Abstract
The present review addresses the literature regarding the sensitivity and specificity of the various diagnostic methods for evaluating non-immediate (ie, occurring more than 1 hour after drug administration) hypersensitivity reactions associated with β-lactams and other antibiotics, anticonvulsants, heparins, iodinated contrast media, etc. Such reactions include several clinical entities, which range from mild reactions, such as maculopapular rash and delayed-appearing urticaria, to severe ones, such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson syndrome, and toxic epidermal necrolysis (TEN). Clinical and laboratory studies indicate that a cell-mediated pathogenic mechanism is often involved in maculopapular rashes. However, this mechanism has also been demonstrated in other non-immediate reactions, such as urticarial and/or angioedematous manifestations, TEN, bullous exanthems, and AGEP. Patch tests, together with delayed-reading intradermal tests, lymphocyte transformation tests, and challenges, are useful tools for evaluating non-immediate drug eruptions. Patch tests can be performed with any form of commercial drugs and are safer than intradermal tests. However, patch tests are less sensitive than intradermal tests, and their sensitivity may vary, depending on the vehicle used.
Keywords: delayed-reading intradermal tests, non-immediate reactions, patch tests
In recent years, increasing attention has been paid to non-immediate (ie, occurring more than 1 hour after drug administration)[1] hypersensitivity reactions to systemically administered drugs. The main non-immediate reactions are maculopapular rashes and delayed-appearing urticaria. In addition, drugs can elicit exfoliative dermatitis, acute generalized exanthematous pustulosis (AGEP), more severe bullous exanthems such as Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). Furthermore, drugs can cause hematologic abnormalities, interstitial nephritis, pneumonitis, hepatitis, and vasculitis. Cutaneous eruptions sometimes occur as part of a generalized syndrome, which is referred to as the hypersensitivity syndrome and is characterized by a triad of fever, skin rash, and internal organ involvement [2-4].
Clinical and laboratory studies indicate that a cellmediated pathogenic mechanism is often involved in maculopapular rashes. However, this mechanism has also been demonstrated in other non-immediate reactions, such as urticarial and/or angioedematous manifestations, TEN, erythema multiforme, bullous exanthems, AGEP, fixed eruptions, and flexural exanthems [3,4].
With regard to the diagnostic tools, patch tests, together with delayed-reading intradermal tests, lymphocyte transformation tests (LTTs), and challenges, can be used for evaluating non-immediate reactions to drugs [1,3-7]. At the beginning of this decade, almost simultaneously, the European Society of Contact Dermatitis (ESCD) and the European Network on Drug Allergy (ENDA; the European Academy of Allergology and Clinical Immunology interest group on drug hypersensitivity) devised the guidelines for performing skin and patch tests in the diagnosis of cutaneous adverse drug reactions (Tables 1, 2, 3, 4) [6,7].
Table 1.
Drug Patch Testing
| Characteristics | ESCD [6] | ENDA [7] |
|---|---|---|
| Time interval* | 6 wk-6 mo | 3 wk-3 mo |
| Site | Upper back | Upper back |
| Reading | 20 min, D2, (D3), D4, D7 | D2, D3, (D4) |
| Scoring | ICDRG criteria† | EECDRG criteria‡ |
D = day; EECDRG = European Environmental Contact Dermatitis Research Group; ENDA = European Network on Drug Allergy; ESCD = European Society of Contact Dermatitis; ICDRG = International Contact Dermatitis Research Group.
*Time interval between the complete healing of cutaneous adverse reactions and the allergologic evaluation.
†0 = no reaction; ? = doubtful reaction; + = weak (non-vesicular) reaction; ++ = strong (edematous or vesicular) reaction; +++ = extreme reaction.
‡0 = no reaction; ? = faint erythema; + = erythema, infiltration, possibly discrete papules; ++ = erythema, infiltration, papules, vesicles; ++++ = intense erythema, infiltration, coalescing vesicles.
Table 2.
Vehicles and Concentrations Suggested by the ESCD[6] for Patch Testing with Specific Drugs
| Drug | Vehicle | Concentration (%) |
|---|---|---|
| Acyclovir | Pet/Aq | 1-10 |
| β-Lactams | Pet | 5-10 |
| Carbamazepine | Pet | 1-10 |
| Celecoxib | Pet | 5-10 |
| Corticosteroids | Aq/Al | Up to 30 |
| Ganciclovir | Aq | 20 |
| Steroid hormones | Pet/Aq/Al | Up to 30 |
Al = alcohol; Aq = water; ESCD = European Society of Contact Dermatitis; Pet = petrolatum.
Table 3.
Patch Test Concentrations Used in the Literature and in Practice
| Antibiotic | DKG | De Groot | Barbaud | Others |
|---|---|---|---|---|
| Penicillin G | 5% Pet | Pure 1% Pet 10,000 IU Pet |
Pure in powder with sodium citrate* | Romano: 5,000 IU/g Pet Bruynzeel: 20% w/w |
| Other penicillins | 5% Pet | Pure 1% Pet |
Pure in powder* | Romano: 5% Pet Bruynzeel: 20% w/w |
| Cephalosporins | 5% Pet | 20% Pet or pure 0.5% Aq |
Pure in powder* | Bruynzeel: 20% w/w |
| Cotrimoxazole | Trimethoprim 5% Pet Sulphamethoxazole 5% Pet |
Sulphonamide (not specified): 5% Pet |
80 mg/mL in Aq | |
| Tetracycline-HCl | 2% Pet | 3% Pet 5% Pet |
Doxycycline: 20 mg/mL in Aq | |
| Gentamicin sulphate Ciprofloxacin, ofloxacin | 20% Pet 5% Pet |
20% Pet | Norfloxacin: in powder from pill* | |
| Erythromycin | 1% Pet | 1% Pet 5% Pet 10% Pet |
Pure in powder* | |
| Pristinamycine Carbamazepine |
Pure in powder* Pure in powder* |
Adapted from Brockow et al. [7]
Aq = water; DKG = German contact allergy group (test concentrations in the German practice); Pet = petrolatum (Vaseline); w/w = watery solution.
*All of these preparations were tested pure and diluted to 30% in water and in petrolatum.
Table 4.
Drug Intradermal Testing
| Characteristics | ESCD [6] | ENDA [7] |
|---|---|---|
| Time interval* | 6 wk-6 mo | 3 wk-3 mo |
| Site | Volar forearm skin | Volar forearm skin |
| Reagents | Sterile solutions (1/10,000 → 1/10) in phenolated saline or in 0.9% saline | Sterile solutions (1/100,000 → 1/1) in 0.9% saline (non-hydrosoluble drugs → in DMSO) |
| Amount | 0.04 mL | 0.02-0.05 mL |
| Reading | 30 min, 6 h, D1, D7 | 20 min, D1, D3 |
| Documentation/scoring | By measuring the diameter of the papule | Infiltrate erythema = positive reaction |
| Contraindications* | Erythema multiforme, SJS, TEN, leukocytoclastic vasculitis | None |
D = day; DMSO = dimethyl sulphoxide; ENDA = European Network on Drug Allergy; ESCD = European Society of Contact Dermatitis; SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis.
*Time interval between the complete healing of cutaneous adverse reactions and the allergologic evaluation.
Patch Tests
Patch, or epicutaneous, testing is useful in diagnosing eczematous contact forms of allergy such as those observed in pharmaceutical workers. Patch-test positivity can also occur in non-immediate cutaneous reactions to systemically administered drugs such as penicillins and anticonvulsants [5-8].
In a patch test, the allergen is usually fixed on the back of the patient for 2 days. Patch tests are done on the upper back on unaffected, untreated, and uncleaned skin using Finn chambers or an equivalent fixed with hypoallergic tape. Systemic glucocorticoids or immunosuppressive therapy should be discontinued at least 1 month before patch testing [6]. Topical glucocorticoids should not be used at the site of patch tests for at least 2 weeks before their application. However, large doses of topical glucocorticoids away from the test site may have the same effect as low doses of systemic glucocorticoids [7]. There are slight differences between the aforementioned guidelines regarding the time interval between the complete healing of cutaneous adverse reactions and the allergologic evaluation, the time of readings, and the scoring (see Table 1). In effect, the criteria of the International Contact Dermatitis Research Group are similar to those of the European Environmental Contact Dermatitis Research Group.
Generally, readings should be done when the patch test is removed (ie, 48 hours after its application) and 2 days later. In negative cases, additional readings in the subsequent days are recommended. Sometimes reactions to patch testing occur earlier than 2 days (eg, after 24 hours), as in the case of abacavir, [9] or much later, as in the case of glucocorticoids and β-lactams [10,11]. In effect, positive responses to patch tests with glucocorticoids or β-lactams have been observed 6 and 7 days after testing, respectively [10,11]. Therefore, patients should be instructed to report any reactions occurring after the physician's last negative reading.
With regard to the drug concentrations, according to the ESCD, pure substances obtained from the manufacturer should be tested at concentrations up to 10% in petrolatum and, if possible, also diluted at 10% in water or alcohol; the powder obtained from tablets and pills should be used at concentrations up to 30% in both petrolatum and water; and liquid preparations should be tested both as is and diluted at 30% in water [6].
Table 2 shows the vehicles and concentrations recommended for patch testing certain specific drugs. β-Lactams should be tested at 5 to 10% in petrolatum; false-negative results were observed by Barbaud and colleagues when water was used as a vehicle [6].
Table 3 displays information on concentrations provided by the ENDA; it is based on the experience of the DKG (the German contact allergy group) and that of some other authors [7]. All concentrations except one (concerning carbamazepine) pertain to antibiotics, such as β-lactams, quinolones, cotrimoxazole, tetracyclines, and gentamicin.
Patch tests can give false-negative results, mainly because of poor penetration of the drug into the epidermis. For this reason, it is crucial to use different vehicles, such as petrolatum, water, and alcohol. Falsenegative results in drug patch testing may also be due to the fact that a drug metabolite is actually responsible for the reaction or that concomitant factors, such as viral infections, are no longer present [6].
False-positive results were observed by Barbaud and colleagues in patch testing with colchicine at 10% in petrolatum, misoprostol at 30% in petrolatum, and drugs containing sodium lauryl sulphate [6].
Intradermal Tests
Intradermal tests are performed by injecting an allergen solution intradermally, raising a small bleb measuring about 3 mm in diameter. Both the ESCD and the ENDA suggest performing such tests on the volar forearm skin. The amount that the ESCD suggests injecting is 0.04 mL, whereas the amount suggested by the ENDA ranges between 0.02 and 0.05 mL. The ESCD suggests that nonhydrosoluble drugs be dissolved with dimethyl sulphoxide; however, in both guidelines, sterile solutions are mandatory. There are some differences between the aforesaid guidelines regarding the timing of readings (see Table 4). In any case, readings should be taken after 20 to 30 minutes if immediate reactions are also analyzed, and after 24 and 72 hours for evaluation of non-immediate (late) reactions. In negative cases, additional readings (eg, after 1 week) are recommended as time intervals between testing and positive test reactions may vary. In a study by Rosso and colleagues, some patients displayed positive responses to intradermal tests with β-lactams 6 days after testing [11].
As far as documentation and scoring are concerned, the ESCD suggests measuring the diameter of the papule, whereas the ENDA considers an infiltrate erythema as a positive reaction and suggests measuring the diameter of the reaction and performing a morphologic description of the erythematous swelling, erythematous infiltrate, erythema only, and eczema with papulation and/or vesicles.
The ESCD considers as contraindications severe cutaneous reactions such as erythema multiforme, TEN, SJS, and leukocytoclastic vasculitis, whereas in such cases, the ENDA advises performing first patch tests and then, in case of negative results, intradermal tests using the highest dilution (see Table 4) [6,7].
Patch tests can be done with any form of drugs and are safer than intradermal tests. In effect, systemic reactions to patch tests are extremely rare. However, patch tests are less sensitive than intradermal tests, and their sensitivity may vary, depending on the vehicle used. For example, Gonçalo and colleagues observed false-positive results when testing estrogens diluted in water or petrolatum but obtained truly positive results when steroid hormones were diluted in alcohol [12]. Moreover, in case of reactions to drugs in the form of syrups, pills, tablets, and capsules, preservatives, colouring agents, and excipients should also be tested. On the other hand, intradermal tests require sterile solutions and are less safe than patch tests, but they are more sensitive.
Specific Drugs
β-Lactam Antibiotics
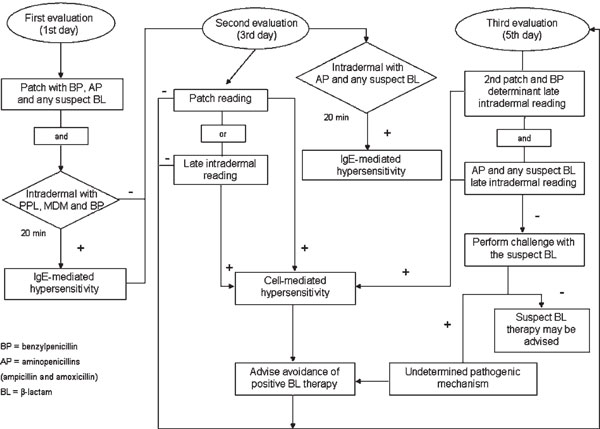
These antibiotics can provoke all kinds of non-immediate reactions, particularly maculopapular rashes. Recently, members of the ENDA devised an algorithm for in vivo allergologic evaluation of non-immediate reactions to β-lactams, which combines skin tests and patch tests with a common panel of reagents--including penicillin determinants (penicilloylpolylysine, minor determinant mixture, and benzylpenicillin) and the two most used aminopenicillins (ampicillin and amoxicillin)--as well as the suspect β-lactam (Figure 1)[8]; provocation tests with the latter are also suggested in selected cases, such as those indicated in the recent position paper of the ENDA group [13].
Figure 1.
Algorithm for in vivo allergologic evaluation of non-immediate reactions to β-lactams. Adapted from Romano A et al. [8]
In the case of severe reactions, such as AGEP, SJS, and TEN, patch tests (and/or LTTs) should be used as the first line of investigation.
In a study by our group that evaluated 241 subjects with non-immediate reactions to penicillins with a protocol identical to that of the ENDA, patch tests with benzylpenicillin were positive in 7.5% of patients, whereas ampicillin and amoxicillin elicited positive reactions in 37.3%.14 Delayed-reading intradermal tests with a minor determinant mixture and benzylpenicillin were positive in 12% of cases, whereas those with ampicillin and amoxicillin were positive in 39%. However, considering only the 166 subjects with aminopenicillin-associated maculopapular exanthems, patch tests and delayed-reading intradermal tests with ampicillin and amoxicillin were positive in 52.4% and 54.2%, respectively. Moreover, all but 1 of the 64 subjects who were negative to the allergologic tests tolerated provocation tests with the suspect aminopenicillin, indicating that most of the results were not falsely negative. However, several cases with non-immediate reactions to β-lactams displaying skin or patch test negativity and challenge positivity have been reported, in particular by Blanca's group [1,14-17]. Therefore, further studies should be performed in large samples of subjects with non-immediate reactions to β-lactams to fully establish the negative predictive value of skin and patch tests.
In our study, the specificity of delayed-reading intradermal tests and patch tests with penicillin determinants, as well as with ampicillin and amoxicillin, was 100%: all 30 healthy subjects, who had previously been treated with one or more of these penicillins, showed negative results [1].
With regard to other β-lactams, there are only a few large studies and no definitive data on skin test sensitivity. In a recent study, cephalosporins elicited a positive patch test reaction in 12 (4.1%) of 290 patients with cutaneous adverse reactions to these β-lactams, whereas meropenem caused positive patch test reactions in one of two patients [18]. It is interesting to note that only 1 of the 75 patients with cutaneous eruptions associated with cephalosporins and negative results in allergologic tests reacted to challenges with the suspect cephalosporins (cefadroxil or cephalexin).
Considering the literature data, delayed-reading intradermal tests appear to be somewhat more sensitive than patch tests but also less specific [8]. In some studies, subjects with delayed intradermal test positivity and patch test negativity were challenged, with positive responses in six of nine cases [1,14,19]. Therefore, false intradermal test positivities have been observed, whereas all 33 reported subjects who displayed patch test positivity and were challenged with the positive drug reacted to the challenge [8].
Non-β-Lactam Antibiotics
Sulphonamides are frequently associated with nonimmediate manifestations such as fixed eruptions and maculopapular rashes. Some reactions may be T cell mediated as positive patch tests have been reported in patients with fixed eruptions caused by cotrimoxazole (trimethoprim + sulphamethoxazole) [20,21]. However, patch tests should be applied to the site of the fixed drug eruption. In the aforementioned study, which evaluated 947 patients with cutaneous adverse drug reactions, sulphamethoxazole and trimethoprim were frequently assessed by patch tests [18]. Sulphamethoxazole elicited a positive reaction in 1 (0.4%) of 215 patients and trimethoprim in 10 (6.2%) of 163 patients. The test with trimethoprim was positive at the previous fixed drug eruption site in 2 of 10 cases. In the same study, clindamycin elicited positive patch test responses in 12 (19%) of 63 patients and gentamicin and isoniazid in 1 of 2 patients. On the other hand, macrolides, tetracyclines, and quinolones were tested in 130, 108, and 32 patients, respectively, but no positive patch test reactions were observed. For patch testing, drugs were diluted to 20 or 30% in white petrolatum and/or normal saline or occasionally in ethanol. In a study by Schmid and colleagues, however, patch tests were positive to responsible quinolones (ciprofloxacin, norfloxacin, or moxifloxacin, diluted to 10 or 25% in white petrolatum) in three of six patients who had experienced exanthems or AGEP, whereas the LTT was positive in all [22].
Anticonvulsants
Anticonvulsant or antiepileptic drugs, particularly aromatic ones (phenytoin, carbamazepine, oxcarbazepine, and phenobarbital), can provoke cutaneous eruptions and a severe hypersensitivity syndrome [23].
Patch tests can be useful tools for diagnosing such hypersensitivity reactions. However, few studies have been carried out with patch tests on samples of at least 10 subjects with adverse reactions to anticonvulsants, [18,24-29] and most of them refer only to carbamazepine [24,26-29]. With regard to this drug, the percentage of positive responses to patch tests ranged from 18.9% (7 of 37 patients)[18] to 66.6% (4 of 6)[25]; when metabolites of carbamazepine were also used in patch testing, the frequency of positive responses increased to 69.2% [29]. Different carbamazepine concentrations (ranging from 1% to pure powder) in different vehicles (petrolatum, distilled water, ethanol) were used, and positive reactions were seen at all concentrations. However, a severe systemic exfoliative eruption after patch testing with crushed 200 mg carbamazepine tablets has been reported [30]. Thus, percentages of carbamazepine up to 20% weight/weight in white petrolatum seem to be sufficient to induce positive patch test reactions and could also be recommended to avoid the risk of systemic reactions. On the other hand, some weak reactions may be missed.
As far as hypersensitivity reactions to anticonvulsants other than carbamazepine are concerned, most studies are reports of single cases. In a previously cited study, Osawa and colleagues patch-tested 23 subjects with cutaneous eruptions associated with anticonvulsant therapy: 6 of them had reacted to carbamazepine, 10 to phenobarbital, 5 to sodium valproate, and 2 to phenytoin [25]. Carbamazepine was tested at a concentration of 1% in white petrolatum, phenobarbital at 1 and 20%, and sodium valproate at 1 and 10%. Thirteen (56.5%) of 23 subjects displayed positive responses to patch tests: specifically, 4 of 6 to carbamazepine, 4 of 10 to phenobarbital, 4 of 5 to sodium valproate, and 1 of 2 to phenytoin. In this study, 9 patients with adverse reactions to phenobarbital and 1 patient with a reaction to phenytoin were also evaluated by delayed-reading intradermal tests. It is interesting to note that intradermal test sensitivity was lower than that of patch tests.
In the study by Lammintausta and Kortekangas-Savolainen, 10 (19.6%) of 51 patients were positive to patch tests: 7 of 37 to carbamazepine, 2 of 6 to phenytoin, 1 of 8 to oxcarbazepine, and none of 5 to lamotrigine [18].
Heparins
Heparins can be classified according to their molecular weight as unfractionated heparins (UFHs; 10-20 kD: heparin calcium, heparin sodium), low-molecular-weight heparins (LMWHs; 4-6 kD: enoxaparin, dalteparin, centoparin, repivarin, nadroparin, tinzaparin), and ultralow-molecular-weight heparins (ULMWHs 1.7 kD: fondaparinux). Delayed hypersensitivity reactions have been reported with UFHs, LMWHs, and heparinoids (danaparoid sodium, glycosaminoglycane polysulphate, and pentosanpolysulphate). Such reactions usually consist of erythematous, infiltrated, or vesicular (eczema-like) itchy plaques usually confined to the injection sites but sometimes accompanied by a maculopapular rash [31-33].
A cell-mediated pathogenic mechanism has been demonstrated in patients who have delayed-type hypersensitivity reactions [31-33]. In evaluating such reactions, delayed-reading intradermal tests are more sensitive than patch tests; generally, patch tests are performed with undiluted compounds, whereas intradermal tests are done with heparins diluted 1 to 10 in normal saline. However, subcutaneous provocation tests are considered to be the most reliable diagnostic method because intradermal testing may produce false-negative results. Subcutaneous provocation tests are performed with 0.1 mL of an undiluted compound, and subjects must be checked until the fifth day [31].
Iodinated Contrast Media
Non-immediate reactions to iodinated contrast media (ICM) consist mainly of cutaneous manifestations, such as maculopapular rashes, fixed eruptions, erythema multiforme, and urticarial eruptions.
Recent data strongly indicate that most of these manifestations are T cell-mediated hypersensitivity reactions. Several investigators have shown positive patch and/or delayed-reading intradermal tests to the culprit ICM in subjects with non-immediate reactions to ICM [34-36]. In particular, positive delayed-reading skin tests and/or patch tests for the responsible compound have been found in about 100 patients with ICM-induced late-onset skin reactions. Approximately 50% of such patients presented positive responses not only to the culprit ICM but also to other, structurally similar compounds [34]. Generally, intradermal tests are performed with ICM diluted 1 to 10 in normal saline, whereas patch tests are performed with undiluted ICM. However, these two methods can display a different sensitivity. In a recent study regarding delayed reactions to ICM, only 2 of 15 patients had positive patch tests, whereas 8 had positive delayed-reading intradermal tests [37]. Thus, it seems that the latter tests are more reliable than patch tests in delayed skin reactions, but larger studies are needed to reach a definitive conclusion.
Glucocorticoids
These drugs are used both topically and systemically. They induce allergic contact dermatitis (ACD) far more often than systemic drug reactions. Most articles dealing with patch testing with glucocorticoids were in the context of ACD, and in this particular context, the sensitivity of patch testing (especially if testing is performed with an extended series of glucocorticoids) is very good [38]. Positive patch tests may be indicative of topical sensitization only, whereas systemic administration may be well tolerated. Many patients with positive patch tests to tixocortol pivalate (a marker of allergy to hydrocortisone) have received systemic hydrocortisone or prednisone without developing a generalized eruption. Of course, the opposite may also be seen, when a patient previously sensitized by topical exposure to a glucocorticoid develops an extensive dermatitis after systemic administration (systemic contact dermatitis), but, fortunately, this occurrence seems to be rare [39].
Non-immediate hypersensitivity reactions to systemic glucocorticoids generally consist of eczematous or exanthematous skin eruptions [40,41]. In effect, together with delayed-appearing urticarial eruptions, maculopapular exanthems were the main non-immediate reactions reported by the 38 patients recently studied by Padial and colleagues [42]. Most of these subjects had been treated for osteoarticular diseases, and glucocorticoids had been administered intralesionally in 71% of cases.
The anti-inflammatory activity of glucocorticoids can cause problems in patch testing. In fact, if the glucocorticoid is tested at too high concentrations, the antiinflammatory effect may predominate, and patch test results may be negative. On the other hand, if the concentration used for patch testing on intact skin is too low, a negative reaction is not uncommon; such a concentration may elicit a positive reaction only when applied on eczematous skin [10].
In most studies, glucocorticoids were tested at a concentration of 1%. However, some authors suggested that lower concentrations should also be used because of the inhibition of hypersensitivity reactions at 1% [39,43]. The choice of vehicles for patch testing is also important [44]. Matura and Goossens used a 1% concentration of tixocortol pivalate in both ethanol and petrolatum and did not observe any statistical difference in the number of positive reactions [39]. However, patients tested with 0.1% budesonide in both ethanol and petrolatum presented significantly more positive reactions to budesonide in ethanol.
Anti-inflammatory and vasoconstrictor effects of glucocorticoids may hide positive reactions after the removal of patch tests, suggesting the need for further readings 2 to 7 days later [45].
Patch tests and delayed-reading intradermal tests can display a different sensitivity, according to the glucocorticoid assessed. Generally, delayed-reading intradermal testing appears to be more sensitive than patch testing [46]. In any case, the sensitivity of patch testing and delayedreading intradermal testing is limited. Therefore, provocation tests are often necessary to diagnose hypersensitivity to glucocorticoids. In the recent study by Padial and colleagues, only 2 of the 38 patients with non-immediate reactions to glucocorticoids displayed positive delayedreading intradermal tests and patch tests to the responsible drugs, whereas 21 of the 32 patients who agreed to undergo challenges reacted to them [42].
Miscellanea
Although many cutaneous reactions to non-steroidal antiinflammatory drugs (NSAIDs) appear to be induced by a non-allergic hypersensitivity pathogenic mechanism, in some non-immediate ones to NSAIDs, such as diclofenac, piroxicam, acetaminophen, and pyrazolones, a cell-mediated hypersensitivity mechanism may be involved, and patch testing can be useful in assessing such reactions [5,18].
The same pathogenic mechanism has been demonstrated in patients who developed delayed hypersensitivity reactions, mainly maculopapular rashes, to drugs such as diltiazem, captopril, pseudoephedrine, and stepronin, on the basis of positive responses to patch tests and/or delayed-reading intradermal tests [5,18].
Conclusion
Patch tests, together with delayed-reading intradermal tests, are useful tools for evaluating non-immediate reactions to systemically administered drugs. The sensitivity of patch testing alone is low (range of 10.8 to 37.5% depending on previous publications)[18,25,47]; therefore, in many cases, provocation tests are necessary for diagnosis.
However, patch test sensitivity varies with the type of eruption (higher in eczematous, maculopapular, and AGEP; lower in urticaria, SJS, and TEN; and nil in vasculitis), [6,48] as well as with the drug involved (higher with diltiazem, abacavir, β-lactam antibiotics, anticonvulsants, tetrazepam, and pseudoephedrine) [6,9,47].
Much research needs to be done to standardize both patch tests and delayed-reading intradermal tests (particularly those performed with non-injectable drugs), improve their sensitivity, and establish their negative predictive value.
References
- Romano A, Quaratino D, Di Fonso M. A diagnostic protocol for evaluating nonimmediate reactions to aminopenicillins. J Allergy Clin Immunol. 1999;103:1186–90. doi: 10.1016/S0091-6749(99)70197-1. [DOI] [PubMed] [Google Scholar]
- Bocquet H, Bagot M, Roujeau J-C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms-DRESS) Semin Cutan Med Surg. 1996;15:250–7. doi: 10.1016/S1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:683–93. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- Posadas S, Pichler WJ. Delayed drug hypersensitivity reactions--new concepts. Clin Exp Allergy. 2007;37:989–99. doi: 10.1111/j.1365-2222.2007.02742.x. [DOI] [PubMed] [Google Scholar]
- Romano A, Torres MJ, Quaratino D. Diagnostic evaluations of delayed hypersensitivity to systemically administered drugs. Allergy. 1999;54(Suppl 58):23–7. [PubMed] [Google Scholar]
- Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45:321–8. doi: 10.1034/j.1600-0536.2001.450601.x. [DOI] [PubMed] [Google Scholar]
- Brockow K, Romano A, Blanca M. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51. doi: 10.1034/j.1398-9995.2002.13027.x. [DOI] [PubMed] [Google Scholar]
- Romano A, Blanca M, Torres MJ. Diagnosis of nonimmediate reactions to β-lactam antibiotics. Allergy. 2004;59:1153–60. doi: 10.1111/j.1398-9995.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- Phillips EJ, Wong GA, Kaul R. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–81. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- Dooms-Goossens A, Verschaeve H, Degreef H, van Berendrocks J. Contact allergy to hydrocortisone and tixocortol pivalate: problems in detection of corticosteroid sensitivity. Contact Dermatitis. 1986;14:94–102. doi: 10.1111/j.1600-0536.1986.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Rosso R, Mattiacci G, Bernardi ML. Very delayed reactions to β-lactam antibiotics. Contact Dermatitis. 2000;42:293–5. [PubMed] [Google Scholar]
- Gonçalo M, Oliveira HS, Monteiro C. Allergic and systemic contact dermatitis from estradiol. Contact Dermatitis. 1999;40:58–9. doi: 10.1111/j.1600-0536.1999.tb05989.x. [DOI] [PubMed] [Google Scholar]
- Aberer W, Bircher A, Romano A. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–63. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- Romano A, Viola M, Mondino C. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169–74. doi: 10.1159/000065876. [DOI] [PubMed] [Google Scholar]
- Aihara M, Ikezawa Z. Evaluation of the skin test reactions with delayed type rash induced by penicillins and cephalosporins. J Dermatol. 1987;14:440–8. doi: 10.1111/j.1346-8138.1987.tb03607.x. [DOI] [PubMed] [Google Scholar]
- Terrados S, Blanca M, Garcia J. Nonimmediate reactions to betalactams: prevalence and role of the different penicillins. Allergy. 1995;50:563–7. doi: 10.1111/j.1398-9995.1995.tb01200.x. [DOI] [PubMed] [Google Scholar]
- Luque I, Leyva L, Torres MJ. In vitro T-cell responses to β-lactam drugs in immediate and nonimmediate allergic reactions. Allergy. 2001;56:611–8. doi: 10.1034/j.1398-9995.2001.000115.x. [DOI] [PubMed] [Google Scholar]
- Lammintausta K, Kortekangas-Savolainen O. The usefulness of skin tests to prove drug hypersensitivity. Br J Dermatol. 2005;152:968–74. doi: 10.1111/j.1365-2133.2005.06429.x. [DOI] [PubMed] [Google Scholar]
- López Serrano C, Vilas F, Cabañas R, Contreras J. Delayed hypersensitivity to β-lactams. J Invest Allergol Clin Immunol. 1994;4:315–9. [PubMed] [Google Scholar]
- Alanko K. Topical provocation of fixed drug eruption. A study of 30 patients. Contact Dermatitis. 1994;31:25–7. doi: 10.1111/j.1600-0536.1994.tb01900.x. [DOI] [PubMed] [Google Scholar]
- Lee AY. Topical provocation in 31 cases of fixed drug eruption: change of causative drugs in 10 years. Contact Dermatitis. 1998;38:258–60. doi: 10.1111/j.1600-0536.1998.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones. Clin Exp Allergy. 2006;36:59–69. doi: 10.1111/j.1365-2222.2006.02402.x. [DOI] [PubMed] [Google Scholar]
- Romano A, Pettinato R, Andriolo M. Hypersensitivity to aromatic anticonvulsants: in vivo and in vitro cross-reactivity studies. Curr Pharm Des. 2006;12:3373–81. doi: 10.2174/138161206778193962. [DOI] [PubMed] [Google Scholar]
- Motley RJ, Reynolds AJ. Carbamazepine and patch testing [letter] Contact Dermatitis. 1989;21:285–6. doi: 10.1111/j.1600-0536.1989.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Osawa J, Naito S, Aihara M. Evaluation of skin test reactions in patients with non-immediate type drug eruptions. J Dermatol. 1990;17:235–9. doi: 10.1111/j.1346-8138.1990.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Prens EP, Troost RJJ, van Parys JAP. The value of the lymphocyte proliferation assay in detection of carbamazepine allergy. Contact Dermatitis. 1990;23:292. doi: 10.1111/j.1600-0536.1990.tb05136.x. [DOI] [Google Scholar]
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254–7. doi: 10.1111/j.1600-0536.1993.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Troost RJJ, van Parys JAP, Hooijkaas H. Allergy to carbamazepine: parallel in vivo and in vitro detection. Epilepsia. 1996;37:1093–9. doi: 10.1111/j.1528-1157.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Lee AY, Choi J, Chey WY. Patch testing with carbamazepine and its main metabolite carbamazepine epoxide in cutaneous adverse drug reactions to carbamazepine. Contact Dermatitis. 2003;48:137–9. doi: 10.1034/j.1600-0536.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- Vaillant L, Camenen I, Lorette G. Patch testing with carbamazepine: reinduction of an exfoliative dermatitis [letter] Arch Dermatol. 1989;125:299. doi: 10.1001/archderm.125.2.299b. [DOI] [PubMed] [Google Scholar]
- Wütschert R, Piletta P, Bounameaux H. Adverse skin reactions to low molecular weight heparins. Drug Saf. 1999;20:515–25. doi: 10.2165/00002018-199920060-00005. [DOI] [PubMed] [Google Scholar]
- Koch P, Münssinger T, Rupp-John C, Uhl K. Delayed-type hypersensitivity skin reactions caused by subcutaneous unfractioned and low-molecular weight heparins: tolerance of a new recombinant hirudins. J Am Acad Dermatol. 2000;42:612–9. doi: 10.1016/S0190-9622(00)90173-7. [DOI] [PubMed] [Google Scholar]
- Bircher AJ, Harr T, Hohenstein L, Tsakiris DA. Hypersensitivity reactions to anticoagulant drugs. Allergy. 2006;61:1432–40. doi: 10.1111/j.1398-9995.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- Christiansen C. Late-onset allergy-like reactions to x-ray contrast media. Curr Opin Allergy Clin Immunol. 2002;2:333–9. doi: 10.1097/00130832-200208000-00007. [DOI] [PubMed] [Google Scholar]
- Brockow K, Christiansen C, Kanny G. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2005;60:150–8. doi: 10.1111/j.1398-9995.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- Guéant-Rodriguez RM, Romano A, Barbaud A. Hypersensitivity reactions to iodinated contrast media. Curr Pharm Des. 2006;12:3359–72. doi: 10.2174/138161206778193999. [DOI] [PubMed] [Google Scholar]
- Vernassiere C, Trechot P, Commun N. Low negative predictive value of skin tests in investigating delayed reactions to radio-contrast media. Contact Dermatitis. 2004;50:359–66. doi: 10.1111/j.0105-1873.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Dooms-Goossens A, Morren M. Results of routine patch tests with corticosteroid series in 2073 patients. Contact Dermatitis. 1992;26:182–91. doi: 10.1111/j.1600-0536.1992.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698–704. doi: 10.1034/j.1398-9995.2000.00121.x. [DOI] [PubMed] [Google Scholar]
- Whitmore SE. Delayed systemic allergic reactions to corticosteroids. Contact Dermatitis. 1995;32:193–8. doi: 10.1111/j.1600-0536.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Ventura MT, Calogiuri GF, Muratore L. Cross-reactivity in cell-mediated and IgE-mediated hypersensitivity to glucocorticoids. Curr Pharm Des. 2006;12:3383–91. doi: 10.2174/138161206778194006. [DOI] [PubMed] [Google Scholar]
- Padial A, Posadas S, Torres MJ. Nonimmediate reactions to systemic corticosteroids suggest an immunological mechanism. Allergy. 2005;60:665–70. doi: 10.1111/j.1398-9995.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- Isaksson M, Bruze M, Goossens A, Lepoittevin JP. Patch testing with budesonide in serial dilutions: the significance of dose, occlusion time and reading time. Contact Dermatitis. 1999;40:24–31. doi: 10.1111/j.1600-0536.1999.tb05972.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson SM, Beck HM. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305–8. doi: 10.1111/j.1600-0536.1996.tb02212.x. [DOI] [PubMed] [Google Scholar]
- Dooms-Goossens A. Corticosteroid contact allergy: a challenge to patch testing. Am J Contact Dermatitis. 1993;4:120–6. [Google Scholar]
- Herbst RA, Lauerma AI, Maibach HI. Intradermal testing in the diagnosis of allergic contact dermatitis: a reappraisal. Contact Dermatitis. 1993;29:1–5. doi: 10.1111/j.1600-0536.1993.tb04527.x. [DOI] [PubMed] [Google Scholar]
- Barbaud A, Reichert-Penetrat S, Tréchot P. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49–58. doi: 10.1046/j.1365-2133.1998.02313.x. [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Chosidow O, Fléchet M-L. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234–6. doi: 10.1111/j.1600-0536.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]