Abstract
The Borrelia burgdorferi Rrp1 protein is a diguanylate cyclase that controls a regulon consisting of ~10% of the total genome. Because Rrp1 lacks a DNA-binding domain, its regulatory capability is most likely mediated through the production of bis-(3′–5′)-cyclic dimeric GMP (c-di-GMP). C-di-GMP binds to and activates the regulatory activity of proteins that harbor a PilZ domain. The occurrence of a PilZ domain within a protein is not in and of itself sufficient to convey c-di-GMP binding, as other structural aspects of the protein are important in the interaction. In this study, we have assessed the expression and c-di-GMP binding ability of the sole PilZ domain-containing protein of B. burgdorferi B31, PlzA. PlzA was determined to be upregulated by tick feeding and to be expressed during mammalian infection. The gene is highly conserved and present in all Borrelia species. Analyses of recombinant PlzA demonstrated its ability to bind c-di-GMP and site-directed mutagenesis revealed that this interaction is highly specific and dependent on Arg residues contained within the PilZ domain. In summary, this study is the first to identify a c-di-GMP effector molecule in a spirochete and provides additional evidence for the existence of a complete c-di-GMP regulatory network in the Lyme disease spirochete, B. burgdorferi.
Keywords: Borrelia, Lyme disease, cyclic-di-GMP, Rrp1, PilZ
Introduction
Lyme disease, caused by Borrelia burgdorferi (Burgdorfer et al., 1982; Benach et al., 1983; Steere et al., 1983), is the most common tick-borne illness in North America with approximately 35 000 cases reported to the Centers for Disease Control and Prevention in 2008. The Lyme disease spirochetes are sustained in an enzootic cycle involving Ixodes ticks and a range of mammals (Burgdorfer et al., 1982; Lane et al., 1991). Differential gene expression is central in the spirochete’s ability to survive the diverse and changing environmental conditions encountered over the course of the enzootic cycle (Revel et al., 2002; Ojaimi et al., 2003, 2005; Fisher et al., 2005; Caimano et al., 2007; Boardman et al., 2008; Ouyang et al., 2008; Rogers et al., 2009b). As in other bacteria, two-component regulatory systems (TCS) are important mediators of adaptive transcriptional responses in B. burgdorferi. Borrelia burgdorferi has only two TCS with demonstrated global regulatory capability (Yang et al., 2003a, b; Rogers et al., 2009b). The Hpk2-Rrp2 TCS works synergistically with RpoN (σ54) to activate the expression of RpoS, which, in turn, positively regulates primarily plasmid-carried genes (Yang et al., 2003a, b; Caimano et al., 2007). In contrast, the Hpk1-Rrp1 TCS, which operates independently of RpoS, primarily regulates genes that are carried by the core Borrelia genome (Rogers et al., 2009b). The core genome consists of the linear chromosome, linear plasmid 54 (lp54) and circular plasmid 26 (cp26) (Rogers et al., 2009b).
The response regulatory protein Rrp1 lacks a DNA-binding domain but harbors a GGDEF domain, which has been associated with diguanylate cyclase (DGC) activity. DGCs convert two molecules of GTP into bis-(3′–5′)-cyclic dimeric GMP (c-di-GMP), an important secondary messenger molecule (Hengge, 2009). C-di-GMP has been demonstrated in several bacteria to regulate biofilm formation, virulence factor production, motility transitions, and cell– cell signaling (reviewed in Cotter & Stibitz, 2007; Hengge, 2009). The study of proteins involved in regulating c-di-GMP levels in other bacteria is complicated by the presence of numerous proteins with these activities. In contrast, in the Borrelia, Rrp1 is the only protein with a GGDEF domain, suggesting that it is solely responsible for the synthesis of c-di-GMP (Ryjenkov et al., 2005). The Borrelia genome also encodes the additional proteins that are required to form a fully functional c-di-GMP regulatory network. ORFs BB0363 and BB0374 encode EAL and HD-GYP domain-containing c-di-GMP phosphodiesterases (PdeA and PdeB, respectively). PDEs, which breakdown c-di-GMP, play a critical role in regulating c-di-GMP levels. C-di-GMP appears to exert its regulatory effects through multiple mechanisms including binding to regulatory proteins and riboswitches (Hickman & Harwood, 2008; Sudarsan et al., 2008). PilZ domain-containing proteins have been demonstrated to bind c-di-GMP and to serve as important downstream effector proteins (Amikam & Galperin, 2006; Benach et al., 2007). Borrelia burgdorferi B31 encodes one protein with a PilZ domain (BB0733; henceforth designated as PlzA). In this study, we demonstrate that PlzA binds specifically to c-di-GMP. The specificity of the interaction was further demonstrated through site-directed mutagenesis. Substitution of specific Arg residues within the PilZ domain completely abolished c-di-GMP binding. Transcriptional analyses revealed that plzA is expressed in spirochetes present in the tissues and organs of infected mice and is upregulated in Ixodes scapularis ticks upon ingestion of the bloodmeal. This study represents an important step forward in the demonstration of a fully functional c-di-GMP regulatory network in the Borrelia, and it represents the first study to identify a c-di-GMP effector protein in any spirochete.
Materials and methods
Bacterial strains and cultivation conditions
Borrelia isolates were cultivated in BSK-H complete media at 33 °C and then shifted to 23, 33, or 37 °C. BSK-H complete media was prepared as described previously using bovine serum albumin from Millipore (lot number 760) (Samuels et al., 1994). Midlog-phase spirochetes were harvested by centrifugation and washed with phosphate-buffered saline (PBS). All isolates used in this study have been described in earlier publications (Rogers & Marconi, 2007; Rogers et al., 2009a, b). A cell pellet of Pseudomonas aeruginosa FRD-1 was kindly provided by Dr Dennis Ohman (Virginia Commonwealth University). Escherichia coli strains and growth conditions are described below as appropriate.
Preparation of B. burgdorferi RNA from cultures and from infected I. scapularis ticks
RNA was isolated from actively growing cultures of B. burgdorferi B31 as described previously (Rogers et al., 2009a, b). To allow for the analysis of specific B. burgdorferi transcript levels in ticks, it was first necessary to infect mice so that naїve ticks could be infected by natural feeding. Mice (C3H/HeJ; Jackson labs) were inoculated with B. burgdorferi B31 A3 (104 cells; intradermal between the shoulder blades) and infection was confirmed by culture of ear biopsies and by serology. The naїve larval ticks, provided by Dr Durland Fish (Yale University), were placed on infected mice (150–200 per mouse) and fed to repletion (48–96 h). The ticks were collected and maintained for 8–10 weeks in desiccator chambers (21 °C; 100% humidity) until molting to the nymphal stage. Two weeks post-molt, pools of 100 infected flat nymphs were used for RNA isolation. The remaining infected flat nymphs (~30) were placed on naїve, uninfected C3H/HeJ mice and fed to repletion. All mice became infected as confirmed by culture positivity of ear tissues. To isolate RNA, 100 flat nymphs or 30 fed nymphs were crushed in glass homogenizers containing 1 mL of TRizol reagent (Invitrogen) and debris was removed by centrifugation (300 g). Supernatants were used for RNA isolation according to the manufacturer’s instructions (Invitrogen). To remove contaminating genomic DNA, RNA samples were treated twice with RNAse-free DNAse using the Ambion DNA free kit.
Preparation of RNA from the organs and tissues of mice infected with B. burgdorferi
Mice were infected as described above with B. burgdorferi B31-5A4 (a clonal derivative of B31 kindly provided by Jon Skare, Texas A&M). Note that for logistical reasons a separate pool of mice were used for these analyses than those described above. Mice were sacrificed at 2, 4, 6 and 13 weeks (five mice per time point) and the urinary bladders were collected and prepared for RNA analyses. RNA was isolated as described previously (Rogers et al., 2009a).
Real-time quantitative reverse trascriptase-PCR (qRT-PCR)
Total RNA isolated from ticks or from urinary bladders of infected mice was reverse transcribed using 10 U AMV reverse transcriptase and random hexamers as described by the supplier (Promega). The cDNA was used as template for qPCR with gene-specific primers (designed using primer express software, version 2.0; Applied Biosystems) as described previously (Rogers et al., 2009a, b). All primers are listed in Table 1. Duplicate or triplicate assays were carried out with each RNA sample and were performed with RNA obtained from two separate pools of flat nymphs, three separate pools of fed nymphs or with RNA from murine bladders. qPCR data were normalized against flaB transcript levels. To verify the purity of the PCR product, melting curve analyses were performed and a no-template negative reaction control was included. The statistical significance of observed expression differences between flat and fed nymphs was evaluated by an unpaired t-test with two-tailed P-values and 95% confidence interval using GRAPHPAD PRISM software (v 5.0) (La Jolla, CA).
Table 1.
Oligonucleotides used in this study
| Primer | Sequence | Description |
|---|---|---|
| Rrp1-QF | CGGGATCGCTTTTTAGCTTT | qRT-PCR analysis of rrp1 in vitro and in vivo |
| Rrp1-QR | TTGAGGTTGCAACAAATGGA | qRT-PCR analysis of rrp1 in vitro and in vivo |
| PlzA-QF | CTTTTGATTATGGGGATGTCG | qRT-PCR analysis of plzA in vitro and in vivo |
| PlzA-QR | AAAGCAATACCAAGCGCAAA | qRT-PCR analysis of plzA in vitro and in vivo |
| FlaB-QF | GCTCCTTCCTGTTGAACACC | qRT-PCR control gene flaB in vitro and in mammalian host |
| FlaB-QR | TTCATGTTGGAGCAAACCAA | qRT-PCR control gene flaB in vitro and in mammalian host |
| FlaB-QF(Tick) | GCAGCTAATGTTGCAAATCTTTTC | qRT-PCR control gene flaB in flat and fed nymphal ticks |
| FlaB-QR(Tick) | TGAGCTCCTTCCTGTTGA | qRT-PCR control gene flaB in flat and fed nymphal ticks |
| Rrp1-pMAL-BamHIF | CGGATCCGTGGAAATGATAATTAAAGATAAA | Cloning of rrp1 into pMAL-c4x |
| Rrp1-pMAL-SalIR | CCCGTCGACTTAATATCTAAACTGATTTCTTCC | Cloning of rrp1 into pMAL-c4x |
| PlzA-pMAL-BamHIF | GGATCCTTGTTTAGTATTTTTATATTCAAAAAAAGGAGAAAGGAGAGGT | Cloning of plzA into pMAL-c4x |
| PlzA-pMAL-SalIR | GTCGACTTAATTGAAATAATCATGGATCAACATAGTATACTCAAGTGGTA | Cloning of plzA into pMAL-c4x |
| PlzA-R150DR154D-F | CCTGGGCAAAATCAGGATATTCATGAGGATAAT | Site directed mutagenesis to R 150D-R154D of PlzA |
| PlzA-R150DR154D-R | ATTATCCTCATGAATATCCTGATTTTGCCCAGG | Site directed mutagenesis to R 150D-R154D of PlzA |
| PlzA-R150KR154K-F | CCTGGGCAAAATCAGAAAATTCATGAGAAAAAT | Site directed mutagenesis to R150K-R154K of PlzA |
| PlzA-R150KR154K-R | ATTTTTCTCATGAATTTTCTGATTTTGCCCAGG | Site directed mutagenesis to R 150K-R154K of PlzA |
| WspRpTyb12-BsmIF | GAATGCTATGCACAACCCTCATGAGAGCAAG | Cloning of wspR into pTyb12 |
| WspRpTyb12-EcoRIR | GAATTCTCAGCCCGCCGGGGCCGG | Cloning of wspR into pTyb12 |
Underlined text in the oligonucleotide sequence indicates a restriction site, and bold text indicates codon changes from the wild-type sequence.
Generation of r-WspR, Rrp1, PlzA and PlzA site-directed mutants
PCR primers were designed to amplify full-length rrp1 and plzA from B. burgdorferi B31-5A4 (Table 1). Site-directed mutations (R150D and R154D; R150K and R154K) were introduced into plzA using an overlap extension mutagenic PCR approach as described previously (McDowell et al., 2004). The amplicons, generated with 5′ BamHI and 3′ SalI sites, were cloned into pCR2.1-TOPO and the resulting plasmids were transformed into One Shot TOP10 E. coli (Invitrogen). Positive clones were identified by PCR screening and plasmids were isolated. The plasmids were digested with BamHI and SalI and the inserts were directionally cloned into pMAL-c4x (New England Biolabs) to yield constructs encoding proteins with N-terminal mannose-binding protein (MBP) fusions. Ligated vectors were transformed into E. coli BL21 (DE3) for protein production. Briefly, cells were grown at 37 °C to an OD600 nm of 0.6 and shifted to 23 °C. Cultures were then induced with isopropyl β-d-1-thiogalactopyranoside (IPTG; 1 mM; 3 h). Following induction, cells were lysed by sonication, and protein in the soluble fraction was purified using amylose affinity chromatography as instructed by the supplier (New England Biolabs). Protein purification was verified by Coomassie blue staining and immunoblotting following sodium dodecyl sulfate polyacrylamide gel electrophoresis and transfer to polyvinylidene fluoride membranes. The blots were screened with antibodies specific for the N-terminal MBP tag. The resulting r-Rrp1 and r-PlzA were 78 kDa and 73 kDa in size, respectively. Note that attempts to generate soluble protein with other vectors including several in the pET series were not successful. Hence, the identity and placement of the tag is an important consideration in the expression and production of Rrp1.
To generate r-WspR, the gene was amplified from P. aeruginosa FRD-1, cloned, and expressed and the protein was purified as described previously (Hickman et al., 2005). Briefly, a full-length wspR amplicon was digested with the appropriate restriction enzymes, ligated into pTYB12 (New England Biolabs) and the plasmid was propagated in E. coli. The resulting plasmid was then transformed into E. coli ER2566 for protein production. Escherichia coli ER2566 was grown at 37 °C to an OD600 nm of 0.3 and induced with 0.3 mM IPTG at 18 °C overnight. Cells were lysed by sonication and protein was purified using chitin affinity chromatography. Intein cleavage by dithiothreitol was allowed to proceed for 48 h at 4°C, and protein was eluted with reaction buffer (75 mM Tris, pH 7.8, 250 mM NaCl, 25 mM KCl, 10 mM MgCl2). All purified r-proteins were dialyzed in reaction buffer with 40% glycerol. The r-WspR was 37 kDa in size.
Synthesis and analysis of radiolabeled c-di-GMP
R-WspR and r-Rrp1 were used to generate radiolabeled and cold c-di-GMP. The r-Rrp1 and r-WspR (5 µM) were incubated with 25 mM acetyl phosphate (37 °C for 30 min) to potentially activate the proteins. To generate cold c-di-GMP, GTP was added to each reaction at a final concentration of 150 µM (37 °C; 60 min). To generate radiolabeled c-di-GMP, the reactions were spiked with [α-32P]GTP (0.33 µM). The production and purity of the c-di-GMP was assessed using HPLC. GTP and unlabeled c-di-GMP (Biogen) served as standards. HPLC was performed essentially as described by Ryjenkov et al. (2005). Briefly, the samples were boiled, filtered (Ultrafree centrifugal filters; Millipore), applied to a 15 × 4.6 cm Supelcosil LC-18-T column (Supelco) and separated on an AKTApurifier (GE Health Sciences) by reverse-phase HPLC. The gradient system utilized buffers A (100 mM KH2PO4, 4 mM tetrabutyl ammonium hydrogen sulfate, pH 5.9) and B (75% buffer A, 25% methanol). Nucleotides were detected at a wavelength of 254 nm.
Analysis of c-di-GMP binding to wild type and site-directed substitution mutants of PlzA
R-PlzA, site-directed substitution mutants (R150D,R154D and R150K,R154K) and control proteins were tested for c-di-GMP binding. The r-proteins (0.25 µg) (including Rrp1 and a MBP-tagged negative control protein) were spotted onto nitrocellulose and dried. The membranes were incubated with [32P]c-di-GMP (2 nM) alone, or in combination with 750 nM of GTP, GMP, ATP, cGMP, or cAMP (PBS with 1% milk; 2 h; room temperature). The membranes were washed three times for 5 min in PBS at room temperature and exposed to film overnight.
Results
Analysis of the distribution of PlzA among pathogenic Borrelia species
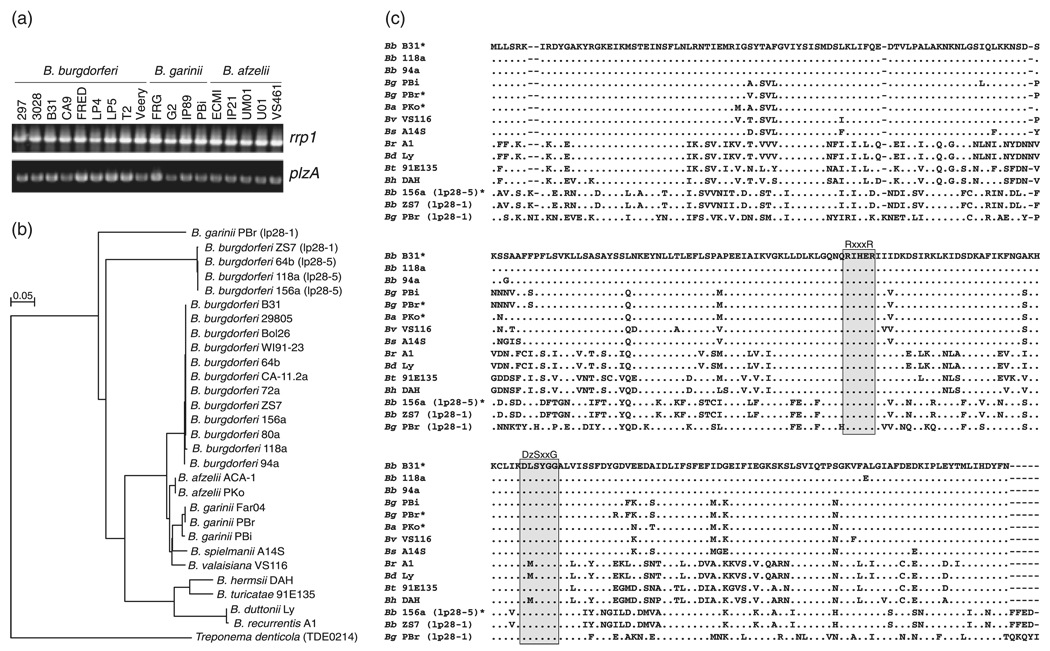
Borrelia burgdorferi B31 possesses a single ORF (BB0733) that encodes a protein with a PilZ domain. The consensus sequence for PilZ domains is RxxxR … D/NzSxxG with x being any amino acid and z being any hydrophobic residue (Amikam & Galperin, 2006; Romling & Amikam, 2006; Ryjenkov et al., 2006). PCR analyses of a panel of Lyme disease isolates previously shown to carry rrp1 (Rogers et al., 2009b) revealed that all harbor BB0733 (Fig. 1a). We have designated this ORF as plzA. Other species of the B. burgdorferi sensu lato complex and the relapsing fever spirochetes also possess a chromosomally encoded PlzA ortholog. Analysis of several recently released B. burgdorferi sensu lato complex genome sequences indicate that some isolates possess a second gene encoding a PilZ domain-containing protein that is present on plasmids of the lp28 group (http://cmr.jcvi.org). Identity and similarity values for the PilZ domain-containing proteins of the Borrelia are indicated in Table 2 and their relationships to one another are displayed in the phylogenetic tree presented in Fig. 1b. An alignment of PlzA sequences, with the PilZ domain residues indicated, is presented in Fig. 1c. To establish a workable nomenclature for the PilZ domain-containing proteins of the Borrelia, gene/protein designations were assigned based on phylogenetic relationships using nomenclature guidelines recommended by the American Society for Microbiology. The chromosomal and plasmid-encoded PilZ proteins of B. burgdorferi sensu lato complex isolates are designated as PlzA and PlzB, respectively. The PilZ domain-containing proteins of the relapsing fever spirochetes are designated as PlzC. The universal distribution of Rrp1 and PilZ domain-containing proteins among the Borrelia supports the existence of a functional c-di-GMP regulatory network in all Borrelia species.
Fig. 1.
Distribution, consensus, and phylogenetic analysis of PlzA in pathogenic Borrelia spp. The distribution of the plzA gene in Borrelia burgdorferi, Borrelia garinii, and Borrelia afzelii was assessed by PCR using full-length primers, and products were visualized by ethidium bromide staining and UV detection (a). A neighbor-joining phylogenetic tree is presented in (b). The scale represents distance as the number of amino acid changes per 100 amino acids. (c) An amino acid alignment with dashes indicating gaps and dots indicating amino acids identical to the reference sequence (B. burgdorferi B31). The following abbreviations are used in (c): Bb, B. burgdorferi; Bg, B. garinii; Ba, B. afzelii; Bv, Borrelia valaisiana; Bs, Borrelia speilmanii; Br, Borrelia recurrentis; Bd, Borrelia duttonii; Bt, Borrelia turicatae; and Bh Borrelia hermsii. Sequences that are derived from plasmid-encoded genes are indicated with the plasmid of origin shown in parentheses. An asterisk following a sequence indicates that several other isolates possess the identical sequence [refer to the tree in (b)]. The grey boxes highlight the residues that define the PilZ domain with the PilZ consensus sequence shown above the alignment (x indicating any amino acid and z any hydrophobic amino acid).
Table 2.
Similarity and identity values for PlzA from Borrelia species
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Bb B31 | 99.6 | 99.6 | 94.3 | 92.0 | 92.0 | 62.8 | 64.4 | 64.4 | 91.6 | 92.0 | 64.5 | 64.9 | 68.2 | 68.6 | |
| 2 Bb 94a | 99.6 | 99.2 | 93.9 | 92.0 | 92.0 | 62.8 | 64.0 | 64.0 | 92.0 | 91.6 | 64.5 | 64.9 | 68.2 | 68.6 | |
| 3 Bb 118a | 99.6 | 99.2 | 93.9 | 91.6 | 91.6 | 62.5 | 64.0 | 64.0 | 91.2 | 91.6 | 64.1 | 64.5 | 67.8 | 68.2 | |
| 4 Ba Pko | 97.7 | 97.3 | 97.3 | 96.2 | 95.8 | 65.1 | 64.8 | 64.8 | 94.6 | 95.4 | 65.6 | 66.0 | 69.0 | 69.0 | |
| 5 Bg PBi | 96.6 | 96.2 | 96.2 | 97.7 | 98.9 | 65.4 | 63.7 | 63.7 | 94.3 | 94.3 | 66.5 | 66.9 | 68.6 | 69.0 | |
| 6 Bg PBr | 95.8 | 95.4 | 95.4 | 96.9 | 99.2 | 65.8 | 63.7 | 63.7 | 94.3 | 94.3 | 66.2 | 66.5 | 69.0 | 69.3 | |
| 7 Bg PBr (lp28-1) | 82.2 | 81.8 | 81.8 | 82.9 | 82.9 | 82.9 | 58.1 | 58.1 | 65.1 | 66.5 | 52.9 | 53.3 | 50.9 | 53.2 | |
| 8 Bb 156a (lp28-5) | 80.5 | 80.1 | 80.1 | 82.3 | 80.8 | 80.1 | 78.4 | 99.6 | 64.0 | 64.0 | 53.2 | 53.6 | 53.0 | 51.9 | |
| 9 Bb ZS7 (lp28-1) | 80.5 | 80.1 | 80.1 | 82.3 | 80.8 | 80.1 | 78.8 | 99.6 | 64.0 | 64.0 | 53.2 | 53.6 | 53.0 | 51.9 | |
| 10 Bs A14S | 96.6 | 96.9 | 96.2 | 97.7 | 97.3 | 96.9 | 82.5 | 80.1 | 80.1 | 93.9 | 64.5 | 64.9 | 68.6 | 68.2 | |
| 11 Bv VS116 | 96.9 | 96.6 | 96.6 | 97.3 | 97.3 | 96.9 | 83.3 | 81.6 | 81.6 | 96.9 | 64.9 | 65.3 | 68.2 | 69.3 | |
| 12 Br A1 | 83.6 | 83.6 | 83.2 | 84.7 | 85.9 | 85.9 | 77.3 | 74.8 | 74.8 | 85.1 | 84.4 | 99.6 | 80.5 | 79.0 | |
| 13 Bd Ly | 84.0 | 84.0 | 83.6 | 85.1 | 86.3 | 86.3 | 77.7 | 75.2 | 75.2 | 85.5 | 84.7 | 99.6 | 80.9 | 79.4 | |
| 14 Bt 91E135 | 84.3 | 84.3 | 83.9 | 84.7 | 84.3 | 84.3 | 74.7 | 73.7 | 73.7 | 83.9 | 83.9 | 92.0 | 92.4 | 90.0 | |
| 15 Bh DAH | 83.9 | 83.5 | 83.5 | 84.7 | 83.9 | 83.9 | 75.1 | 74.4 | 74.4 | 83.1 | 83.9 | 93.1 | 93.5 | 96.2 |
Transcriptional analysis of plzA during in vitro cultivation, in ticks, and in infected C3H/HeJ mice
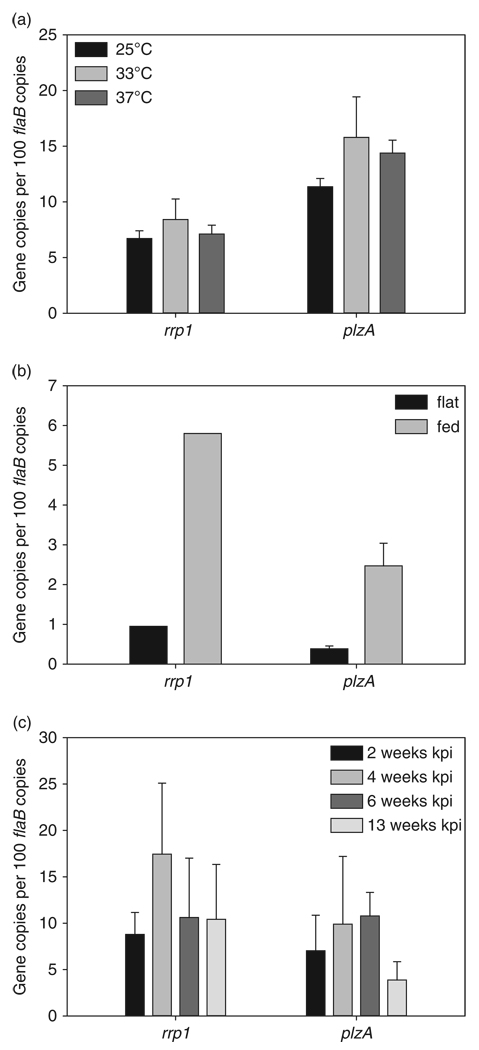
To determine whether plzA is transcriptionally active during in vitro cultivation, in ticks and in mammals, expression was assessed using qRT-PCR. Focusing first on in vitro analyses, expression was determined to be constitutive and not influenced by temperature (Fig. 2a). The constitutive expression reported here is consistent with earlier microarray analyses (Ojaimi et al., 2002; Revel et al., 2002; Brooks et al., 2003). plzA expression was also assessed and detected in flat and fed infected nymphal ticks. Expression was significantly upregulated upon feeding (Fig. 2b) (P = 0.0165). As a control for transcriptional transitions that occur in B. burgdorferi upon exposure to the bloodmeal, we also determined the expression levels of the ospC gene. As expected, a significant increase in ospC transcript levels was observed upon feeding (data not shown). To determine whether plzA and rrp1 are produced by spirochetes during murine infection, urinary bladders were harvested from infected mice at 2, 4, 6 or 13 weeks. The urinary bladder has been demonstrated to be the most consistent organ for the detection of spirochetes (Schwan et al., 1988), and hence transcriptional analyses were focused on RNA extracted from this organ. Detection of the flaB transcript served as the positive control for the detection of spirochetal RNA. Both plzA and rrp1 mRNA were detected in the bladder at 2 weeks (Fig. 2c), with transcript also detected at week 13 (the last time point to be tested). In summary, it can be concluded that plzA expression is constitutive in vitro, is upregulated by the bloodmeal, and its expression is maintained throughout the enzootic cycle. The expression patterns for plzB and plzC remain to be determined.
Fig. 2.
In vitro and in vivo transcriptional analyses of rrp1 and plzA. (a) Borrelia burgdorferi B31 grown at 23, 33, and 37 °C were assessed for rrp1 and plzA expression using qRT-PCR as detailed in the text. In all panels, the number of rrp1 or plzA transcripts is presented as the number of copies per 100 copies of flaB. (b) The results of qRT-PCR analysis of rrp1 and plzA in flat or fed nymphal ticks. Note that the rrp1 data in (b) are derived from an earlier analysis (Rogers et al., 2009a, b) and is included solely for comparative reference. The absence of error bars for that specific set of data was due to the limited amount of RNA that was available for analysis. (c) The results of qRT-PCR analysis of rrp1 and plzA in the bladders of infected C3H/HeJ mice after 2, 4, 6 or 13 weeks of infection. pi, postinfection.
Synthesis of c-di-GMP using recombinant DGCs derived from B. burgdorferi and P. aeruginosa
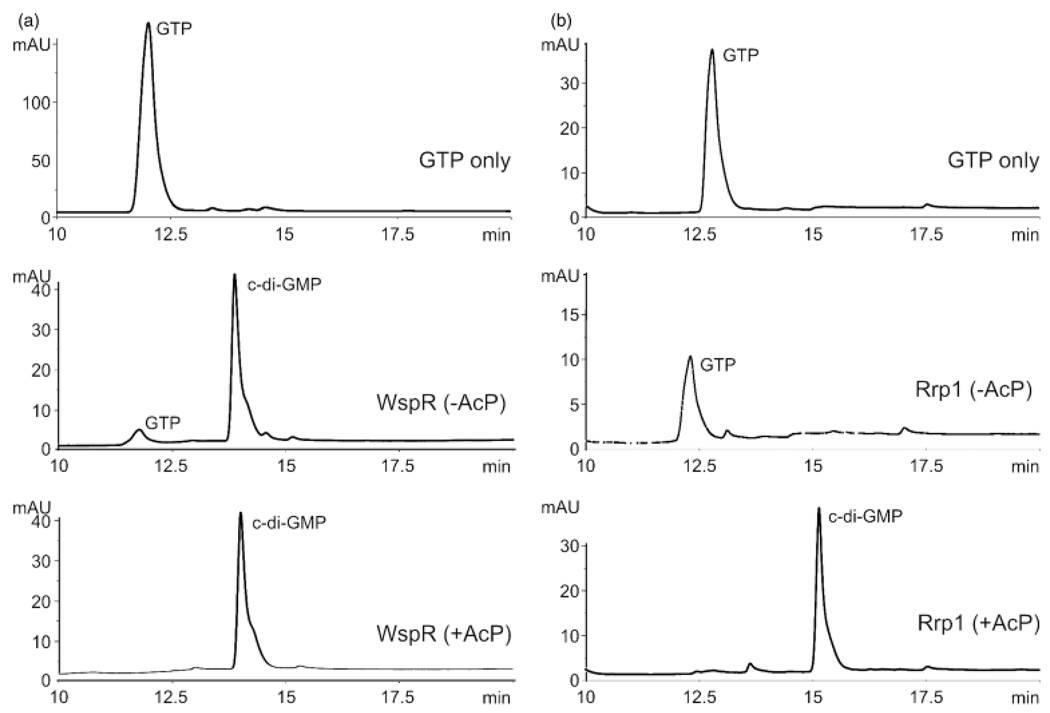
To allow for the assessment of c-di-GMP binding to PlzA, c-di-GMP was produced using B. burgdorferi Rrp1 or P. aeruginosa WspR. C-di-GMP production by WspR was phosphate independent, while synthesis from Rrp1 was determined to be phosphate dependent (Fig. 3; note that due to minor differences in the HPLC protocols used during the analysis of c-di-GMP production by Rrp1 and WspR, there are slight differences in the retention time, hence, the GTP alone controls are shown for both). These findings are consistent with earlier reports regarding the phosphate activation requirements of these DGCs (Ryjenkov et al., 2005; De et al., 2008). Both WspR and phosphate-activated Rrp1 efficiently converted GTP to c-di-GMP, with no significant nucleotide contaminants or residual GTP evident in the c-di-GMP preparations (as determined by HPLC; Fig. 3). The fractions containing the c-di-GMP were pooled and used in the analyses below.
Fig. 3.
HPLC analysis of c-di-GMP synthesis by r-WspR and r-Rrp1. The ability of r-WspR (panel a) and r-Rrp1 (panel b) to produce c-di-GMP was assessed using HPLC. The top graph demonstrates the retention time of GTP with no protein added. This control is presented for both WspR and Rrp1 since slightly different solvent conditions were used with each protein. r-WspR and r-Rrp1 were incubated with or without acetyl phosphate (+AcP or − AcP, as indicated in the figure) before the addition of GTP to assess the dependence of c-di-GMP production on phosphate activation. The r-proteins were then incubated with GTP and the nucleotide products were analyzed by HPLC as detailed in the text. Note that the slight differences in the retention times for c-di-GMP generated with r-Rrp1 and r-WspR are the result of slight differences in solvent conditions for each run.
Generation of r-PlzA and r-PlzA site-directed mutants and analysis of their c-di-GMP binding ability
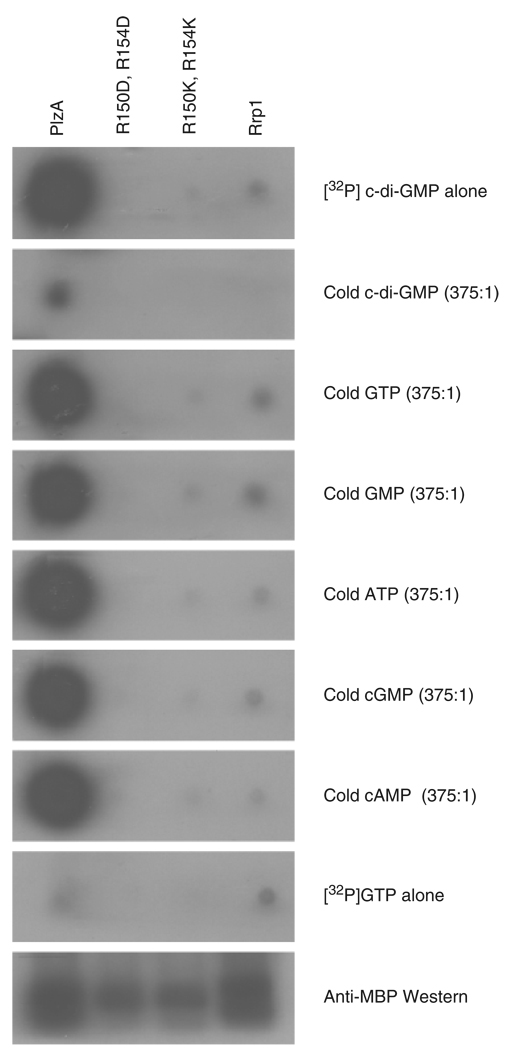
It has been demonstrated that R residues within the RxxxR … D/NzSxxG PilZ domain are critical determinants for c-di-GMP binding (Christen et al., 2005; Ryjenkov et al., 2006; Merighi et al., 2007). To gain further insight into the determinants required for c-di-GMP binding by PlzA and to verify that the interaction of PlzA with c-di-GMP is specific, wild type and double substitution mutants (R150D-R154D and R150K-R154K) were tested for their ability to bind [32P]c-di-GMP. While strong binding of [32P]c-di-GMP was observed with wild-type PlzA, no significant binding of [32P]c-di-GMP to the mutant proteins (or negative control) was observed. The complete loss of [32P]c-di-GMP binding by the RxxxR site-directed mutants demonstrates the absolute requirement for these residues of the PilZ domain in the interaction between PlzA and c-di-GMP and demonstrates that the interaction of c-di-GMP with PlzA is specific. Note that no binding of [α-32P]GTP was observed to any of the proteins.
To determine whether other nucleotides can compete with [32P]c-di-GMP for binding to PlzA, identical binding reactions were performed in the presence of excess unlabeled cyclic-di-GMP, GTP, GMP, ATP, cGMP, and cAMP. Unlabeled c-di-GMP significantly reduced binding of [32P]c-di-GMP, consistent with competitive inhibition. In contrast, excess cold GTP, GMP, ATP, cGMP, or cAMP had no inhibitory effect on binding, demonstrating that the interaction is specific for c-di-GMP (Fig. 4). In summary, these data represent the first direct demonstration of c-di-GMP binding to a spirochetal protein and support the hypothesis that PlzA is a functional component of the c-di-GMP regulatory network.
Fig. 4.
C-di-GMP binds to PlzA of Borrelia burgdorferi with high specificity. R-PlzA, r-PlzA mutants R150D,R154D and R150K,R154K, and r-Rrp1 were expressed as N-terminal MBP fusions and were assessed for their ability to bind to [32P]c-di-GMP. The r-proteins used in the analyses are designated across the top of the panels. The ratio of competing nucleotide to [32P]c-di-GMP is indicated to the right (no competitor was added in the top panel). The second panel from the bottom was screened with [α-32P]GTP alone (with no c-di-GMP added) and the bottom panel is a Western blot using anti-MBP antibody.
Discussion
C-di-GMP has only recently been recognized for its potential to serve as a major regulator of cellular processes (reviewed in Cotter & Stibitz, 2007; Hengge, 2009). While DGC, PDE, and PilZ domain-containing proteins are widespread among bacteria, most remain uncharacterized. For example, among the spirochetes, only a single DGC has been partially characterized, and there are no published analyses of PDE or PilZ proteins. Efforts to study the influence of specific proteins of the c-di-GMP regulatory network on bacterial pathophysiology using approaches such as gene inactivation or deletion are complicated by the existence of extensive functional redundancy. Vibrio cholerae, for example, encodes 53 DGC proteins (Lim et al., 2006), while Shewanella oneidensis encodes 98 DGCs (Thormann et al., 2006). The Borrelia could serve as an excellent model system for studying c-di-GMP because the composition of its c-di-GMP regulatory network is relatively simple. The B. burgdorferi B31 genome encodes one DGC (BB0419 – Rrp1), two PDEs (BB0363 – PdeA and BB0374 – PdeB) and one PilZ domain-containing protein (BB0733 – PlzA). The primary goals of this study were to determine whether PlzA is universal among B. burgdorferi sensu lato complex isolates, expressed during distinct stages of the enzootic cycle and capable of serving as a potential downstream effector protein in the c-di-GMP regulatory network through the binding of c-di-GMP.
PCR analyses conducted here and analyses of recently released genome sequences revealed that PlzA is universal among Borrelia isolates and is present in all Borrelia species for which genome sequence information is currently available. Interestingly, some isolates of B. garinii and B. burgdorferi encode two PilZ domain-containing proteins, with one present on the chromosome and the other on a linear plasmid of the lp28 plasmid group. The chromosomally encoded PilZ domain-containing proteins are highly conserved among species of the B. burgdorferi sensu lato complex (identity values > 90%) and, hence, represent clear PlzA orthologs. Interestingly, the plasmid-carried PlzA sequences are as distant from the chromosomally encoded B. burgdorferi B31 PlzA (amino acid identity values of ~64%) as the relapsing fever spirochete PlzC sequences are to the B31 PlzA sequence (identity values of ~65–69%). The plasmid-carried PilZ domain-encoding genes of B. burgdorferi sensu lato species and those of the relapsing fever spirochetes are of sufficient divergence to warrant distinct gene designations for which we propose PlzB and PlzC, respectively. It is important to note the domains associated with the regulation of c-di-GMP levels or effector functions are often found in proteins that possess other functional domains. This suggests that these proteins can receive and respond to many different stimuli through a variety of mechanisms. PlzA, PlzB, and PlzC do not harbor identifiable functional domains other than PilZ and hence they appear to be ‘stand alone’ c-di-GMP effector proteins.
It has recently been demonstrated that B. burgdorferi rrp1 is constitutively expressed in vitro and is upregulated in ticks upon ingestion of the bloodmeal (Rogers et al., 2009b). We speculated that, because Rrp1 and PlzA may be functionally linked, PlzA and Rrp1 would have similar transcriptional expression patterns. In vitro, plzA transcript was detected under all environmental conditions tested with no significant differences in expression levels. However, in ticks, plzA expression was significantly upregulated in fed vs. flat nymphal ticks, indicating that factors in the bloodmeal trigger transcription. The elevated temperature in the tick upon feeding does not appear to be a factor because temperature had no effect on expression of plzA in in vitro-cultivated bacteria. The molecular signals that result in upregulation of plzA upon tick feeding are not yet known, but it is conceivable that rrp1, which is also upregulated by the bloodmeal, and thus putatively increases the pool of intracellular c-di-GMP, may directly or indirectly influence plzA transcription. Note that in an earlier microarray study, the inactivation of rrp1 did not lead to a repression of plzA. We hypothesize that Rrp1, c-di-GMP, or an unidentified effector protein in the c-di-GMP regulon serves as a transcriptional activator of plzA that increases its expression beyond its normal basal level. We also assessed expression of plzA and rrp1 during infection in mice. Expression of plzA and rrp1 was demonstrated in the urinary bladders of infected mice throughout the 13-week infection period. Collectively, these analyses suggest that the c-di-GMP regulatory network contributes to regulation of cellular processes throughout the enzootic cycle.
While the presence of a PilZ domain is suggestive of c-di-GMP binding activity, an important component of this study was to directly demonstrate this activity for PlzA. Toward this goal, we produced r-DGC proteins and used them to produce radiolabeled c-di-GMP, which could in turn be used to assess binding to PlzA. C-di-GMP binding to r-PlzA was readily demonstrated using a membrane-based approach. Binding was specific as only trace binding of c-di-GMP to Rrp1 was observed. The nucleotide-binding specificity of PlzA was assessed using several different nucleotides as potential competitors. As predicted for a specific interaction, the addition of excess unlabeled c-di-GMP drastically decreased the binding of radiolabeled c-di-GMP, indicating saturable binding and competitive inhibition. In contrast, none of the other nucleotides tested, even when present in vast excess, influenced the binding of radiolabeled c-di-GMP. This series of binding experiments confirms the highly specific nature of the interaction between c-di-GMP and PlzA.
To further assess the molecular determinants involved in c-di-GMP binding, site-directed mutants of PlzA were generated and tested for c-di-GMP binding. The residues targeted were chosen based on similar analyses of the YcgR PilZ domain-containing protein of E. coli in which Arg residues within the PilZ domain were demonstrated to be essential for binding. We generated r-proteins harboring two double-amino acid substitution mutants: R150D-R154D and R150K-R154K. Both mutants were readily expressed by E. coli and were soluble. Neither mutant bound c-di-GMP, indicating that the amino acid substitutions perturb critical binding determinants in PlzA. These data verify the importance of the Arg residues in the PilZ domain and provide further evidence that the interaction of c-di-GMP with PlzA is highly specific.
In summary, in this study, we demonstrate that B. burgdorferi produces a functional c-di-GMP-binding protein that we have designated as PlzA. PlzA expression was demonstrated during both the tick and the mammalian stages of the enzootic cycle. Binding analyses using site-directed mutants of PlzA, along with competitive-binding analyses using other nucleotides, demonstrate that the interaction between PlzA and c-di-GMP is highly specific. The characterization of PlzA is an important step forward in demonstrating a fully functional c-di-GMP regulatory network in the Lyme disease spirochetes. The analyses detailed here provide insight into the initial steps in the regulatory cascade driven by c-di-GMP. The specific role of PlzA and the processes that it influences remain to be determined.
Acknowledgements
We thank Durland Fish (Yale University) for providing I. scapularis larvae and Christopher G. Earnhart (Virginia Commonwealth University) for providing the pTYB12-wspR vector for expression of WspR. Supported in part by grant AI45801 (to I.S.).
References
- Amikam D, Galperin MY. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics. 2006;22:3–6. doi: 10.1093/bioinformatics/bti739. [DOI] [PubMed] [Google Scholar]
- Benach J, Swaminathan SS, Tamayo R, et al. The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 2007;26:5153–5166. doi: 10.1038/sj.emboj.7601918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, et al. Spirochetes isolated from the blood of two patients with Lyme disease. New Eng J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. 2008;76:3844–3853. doi: 10.1128/IAI.00467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Hefty PS, Joliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun. 2003;71:3371–3383. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease – a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M, Christen B, Folcher M, Schauerte A, Jenal U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J Biol Chem. 2005;280:30829–30837. doi: 10.1074/jbc.M504429200. [DOI] [PubMed] [Google Scholar]
- Cotter PA, Stibitz S. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol. 2007;10:17–23. doi: 10.1016/j.mib.2006.12.006. [DOI] [PubMed] [Google Scholar]
- De N, Pirruccello M, Krasteva PV, Bae N, Raghavan RV, Sondermann H. Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol. 2008;6:e67. doi: 10.1371/journal.pbio.0060067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. From the cover: Borrelia burgdorferi {sigma}54 is required for mammalian infection and vector transmission but not for tick colonization. PNAS. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. P Natl Acad Sci USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- Lim B, Beyhan S, Meir J, Yildiz FH. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol Microbiol. 2006;60:331–348. doi: 10.1111/j.1365-2958.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- McDowell JV, Wolfgang J, Senty L, Sundy CM, Noto MJ, Marconi RT. Demonstration of the involvement of outer surface protein E coiled coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H. J Immunol. 2004;173:7471–7480. doi: 10.4049/jimmunol.173.12.7471. [DOI] [PubMed] [Google Scholar]
- Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. The second messenger bis-(3′–5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2007;65:876–895. doi: 10.1111/j.1365-2958.2007.05817.x. [DOI] [PubMed] [Google Scholar]
- Ojaimi C, Brooks C, Akins D, et al. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Method Enzymol. 2002;358:165–177. doi: 10.1016/s0076-6879(02)58088-5. [DOI] [PubMed] [Google Scholar]
- Ojaimi C, Brooks C, Casjens S, et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun. 2003;71:1689–1705. doi: 10.1128/IAI.71.4.1689-1705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaimi C, Mulay V, Liveris D, Iyer R, Schwartz I. Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect Immun. 2005;73:6791–6802. doi: 10.1128/IAI.73.10.6791-6802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. P Natl Acad Sci USA. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Marconi RT. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun. 2007;75:5272–5281. doi: 10.1128/IAI.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Abdunnur SV, McDowell JV, Marconi RT. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun. 2009a;77:4396–4405. doi: 10.1128/IAI.00393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009b;71:1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Amikam D. Cyclic di-GMP as a second messenger. Curr Opin Microbiol. 2006;9:218–228. doi: 10.1016/j.mib.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov DA, Simm R, Romling U, Gomelsky M. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem. 2006;281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- Samuels DS, Mach K, Garon CF. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J Bacteriol. 1994;176:6045–6049. doi: 10.1128/jb.176.19.6045-6049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus) J Clin Microbiol. 1988;26:893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. The spirochetal etiology of Lyme disease. New Eng J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormann KM, Duttler S, Saville RM, Hyodo M, Shukla S, Hayakawa Y, Spormann AM. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J Bacteriol. 2006;188:2681–2691. doi: 10.1128/JB.188.7.2681-2691.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. P Natl Acad Scie USA. 2003a;100:11001–11006. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Hubner A, Popova TG, Hagman KE, Norgard MV. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect Immun. 2003b;71:5012–5020. doi: 10.1128/IAI.71.9.5012-5020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]