Abstract
Dysregulated glutamate neurotransmission has been implicated in the pathophysiology of schizophrenia. In particular, hypofunction of the NMDA glutamate receptor has been proposed to play an important role in mediating cognitive deficits in patients. The two NMDA receptor subunits, NR2A and NR2B, are distinctly regulated during development and are associated with different intracellular pathways and functions, which suggest that these receptors play separate roles in the control of higher cognitive functions such as learning and memory. Trafficking of the NR2B subunit-containing receptor is regulated by a microtubule-associated trafficking complex consisting of the KIF17, APBA1, CASK, mLin7 proteins. Several studies have demonstrated an integrated functional regulation of this trafficking complex with NR2B receptor subunit expression, which in turn has been linked to higher cognitive functions. In the present work, we investigated whether expression of this NR2B-associated trafficking complex might be abnormal in schizophrenia. We analyzed the expression of KIF17, APBA1, CASK, mLin7A and mLin7C in postmortem brain from patients with schizophrenia a comparison group. Analysis of transcripts for all of these proteins revealed particularly prominent expression in cortical layer III and layer IV, which overlapped with NR2B but not NR2A transcripts. We found altered expression of transcripts for the CASK, ABPA1, and mLin7 molecules and the CASK, mLin7 proteins, suggesting that NR2B-containing NMDA receptor transport could be selectively compromised in schizophrenia, and that these changes likely involve altered NR2B function in a subset of cortical neurons.
Keywords: glutamate, dendrite, mRNA, protein expression, postmortem, postsynaptic density
Introduction
Schizophrenia is a devastating psychiatric illness that is characterized by positive, negative, and cognitive symptoms (Tamminga and Holcomb 2005). Evidence for altered glutamate neurotransmission in schizophrenia suggests a role for altered NMDA receptor-mediated neurotransmission, which has led to the formulation of a general glutamate hypofunction hypothesis of this illness (Lindsley and others 2006).
The NMDA receptor consists of a tetrameric or pentameric assembly of obligate NR1 subunits in region- and development-specific constellations with the NR2 (A-D) or NR3 (A-B) subunits (Ozawa and others 1998; Stephenson 2001). In prefrontal cortex (PFC) and hippocampus, the majority of NMDA receptors include either the NR2A or NR2B subunit (Hynd and others 2003; Law and others 2003; Sheng and others 1994; Takai and others 2003). Although co-expressed in dendritic spines, NR2A- and NR2B-containing NMDA receptors are generally associated with distinct ultrastructural compartments, with NR2A expressed directly at synaptic and NR2B-receptor at perisynaptic sites (Janssen and others 2005). Thus, differences in the expression of NR2A and NR2B subunits suggest unique roles in the regulation of complex brain functions (Yashiro and Philpot 2008).
As an integrated member of the postsynaptic density (PSD), NMDA receptors interact through direct and specific interactions with several downstream signaling pathways (Beresewicz 2007; Cuthbert and others 2007; Tu and others 1999). NR2A or NR2B knockout animals have highly complex phenotypes with evidence for diverse functional implications for each NR2 subunit (Boyce-Rustay and Holmes 2005; Zhao and Constantine-Paton 2007). Henceecent studies indicate that differential interactions between PSD-associated signaling proteins and NR2A- and NR2B-type subunits might be involved in subtype-selective regulation of different, and often opposing, NMDA receptor functions (Cousins and others 2008; Duffy and others 2008; Iwamoto and others 2004; Kim and others 2005). Interestingly, NR2B-containing receptors interact differentially with several signaling pathways that are central to higher brain function (Chen and others 2007; Kim and others 2005; Sessoms-Sikes and others 2005; Tran and others 2007; Xu and others 2006; Zhou and others 2007), and abnormalities of NR2B receptor regulation has been proposed as an important mechanism for compromised learning and memory deficits in schizophrenia (Loftis and Janowsky 2003)
Several studies have analyzed expression of the NMDA receptor complex in postmortem brain in schizophrenia. Often, these studies have found normal expression of transcripts and protein for the NR2A and NR2B subunits (Akbarian and others 1996; Beneyto and others 2007a; Hemby and others 2002; Kristiansen and others 2006). However, two studies have reported abnormal splicing of the C1 and C2 alternatively spliced exons of the NR1 subunit (Kristiansen and others 2006; Le Corre and others 2000), suggestive of altered dendritic trafficking of NMDA receptors in schizophrenia (Cull-Candy and others 2001; Kristiansen and others 2007). Compromised dendritic trafficking might lead to decreased functional expression of NMDA receptors at the PSD without necessarily affecting overall expression levels of these receptors in the brain.
Trafficking of newly assembled NR2B-containing receptors from their cellular region of synthesis close to the soma to synaptic spines on neuronal dendrites requires specific interaction with a microtubule-associated trafficking complex, which consists of the APBA1/Mint1/X11, CASK/Lin2 and Lin7(A-C)/Veli(1-3) proteins bound to the microtubule-associated ATPase, KIF17 (Setou and others 2000). This molecular complex is selectively required for dendritic /microtubular trafficking of NR2B-containing NMDA receptors (Guillaud and others 2003; Guillaud and others 2008; Yuen and others 2005). A study by Wong and coworkers supports an important role for trafficking of NR2B-containing receptors in regulation of higher cognitive functions. Hence, increased expression of KIF17 in transgenic mice, which is linked to a simultaneous increase in NR2B expression, was demonstrated to improve spatial and working memory formation (Wong and others 2002).
Based on a hypothesis of compromised NMDA receptor function in schizophrenia and our recent observation that total cellular expression of NR2 proteins is not consistently altered in postmortem brain (Kristiansen and others 2006), we hypothesized that an important aspect of understanding compromised NMDA receptor function in schizophrenia might be linked to altered NR2B trafficking (Kristiansen and others 2007). Hence, compromised forward transport of NR2B-type receptors due to altered expression of the KIF17-associated trafficking complex would combine observations of unaltered NR2B expression in postmortem brain with the general hypothesis of compromised NMDA receptor function in schizophrenia. To test this hypothesis, we analyzed postmortem expression of transcripts and proteins for the NR2B receptor-associated trafficking complex in two frontal cortical regions known to be involved in cognitive dysfunction in patients with schizophrenia (Glahn and others 2005; Ragland and others 2007).
Materials and methods
Human brain tissue
Brain samples from a group of elderly patients with schizophrenia and comparison subjects were obtained from the Mount Sinai VA Medical Center brain collection (table 1). None of these brains showed any signs of neurodegenerative disorders or other discernable neuropathologies, including Alzheimer’s Disease (Purohit and others 1993). For protein studies, 27 comparison subjects and 23 patients and for in-situ hybridization transcript analyses, 11 comparison subjects and 18 patients were included (table 1). There was an overlap of 20% (13/66) between subjects included in both protein and transcript studies, which consisted of 5 comparison and 8 schizophrenia subjects. There was no difference in gender distribution between subjects used for protein and transcript studies (p=0.19).
Table 1.
Table of subject characteristics. ISH: in-situ hybridization; WB: western blot; F: female; M: male; AOD: age at death; PMI: postmortem interval indicated in hours; Rx<6wk: medication with antipsychotic drugs within 6 weeks of death; A.S.H.D: arteriosclerotic heart disease; CHF: chronic heart failure; NIDD: noninsulin-dependent diabetes; HTN: hypertension; COPD: Chronic obstructive pulmonary disease.
| Comparison subjects | |||||||
|---|---|---|---|---|---|---|---|
| # | Methods | Sex | AOD | PMI | pH | Rx<6wk | Cause of death |
| 1 | WB | F | 86 | 4.7 | 6,5 | NA | Unknown |
| 2 | IS | F | 79 | 3.0 | 6,3 | NA | Cardiopulmonary failure |
| 3 | IS | F | 96 | 3.3 | 6,7 | NA | Cardio respiratory failure |
| 4 | WB/IS | F | 90 | 4.2 | 6,0 | NA | Cardiopulmonary failure |
| 5 | WB | F | 74 | 3.0 | 6,0 | NA | Cardio respiratory failure |
| 6 | WB | F | 88 | 5.1 | 6.4 | NA | COPD/Pneumonia/Arthritis |
| 7 | WB | F | 98 | 1.4 | 6.6 | NA | Myocardial infarction, aortic aneurysm |
| 8 | WB/IS | M | 69 | 4.3 | 6,3 | NA | Cancer of lung |
| 9 | WB | F | 82 | 5.7 | 6.1 | NA | Cardiopulmonary arrest |
| 10 | WB | F | 80 | 4.8 | 6.2 | NA | Sepsis |
| 11 | IS | F | 64 | 19.1 | 6,1 | NA | Pulmonary edema |
| 12 | IS | M | 93 | 19.0 | 6,4 | NA | Congestive heart failure |
| 13 | IS | F | 102 | 7.1 | 6,5 | NA | Acute myocardial infarction |
| 14 | WB/IS | F | 73 | 3.4 | 6,3 | NA | Acute myocardial infarction |
| 15 | IS | F | 79 | 7.7 | 6,5 | NA | Acute myocardial infarction |
| 16 | WB/IS | F | 84 | 18.5 | 6,2 | NA | Myocardial infarction |
| 17 | WB/IS | M | 101 | 4.7 | 6,8 | NA | Coronary Artery Disease |
| 18 | WB | M | 95 | 4.1 | 6.5 | NA | Chronic renal failure, N.I.D.D., HTN |
| 19 | WB | M | 65 | 3.8 | 6.8 | NA | Renal failure, Diabetes-Cirrhosis of liver |
| 20 | WB | F | 89 | 2.3 | 6.7 | NA | Bronchopneumonia, Asthma, COPD |
| 21 | WB | F | 83 | 6.2 | 6.8 | NA | Cardiopulmonary arrest |
| 22 | WB | M | 66 | 7.6 | 6.6 | NA | Cardiac arrest |
| 23 | WB | F | 75 | 6.5 | 6.0 | NA | Cardiopulmonary arrest |
| 24 | WB | M | 69 | 7.4 | 6.7 | NA | Septic shocks |
| 25 | WB | M | 74 | 16.6 | 6.7 | NA | Cardiopulmonary arrest |
| 26 | WB | F | 62 | 7.0 | 6.6 | NA | Acute myocardial infarction |
| 27 | WB | M | 76 | 2.9 | 6.3 | NA | Bronchopneumonia bilateral. Atherosclerotic heart disease |
| 28 | WB | M | 60 | 28.8 | 6.6 | NA | Acute myocardial infarction |
| 29 | WB | M | 64 | 4.2 | 6.4 | NA | Acute myocardial infarction |
| 30 | WB | M | 93 | 4.2 | 6.3 | NA | Acute myocardial infarction |
| 31 | WB | M | 85 | 5.3 | 6.5 | NA | Cancer of bladder w Metastasis |
| 32 | WB | M | 59 | 20.4 | 6.7 | NA | CAD; hypertension, deep venous thrombosis |
| 33 | WB | M | 92 | 20.0 | 6.4 | NA | Arrhythmia |
| Schizophrenia subjects | |||||||
|---|---|---|---|---|---|---|---|
| # | Methods | Sex | AOD | PMI | pH | Rx<6wk | Cause of death |
| 1 | IS | F | 86 | 6.9 | 5,8 | Yes | Respiratory insufficiency, renal failure |
| 2 | IS | F | 84 | 15.6 | 6,2 | no | Unknown |
| 3 | WB | M | 58 | 6.7 | 6,2 | Yes | Cardio pulmonary arrest |
| 4 | WB | M | 52 | 21.2 | 6,3 | No | Cardio respiratory failure |
| 5 | WB/IS | M | 84 | 6.2 | 6,5 | Yes | Cardiopulmonary failure |
| 6 | IS | M | 69 | 4.5 | 6,4 | Yes | Cardic Infarction, renal failure |
| 7 | WB | M | 58 | 13.3 | 6,9 | Yes | Cardio respiratory failure |
| 8 | WB | M | 57 | 13.7 | 6,1 | Yes | Cardio respiratory arrest |
| 9 | IS | F | 65 | 5.8 | 5,9 | Yes | Cardiopulmonary failure |
| 10 | WB | M | 63 | 6.2 | 5,9 | No | Cardiopulmonary failure |
| 11 | WB/IS | F | 69 | 13.7 | 6,2 | Yes | Cardio respiratory failure |
| 12 | WB/IS | M | 87 | 11.2 | 6,5 | No | Cardiopulmonary failure |
| 13 | IS | M | 68 | 5.6 | 6,8 | No | Cardiopulmonary failure |
| 14 | WB | M | 86 | 7.0 | 6.3 | Yes | Cardio pulmonary arrest |
| 15 | WB/IS | F | 79 | 20.4 | 7,1 | Yes | Cardiopulmonary arrest, cancer of pancreas |
| 16 | WB/IS | M | 85 | 5.3 | 6,3 | Yes | Cardiopulmonary arrest |
| 17 | WB/IS | M | 73 | 7.9 | 6,5 | Yes | Cardio respiratory failure |
| 18 | IS | M | 66 | 12.1 | 6,5 | No | Acute cardiac failure |
| 19 | IS | F | 76 | 21.2 | 6,1 | Yes | Cardiogenic shock |
| 20 | IS | M | 97 | 9.3 | 6,5 | No | Cardiopulmonary arrest |
| 21 | WB | M | 86 | 14.1 | 6.7 | Yes | A.S.H.D. |
| 22 | IS | M | 66 | 8.4 | 6,7 | Yes | Cardiopulmonary arrest |
| 23 | IS | F | 82 | 18.8 | 6,6 | No | Cardiopulmonary arrest |
| 24 | WB/IS | F | 79 | 9.9 | 6,8 | No | Cardiac arrest |
| 25 | WB/IS | M | 68 | 17.3 | 6,6 | Yes | Cardiopulmonary arrest |
| 26 | WB | M | 69 | 40.2 | 6.7 | Yes | Acute renal failure |
| 27 | WB | M | 76 | 16.6 | 6.7 | No | CHF, Coronary arterial disease, A.S.H.D. |
| 28 | WB | F | 74 | 7.0 | 6.3 | Yes | Cardiopulmonary arrest |
| 29 | WB | M | 57 | 21.4 | 6.4 | Yes | Small cell cancer of lung |
| 30 | WB | F | 81 | 12.5 | 5.9 | No | Acute myocardial infarction |
| 31 | WB | F | 77 | 9.7 | 6.0 | Yes | Cardiopulmonary arrest |
| 32 | WB | M | 56 | 13.5 | 6.5 | No | Cardiopulmonary arrest |
| 33 | WB | F | 81 | 15.1 | 6.7 | No | Cardiopulmonary arrest |
Comp: 18F/15M 80.2 ±12.5 8.1 ±6.9 6.4 ±0.3
Schz: 12F/21M 73.2±11.2 12.1 ±7.2 6.4 ±0.3
There were no significant differences in age, pH, and postmortem interval (PMI) between the comparison and schizophrenia subjects that were included in the transcript studies (p=0.103; 0.507; 0.304 respectively). For subjects included in analyses of protein expression, average values for age and PMI were significantly different (p=0.04 and p=0.007) with no significant difference in pH (p=0.301). To the degree it has been deemed necessary, patients have received medical treatment in the form of typical antipsychotic medication throughout their life.
Animals treated with haloperidol
22 adult male Sprague Dawley rats were treated daily with haloperidol (intramuscular injection; 1 mg/kg/day) or vehicle (DMSO) for 28 consecutive days. Animals were kept in the animal housing facility with free access to food and water throughout the experimental period. Twenty-four hours after the last injection, the animals were euthanized by decapitation and brains were quickly extracted, frontal cortex removed and frozen in isopentane (−25°C). Tissue was kept at −80°C until further processing.
Tissue preparation
Dissected blocks of postmortem brain containing dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) were kept at −80°C. For in-situ hybridization, tissue was thawed to −20°C, cryosectioned at 15 μm onto Fisherbrand Superfrost /Plus positively charged microscope slides (Fisher Scientific), and returned to storage at −80°C. For Western blot experiments, brain tissue from human (DLPFC and ACC) or rat (frontal cortex), was homogenized in buffer (10% W/V; 50mM Tris-HCl (pH 7.0) containing protease inhibitors (Roche Applied Sciences)) for 30 seconds with a polytron homogenizer and stored at −80°C. Protein concentration was determined by the Bradford method (Bradford 1976).
In-situ hybridization
Clones corresponding to specific regions of the KIF17, CASK, mLin7A, mLin7C and ABPA1 transcripts were generated by PCR amplification of specific sequences within the coding regions of each molecule using a fetal brain cDNA library as template. Following amplification (300-600 bp/clone), PCR-generated fragments were sub-cloned into the TOPO vector using the TOPO TA cloning kit (Invitrogen). Inserted sequences were verified by nucleotide sequencing and target specificity analyzed using the nucleotide BLAST database through the National Center for Biotechnology Information (NCBI). Generation of specific clones for the NR2A- and NR2B-NMDA receptor subunits have previously been described (Ibrahim and others 2000b). [35S] labeled sense and antisense probes were prepared from each clone using linearized, purified plasmids, as previously described (Clinton and Meador-Woodruff 2002; Ibrahim and others 2000b).
For in-situ hybridization, two slides per subject were fixed in 4% (W/V) formaldehyde for 1 hour and processed according to our previously published protocol (Clinton and Meador-Woodruff 2004a; Ibrahim and others 2000a). Slides from each in-situ experiment were apposed to autoradiography film (Kodak Biomax MR) until specific labeling of cortical layers was above background levels and within the dynamic detection range (≈ 14 days – 3 months). Sense strand probes were used as a negative control and did not show specific binding for any molecule.
Digital capturing of images was performed using a Northern Light Illuminator (Imaging Research Inc) with a XC-77 CCD video camera module (Sony Corporation) connected to an image acquisition and analysis software package (Scion Corporation). For each subject, tissue background values from adjacent white matter were subtracted from gray scale values (GSVs) and converted to optical density units (OD). Values for two slides were averaged to give one value per subject. The amount of radioactivity per gram of tissue (nCi/g) was calculated using a [14C] microscale standard (Amersham Biosciences) that was exposed onto the same film as the slides in each study (Miller 1991). Bound radioactivity was converted to concentration of transcript per gram of tissue (fmol/g) by taking into account the number of radioactively labeled uridine nucleotides in each probe.
Nissl staining
Slides were removed from −80°C storage and air-dried for 30 minutes before transfer into xylene (2×15 minutes) and rehydration in graded solutions of ethanol (2×100%, 1×95%, 1×70%). Tissue was fixed in 4% formaldehyde (15 minutes) and briefly washed in water before incubation in thionin stain (30 minutes). Finally, slides were washed in water and dehydrated in graded ethanols (1×70%, 1×95%, 1×100%), followed by a final rinse in xylene (2x) before being cover slipped.
Western blotting
For preparation of samples for SDS-PAGE electrophoresis, tissue homogenates (20 μg per lane) were mixed with 6x sample buffer (4.5% sodium dodecyl sulfate, 170 mM tris-HCl (pH 6.8), 36% glycerol, 15% β-mercaptoethanol, 0.018% bromphenol blue) and heated at 95°C for 4 minutes. Samples were loaded in duplicate onto 7.5% 15 well precast Tris-HCl gels in a mini-protean 3 electrophoresis module (Bio-Rad) and separated at 120 volts in electrophoresis buffer (25mM Tris-base, 192mM glycine, 0.1% SDS). Subsequently, proteins were blotted onto PVDF membranes using a semi-dry transfer cell (Bio-Rad). Protein-containing membranes were rinsed briefly in water before blocking (30 minutes in PBS containing 3-5% dry milk powder). Subsequently, blots were incubated overnight with primary antibodies diluted in blocking buffer (4°C on a rocking platform). The following antibodies were used: KIF17 (Abcam; 1:1000), APBA1 (Affinity Bioreagents; 1:250), CASK (Millipore; 1:1000), mLin7A (Santa Cruz; 1:200), mLin7C (Abcam; 1:1000), β-tubulin (Millipore; 1:10000). For each antibody, protocols for buffers (washing, blocking) and dilution were optimized prior to the actual experiment. Following several washes in PBS or TBS, membranes were incubated with horseradish peroxidase-coupled secondary antibody for 2 hours (goat-anti-mouse or goat-anti-rabbit, Millipore; 1:1000)) washed and developed using enhanced chemiluminescence (ECL; Amersham Biosciences). To ensure non-saturated film exposures within the dynamic range of detection, several exposures for each protein were performed. Film exposures for immunopositive bands were digitally captured for each subject, using the image acquisition package described above. Film background was subtracted from specific GSVs and averaged for each subject. In addition to proteins of interest, each blot was probed for expression of β-tubulin, which was used as an internal loading/lane control. Experimental values were expressed as the ratio of protein of interest to β-tubulin.
Statistical analysis
Data analysis for in-situ hybridization and protein quantification experiments was performed using the Statistica software package 7 (Statsoft, Inc). Analysis of variance (ANOVA) was used for all experiments except when significant correlation was detected with age, post-mortem interval (PMI) or pH, in which case analysis of covariance (ANCOVA) was used. For the transcript data, to avoid artificial inflation of statistical power, regression analyses for postmortem samples were based on GSVs for the entire cortical depth for each subject (cortical wedge). Distribution pattern of transcript expression (cortical wedge) was analyzed by the Shapiro-Wilk W test and Grubb’s test was applied to identify significant outliers. To analyze for diagnosis-specific changes in transcript expression and to test the a-priori hypothesis of diagnosis-specific changes in specific cortical isodense bands, factorial two-way ANOVA was performed with isodense cortical bands and diagnosis as categorical independent variables. For posthoc analysis Tukey HSD was used. For western blot analysis, data were analyzed by the Mann–Whitney–Wilcoxon test. To test for the overall significant differences in age, PMI and pH between subjects, Students t-test was used. Gender distribution between cohorts was tested with a Chi-Square 2×2 analysis. For all tests α = 0.05.
Results
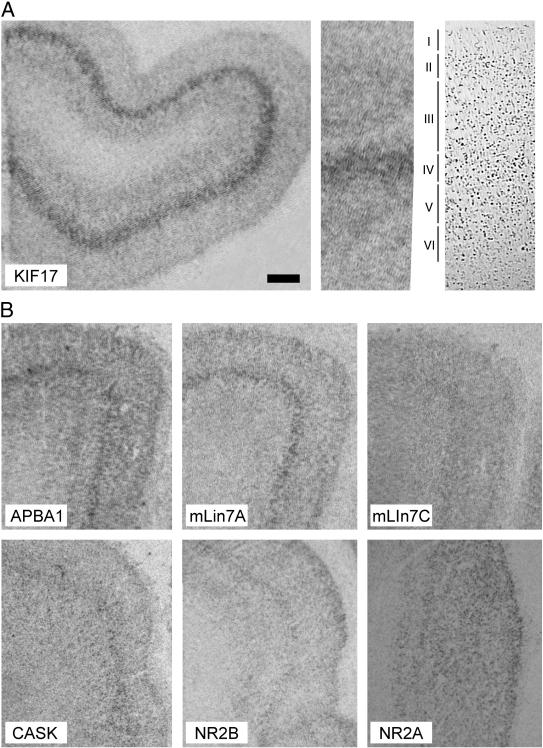
We detected specific cortical labeling of transcripts encoding KIF17, ABPA1, mLin7A, mLin7C, and CASK in DLPFC and ACC in all subjects. For all transcripts, expression profiles were divided into different isodense bands of distinct intensity across the entire cortical depth, with one isodense band being particularly prominent in a deeper cortical layer as especially noted for KIF17 (figure 1A). By comparison to nissl-stained adjacent sections, this isodense band was identified to correspond to deep layer III and layer IV (figure 1A). Further, this prominent layer III/IV isodense band was found to be overlapping for all transcript expression profiles. Consistent with its role in NR2B-containing NMDA receptor trafficking, the expression profile of KIF17 coincided with layer III/IV NR2B transcript expression (figure 1B). Contrary to NR2B, cortical expression of transcripts for the NR2A subunit was homogenous across the entire cortical depth, with no prominent labeling associated any specific cortical layer (figure 1B).
Figure 1.
Cortical expression of transcripts for KIF17, APBA1, mLin7A, mLin7C, CASK, NR2B, and NR2A. A: expression of KIF17 transcripts in a control subject. For cortical orientation, wedge of KIF17 transcript expression is localized next to nissl-stained parallel section from the same subject. B: Representative examples of cortical expression profiles for APBA1, mLin7A, mLin7C, CASK, NR2B, and NR2A. Scale bar in A corresponds to 1.0 mm in A and 0.7 mm in B.
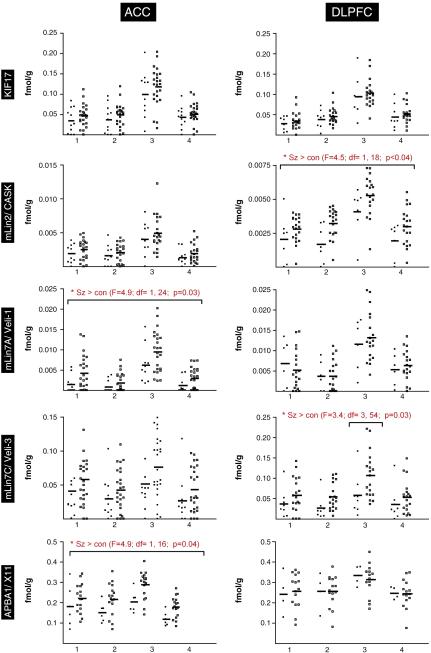
For all transcripts, four isodense bands were clearly distinguishable in all subjects. Two superficial isodense bands corresponded to cortical layer I-III (isodense band 1=layer I-II; isodense band 2=external layer III), one prominent isodense band corresponding to layer III/IV, and one deep isodense band corresponding to layer V/VI (figure 2). For all transcripts, expression levels in isodense band 3 were significantly higher than expression levels in isodense bands 1, 2, and 4. The ABPA1/X11 transcript was expressed at the highest (0.12 – 0.33 fmol/g) and CASK/mLin2 and mLin7A at the lowest levels (≈0.002 fmol/g). KIF17 and mLin7C were expressed at intermediate levels (≈0.03 fmol/g).
Figure 2.
Quantification of transcript expression of transcripts for KIF17, CASK, mLin7A, mLin7C, and APBA1 in ACC and DLPFC. For each probe, sets of black (control) and white boxes (schizophrenia) indicate transcript expression levels in 4 discrete isodense bands. Values are means ± SEM. Significant findings across all isodense layers or within an isodense band (mLin7C in DLPFC) are indicated by * (*p<0.05).
Significant interactions between PMI and expression of mLin7A in DLPFC (r=0.59, p=0.03) and ACC (r=0.52, p=0.02) were detected. There was no significant interaction between other transcripts and age, pH, or PMI in either DLPFC or ACC. No outliers and evidence of non-normal distribution pattern was identified for any transcript. Using ANOVA or ANCOVA (PMI as covariate for mLin7A), we identified a significant effect of diagnosis for APBA1 and mLin7A in ACC (F=4.9; df=1, 16; p=0.041 and F=4.9; df=1, 24; p=0.037 respectively) with significantly higher expression of both transcripts in schizophrenia. Further, we detected significantly increased expression of the CASK/mLin2 transcript in DLPFC (F=4.5; df=1, 18; p=0.048). Although no main effect for diagnosis was detected for mLin7C expression in DLPFC (F=2.8; df=1, 18; p=0.109), a significant band x diagnosis interaction was detected (F=3.4; df=3, 54; p=0.025). Post-hoc analysis revealed that this was due to significantly higher expression in isodense band 3 in schizophrenia.
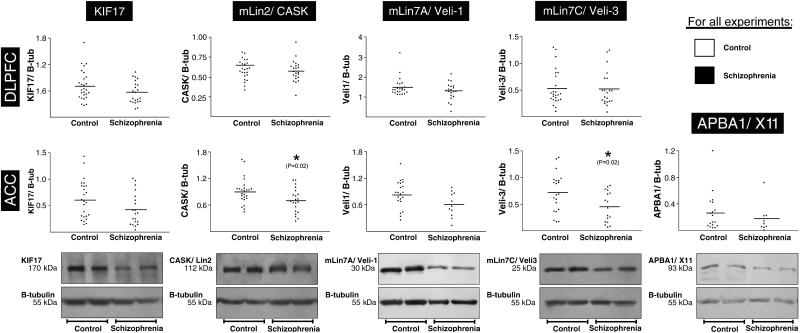
We next analyzed expression of KIF17, CASK, mLin7A, mLin7C at the protein level using western blot analysis in DLPFC and ACC. Due to limited availability of tissue, APBA1 expression was only analyzed in ACC. Prior to analysis for diagnosis-specific changes, we tested for significant interactions with the covariates age and PMI, as these have been suggested to influence protein stability at extended time periods in a protein-specific manner (Halim and others 2003; Harrison and others 1995; Hilbig and others 2004; Siew and others 2004). Regression analysis did not reveal any significant interactions. Using the Mann–Whitney–Wilcoxon statistical test, we detected significant effects of diagnosis on CASK (p=0.022) and mLin7C (p=0.017) in ACC with decreased expression in schizophrenia (figure 3). Expression of mLin7A in ACC was just outside significance detection (p=0.057). We did not detect any significant changes in the expression of KIF17 or ABPA1 in ACC and of any of the KIF17, CASK, mLin7A, mLin7C proteins in DLPFC.
Figure 3.
Protein expression of KIF17, CASK, mLin7A, mLin7C, and APBA1 in ACC and DLPFC. black boxes and triangles for each protein indicate protein expression levels corrected for B-tubulin. Below, representative images of duplicate detection of each specific protein and the associated B-tubulin in control and schizophrenia subjects, as indicated. Detection of APBA1 was only possible in ACC. For each protein, the molecular weight in kilodaltons (kDa) is indicated. *p<0.05.
To analyze the effect that antipsychotic medication potentially has on expression of the NR2B-associated receptor trafficking complex, we treated adult rats daily with haloperidol for 28 days (1 mg/kg/day) and examined protein expression in the frontal cortex of this trafficking complex by quantitative western blot. Following chronic treatment with haloperidol, we found significantly increased protein expression of KIF17 (p=0.027) and mLin7A (F=8.5; df=1, 18; p=0.01) in the haloperidol-treated animals (figure 4), while expression of mLin7C, CASK, and APBA1 were not significantly altered.
Discussion
In this study we have analyzed expression of the KIF17-associated trafficking complex that is responsible for NR2B-containing NMDA receptor trafficking in neuronal dendrites. We found increased expression of transcripts for CASK, mLin7A, mLin7C, and ABPA1 in schizophrenia. We found decreased expression of the CASK and mLin7C proteins in ACC. Analysis of protein expression in rats demonstrated that chronic treatment with typical antipsychotics increased expression of KIF17 and mLin7A in frontal cortex without significantly affecting expression of APBA1, CASK and mLin7C.
The frontal cortex is organized into 6 distinct layers, which serve as a stereotyped framework for the complex heterogeneous synaptic organization that is required for higher cortical functions (Somogyi and others 1998; White 2007). In the present study, we found expression of the NR2B subunit and the molecules involved in its microtubular dendritic transport localized within a particularly prominent isodense band in deep layer III and layer IV. The laminar organization of the frontal cortex is characterized by the combined expression of specialized inhibitory and excitatory neurons. Hence, excitatory neurons in deep layer III and IV include pyramidal cells in deep layer III and the predominant localization of spiny stellate neurons and star pyramidal cells in layer IV (Staiger and others 2004) as well as inhibitory parvalbumin-positive GABAergic neurons of the chandelier and basket cells types (Hendry and others 1989; Staiger and others 1996). Spiny stellate neurons in layer IV likely represent a principal target for glutamatergic thalamo-cortical afferents, which function to amplify thalamic input to inhibitory neurons. However, GABAergic neurons in this layer also receive significant direct innervation from the thalamus (Benshalom and White 1986; Freund and others 1989; Stratford and others 1996). Whereas spiny stellate and parvalbumin-positive neurons in cortical layer IV are anatomically restricted to this layer (Lubke and others 2000), deep layer III pyramidal neurons, which also receive direct projections from the thalamus, branch throughout layers I-IV to establish cortico-cortical pathways through an extensive arborization. The predominant expression of transcripts for NR2B and its trafficking complex in layers III and IV therefore might indicate a particularly important role for NR2B receptors in direct and indirect modulation of excitatory thalamic input to the frontal cortex. However, from the present data we cannot determine the exact neuronal subpopulations that are principally associated with increased expression of the NR2B-related transcripts in PFC and therefore we can only speculate as to the particular neuronal circuits involved. Furthermore, despite the fact that increased expression of KIF17 transcripts was very enriched in layer III/IV neurons, expression of this and other transcripts was not restricted to these layers, indicating, as expected, that NR2B expression is involved in mediating synaptic functions across the entire cortical column.
Differences in the expression levels and profiles of transcripts for the NR2B receptor trafficking molecules likely indicates the involvement of some or all of these proteins in processes other than dendritic trafficking of NR2B-containing receptors. Hence, the multidomain protein CASK, in addition to its involvement with NR2B transport, is involved in regulating nuclear transcription from the genes encoding NR2B and Neuregulin through its interaction with the CINAP and Tbr-1 transcriptional complex. Additionally, CASK was recently demonstrated to be essential for synaptic development and maturation (Atasoy and others 2007; Hsueh 2006). In a similar manner, the neuronal APBA1/X11 protein possibly plays a role in presynaptic vesicle release from inhibitory GABA neurons (Biederer and Sudhof 2000; Ho and others 2003) and the expression of the small mLin7/Veli adapter proteins in cortical dendrites as well as axons points to the involvement of these proteins in both pre- and postsynaptic cellular functions (Misawa and others 2001). Finally, although the known primary function of dendritic KIF17 appear to involve microtubule-based dendritic delivery of NR2B-containing receptors (Guillaud and others 2008; Hirokawa and Takemura 2004), KIF17 has also been associated trafficking of GluR5-containing kainate receptors and Kv4.2 potassium channels (Chu and others 2006; Kayadjanian and others 2007). Hence, in addition to compromised synaptic delivery of NR2B receptors, decreased expression of the KIF17-associated protein complex in schizophrenia might affect other cellular functions such as transcriptional regulation, trafficking and presynaptic vesicle release. Interestingly, cortical expression of the GluR5 kainate receptor has been reported decreased in schizophrenia by several postmortem studies (Beneyto and others 2007b; Garey and others 2006; Scarr and others 2005) and decreased expression of the KIF17-associated trafficking complex might also be involved in these changes.
The observed increase in cortical expression of transcripts for APBA1, mLin7A, and mLin7C in ACC, as well as increased expression of CASK in DLPFC, suggests abnormal regional regulation of these molecules in schizophrenia. In particular, the selective increase in mLin7C transcript in isodense layer 3 indicates that these changes involve NR2B-relevant cellular abnormalities. Due to their integrated function as trafficking complex for newly synthesized NR2B-containing receptors, altered expression of any one or several of these molecules in schizophrenia could affect delivery of this NMDA receptor subunit to the PSD. Combined, the above observations of altered transcript expression in schizophrenia most likely suggest altered NR2B trafficking.
Analysis at the protein level indicated decreased expression of CASK and mLin7C in ACC in schizophrenia, with mLin7A expression also approaching a significant decrease in ACC. No significant changes in the expression of these proteins were detected in DLPFC. Hence, although not entirely matching the individual changes in transcript expression, changes in protein levels were, similar to transcriptional changes, principally associated with ACC. Divergence in polarity and degree of overlap between transcript and protein expression in ACC might be interpreted in several ways. First, this might occur as a result of the large group of non-overlapping subjects used in the transcript and protein analyses for this study. However, we consider this possibility as unlikely because the tissue used for each of these techniques originated from the same general group of elderly patients and comparison subjects (table 1). Second, differences in the polarity between transcript and protein expression could be caused by opposite changes in different cellular populations, originating in different cortical areas. Hence, cells with altered transcript expression in ACC could belong to a population of neurons projecting outside of the ACC. Similarly, altered protein expression in ACC could be due to altered transcript synthesis in cells located outside of the ACC. As discussed above, analysis of transcripts for the NR2B trafficking molecules indicate high expression in layer IV, which does not contain neurons that typically project outside this area. Opposite regulation of transcript and protein expression, such as those found for the mLin7 molecules in this study, therefore most likely involves the same neuronal populations. A plausible explanation for these opposite changes in expression of transcripts and proteins could involve a compensatory mechanism to a primary deficit in functional protein expression by which transcript synthesis is up-regulated. We recently proposed a similar mechanism to be involved in the altered regulation of several PSD proteins in ACC in schizophrenia (Kristiansen and others 2006).
Based on numerous studies in postmortem brain and animal models, decreased expression of parvalbumin-containing GABA interneurons has been proposed as a central pathophysiological feature of schizophrenia (Beasley and Reynolds 1997; Bloomfield and others 2008; Harte and others 2007; Hashimoto and others 2008; Lewis and Moghaddam 2006; Pratt and others 2008; Tseng and others 2008). Regional and coordinated inhibition by parvalbumin-positive cells in DLPFC and ACC has been recognized as centrally important for synchronized gamma-oscillation in cortical pyramidal cells, which likely is a mechanism that helps filter specific and relevant sensory information from background noise (Gray and Roth 2007; Lee and others 2003; Lewis and others 2005). Due to the principal association of the NR2B trafficking complex with layer IV where spiny stellate and GABAergic neurons are predominant, altered trafficking and expression of this receptor could negatively affect direct and indirect modulation of these inhibitory cortical circuits by thalamo-cortical afferents. Thalamo-cortical processing as well as the inhibitory regulation in PFC of pyramidal neurons have been proposed as central mechanisms for mediating cognitive dysfunction in schizophrenia (Behrendt 2006; Clinton and Meador-Woodruff 2004b; Coyle 2004). Compromised trafficking of NR2B-type receptors in layer IV, due to decreased expression of the CASK and mLin7 proteins in ACC therefore might, in addition to other biochemical changes in these neurons, such as altered receptor phosphorylation, lead to altered thalamic activation of local inhibitory circuits and possibly cause altered prefrontal gamma-synchronization of pyramidal cells. Glutamate has previously been implicated in these changes and has been proposed, with dopaminergic and cholinergic systems, to play an important role in dysregulated inhibitory control in frontal cortex (Coyle 2004; Ford and Mathalon 2008; Winterer 2006).
The effect of antipsychotic medication on cellular function and expression of biochemical markers, including the expression of transcripts and proteins in postmortem brain, should be evaluated since such changes potentially could be associated with exposure to these drugs rather than the illness. Hence, typical neuroleptics such as haloperidol have been linked to altered cellular metabolism in neurons and altered glutamatergic neurotransmission (Hanaoka and others 2003; Konradi and Heckers 2001; Leveque and others 2000). Given that all patients that were included in the present study at some stage of their illness were treated with typical antipsychotics, we included an animal study to analyze whether chronic haloperidol administration altered expression of the KIF17-associated protein complex. The observation that chronic administration of haloperidol increased expression of KIF17 and mLin7A indicates that this drug might, in a similar manner, modulate the NR2B-trafficking potential in patients. Due to the fact that expression of KIF17 and NR2B is co-regulated, which is a mechanism that might play an important role in improving cognition (Wong and others 2002), increased expression of KIF17 following chronic haloperidol administration indicates that increased KIF17 potentially could be part of a beneficial therapeutic mechanism. However, studies of haloperidol’s functional interaction with glutamate neurotransmission and its regulation of the NMDA receptor system has proven to be highly complex.
Expression of KIF17 in this study and NR2B in a previous study was not altered in patients (Kristiansen and others 2006). Furthermore, contrary to the animal experiment, expression of mLin7A was decreased in schizophrenia. Combined, these studies therefore suggest that the observed changes in protein expression of the NR2B trafficking complex in schizophrenia form part of specific pathophysiological mechanisms and are not secondary to treatment history.
In summary, we have analyzed expression of the NR2B-associated trafficking proteins KIF17, APBA1, CASK, mLin7A, and mLin7C at transcript and protein levels in postmortem brain from patients with schizophrenia and comparison subjects. Altered expression was somewhat overlapping between transcript and protein and was primarily associated ACC, although expression of CASK mRNA was significantly increased in DLPFC. Expression of these proteins is enriched in deep aspects of cortical layer III and layer IV cells, likely corresponding to both inhibitory and excitatory populations of mostly non-pyramidal neurons. This suggests a prominent role for NR2B-NMDA receptors in regulation of thalamic afferent input to the cortex. Altered expression of the CASK, mLin7A, and mLin7C proteins in ACC suggests a primary role for altered NR2B trafficking in this region, which at the behavioral level might correspond to monitoring of higher cognitive functions.
Acknowledgements
The authors wish to acknowledge the technical assistance of Charlotte Hammond. The authors acknowledge financial support from: The Stanley Foundation (JMW), MH53327 (JMW), NIH MH064673 (VH) and MH066392 (VH)
Role of funding sources Funding for this study was provided by: The Stanley Foundation (JMW), MH53327 (JMW), NIH MH064673 (VH) and MH066392 (VH). Authors have no further disclosures. These funding organizations had no further role in study design; collection, analysis and interpretation of the data; in writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest There are no conflicts of interest to disclose for any author in respect to the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr., Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16(1):19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104(7):2525–30. doi: 10.1073/pnas.0611003104. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24(3):349–55. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Behrendt RP. Dysregulation of thalamic sensory “transmission” in schizophrenia: neurochemical vulnerability to hallucinations. J Psychopharmacol. 2006;20(3):356–72. doi: 10.1177/0269881105057696. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal Glutamate Receptor Expression in the Medial Temporal Lobe in Schizophrenia and Mood Disorders. Neuropsychopharmacology. 2007a doi: 10.1038/sj.npp.1301312. In press. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007b;32(9):1888–902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Benshalom G, White EL. Quantification of thalamocortical synapses with spiny stellate neurons in layer IV of mouse somatosensory cortex. J Comp Neurol. 1986;253(3):303–14. doi: 10.1002/cne.902530303. [DOI] [PubMed] [Google Scholar]
- Beresewicz M. Scaffold proteins (MAGUK, Shank and Homer) in postsynaptic density in the central nervous system. Postepy Biochem. 2007;53(2):188–97. [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275(51):39803–6. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Bloomfield C, French SJ, Jones DN, Reavill C, Southam E, Cilia J, Totterdell S. Chandelier cartridges in the prefrontal cortex are reduced in isolation reared rats. Synapse. 2008;62(8):628–31. doi: 10.1002/syn.20521. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A andNR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56(4):222–5. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007;27(3):542–52. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281(1):365–73. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Nucleus-specific expression of NMDA receptor-associated postsynaptic density proteins in primate thalamus. Thalamus and Related Systems. 2002;1(4):303–316. [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2004a;29(7):1353–62. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004b;69(2-3):237–53. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Cousins SL, Papadakis M, Rutter AR, Stephenson FA. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J Neurochem. 2008;104(4):903–13. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68(8):1507–14. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–35. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O’Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27(10):2673–82. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. D-Serine Augments NMDA-NR2B Receptor-Dependent Hippocampal Long-Term Depression and Spatial Reversal Learning. Neuropsychopharmacology. 2008;33(5):1004–18. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH. Neural synchrony in schizophrenia. Schizophr Bull. 2008;34(5):904–6. doi: 10.1093/schbul/sbn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Soltesz I, Somogyi P, Whitteridge D. Arborisation pattern and postsynaptic targets of physiologically identified thalamocortical afferents in striate cortex of the macaque monkey. J Comp Neurol. 1989;289(2):315–36. doi: 10.1002/cne.902890211. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Von Bussmann KA, Hirsch SR. Decreased numerical density of kainate receptor-positive neurons in the orbitofrontal cortex of chronic schizophrenics. Exp Brain Res. 2006;173(2):234–42. doi: 10.1007/s00221-006-0396-8. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–9. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33(5):1100–19. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23(1):131–40. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10(1):19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, Lipska BK. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8(9):797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- Hanaoka T, Toyoda H, Mizuno T, Kikuyama H, Morimoto K, Takahata R, Matsumura H, Yoneda H. Alterations in NMDA receptor subunit levels in the brain regions of rats chronically administered typical or atypical antipsychotic drugs. Neurochem Res. 2003;28(6):919–24. doi: 10.1023/a:1023231611616. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200(3):151–4. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114(7):893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165(4):479–89. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Arnold SE, Trojanowski JQ, Eberwine JH. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch Gen Psychiatry. 2002;59(7):631–40. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 1989;76(2):467–72. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- Hilbig H, Bidmon HJ, Oppermann OT, Remmerbach T. Influence of post-mortem delay and storage temperature on the immunohistochemical detection of antigens in the CNS of mice. Exp Toxicol Pathol. 2004;56(3):159–71. doi: 10.1016/j.etp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res. 2004;301(1):50–9. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Ho A, Morishita W, Hammer RE, Malenka RC, Sudhof TC. A role for Mints in transmitter release: Mint 1 knockout mice exhibit impaired GABAergic synaptic transmission. Proc Natl Acad Sci U S A. 2003;100(3):1409–14. doi: 10.1073/pnas.252774899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YP. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem. 2006;13(16):1915–27. doi: 10.2174/092986706777585040. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Quantitation of NMDA receptor NR2 mRNA transcripts in human brain by competitive RT-PCR. Brain Res Brain Res Protoc. 2003;11(1):67–79. doi: 10.1016/s1385-299x(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Healy DJ, Hogg AJ, Jr., Meador-Woodruff JH. Nucleus-specific expression of ionotropic glutamate receptor subunit mRNAs and binding sites in primate thalamus. Brain Res Mol Brain Res. 2000a;79(1-2):1–17. doi: 10.1016/s0169-328x(00)00072-3. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Hogg AJ, Jr., Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry. 2000b;157(11):1811–23. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Yamada Y, Hori K, Watanabe Y, Sobue K, Inui M. Differential modulation of NR1-NR2A and NR1-NR2B subtypes of NMDA receptor by PDZ domain-containing proteins. J Neurochem. 2004;89(1):100–8. doi: 10.1046/j.1471-4159.2003.02293.x. [DOI] [PubMed] [Google Scholar]
- Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, Morrison JH. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–44. doi: 10.1016/j.expneurol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Kayadjanian N, Lee HS, Pina-Crespo J, Heinemann SF. Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17. Mol Cell Neurosci. 2007;34(2):219–30. doi: 10.1016/j.mcn.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46(5):745–60. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry. 2001;50(10):729–42. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11(8):737–47. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7(1):48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur J Neurosci. 2003;18(5):1197–205. doi: 10.1046/j.1460-9568.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- Le Corre S, Harper CG, Lopez P, Ward P, Catts S. Increased levels of expression of an NMDARI splice variant in the superior temporal gyrus in schizophrenia. Neuroreport. 2000;11(5):983–6. doi: 10.1097/00001756-200004070-00017. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41(1):57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2000;20(11):4011–20. doi: 10.1523/JNEUROSCI.20-11-04011.2000. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–6. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr., Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6(8):771–85. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97(1):55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Lubke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci. 2000;20(14):5300–11. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA. The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB Ultrofilm or Amersham Hyperfilm. Neurosci Lett. 1991;121(1-2):211–4. doi: 10.1016/0304-3940(91)90687-o. [DOI] [PubMed] [Google Scholar]
- Misawa H, Kawasaki Y, Mellor J, Sweeney N, Jo K, Nicoll RA, Bredt DS. Contrasting localizations of MALS/LIN-7 PDZ proteins in brain and molecular compensation in knockout mice. J Biol Chem. 2001;276(12):9264–72. doi: 10.1074/jbc.M009334200. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54(5):581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. Br J Pharmacol. 2008;153(Suppl 1):S465–70. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, McCrystal J, Losonczy M, Davis KL. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry. 1993;33(4):255–60. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19(4):417–27. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr E, Beneyto M, Meador-Woodruff JH, Deans B. Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology. 2005;30(8):1521–31. doi: 10.1038/sj.npp.1300758. [DOI] [PubMed] [Google Scholar]
- Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. CaMKIIalpha enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Mol Cell Neurosci. 2005;29(1):139–47. doi: 10.1016/j.mcn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288(5472):1796–802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368(6467):144–7. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Siew LK, Love S, Dawbarn D, Wilcock GK, Allen SJ. Measurement of pre- and post-synaptic proteins in cerebral cortex: effects of post-mortem delay. J Neurosci Methods. 2004;139(2):153–9. doi: 10.1016/j.jneumeth.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26(2-3):113–35. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Flagmeyer I, Schubert D, Zilles K, Kotter R, Luhmann HJ. Functional diversity of layer IV spiny neurons in rat somatosensory cortex: quantitative morphology of electrophysiologically characterized and biocytin labeled cells. Cereb Cortex. 2004;14(6):690–701. doi: 10.1093/cercor/bhh029. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Zilles K, Freund TF. Distribution of GABAergic elements postsynaptic to ventroposteromedial thalamic projections in layer IV of rat barrel cortex. Eur J Neurosci. 1996;8(11):2273–85. doi: 10.1111/j.1460-9568.1996.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Stephenson FA. Subunit characterization of NMDA receptors. Curr Drug Targets. 2001;2(3):233–9. doi: 10.2174/1389450013348461. [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382(6588):258–61. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Takai H, Katayama K, Uetsuka K, Nakayama H, Doi K. Distribution of N-methyl-D-aspartate receptors (NMDARs) in the developing rat brain. Exp Mol Pathol. 2003;75(1):89–94. doi: 10.1016/s0014-4800(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10(1):27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- Tran DH, Gong R, Tang SJ. Differential roles of NR2A and NR2B subtypes inNMDA receptor-dependent protein synthesis in dendrites. Neuropharmacology. 2007;53(2):252–6. doi: 10.1016/j.neuropharm.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28(48):12691–9. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23(3):583–92. doi: 10.1016/s0896-6273(00)80810-7. others. [DOI] [PubMed] [Google Scholar]
- White EL. Reflections on the specificity of synaptic connections. Brain Res Rev. 2007;55(2):422–9. doi: 10.1016/j.brainresrev.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Winterer G. Cortical microcircuits in schizophrenia--the dopamine hypothesis revisited. Pharmacopsychiatry. 2006;39(Suppl 1):S68–71. doi: 10.1055/s-2006-931498. [DOI] [PubMed] [Google Scholar]
- Wong RW, Setou M, Teng J, Takei Y, Hirokawa N. Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci U S A. 2002;99(22):14500–5. doi: 10.1073/pnas.222371099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Plummer MR, Len GW, Nakazawa T, Yamamoto T, Black IB, Wu K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121(1):22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55(7):1081–94. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Feng J, Yan Z. Microtubule regulation of N-methyl-D-aspartate receptor channels in neurons. J Biol Chem. 2005;280(33):29420–7. doi: 10.1074/jbc.M504499200. [DOI] [PubMed] [Google Scholar]
- Zhao JP, Constantine-Paton M. NR2A-/- mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus. J Neurosci. 2007;27(50):13649–54. doi: 10.1523/JNEUROSCI.3153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27(50):13843–53. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]