro AM, Chaimovich H. Salt-Induced aggregation and fusion of dioctadecyldimeethylammonium chloride and sodium dihexadecylphosphate vesicles. Biophys. J. 1986;50:621–628. doi: 10.1016/S0006-3495(86)83501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocera M, Lopez O, Coderch L, Parra JL, de la Maza A. Sublytic alterations caused by the nonionic surfactant dodecyl maltoside in stratum corneum lipid liposomes. Langmuir. 2002;18:297–300. [Google Scholar]
- Chern CS, Chiu HC, Yang YS. Interactions between nonionic Triton X surfactants and cholesterol-containing phosphatidylcholine liposomes. J. Colloid Interf. Sci. 2006;302:335–340. doi: 10.1016/j.jcis.2006.06.028. [DOI] [PubMed] [Google Scholar]
- de la Maza A, Parra JL. Disintegration Liposomes by Anionic Surfactants and Formation Mixed Micelles. Langmuir. 1993a;9:870–873. [Google Scholar]
- de la Maza A, Parra JL. Solubilization of phospholipid bilayer caused by surfactants. J. Am. Oil Chem. Soc. 1993b;70:699–706. [Google Scholar]
- Deo N, Somasundaran P. Disintegration of liposomes by surfactants: Mechanism of protein and cholesterol effects. Langmuir. 2003;19:2007–2012. [Google Scholar]
- Deo N, Somasundaran P, Itagaki Y. Mechanisms of solubilization of mixed liposomes: Preferential dissolution of liposome components. Ind. Eng. Chem. Res. 2005;44:1181–1186. [Google Scholar]
- El Kirat K, Morandat S. Cholesterol modulation of membrane resistance to Triton X- 100 explored by atomic force microscopy. Biochim. Biophys. Acta-Biomembr. 2007;1768:2300–2309. doi: 10.1016/j.bbamem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Encinas MV, Lemp E, Lissi EA. Vesicular Effect on the Reactivity of Anthracene- Derivatives Towards Singlet Molecular-Oxygen. J. Photochem. Photobiol. B Biology. 1989;3:113–122. [Google Scholar]
- Granizo N, Thunig C, Valiente M. The effect of octyl glucoside on the lamellar phase of diluted C12E4 and alcohol systems. J. Colloid Interf. Sci. 2004;273:638–644. doi: 10.1016/j.jcis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Harris FM, Best KB, Bell JD. Use of laurdan fluorescence intensity andpolarization to distinguish between changes in membrane fluidity and phospholipid order. BBA. 2002;1565:123–128. doi: 10.1016/s0005-2736(02)00514-x. [DOI] [PubMed] [Google Scholar]
- Heerklotz H, Binder H, Lantzsch G. Determination of the Partition Coefficients of the Nonionic Detergent C12E6 Between Lipid-Detergent Mixed Membranes and water by Means of Laurdan Fluorescence Spectroscopy. J. Fluoresc. 1994;4:319–352. doi: 10.1007/BF01881454. [DOI] [PubMed] [Google Scholar]
- Helenius A, Simons K. Solubilization of membranes by detergents. Biochim. Biophys. Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Inoue T, Muraoka Y, Fukushima K, Shimozawa R. Interaction of Surfactants with Vesicle Membrane of Dipalmitoylphosphatidylcholine - Fluorescence Depolarization Study. Chem. Phys. Lip. 1988;46:107–115. doi: 10.1016/0009-3084(88)90120-x. [DOI] [PubMed] [Google Scholar]
- Jackson ML, Schmidt CF, Lichtenberg D, Litman BJ, Albert AD. Solubilization of phosphatidilcholine by octyl glucoside. Biochemistry. 1982;21:4576–4582. doi: 10.1021/bi00262a010. [DOI] [PubMed] [Google Scholar]
- Koynova R, Caffrey M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta-Rev. Biomembr. 1998;1376:91–145. doi: 10.1016/s0304-4157(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U, le Maire M, Möller JV. The Mechanism of Detergent Solubilization of Liposomes and Protein-Containing Membranes. Biophys. J. 1998;75:2932. doi: 10.1016/S0006-3495(98)77735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemp E, Zanocco AL, Gunther G. Structural changes in DODAC unilamellar liposomes by addition of sucrose esters monitored by using fluorescent techniques. Colloid Surface A. 2003;229:63–73. [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. BBA. 1985;821:470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lissi EA, Abuin E, Saez M, Zanocco A, Disalvo A. Anomalous Dependence of Pyrene Spectra and Lifetimes with Temperature in Large Unilamellar Vesicles from Dioctadecyldimethylammonium Chloride and Dipalmitoylphosphatidylcholine. Langmuir. 1992;8:348–350. [Google Scholar]
- Lopez O, Walther P, Cocera M, de la Maza A, Coderch L, Parra JL. Structural modifications in the stratum corneum by effect of different solubilizing agents: A study based on high-resolution low-temperature scanning electron microscopy. Skin Pharmacol. App. Skin Phys. 2000a;13:265–272. doi: 10.1159/000029932. [DOI] [PubMed] [Google Scholar]
- Lopez O, Cocera M, Parra JL, de la Maza A. Solubilization of stratum corneum lipid liposomes by C-14-betain/sodium dodecyl sulfate mixtures. Influence of the level of ceramides in the solubilization process. Colloid Surface A. 2000b;162:131–140. doi: 10.1016/s0378-5173(99)00188-x. [DOI] [PubMed] [Google Scholar]
- Lopez O, Cocera M, Walther P, de la Maza A, Coderch L, Parra JL. Octyl glucoside as a tool to induce structural modifications in the stratum corneum. Colloid Surface A. 2000c;168:115–123. [Google Scholar]
- Lopez O, Cocera M, Parra JL, de la Maza A. Influence of the hydrophobic tail of alkyl glucosides on their ability to solubilize stratum corneum lipid liposomes. Colloid Polym. Sci. 2001a;279:909–915. [Google Scholar]
- Lopez O, Cocera M, Parra JL, de la Maza A. Influence of the alkyl chain length of alkyl glucosides on their ability to solubilize phosphatidylcholine liposomes. Colloid Surface A. 2001b;193:221–229. [Google Scholar]
- Lopez O, Cocera M, Lopez-Iglesias C, Walter P, Coderch L, Parra JL, de la Maza A. Reconstitution of liposomes inside the intercellular lipid domain of the stratum corneum. Langmuir. 2002;18:7002–7008. [Google Scholar]
- Melchior DL, Scavitto FJ, Steim JM. Dilatometry of Dipalmitoyllecithin- Cholesterol Bilayers. Biochemistry. 1980;19:4828–4834. doi: 10.1021/bi00562a018. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: ellusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Nascimento DB, Rapuano R, Lessa MM, Carmona-Ribeiro AM. Counterion effects on properties of cationic vesicles. Langmuir. 1998;14:7387–7391. [Google Scholar]
- Needham D, McIntosh TJ, Evans E. Thermomechanical and Transition Properties of Dimyristoylphosphatidylcholine Cholesterol Bilayers. Biochem. 1988;27:4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- Nemeczt G, Schroeder F. Time-Resolved Fluorescence Investigation Membrane Cholesterol Heterogeneity and Exchange. Biochemistry. 1988;27:7740–7749. doi: 10.1021/bi00420a024. [DOI] [PubMed] [Google Scholar]
- Ollivon M, Eidelman O, Blumenthal R, Walter A. Micelle-Vesicle Transition of Egg Phosphatidylcholine and Octyl Glucoside. Biochemistry. 1988;27:1695–1703. doi: 10.1021/bi00405a047. [DOI] [PubMed] [Google Scholar]
- Osipow L, Snell FD, York WC, Finchler A. Methods of preparation of fatty acid esters of sucrose Ind. Eng. Chem. 1956;48:1459. [Google Scholar]
- Parasassi T, De Stasio G, Ravagnan G, Rusch RM, Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of LAURDAN fluorescence. Biophys. J. 1991;60:179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T, Krasnowska EK, Bagatolli L, Gratton E. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998;8:365–373. [Google Scholar]
- Paternostre M, Meyer O, GrabielleMadelmont C, Lesieur S, Ghanam M, Ollivon M. Partition coefficient of a surfactant between aggregates and solution: Application to the micelle-vesicle transition of egg phosphatidylcholine and octyl beta-Dglucopyranoside. Biophys. J. 1995;69:2476–2488. doi: 10.1016/S0006-3495(95)80118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternostre MT, Roux M, Rigaud JL. Mechanisms of Membrane-Protein Insertion into Liposomes During Reconstitution Procedures Involving the Use of Detergents .1.Solubilization of Large Unilamellar Liposomes (Prepared by Reverse-Phase Evaporation) by Triton X-100, Octyl Glucoside, and Sodium Cholate. Biochemistry. 1988;27:2668–2677. doi: 10.1021/bi00408a006. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. BBA. 2005;1476:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the keystone symposium on lipids rafts and cell function. J. Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophys. J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SA, Bagatolli LA, Gratton E, Hazlett TL. A two-photon view of an enzyme at work: Crotalus atrox venom PLA(2) interaction with single-lipid and mixed-lipid giant unilamellar vesicles. Biophys. J. 2002;82:2232–2243. doi: 10.1016/S0006-3495(02)75569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer E, Kozlov MM, Lichtenberg D. The effect of cholesterol on the solubilization of phosphatidylcholine bilayers by the non-ionic surfactant Triton X-100. Chem. Phys. Lipids. 2005;135:69–82. doi: 10.1016/j.chemphyslip.2005.02.002. [DOI] [PubMed] [Google Scholar]
- So PTC, French T, Yu WM, Berland KM, Dong CY, Gratton E. Time resolved fluorescence microscopy using two photon excitation. Bioimaging. 1995;3:49–63. [Google Scholar]
- Sot J, Collado MI, Arrondo JLR, Alonso A, Goni FM. Triton X-100-resistant bilayers: Effect of lipid composition and relevance to the raft phenomenon. Langmuir. 2002;18:2828–2835. [Google Scholar]
- Thewalt JL, Bloom M. Phosphatidylcholine - Cholesterol Phase-Diagrams. Biophys.J. 1992;63:1176–1181. doi: 10.1016/S0006-3495(92)81681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov IR, Vlahova PI, Lindhart RJ. Regioselective synthesis of sucrose monoesters as surfactants. J. Carbohyd. Chem. 1997;16:1–10. [Google Scholar]
- Williams EE, Cooper JA, Stillwell W, Jenski LJ. The curvature and cholesterol content of phospholipid bilayers alter the transbilayer distribution of specific molecular species of phosphatidylethanolamine. Molec. Memb. Biol. 2000;17:157–164. doi: 10.1080/09687680050197383. [DOI] [PubMed] [Google Scholar]
- Yakushi T, Kojima M, Homma K. Isolation of Vibrio alginolyticus sodium-driven flagellar motor complex composed PomA and PomB solubilized by sucrose monocaprate. Microbiology-Sgm. 2004;150:911–920. doi: 10.1099/mic.0.26577-0. [DOI] [PubMed] [Google Scholar]
- York WC, Finchler A, Osipow L, Snell FD. Structural studies on sucrose monolaurate. J. Am. Oil Chem. Soc. 1956;33:424. [Google Scholar]
- Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. BBA. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]