Abstract
A proximity map showing the three-dimensional arrangement of 12 chemically defined points in actomyosin subfragment 1 is developed and roughly correlated with published electron microscope reconstruction of others. Several additional points and topological relationships in the primary polypeptide chain folding are assimilated into this model. Certain crosslinkings and distance change observations are interpreted as indicators of transmission of force/displacement between the nucleotide-binding and an actin-binding site--i.e., as indications of how energy is transduced in this system.
Full text
PDF
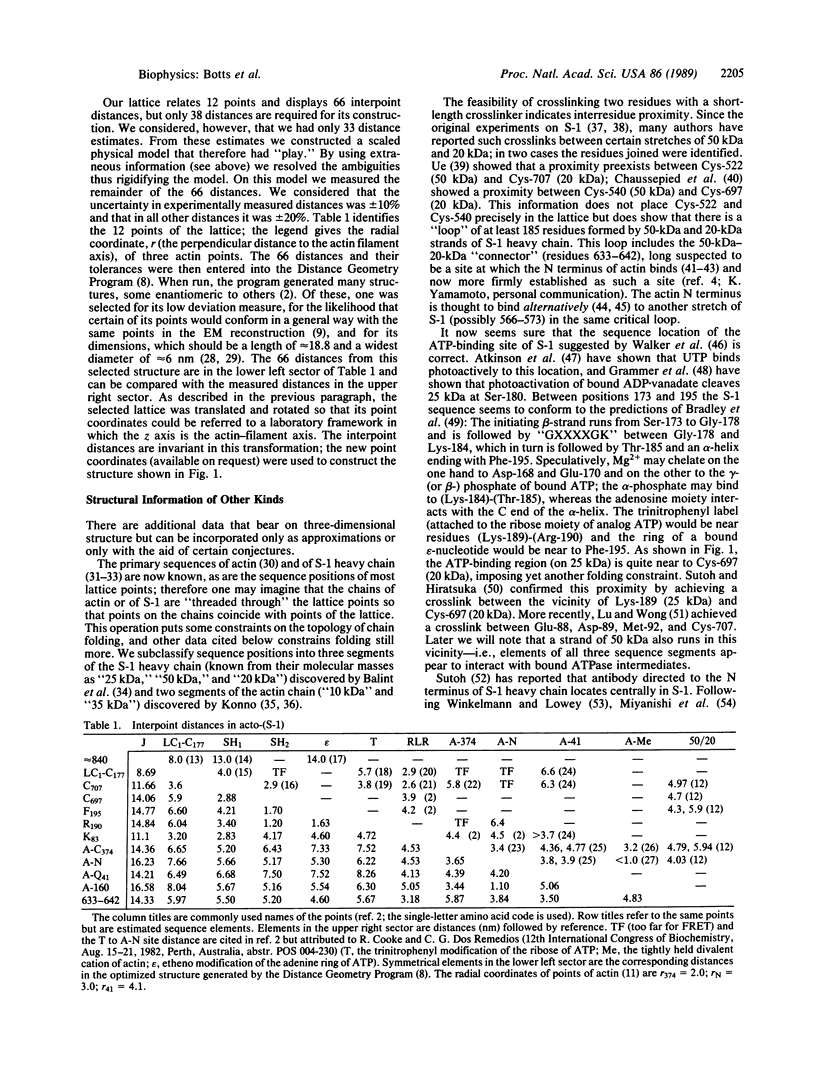
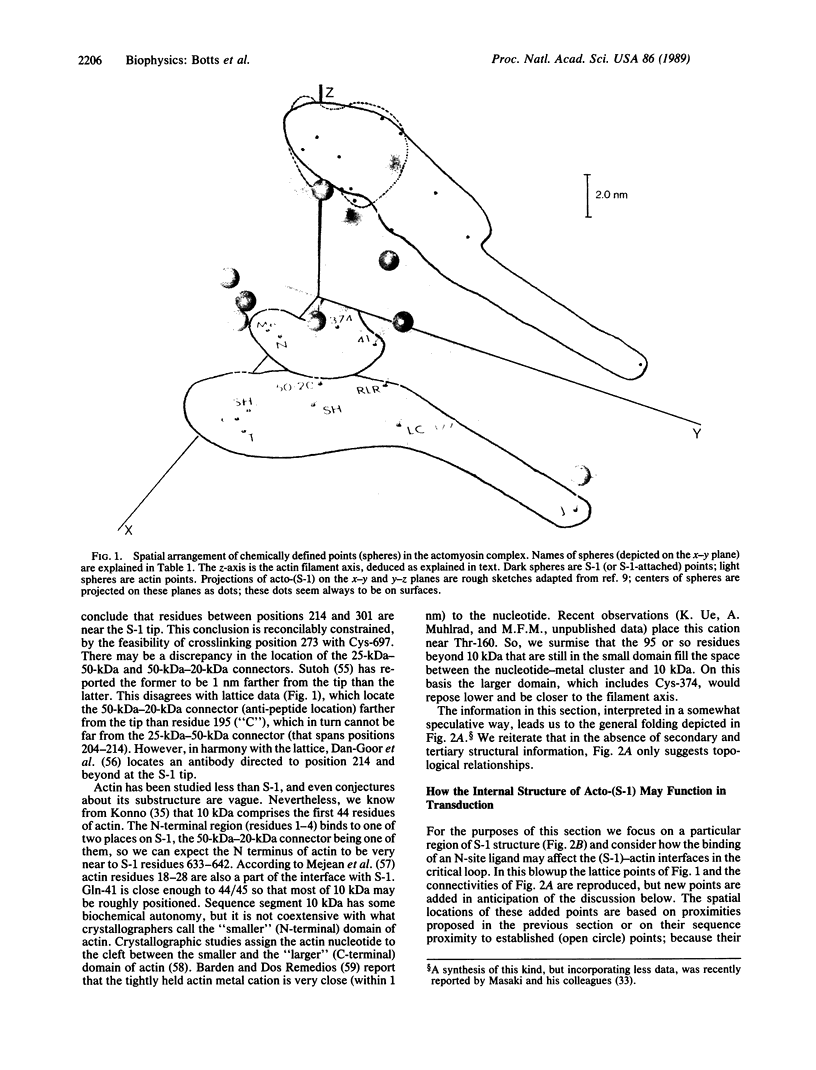
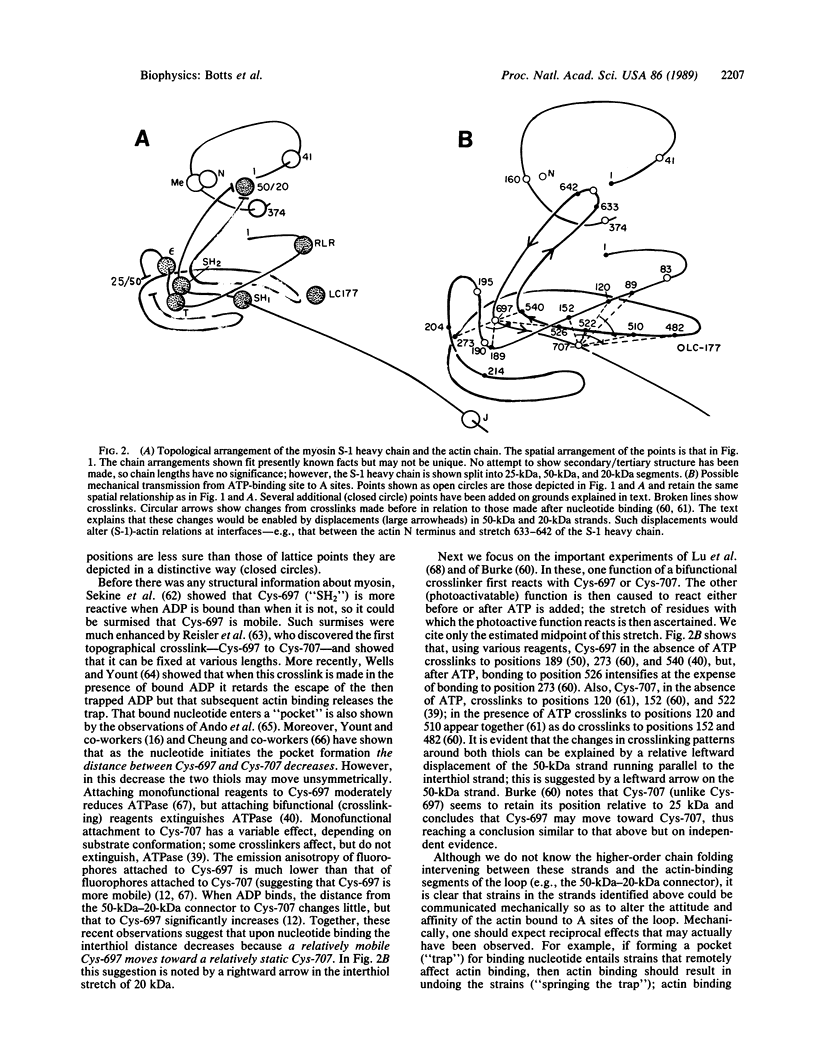

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre R., Gonsoulin F., Cheung H. C. Interaction of fluorescently labeled myosin subfragment 1 with nucleotides and actin. Biochemistry. 1986 Nov 4;25(22):6827–6835. doi: 10.1021/bi00370a015. [DOI] [PubMed] [Google Scholar]
- Ando T., Duke J. A., Tonomura Y., Morales M. F. Spectroscopic isolation of ES complexes of myosin subfragment-1 ATPase by fluorescence quenching. Biochem Biophys Res Commun. 1982 Nov 16;109(1):1–6. doi: 10.1016/0006-291x(82)91557-1. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Robinson E. A., Appella E., Korn E. D. Amino acid sequence of the active site of Acanthamoeba myosin II. J Biol Chem. 1986 Feb 5;261(4):1844–1848. [PubMed] [Google Scholar]
- Barden J. A., dos Remedios C. G. Fluorescence resonance energy transfer between sites in G-actin. The spatial relationship between Cys-10, Tyr-69, Cys-374, the high-affinity metal and the nucleotide. Eur J Biochem. 1987 Oct 1;168(1):103–109. doi: 10.1111/j.1432-1033.1987.tb13393.x. [DOI] [PubMed] [Google Scholar]
- Barden J. A., dos Remedios C. G. Fluorescence resonance energy transfer between sites in G-actin. The spatial relationship between Cys-10, Tyr-69, Cys-374, the high-affinity metal and the nucleotide. Eur J Biochem. 1987 Oct 1;168(1):103–109. doi: 10.1111/j.1432-1033.1987.tb13393.x. [DOI] [PubMed] [Google Scholar]
- Botts J., Muhlrad A., Takashi R., Morales M. F. Effects of tryptic digestion on myosin subfragment 1 and its actin-activated adenosinetriphosphatase. Biochemistry. 1982 Dec 21;21(26):6903–6905. doi: 10.1021/bi00269a043. [DOI] [PubMed] [Google Scholar]
- Botts J., Takashi R., Torgerson P., Hozumi T., Muhlrad A., Mornet D., Morales M. F. On the mechanism of energy transduction in myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2060–2064. doi: 10.1073/pnas.81.7.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. K., Smith T. F., Lathrop R. H., Livingston D. M., Webster T. A. Consensus topography in the ATP binding site of the simian virus 40 and polyomavirus large tumor antigens. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4026–4030. doi: 10.1073/pnas.84.12.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint M., Wolf I., Tarcsafalvi A., Gergely J., Sréter F. A. Location of SH-1 and SH-2 in the heavy chain segment of heavy meromyosin. Arch Biochem Biophys. 1978 Oct;190(2):793–799. doi: 10.1016/0003-9861(78)90339-9. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Morales M. F., Kassab R. The myosin SH2-50-kilodalton fragment cross-link: location and consequences. Biochemistry. 1988 Mar 8;27(5):1778–1785. doi: 10.1021/bi00405a059. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Morales M. F. Modifying preselected sites on proteins: the stretch of residues 633-642 of the myosin heavy chain is part of the actin-binding site. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7471–7475. doi: 10.1073/pnas.85.20.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. Fluorescence as a probe of contractile systems. Methods Enzymol. 1982;85(Pt B):574–593. doi: 10.1016/0076-6879(82)85053-2. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Weiel J., Yount R. G. Förster energy transfer measurements of thiol 1 to thiol 2 distances in myosin subfragment 1. Biochemistry. 1983 Sep 27;22(20):4696–4706. doi: 10.1021/bi00289a014. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios C. G., Cooke R. Fluorescence energy transfer between probes on actin and probes on myosin. Biochim Biophys Acta. 1984 Jul 31;788(2):193–205. doi: 10.1016/0167-4838(84)90262-0. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D. Three-dimensional structure of the complex of actin and DNase I at 4.5 A resolution. EMBO J. 1985 Aug;4(8):2113–2118. doi: 10.1002/j.1460-2075.1985.tb03900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak A. A., Takashi R., Morales M. F. Orientation of actin monomer in the F-actin filament: radial coordinate of glutamine-41 and effect of myosin subfragment 1 binding on the monomer orientation. Biochemistry. 1988 Jun 14;27(12):4512–4522. doi: 10.1021/bi00412a044. [DOI] [PubMed] [Google Scholar]
- Konno K. Functional, chymotryptically split actin and its interaction with myosin subfragment 1. Biochemistry. 1987 Jun 16;26(12):3582–3589. doi: 10.1021/bi00386a050. [DOI] [PubMed] [Google Scholar]
- Konno K. G-actin structure revealed by chymotryptic digestion. J Biochem. 1988 Feb;103(2):386–392. doi: 10.1093/oxfordjournals.jbchem.a122279. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Newton G. L., Ranney H. M. Bimane fluorescent labels: labeling of normal human red cells under physiological conditions. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3382–3386. doi: 10.1073/pnas.76.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R. C., Moo L., Wong A. G. Both the 25-kDa and 50-kDa domains in myosin subfragment 1 are close to the reactive thiols. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6392–6396. doi: 10.1073/pnas.83.17.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maita T., Hayashida M., Tanioka Y., Komine Y., Matsuda G. The primary structure of the myosin head. Proc Natl Acad Sci U S A. 1987 Jan;84(2):416–420. doi: 10.1073/pnas.84.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. J., Lowey S. Fluorescence energey transfer in myosin subfragment-1. Biochemistry. 1980 Feb 19;19(4):774–784. doi: 10.1021/bi00545a025. [DOI] [PubMed] [Google Scholar]
- Miki M., Wahl P. Fluorescence energy transfer between points in G-actin: the nucleotide-binding site, the metal-binding site and Cys-373 residue. Biochim Biophys Acta. 1985 Apr 5;828(2):188–195. doi: 10.1016/0167-4838(85)90056-1. [DOI] [PubMed] [Google Scholar]
- Miyanishi T., Toyoshima C., Wakabayashi T., Matsuda G. Electron microscopic study on the location of 23 kDa and 50 kDa fragments in skeletal myosin head. J Biochem. 1988 Mar;103(3):458–462. doi: 10.1093/oxfordjournals.jbchem.a122292. [DOI] [PubMed] [Google Scholar]
- Morales M. F., Botts J. On the molecular basis for chemomechanical energy transduction in muscle. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3857–3859. doi: 10.1073/pnas.76.8.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet D., Pantel P., Audemard E., Kassab R. The limited tryptic cleavage of chymotryptic S-1: an approach to the characterization of the actin site in myosin heads. Biochem Biophys Res Commun. 1979 Aug 13;89(3):925–932. doi: 10.1016/0006-291x(79)91867-9. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K., Chaussepied P., Morales M. F. Modification of the actin interface of skeletal myosin subfragment-1 by treatment with dibromobimane. Eur J Biochem. 1986 Sep 15;159(3):555–561. doi: 10.1111/j.1432-1033.1986.tb09922.x. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K. Incorporation of 6-carboxyfluorescein into myosin subfragment 1. Biochemistry. 1985 Feb 12;24(4):840–846. doi: 10.1021/bi00325a005. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K., Morales M. F. Stabilization of a primary loop in myosin subfragment 1 with a fluorescent crosslinker. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1658–1662. doi: 10.1073/pnas.82.6.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Trentham D. R. Distance measurement between the active site and cysteine-177 of the alkali one light chain of subfragment 1 from rabbit skeletal muscle. Biochemistry. 1983 Nov 8;22(23):5261–5270. doi: 10.1021/bi00292a004. [DOI] [PubMed] [Google Scholar]
- Méjean C., Boyer M., Labbé J. P., Derancourt J., Benyamin Y., Roustan C. Antigenic probes locate the myosin subfragment 1 interaction site on the N-terminal part of actin. Biosci Rep. 1986 May;6(5):493–499. doi: 10.1007/BF01116141. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Himmelfarb S., Harrington W. F. Spatial proximity of the two essential sulfhydryl groups of myosin. Biochemistry. 1974 Sep 10;13(19):3837–3840. doi: 10.1021/bi00716a001. [DOI] [PubMed] [Google Scholar]
- SEKINE T., BARNETT L. M., KIELLEY W. W. The active site of myosin adenosine triphosphatase. I. Localization of one of the sulfhydryl groups. J Biol Chem. 1962 Sep;237:2769–2772. [PubMed] [Google Scholar]
- Sutoh K., Hiratsuka T. Spatial proximity of the glycine-rich loop and the SH2 thiol in myosin subfragment 1. Biochemistry. 1988 Apr 19;27(8):2964–2969. doi: 10.1021/bi00408a045. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Lu R. C. Identification of two segments, separated by approximately 45 kilodaltons, of the myosin subfragment 1 heavy chain that can be cross-linked to the SH-1 thiol. Biochemistry. 1987 Jul 14;26(14):4511–4516. doi: 10.1021/bi00388a051. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Tokunaga M., Wakabayashi T. Electron microscopic visualization of the N terminus of the myosin heavy chain using a site-directed antibody. J Mol Biol. 1987 Jun 20;195(4):953–956. doi: 10.1016/0022-2836(87)90500-6. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Yamamoto K., Wakabayashi T. Electron microscopic visualization of the ATPase site of myosin by photoaffinity labeling with a biotinylated photoreactive ADP analog. Proc Natl Acad Sci U S A. 1986 Jan;83(2):212–216. doi: 10.1073/pnas.83.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K., Yamamoto K., Wakabayashi T. Electron microscopic visualization of the SH1 thiol of myosin by the use of an avidin-biotin system. J Mol Biol. 1984 Sep 15;178(2):323–339. doi: 10.1016/0022-2836(84)90147-5. [DOI] [PubMed] [Google Scholar]
- Takashi R. Fluorescence energy transfer between subfragment-1 and actin points in the rigor complex of actosubfragment-1. Biochemistry. 1979 Nov 13;18(23):5164–5169. doi: 10.1021/bi00590a021. [DOI] [PubMed] [Google Scholar]
- Takashi R., Kasprzak A. A. Measurement of interprotein distances in the acto-subfragment 1 rigor complex. Biochemistry. 1987 Nov 17;26(23):7471–7477. doi: 10.1021/bi00397a041. [DOI] [PubMed] [Google Scholar]
- Takashi R., Muhlrad A., Botts J. Spatial relationship between a fast-reacting thiol and a reactive lysine residue of myosin subfragment 1. Biochemistry. 1982 Oct 26;21(22):5661–5668. doi: 10.1021/bi00265a042. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Reidler J., Spudich J. A., Stryer L. Detection of actin assembly by fluorescence energy transfer. J Cell Biol. 1981 May;89(2):362–367. doi: 10.1083/jcb.89.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Sutoh K., Toyoshima C., Wakabayashi T. Location of the ATPase site of myosin determined by three-dimensional electron microscopy. Nature. 1987 Oct 15;329(6140):635–638. doi: 10.1038/329635a0. [DOI] [PubMed] [Google Scholar]
- Tong S. W., Elzinga M. The sequence of the NH2-terminal 204-residue fragment of the heavy chain of rabbit skeletal muscle myosin. J Biol Chem. 1983 Nov 10;258(21):13100–13110. [PubMed] [Google Scholar]
- Torgerson P. M., Morales M. F. Application of the Dale-Eisinger analysis to proximity mapping in the contractile system. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3723–3727. doi: 10.1073/pnas.81.12.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer H. R., Trayer I. P. Fluorescence resonance energy transfer within the complex formed by actin and myosin subfragment 1. Comparison between weakly and strongly attached states. Biochemistry. 1988 Jul 26;27(15):5718–5727. doi: 10.1021/bi00415a049. [DOI] [PubMed] [Google Scholar]
- Ue K. Intramolecular cross-linking of myosin subfragment 1 with bimane. Biochemistry. 1987 Apr 7;26(7):1889–1894. doi: 10.1021/bi00381a016. [DOI] [PubMed] [Google Scholar]
- Vibert P. J. Domain structure of the myosin head in correlation-averaged images of shadowed molecules. J Muscle Res Cell Motil. 1988 Apr;9(2):147–155. doi: 10.1007/BF01773736. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Trinick J. Visualization of domains in native and nucleotide-trapped myosin heads by negative staining. J Muscle Res Cell Motil. 1988 Aug;9(4):359–366. doi: 10.1007/BF01773879. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Yount R. G. Reaction of 5,5'-dithiobis(2-nitrobenzoic acid) with myosin subfragment one: evidence for formation of a single protein disulfide with trapping of metal nucleotide at the active site. Biochemistry. 1980 Apr 15;19(8):1711–1717. doi: 10.1021/bi00549a030. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Lowey S. Probing myosin head structure with monoclonal antibodies. J Mol Biol. 1986 Apr 20;188(4):595–612. doi: 10.1016/s0022-2836(86)80009-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Tokunaga M., Sutoh K., Wakabayashi T., Sekine T. Location of the SH group of the alkali light chain on the myosin head as revealed by electron microscopy. J Mol Biol. 1985 May 25;183(2):287–290. doi: 10.1016/0022-2836(85)90222-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]
- dos Remedios C. G., Miki M., Barden J. A. Fluorescence resonance energy transfer measurements of distances in actin and myosin. A critical evaluation. J Muscle Res Cell Motil. 1987 Apr;8(2):97–117. doi: 10.1007/BF01753986. [DOI] [PubMed] [Google Scholar]