Abstract
Although improved supportive care has reduced mortality associated with febrile neutropenia, it continues to cause chemotherapy limitations, morbidity, mortality, and cost among patients with cancer.
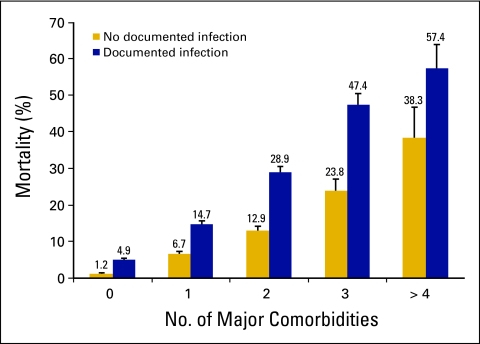
Fever in the setting of neutropenia should be considered a medical emergency requiring immediate evaluation and administration of empiric broad-spectrum antibiotics.1 Early studies demonstrated that patients with febrile neutropenia not promptly treated often experience a rapidly fatal outcome, most notably because of Gram-negative bacteremia.2 Recent studies of unselected patients with cancer with febrile neutropenia have reported rates of mortality of 5% to 20%, increasing in direct proportion to the number of major infectious complications and comorbid medical conditions (Fig 1).3,4 In fact, mortality in excess of 50% despite prompt antibiotic treatment has been reported in neutropenic patients presenting with septic shock or pneumonia and documented bacteremia.4–12 Although improved supportive care has reduced the mortality associated with febrile neutropenia, it remains a major cause of morbidity, mortality, and cost among patients with cancer and may limit the delivery of full-dose chemotherapy on schedule.4 The risk of febrile neutropenia increases in direct proportion to the severity and duration of neutropenia and occurs most frequently early in a course of chemotherapy.13 Early neutropenic events often prompt chemotherapy dose reductions or delays or the addition of prophylactic myeloid growth factors or antibiotics in subsequent treatment cycles.14,15 Optimal management of patients with cancer at risk for febrile neutropenia includes immediate evaluation, prompt initiation of broad-spectrum antibiotics, and careful monitoring in those with documented febrile neutropenia as well as the appropriate use of myeloid growth factor prophylaxis in patients at increased risk, consisting of primary prophylaxis starting in cycle 1 when risk is > 20% and an equivilent but less myelosuppressive regimen is not available or secondary prophylaxis in cycle after a febrile neutropenia event.1,16,17
Figure 1.
Risk of mortality among patients with cancer hospitalized with febrile neutropenia based on the reported number of major associated comorbidities and infectious complications, including Gram-negative or Gram-positive sepsis, invasive aspergillosis or candidiasis, pneumonia, hypotension, pulmonary embolism, or underlying diseases of the heart, lung, kidney, liver, or CNS. Data adapted.4
Clinical and Laboratory Evaluation
Patient evaluation should include a complete history and physical examination, with particular attention directed to the oropharynx/periodontium, sites of catheters and recent procedures, the perineum/perirectal area, nail beds, nares, and external auditory canals. Laboratory evaluation should include a complete blood cell count with differential, basic chemistries and liver function tests, and cultures from all potential sites, along with at least two sets of blood cultures from peripheral veins or one set each from a peripheral vein and a central venous catheter. Although a specific organism will not be found in half of patients with fever and neutropenia, a presumptive diagnosis of infection is important because of the risk of withholding antimicrobial therapy. Chest radiographs are not mandatory but should be obtained when clinical evidence of a respiratory infection is present. We recommend a computed tomography scan of the chest to detect signs of fungal infection in high-risk patients with unexplained fever who have not responded to 3 to 5 days of antibacterial therapy.18
Initial Antibiotic Therapy for Febrile Neutropenia
Currently, Gram-positive organisms are the predominant bacterial pathogens in this setting, with coagulase-negative staphylococci, Staphylococcus aureus, Enterococcus spp., and viridans group streptococci being isolated most often.11,19 Among Gram-negative bacilli, Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa remain the predominant species, although other Enterobacteriaceae, Stenotrophomonas maltophilia, and Acinetobacter spp. are isolated frequently.20,21 Approximately 10% to 15% of bacteremias are polymicrobial, with Gram-negative bacilli being isolated from more than 80% of these infections.22,23 Resistance to common antimicrobial agents is being encountered increasingly at most centers, in part because of heavy usage of these agents.24 Of particular concern, more than 50% of Staphylococcus aureus isolates are methicillin-resistant Staphylococcus aureus, and vancomycin resistance among enterococci constitutes nearly 30% of enterococcal isolates nationwide. Additionally, reduced susceptibility or resistance to penicillin is being reported increasingly with viridans group streptococci and in Streptococcus pneumoniae isolates.25,26 Invasive fungal infections are most often seen in patients with prolonged neutropenia and after stem-cell transplantation.27
Empiric, broad-spectrum antibiotic therapy should be administered promptly to all patients with febrile neutropenia.1 Patient risk should be quickly assessed to determine the number and spectrum of the antibiotic regimen (monotherapy or combination therapy), route of administration (parenteral or oral), and location of treatment (hospital v outpatient).28,29 Low-risk patients may be defined as ambulatory patients with good performance status and no serious comorbidity and anticipated short duration of severe neutropenia or those with an index score of 21 or more using the predictive model developed and validated by the Multinational Association for Supportive Care in Cancer (Table 1). The development of reasonably accurate risk prediction rules and the availability of suitable antimicrobial agents, ambulatory centers, and evidence of safety and efficacy have made the treatment of low-risk patients with febrile neutropenia in the ambulatory setting feasible.30,31 Such an approach requires appropriate infrastructure and a multidisciplinary team of health care providers. Patients managed in the ambulatory setting should be tracked for readmission, important complications, or mortality. Many clinicians prefer an initial 24- to 48-hour period of hospitalization for evaluation and initiation of empiric broad-spectrum antibiotics followed by outpatient treatment for the duration of the neutropenic episode.32,33
Table 1.
MASCC Scoring Index Identifying Low-Risk Febrile Neutropenic Patients
| Characteristic | Score |
|---|---|
| Burden of illness | |
| No or mild symptoms | 5 |
| Moderate symptoms | 3 |
| No hypotension | 5 |
| No chronic obstructive pulmonary disease | 4 |
| Solid tumor or no previous fungal infection | 4 |
| No dehydration | 3 |
| Outpatient at onset of fever | 3 |
| Age < 60 years | 2 |
NOTE. Score range, 0-26; risk score ≥ 21 indicates low-risk patient for medical complications and mortality.28
Abbreviation: MASCC, Multinational Association of Supportive Care in Cancer.
Although there is little to distinguish the many empiric regimens that have been studied in randomized controlled trials, patients at higher risk should be hospitalized immediately to receive parenteral antibiotic therapy.1 Monotherapy with an antipseudomonal beta-lactam, such as the extended spectrum cephalosporins or a carbapenem, is adequate for uncomplicated episodes. Combination regimens with a beta-lactam and either an aminoglycoside or a fluoroquinolone should be considered in the treatment of complicated infections, including those with pneumonia or neutropenic enterocolitis or in the setting of hypotension or shock. The myeloid growth factors should not be used routinely in the treatment of established febrile neutropenia, although they may be considered in patients at increased risk for serious complications including mortality.16,34
Patients who defervesce rapidly without identification of a specific organism should be continued on antibiotics through the period of neutrophil recovery.1 When a specific organism has been identified, patients should be treated for 7 to 14 days. Vancomycin should be considered with catheter-related infection, Gram-positive organisms on blood culture, hypotension, mucosal damage, or colonization with penicillin-resistant pneumococci or methicillin-resistant Staphylococcus aureus.1 For neutropenic patients with persistent fever after 3 to 5 days, empiric antifungal therapy may be considered.1
Prevention of Febrile Neutropenia
Strategies aimed at reducing the risk and consequences of febrile neutropenia should include appropriate antimicrobial stewardship, which significantly limits the emergence of resistant microorganisms while preserving the utility of available antimicrobial agents.35,36 Prophylactic antibiotics have demonstrated some efficacy in reducing the risk of febrile episodes in neutropenic patients with cancer; however, they have been associated with additional toxicity and the emergence of antibiotic-resistant bacteria.1,37 Although potentially efficacious in high-risk patients and in those who develop serious neutropenic complications despite hematopoietic growth factor support, the Infectious Disease Society of America and ASCO recommend against routine antibacterial prophylaxis.1,16
The myeloid growth factors administered prophylactically after cancer chemotherapy but before the onset of neutropenia have been shown to reduce the risk of febrile neutropenia and documented infection.38 A meta-analysis of randomized controlled trials of granulocyte colony-stimulating factor has also demonstrated a significant reduction in infection-related and early all-cause mortality while improving delivery of chemotherapy dose intensity.39 Current guidelines recommend primary prophylaxis with a myeloid growth factor in patients with cancer receiving chemotherapy associated with a 20% or greater risk of febrile neutropenia as well as in those with certain high-risk factors.16,17,40
Conclusion
Myelosuppression and its complications, including febrile neutropenia, continue to represent major dose-limiting toxicities associated with systemic cancer chemotherapy resulting in considerable morbidity, mortality, and costs. The management of febrile neutropenia requires immediate evaluation, risk assessment, and treatment with empiric broad-spectrum antibiotics. Efforts to reduce the risk of febrile neutropenia should be based on appropriate antimicrobial stewardship and the prophylactic use of myeloid growth factors where indicated.
Requirements for a Successful Program of Outpatient Therapy for Low-Risk* Neutropenic Patients With Fever
Dedicated team of health care providers (physicians, nurses, pharmacists, infusion therapists, midlevel providers) familiar with risk-based therapy
Adequate institutional infrastructure
Availability of real-time local epidemiologic and susceptibility/resistance data
Adequate monitoring and follow-up (eg, febrile neutropenia clinic)
Motivated, compliant patients and families and other caregivers (ie, careful patient selection)
Adequate transportation and communication for patients, and proximity (eg, 30-mile radius) to the cancer treatment center
Access 24 hours a day to management team (eg, hotline, emergency departments, pharmacy, infusion, other support services)
*Low-risk patient: ambulatory with good performance status, no serious comorbidity, and anticipated short duration of severe neutropenia or a Multinational Association of Supportive Care in Cancer risk index score of ≥ 21.28
Risk-Based Treatment of Febrile Neutropenic Patients: Common Regimens for Empiric Antibiotic Therapy
Regimens Used in Low-Risk Patients
Oral Regimens
Ciprofloxacin + amoxicillin/clavulanate
Ciprofloxacin + clindamycin or azithromycin
Moxifloxacin or levofloxacin
Parenteral Regimens
Ceftriaxone ± amikacin
Aztreonam + clindamycin or fluoroquinolone
Ceftazidime (or other cephalosporins)
Regimens Used in High-Risk Patients
Monotherapy
Extended-spectrum cephalosporins (cefepime, ceftazidime)
Carbapenems (imipenem, meropenem)
Other agents (piperacillin/tazobactam)
Combination Regimens
Aminoglycoside + extended-spectrum cephalosporins
Aminoglycoside + carbapenem or piperacillin/tazobactam
Vancomycin (or linezolid) + extended-spectrum cephalosporin, or carbapenem, or piperacillin/tazobactam, or aztreonam
Vancomycin + fluoroquinolone or aztreonam (beta-lactam allergic patients)
Acknowledgment
Supported by Grants No. RC2CA148041-01 from the National Cancer Institute and 1R01HL095109-01 from the National Heart, Lung and Blood Institute (G.H.L.).
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Gary H. Lyman, Amgen; Kenneth V.I. Rolston, Merck, Astellas Pharma, Cubist Pharmaceuticals Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Gary H. Lyman, Kenneth V.I. Rolston
Administrative support: Gary H. Lyman
Collection and assembly of data: Gary H. Lyman, Kenneth V.I. Rolston
Data analysis and interpretation: Gary H. Lyman, Kenneth V.I. Rolston
Manuscript writing: Gary H. Lyman, Kenneth V.I. Rolston
Final approval of manuscript: Gary H. Lyman, Kenneth V.I. Rolston
References
- 1.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 2.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Yahav D, Fraser A, et al. Empirical antibiotic monotherapy for febrile neutropenia: Systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2006;57:176–189. doi: 10.1093/jac/dki448. [DOI] [PubMed] [Google Scholar]
- 4.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 5.González-Barca E, Fernández-Sevilla A, Carratalá J, et al. Prognostic factors influencing mortality in cancer patients with neutropenia and bacteremia. Eur J Clin Microbiol Infect Dis. 1999;18:539–544. doi: 10.1007/s100960050345. [DOI] [PubMed] [Google Scholar]
- 6.Malik I, Hussain M, Yousuf H. Clinical characteristics and therapeutic outcome of patients with febrile neutropenia who present in shock: Need for better strategies. J Infect. 2001;42:120–125. doi: 10.1053/jinf.2001.0798. [DOI] [PubMed] [Google Scholar]
- 7.Darmon M, Azoulay E, Alberti C, et al. Impact of neutropenia duration on short-term mortality in neutropenic critically ill cancer patients. Intensive Care Med. 2002;28:1775–1780. doi: 10.1007/s00134-002-1528-7. [DOI] [PubMed] [Google Scholar]
- 8.Carratalà J, Rosón B, Fernández-Sevilla A, et al. Bacteremic pneumonia in neutropenic patients with cancer: Causes, empirical antibiotic therapy, and outcome. Arch Intern Med. 1998;158:868–872. doi: 10.1001/archinte.158.8.868. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 10.Schiel X, Hebart H, Kern WV, et al. Sepsis in neutropenia: Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82:S158-S166. doi: 10.1007/s00277-003-0770-6. suppl 2. [DOI] [PubMed] [Google Scholar]
- 11.Wisplinghoff H, Seifert H, Wenzel RP, et al. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103–1110. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 12.Elting LS, Rubenstein EB, Rolston KV, et al. Outcomes of bacteremia in patients with cancer and neutropenia: Observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 13.Lyman GH, Morrison VA, Dale DC, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44:2069–2076. doi: 10.1080/1042819031000119262. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: A nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 16.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 17.Crawford J, Armitage J, Balducci L, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2009;7:64–83. doi: 10.6004/jnccn.2009.0006. [DOI] [PubMed] [Google Scholar]
- 18.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 19.Yadegarynia D, Tarrand J, Raad I, et al. Current spectrum of bacterial infections in patients with cancer. Clin Infect Dis. 2003;37:1144–1145. doi: 10.1086/378305. [DOI] [PubMed] [Google Scholar]
- 20.Rolston KV, Yadegarynia D, Kontoyiannis DP, et al. The spectrum of Gram-positive bloodstream infections in patients with hematologic malignancies, and the in vitro activity of various quinolones against Gram-positive bacteria isolated from cancer patients. Int J Infect Dis. 2006;10:223–230. doi: 10.1016/j.ijid.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Safdar A, Rolston KV. Immunocompromised hosts: Stenotrophomonas maltophilia—Changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis. 2007;45:1602–1609. doi: 10.1086/522998. [DOI] [PubMed] [Google Scholar]
- 22.Rolston KV, Bodey GP, Safdar A. Polymicrobial infection in patients with cancer: An underappreciated and underreported entity. Clin Infect Dis. 2007;45:228–233. doi: 10.1086/518873. [DOI] [PubMed] [Google Scholar]
- 23.Klastersky J, Ameye L, Maertens J, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007;30:S51-S59. doi: 10.1016/j.ijantimicag.2007.06.012. suppl 1. [DOI] [PubMed] [Google Scholar]
- 24.Rolston KV. Challenges in the treatment of infections caused by gram-positive and gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40:S246-S252. doi: 10.1086/427331. suppl 4. [DOI] [PubMed] [Google Scholar]
- 25.Han XY, Kamana M, Rolston KV. Viridans streptococci isolated by culture from blood of cancer patients: Clinical and microbiologic analysis of 50 cases. J Clin Microbiol. 2006;44:160–165. doi: 10.1128/JCM.44.1.160-165.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumashi P, Girgawy E, Tarrand JJ, et al. Streptococcus pneumoniae bacteremia in patients with cancer: Disease characteristics and outcomes in the era of escalating drug resistance (1998–2002) Medicine (Baltimore) 2005;84:303–312. doi: 10.1097/01.md.0000180045.26909.29. [DOI] [PubMed] [Google Scholar]
- 27.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 28.Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18:3038–3051. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 29.Talcott JA, Siegel RD, Finberg R, et al. Risk assessment in cancer patients with fever and neutropenia: A prospective, two-center validation of a prediction rule. J Clin Oncol. 1992;10:316–322. doi: 10.1200/JCO.1992.10.2.316. [DOI] [PubMed] [Google Scholar]
- 30.Kern WV. Risk assessment and treatment of low-risk patients with febrile neutropenia. Clin Infect Dis. 2006;42:533–540. doi: 10.1086/499352. [DOI] [PubMed] [Google Scholar]
- 31.Rolston KVI, Frisbee-Hume SE, Patel S, et al. Oral moxifloxacin for outpatient treatment of low-risk, febrile neutropenic patients. Support Care Cancer. 2010;18:89–94. doi: 10.1007/s00520-009-0634-2. [DOI] [PubMed] [Google Scholar]
- 32.Klastersky J, Paesmans M, Georgala A, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006;24:4129–4134. doi: 10.1200/JCO.2005.03.9909. [DOI] [PubMed] [Google Scholar]
- 33.Sung L, Nathan PC, Lange B, et al. Prophylactic granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor decrease febrile neutropenia after chemotherapy in children with cancer: A meta-analysis of randomized controlled trials. J Clin Oncol. 2004;22:3350–3356. doi: 10.1200/JCO.2004.09.106. [DOI] [PubMed] [Google Scholar]
- 34.Clark OA, Lyman GH, Castro AA, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: A meta-analysis of randomized controlled trials. J Clin Oncol. 2005;23:4198–4214. doi: 10.1200/JCO.2005.05.645. [DOI] [PubMed] [Google Scholar]
- 35.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 36.Moellering RC, Jr, Graybill JR, McGowan JE, Jr, et al. Am J Med; Antimicrobial resistance prevention initiative: An update—Proceedings of an expert panel on resistance.; 2007. pp. S4–S25. quiz S26-S28. [DOI] [PubMed] [Google Scholar]
- 37.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–987. doi: 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 38.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 39.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 40.Lyman GH, Kleiner JM. Summary and comparison of myeloid growth factor guidelines in patients receiving cancer chemotherapy. J Natl Compr Canc Netw. 2007;5:217–228. doi: 10.6004/jnccn.2007.0021. [DOI] [PubMed] [Google Scholar]