Abstract
β-synemin was previously identified as an α-dystrobrevin-interacting protein in muscle. To better understand its function in neural tissue, in situ and immunohistochemical analyses were performed to identify where the synemin isoforms are expressed in the spinal cord of C57BL/6 and dystrophin-deficient (mdx) C57BL/10 mice. These analyses show that synemin transcript and its encoded protein co-localize in the anterior horn cells and that no differences in synemin expression were found in nerve tissue from C57BL/6 or mdx mice. The expression of synemin mRNA and protein predominantly in the anterior horn cells suggests that synemin performs an essential function in those cells. Since synemin is more highly expressed in the midbrain and pons, its function in neurological cells was further pursued by identifying co-expressed proteins in cells from those regions of the brain. These results show that neurons which express synemin also express tryptophan hydroxylase-1, a marker of serotoninergic nerve fibers.
Keywords: synemin, α-dystrobrevin, anterior horn cell, spinal cord, tryptophan hydroxylase
Introduction
Synemin is a type VI member of the intermediate filament (IF) protein superfamily 13. IF proteins are composed of three common structural regions including the N-terminus head domain, the central α-helical rod domain, and the C-terminus tail domain. Among type VI IF proteins, synemin is unique in that it has a very short N-terminus and a long C-terminus,13 thus the C-terminus may be important for protein-protein interactions.
In mammals, there are two synemin isoforms (α- and β-), whereas only one synemin isoform is expressed in birds. The mammalian α-synemin 28, 32 mRNA is structurally identical 13, 28 to β-synemin (also called desmuslin) 13 with the exception that intron 4 of β-synemin is not spliced from the α-synemin transcript. This difference results in an additional α-synemin coding region which is 906 bp long in mice 32 and 936 bp in humans 13. While the function of α-synemin is unknown, it is expressed predominantly in brain tissue whereas β-synemin is strongly expressed in skeletal and cardiac muscle.
In muscle, the synemin protein forms heteropolymeric IFs with desmin and vimentin 7, which are two different type III IF proteins. Desmin and vimentin encircle and connect the myofibrillar Z-lines of the neighboring myofibrils, and they also bind the Z-lines of myofibrils to the sarcolemma at the costamere. In addition, β-synemin was originally identified as an α-dystrobrevin-binding protein through a yeast two-hybrid screen 13. Although β-synemin can theoretically bind either α-dystrobrevin-1 or -2 13. it is thought that β-synemin preferentially associates with α-dystrobrevin-1 in skeletal muscle 15. There is considerable evidence for this association. For example, it has been shown that α-dystrobrevin-1 (rather than α-dystrobrevin-2) preferentially co-immunoprecipitates with β-synemin in rat skeletal muscle.16 β-synemin and α-dystrobrevin-1 are expressed at the same developmental time (in mice, 5 days post-birth), whereas α-dystrobrevin-2 is detectable before birth.16 β-synemin and α-dystrobrevin-1 are expressed at the same time in regenerating adult muscle (day 7 following cardiotoxin injection), whereas α-dystrobrevin-2 is observed as early as day 1 post-injection.16 α-dystrobrevin-1 and β-synemin expression levels are similar between control and dystrophin-deficient (mdx) mice skeletal muscle, whereas α-dystrobrevin-2 expression is greatly decreased in mdx mice 15. Taken together, these data suggest that β-synemin participates in the stabilization of muscle by linking the myofibril Z-lines with the membrane-associated α-dystrobrevin-1 protein at the costameres.
This role for synemin in muscle is further strengthened by its interactions with other muscle proteins. For example, birds express only one synemin isoform (α-synemin), which has been shown to bind α-actinin (an actin-binding protein) and vinculin (a costameric protein) 1. Recent studies have also shown that human α-synemin interacts directly with both vinculin and metavinculin 26. Human β-synemin was originally identified as an α-dystrobrevin-interacting protein 13 and α-dystrobrevin is a known member of the dystrophin-associated protein complex 21, 22, 23. The association with dystrophin is also strengthened by recent evidence that has shown avian synemin binds directly to dystophin and utrophin (a dystrophin homologue) 3. Given that synemin interacts with a number of different muscle structural proteins and is present at adhesion-type junctions such as costameres 3, these findings suggest that synemin likely functions in maintaining cell structural integrity and stabilizing the cytoskeleton in muscle cells during repeated cycles of cell relaxation and contraction.
In addition to muscle, synemin isoforms are also expressed in other tissues. For example, additional in situ hybridization and immunohistochemical experiments confirmed that the synemin transcript was translated into protein in neurons located in regions of the dorsal side of the midbrain and pons 17. Using an isoform specific synemin RNA in situ probe, it has been shown that the α-synemin isoform is expressed in these same neurons 17. The finding that synemin is expressed in such a limited subset of nerve cells suggests that this gene plays a specific role in neuronal tissue, which is likely much different than that performed in skeletal muscle.
Given that synemin is expressed in specific neurons within the brain, it seemed likely that this protein would also be expressed in the spinal cord, a region central to many debilitating human neurological diseases. Previous results from Izmiryan et al. suggested that synemin was not highly expressed in the adult mouse spinal cord 8. Since their findings8 were based on an immunohistochemical analysis, this analysis was repeated using both RNA-based (in situ hybridization) and additional protein-based (immunohistochemistry) approaches to reanalyze if the synemin transcript and protein are expressed in the spinal cord. Since β-synemin is strongly expressed in muscle and has been shown to interact with dystrophin in vitro 3, synemin expression in spinal cord from 8-week-old control C57BL/6 mice and dystrophin-deficient C57BL/10 mice was also examined. Sections were analyzed using a synemin RNA probe with homology to a non-coding region common to both synemin isoforms, and a human anti-synemin antibody that recognizes both the α- and β-synemin proteins. Since these findings showed that synemin was only weakly expressed in the spinal cord, we returned to brain to better characterize synemin expressing neurons in the midbrain and pons. Our results in brain show that synemin is co-expressed with tryptophan hydroxylase-1, a marker of serotoninergic nerve cells.
Materials and Methods
RNA probe Design and preparation
The in situ probe used was synemin RNA probe-3 [479-bp; position 5972-6450 (5907-6385 bp from the initiation codon)]. The position designation is relative to the mouse muscle cDNA for synemin (GenBank accession number: NM_201639). This probe was created by subcloning the synemin fragment into a pGEM-T vector (Promega; Madison, Wisconsin, USA) and generating digoxigenin-RNA sense and anti-sense probes from the flanking T7 and S6 promoters (Roche; Basel, Switzerland).
In situ hybridization
All in situ hybridization protocols were performed by Genostaff, Inc (Tokyo, Japan), and all results were confirmed through multiple replications. Mouse brain was isolated from both 8-week-old C57BL/6 and dystrophin-deficient (mdx) C57BL/10 mice, fixed with Tissue Fixative, and embedded in paraffin blocks. Dystrophic (mdx) mice were provided by the Central Institute for Experimental Animals (Kanagawa, Japan). Tissue sections (6 μm) were prepared for in situ hybridization by de-waxing with xylene and rehydrating through a series of washes with ethanol and phosphate-buffered saline (PBS). Sections were fixed in PBS with 4% paraformaldehyde for 15 min and then washed with PBS. Sections were then treated with 10μg/ml Proteinase K in PBS for 30 min at 37°C, washed with PBS, refixed with 4% paraformaldehyde in PBS, washed again with PBS, and placed in 0.2M HCl for 10 min. After washing with PBS, sections were acetylated by incubation in 0.1M triethanolamine-HCl (pH 8.0), 0.25% acetic anhydride for 10 min. Sections were then washed with PBS and dehydrated through a series of ethanol washes. Hybridization was performed with RNA probe-3 (100ng/ml) in probe diluent at 60°C for 16 hr. After hybridization, sections were washed in 5 × HybriWash (Genostaff) at 60°C for 20 min and then in 50% formamide, 2 × HybriWash at 60°C for 20 min, followed by RNase treatment in 50ug/ml RNaseA in 10mM Tris-HCl (pH 8.0), 1M NaCl and 1mM ethylenediamine tetraacetic acid (EDTA) for 30 min at 37°C. The sections were then washed twice with 2 × HybriWash at 60°C for 20 min, twice with 0.2 × HybriWash at 60°C for 20 min, and once with TBST [0.1% Tween20 in Tris-buffered saline (TBS)]. After treatment with 0.5% blocking reagent (Roche; Basel, Switzerland) in TBST for 30 min, sections were incubated with anti-digoxigenin-AP conjugate (Roche) diluted 1:1000 with TBST for 2 hr. The sections were washed twice with TBST and incubated in 100mM NaCl, 50mM MgCl2, 0.1% Tween20, 100mM Tris-HCl (pH 9.5). Coloring reactions were performed with BM purple AP substrate (Roche) overnight and washed with PBS. The sections were counterstained with Kernechtrot stain solution (Muto Pure Chemicals; Tokyo, Japan), dehydrated, and mounted with malinol (Muto Pure Chemicals).
Immunohistochemistry
For immunohistochemistry, tissue sections were de-waxed with xylene, and rehydrated through a series of ethanol and TBS washes. Antigen retrieval was performed by microwaving the samples for 20 min with citrate buffer (pH 6.0). The sections were treated with 3% hydrogen peroxide in methanol for 30 min, and Protein Block (Dako; Kyoto, Japan) for 10 min. The sections were treated with anti-human synemin rabbit polyclonal antibody 15, 16, 17 at a final concentration of 0.4 μg/ml at 2-8°C overnight or anti-tryptophan hydroxylase-1 (TPH-1) rabbit monoclonal antibody (Epitomics; California, USA) at a final concentration of 2 μg/ml at 4°C overnight. The 12 amino acid peptide sequence used to generate the anti-human synemin antibody is conserved in mice (amino acids #857-868 in human 13 and #848-859 in mouse 32). For synemin staining, sections were treated with Histofine Simplestain mouse MAX-PO (R) (Nichirei Biosciences; Tokyo, Japan) for 30 min and incubated with 3,3′-diaminobenzidine, tetrahydrochloride (Wako Pure Chemical Industries; Osaka, Japan). For TPH-1 staining, sections were treated with Biotin blocking system (Dako) and successively incubated with biotinylated anti-rabbit immunoglobulins (Dako) diluted 1:600 for 30 min, peroxidase-conjugated streptavidin (Nichirei Biosciences) for 5 min, and 3,3′-diaminobenzidine, tetrahydrochloride (Wako Pure Chemical Industries). Sections were counterstained with Mayer’s hematoxylin (Muto Pure Chemicals), dehydrated, and mounted with malinol (Muto Pure Chemicals). To better highlight signal differences, digital pictures taken under identical light conditions were modified together as a group by making a minor adjustment to the contrast level in Photoshop (Adobe Systems Incorporated, California, USA).
Results
Localization of the synemin transcript and its protein in spinal cord in sagittal sections
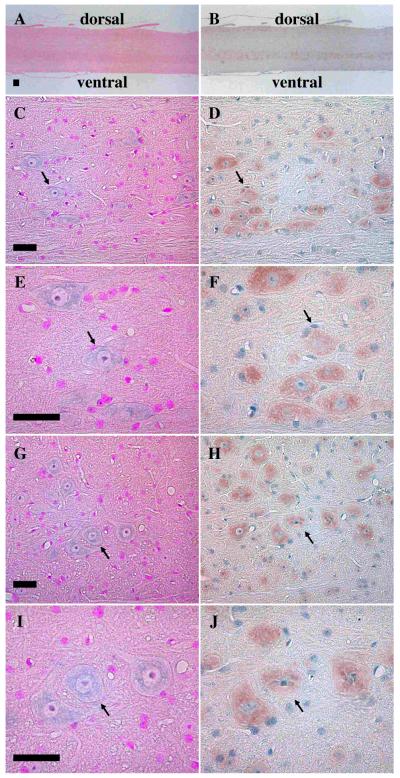
To investigate synemin expression in the mouse spinal cord, in situ analysis was performed on sagittal sections using the general synemin probe-3. Positive structures were visible on the ventral side of the spinal cord, but it was hard to discriminate individual neurons expressing synemin transcript when they were viewed at low magnification (Fig. 1A). Higher magnification of the ventral side showed more obvious staining of synemin transcript (Fig. 1C), and even further magnification of the area in Fig. 1C makes it possible to clearly discern individual reactive neurons (Fig. 1E). Mirror sections were used to determine co-localization of the synemin transcript and protein as a means of showing probe specificity. Immunohistochemical analysis using an antibody derived against human synemin showed ventral alignment of synemin-positive neurons (Fig. 1B). At high magnification, comparisons of the in situ and immunohistochemical analysis confirmed co-localization of the synemin transcript (Fig. 1C and 1E) and protein (Fig. 1D and 1F). Additional control experiments using sense probes were negative (data not shown). This suggests that the labeling patterns for the anti-sense probes were specific.
Fig. 1.
Colocalization of the synemin transcript and protein in mouse spinal cord on sagittal sections. Panels A, C, E, G, and I are in situ experiments hybridized with the synemin probe-3, whereas panels B, D, F, H, and J are mirror sections immunostained with an anti-synemin antibody. Panels A to F are from control mice, whereas panels G to J are from mdx mice. Light purple shows in situ positive structures and orange indicates those immunostained with the antibody. Signals with probe-3 in A are not clearly detectable, while immunohistochemical positive structures seem to be more pronounced toward the ventral side of B. Panels C and D are magnified images of the same ventral side shown in panels A and B, respectively. The signals of synemin transcript seen in panel C are weak, but they are clearly visible with increased magnification (panel E). Immunohistochemical analysis shows a clear colocalization of synemin protein (D and F). In the mdx mice spinal cord, the same synemin staining patterns are seen at the same magnifications (panels G-J). Arrows designate the anterior horn cells. Bar, 50μm
The synemin protein was initially identified as a α-dystrobrevin-interacting protein in muscle. To determine whether synemin expression was altered in nerve cells of a dystrophin-deficient (mdx) mouse, in situ hybridization and immunohistochemical experiments were performed simultaneously on control and mdx mouse spinal cord sections. Synemin staining intensities of the in situ hybridization (Fig. 1G and 1I) and immunohistochemical experiments (Fig. 1H and 1J) in mdx mice were similar to that of control mice, thus the dystrophic condition of the mdx mouse had no measureable effect on synemin expression in the spinal cord nerve cells.
Localization of the synemin transcript and its protein in spinal cord using transverse sections
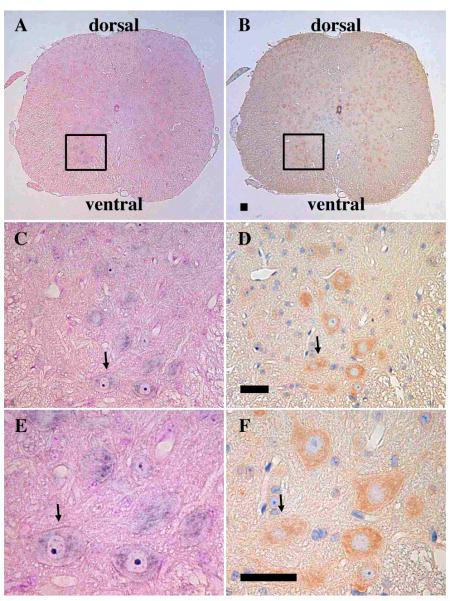
In situ hybridization and immunohistochemical analyses were performed on spinal cord transverse sections to better localize synemin expression. In situ hybridization experiments were performed using similar conditions as were described for the sagittal sections (Fig. 2A). Detailed examination of the region shown in the square in Fig. 2A highlights staining of individual anterior horn cells (Fig. 2C). Further magnification of this region showed weak and irregular synemin signals in the cytoplasm of these neurons (Fig. 2E). In contrast to synemin staining in the midbrain and pons, the intensity of synemin staining in the spinal cord was relatively weak, suggesting that synemin is only expressed at low levels in these cells. Control experiments with synemin sense probes were negative (data not shown), suggesting that the labeling patterns for the anti-sense probes were specific. To determine if synemin transcript and protein was expressed in the same neurons, mirror sections were examined using an anti-synemin antibody. The same anterior horn cells which expressed synemin transcript (Fig. 2C and 2E) also stained positively for the synemin protein (Fig. 2D and 2F). Synemin positive cells were widely distributed in the lateral and posterior horns of the transverse section (Fig. 2B).
Fig. 2.
Colocalization of synemin transcript and protein in mouse spinal cord on transverse sections. Panels A, C, and E are in situ experiments hybridized with the synemin probe-3, whereas panels B, D, and F are mirror sections immunostained with an anti-synemin antibody. Light purple is indicative of in situ positive structures whereas orange indicates immunostained structures. The squares shown in panels A and B are magnified and shown in C and D to more clearly see the transcript-positive and protein-positive structures. Panels E and F are further magnifications of panels C and D to better highlight synemin. A single arrow in panels C to F denotes the same neurons. Bar, 50μm
Localization of the synemin transcript and tryptophan hydroxylase-1
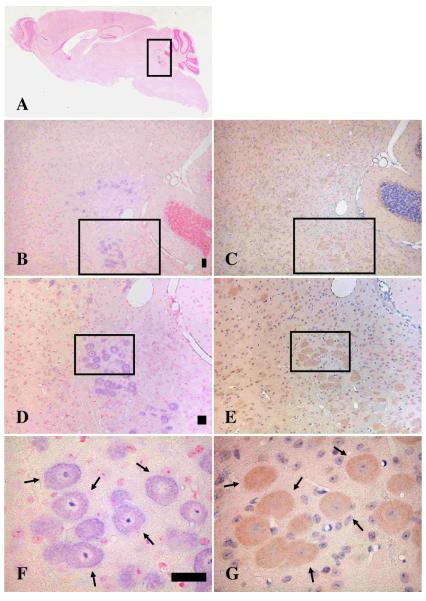
Since synemin was only weakly expressed in the mouse spinal cord, its function was further investigated in the brain, where it is more highly expressed. By identifying co-expressed proteins, one can begin to identify neurons that express synemin and hypothesize as to its function in vivo. To determine if synemin was expressed in serotoninergic neurons, immunohistochemical analysis was performed using an antibody against tryptophan hydroxylase-1 (TPH-1), an enzyme involved in the biosynthesis of serotonin. As previously reported, in situ analysis of brain sections using the synemin probe-3 demonstrated that the synemin transcript was expressed in neurons scattered within a square in Fig. 3A. This region contains the midbrain and pons. As a means of quantitating the number of cells that express synemin, synemin-transcript-positive neurons from the midbrain and pons were counted from 7 different brain sections. The mean number of neurons was 33.1 (range 10-59). Obvious synemin staining was most apparent at high magnification (Fig. 3B). At even higher magnification (100X), large numbers of synemin positive neurons are apparent on the dorsal side of midbrain and pons (Fig. 3D). Detailed analysis of the square designated in Fig. 3D showed synemin expression in large round neurons (Fig. 3F). Mirror sections were used to determine co-localization with immunostained proteins, since these sections provide greater resolution than serial sections when the positively labeled structures are small. In mirror sagittal sections, neurons demonstrated convincing co-localization of synemin transcript (Figs. 3B, 3D, and 3F) with TPH-1 protein (Figs. 3C, 3E, and 3G).
Fig. 3.
Colocalization of synemin transcript and tryptophan hydroxylase-1 protein (TPH-1) in brain sagittal sections. Panels A, B, D, and F are in situ experiments hybridized with the synemin probe-3, whereas panels C, E, and G are mirror sections of panels B, D, and F and are immunostained with an anti-tryptophan hydroxylase-1 antibody. Light purple designates in situ positive structures whereas orange indicates immunostaining with the anti-TPH-1 antibody. Panel A shows a whole sagittal brain where signals with probe-3 are seen inside the square. Panels B, D, and F are magnified of squares in panels A, B, and D, respectively. Squares in B and C show the same area as well as those in D and E. Arrows in F and G indicate the same neurons. Panels B and C are enlarged 50X, D and E are 100X, and F and G are 400X. Bar, 50μm
Discussion
Previous studies examined synemin expression in muscle 15 or whole brain 17. In the brain, synemin transcript and protein are expressed in the large and round neurons of the midbrain and pons 17. The purpose of the present study was to examine synemin expression in the spinal cord using in situ and immunohistochemical analyses to help hypothesize what its function might be in neuronal tissue. In contrast to a previous report 8 which did not detect synemin protein in the anterior horn cells of the mouse spinal cord, Figures 1 and 2 show by both in situ hybridization and immunohistochemical analyses that synemin is weakly, but specifically expressed in those cells. It is possible that the difference between these studies is due to the varying sensitivities of the two protocols used. For example, different antibodies and tissue conditions were tested in these analyses.
Since synemin has been shown to interact in vitro with dystrophin 3 and α-dystrobrevin-1 (a known dystrophin-binding protein), its expression in the spinal cord of dystrophic mice (dystrophin null) was examined. The mdx mouse is a naturally isolated mutant which lacks dystrophin 24 and has been used to show that the expression of other muscle proteins is effected in the diseased state. For example, the sarcoglycans 19 are partially down-regulated due to the loss of dystrophin and the destabilization of the dystrophin-associated protein complex 18, whereas utrophin, a dystrophin homolog, is more highly expressed and can partially compensate for the lack of dystrophin 12. In addition to muscle, dystrophin isoforms are also expressed in other tissues, including brain. For example, (1) Dp260 is expressed in the retina, brain, and cardiac tissue 6, (2) Dp140 is expressed throughout the central nervous system 10, and (3) Dp71 is expressed more ubiquitously with the exception of skeletal muscle 9. To determine whether synemin expression was altered in dystrophin-deficient neurological tissue, its expression was investigated in normal and mdx mouse spinal tissue, and no significant differences were noted. This could be because synemin does not interact with dystrophin in the spinal cord or because synemin is not as highly expressed in spinal cord as compared to other tissues. Experiments to colocalize synemin with specific dystrophin isoforms such as Dp71 9, Dp140 10, and Dp2606 were unsuccessful, namely because the unique sequences between these isoforms are in exon 1 and are too short to make isoform-specific probes necessary for in situ hybridization.
Previous studies have also shown that β-synemin can interact with α-dystrobrevin-1 (as opposed to α-dystrobrein-2) in skeletal muscle 16. As such, it is possible that α- and β-synemin could specifically interact with α-dystrobrein-1 in neurons of the midbrain and pons. This was tested using in situ hybridization experiments with an α-dystrobrevin-1 specific probe (321-bp; position 2,068-2,388 of GenBank accession number: NM_207650) corresponding to the last 106 amino acids and its stop codon 4. Signals from this experiment were weak, suggesting that α-dystrobrevin-1 is not highly expressed in neurons of the midbrain and pons. Since synemin is expressed in those cells, this suggests that synemin likely associates with some other protein(s) in nerve tissue.
Some of the best evidence available for synemin function has been derived from its expression pattern. In the midbrain and pons, synemin is highly expressed in a limited number of neurons that co-express various neurotransmitters such as serotonin. Serotoninergic nerve fibers are derived from the midline raphé nucleus between the medulla oblongata and midbrain, while cholinergic neurons are abundant in the basal nucleus of Meynert, nucleus of the diagonal band, medial septal nucleus of basal forebrain, laterodorsal tegmental nucleus, and pedunculopontine tegmental nucleus of the midbrain and pons. Since cholinergic neuron specific antibodies (i.e. antibodies against choline-O-acetyltransferase) were unavailable for use in paraffin-embedded sections, mouse sections were analyzed using an antibody against tryptophan hydroxylase-1 (TPH-1) to distinguish serotoninergic nerve fibers. TPH is a rate-limiting enzyme involved in the synthesis of serotonin 5. This enzyme hydroxylates the 5-position of tryptophan to form 5-hydroxytryptophan, which is then converted to serotonin by an aromatic L-amino acid decarboxylase. In humans, as well as in other mammals, there are two isoforms of TPH. These isoforms (type 1 and 2) are encoded by two different, but homologous genes 29. TPH-1 is phosphorylated by cAMP-dependent protein kinase A 11 and is present in peripheral tissues such as heart, lung, kidney, duodenum, and adrenal gland, but is also expressed in the central nervous system (CNS) 33. On the other hand, TPH-2 is exclusively expressed in neurons of the CNS 33. The finding that synemin and TPH-1 are expressed in the same neurons suggests that these cells are serotoninergic nerve fibers, although synemin appears to be more widely distributed than the area of raphe nuclei suggesting it may be expressed in other cells as well.
It is unknown what role synemin may play in neurological diseases, but dysbindin is a coiled-coil-containing and α-dystrobrein-binding protein 2. Mutations in the dysbindin gene have been linked to schizophrenia 20, 25. The association between dysbindin and neurological disease is supported by results in schizophrenic patients that show: (1) dysbindin mRNA levels are reduced in the dorsolateral prefrontal cortex;30 and (2) dysbindin protein levels are reduced in the hippocampus 27. In schizophrenic patients, the dorsolateral prefrontal cortex has been consistently found to be dysfunctional 31. In normal brain, dysbindin mRNA is expressed in temporal cortex, frontal cortex, substantia nigra, basal ganglia, and amygdala 30, indicating a wide distribution of dysbindin expression. In contrast, synemin expression in the brain is restricted to the midbrain and pons 17. In addition, the finding of Talbot K et al. that dysbindin is present in intrinsic glutamatergic neurons in the hippocampus 27 also contrasts with synemin, which associates with serotoninergic nerve fibers. These results suggest that, even though the dysbindin and synemin proteins both bind to α-dystrobrevin in muscle, the two proteins likely perform very diverse roles in the brain.
As its function and localization in the brain is not well understood, it is difficult to predict what effect mutations in synemin would have on brain function. Since β-synemin is thought to function as a structural protein in muscle, it is possible that synemin also serves a structural role in neurons of midbrain and pons. To search for potential pathological implications, it will be important to elucidate whether synemin is localized to either presynaptic or postsynaptic sites (or both). Immunoelectron microscopic analysis and the identification of synemin-binding partners in the brain will provide useful information.
In summary, this report shows that synemin transcript and its protein are expressed in anterior horn cells in the mouse spinal cord, although the signals were relatively weak. This suggests that expression was not as high as that seen in regions of the midbrain and pons. If synemin expression was proportionate to its importance, these results would suggest a more influential role for this protein in the midbrain and pons. To investigate the role of synemin in nerve tissue, we returned to brain to identify other co-localizing proteins as a means to better characterize synemin expressing cells. Our results show that synemin and TPH-1 are co-expressed in the same neurons. We may thus begin to speculate as to synemin’s function. Continued characterization of synemin expressing cells will allow us to begin to understand synemin’s function and to help ascertain whether mutations in this gene could contribute to human muscle or psychiatric diseases 14.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (20591016) from the Japan Society for the Promotion of Science to Y.M. L.M.K. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- CNS
central nerve system
- EDTA
ethylenediamine tetraacetic acid
- PBS
phosphate-buffered saline
- TBS
Tris-buffered saline
References
- 1.Bellin RM, Huiatt TW, Critchley DR, Robson RM. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J Biol Chem. 2001;276:32330–32337. doi: 10.1074/jbc.M104005200. [DOI] [PubMed] [Google Scholar]
- 2.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- 3.Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM. Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem Biophys Res Commun. 2006;346:768–777. doi: 10.1016/j.bbrc.2006.05.192. [DOI] [PubMed] [Google Scholar]
- 4.Blake DJ, Nawrotzki R, Peters MF, Froehner SC, Davies KE. Isoform diversity of dystrobrevin, the murine 87-kDa postsynaptic protein. J Biol Chem. 1996;271:7802–7810. doi: 10.1074/jbc.271.13.7802. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JR, Melcer I. The enzymic oxidation of tryptophan to 5-hydroxytryptophan in the biosynthesis of serotonin. J Pharmacol Exp Ther. 1961;132:265–268. [PubMed] [Google Scholar]
- 6.D’Souza VN, Nguyen TM, Morris GE, Karges W, Pillers DA, Ray PN. A novel dystrophin isoform is required for normal retinal electrophysiology. Hum Mol Genet. 1995;4:837–842. doi: 10.1093/hmg/4.5.837. [DOI] [PubMed] [Google Scholar]
- 7.Granger BL, Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980;22:727–738. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- 8.Izmiryan A, Cheraud Y, Khanamiryan L, Leterrier JF, Federici T, Peltekian E, et al. Different expression of synemin isoforms in glia and neurons during nervous system development. Glia. 2006;54:204–213. doi: 10.1002/glia.20378. [DOI] [PubMed] [Google Scholar]
- 9.Lederfein D, Levy Z, Augier N, Mornet D, Morris G, Fuchs O, et al. A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscle tissues. Proc Natl Acad Sci USA. 1992;89:5346–5350. doi: 10.1073/pnas.89.12.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lidov HG, Selig S, Kunkel LM. Dp140: a novel 140 kDa CNS transcript from the dystrophin locus. Hum Mol Genet. 1995;4:329–335. doi: 10.1093/hmg/4.3.329. [DOI] [PubMed] [Google Scholar]
- 11.McKinney J, Knappskog PM, Haavik J. Different properties of the central and peripheral forms of human tryptophan hydroxylase. J Neurochem. 2005;92:311–320. doi: 10.1111/j.1471-4159.2004.02850.x. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno Y, Nonaka I, Hirai S, Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993;119:43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, et al. Desmuslin, an intermediate filament protein that interacts with alpha-dystrobrevin and desmin. Proc Natl Acad Sci USA. 2001;98:6156–6161. doi: 10.1073/pnas.111153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuno Y, Puca AA, O’Brien KF, Beggs AH, Kunkel LM. Genomic organization and single-nucleotide polymorphism map of desmuslin, a novel intermediate filament protein on chromosome 15q26.3. BMC Genet. 2001;2:8. doi: 10.1186/1471-2156-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno Y, Guyon JR, Watkins SC, Mizushima K, Sasaoka T, Imamura M, et al. Beta-synemin localizes to regions of high stress in human skeletal myofibers. Muscle Nerve. 2004;30:337–346. doi: 10.1002/mus.20111. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno Y, Guyon JR, Ishii A, Hoshino S, Ohkoshi N, Tamaoka A, et al. Beta-synemin expression in cardiotoxin-injected rat skeletal muscle. BMC Musculoskelet Disord. 2007;8:40. doi: 10.1186/1471-2474-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuno Y, Guyon JR, Okamoto K, Kunkel LM. Synemin expression in brain. Muscle Nerve. 2007;36:497–504. doi: 10.1002/mus.20847. [DOI] [PubMed] [Google Scholar]
- 18.Ozawa E, Yoshida M, Suzuki A, Mizuno Y, Hagiwara Y, Noguchi S. Dystrophin-associated proteins in muscular dystrophy. Hum Mol Genet. 1995;4:1711–1716. doi: 10.1093/hmg/4.suppl_1.1711. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa E, Mizuno Y, Hagiwara Y, Sasaoka T, Yoshida M. Molecular and cell biology of the sarcoglycan complex. Muscle Nerve. 2005;32:563–576. doi: 10.1002/mus.20349. [DOI] [PubMed] [Google Scholar]
- 20.Owen MJ, Craddock N, O’Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Sadoulet-Puccio HM, Khurana TS, Cohen JB, Kunkel LM. Cloning and characterization of the human homologue of a dystrophin related phosphoprotein found at the Torpedo electric organ post-synaptic membrane. Hum Mol Genet. 1996;5:489–496. doi: 10.1093/hmg/5.4.489. [DOI] [PubMed] [Google Scholar]
- 22.Sadoulet-Puccio HM, Feener CA, Schaid DJ, Thibodeau SN, Michels VV, Kunkel LM. The genomic organization of human dystrobrevin. Neurogenetics. 1997;1:37–42. doi: 10.1007/s100480050006. [DOI] [PubMed] [Google Scholar]
- 23.Sadoulet-Puccio HM, Rajala M, Kunkel LM. Dystrobrevin and dystrophin: an interaction through coiled-coil motifs. Proc Natl Acad Sci USA. 1997;94:12413–12418. doi: 10.1073/pnas.94.23.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 25.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun N, Critchley DR, Paulin D, Li Z, Robson RM. Human alpha-synemin interacts directly with vinculin and metavinculin. Biochem J. 2008;409:657–667. doi: 10.1042/BJ20071188. [DOI] [PubMed] [Google Scholar]
- 27.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titeux M, Brocheriou V, Xue Z, Gao J, Pellissier JF, Guicheney P, et al. Human synemin gene generates splice variants encoding two distinct intermediate filament proteins. Eur J Biochem. 2001;268:6435–6448. doi: 10.1046/j.0014-2956.2001.02594.x. [DOI] [PubMed] [Google Scholar]
- 29.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 30.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351:1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- 32.Xue ZG, Cheraud Y, Brocheriou V, Izmiryan A, Titeux M, Paulin D, et al. The mouse synemin gene encodes three intermediate filament proteins generated by alternative exon usage and different open reading frames. Exp Cell Res. 2004;298:431–444. doi: 10.1016/j.yexcr.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Zill P, Büttner A, Eisenmenger W, Bondy B, Ackenheil M. Regional mRNA expression of a second tryptophan hydroxylase isoform in postmortem tissue samples of two human brains. Eur Neuropsychopharmacol. 2004;14:282–284. doi: 10.1016/j.euroneuro.2003.10.002. [DOI] [PubMed] [Google Scholar]