Abstract
Due to its unique properties, silk fibroin was studied as a biodegradable polymer vehicle for sustained, local delivery of the anticonvulsant adenosine from encapsulated reservoirs. Silk is a biologically derived protein polymer that is biocompatible, mechanically strong and degrades to non-toxic products in vivo. To achieve local, sustained, controlled adenosine release from fully degradable implants, solid adenosine powder reservoirs were coated with silk fibroin. Material properties of the silk coating including thickness, crystallinity and morphology were investigated to assess the relationships between silk coating biomaterial features and adenosine release from silk encapsulated reservoirs. Reservoir coating thickness was varied through manipulation of the silk coating solution concentration and number of coatings applied. Release studies were also performed in proteinase type XIV to model the effects of degradation. Increasing the barrier to diffusion, either by increasing coating thickness or crystallinity was found to delay adenosine burst, decrease average daily release rate, and increase duration of release. In the case of encapsulated reservoirs coated with eight layers of 8% (w/v) silk, a linear release profile was observed and adenosine release was sustained for 14 days. The ability to achieve nearly constant release for two weeks for adenosine via control of the silk coating suggests these encapsulated reservoirs represent a novel system for delivering adenosine. We anticipate that this approach could also be extended to other implant needs and small molecule drugs to treat a range of clinical needs.
Keywords: controlled release, silk fibroin, biomaterials, adenosine, epilepsy, encapsulated reservoir
1. Introduction
In the field of controlled drug delivery, sustained zero order release remains a major unmet challenge. Sustained zero order release signifies that at the steady state, the rate of drug release remains constant [1]. Sustained zero order release is critical when therapeutic efficacy and safety are dependent on maintaining a drug concentration within the range specified by the maximum safe concentration (Cmax) and the minimum effective concentration (Cmin). For drugs with a narrow therapeutic index (the ratio of Cmax/Cmin), a tightly controlled, nearly constant release rate is necessary [2]. Based on geometry and diffusion-driven release mechanisms, cumulative drug release from rectangular slab polymer devices is inversely proportional to the square root of time [3]. These diffusion-controlled polymer devices are frequently criticized for their inability to achieve zero-order release [1], making zero order drug release from polymer implants a critical goal for the field.
Current options for achieving sustained drug delivery include repeated injections and implantation of non-biodegradable pumps, which require eventual surgical retrieval or refill procedures [2, 4]. Moreover, many drugs would benefit from site-specific delivery to maximize efficacy and bypass systemic side-effects. Thus, current options are suboptimal because repeated injections are inconvenient, painful, can induce undesirable side-effects [4] and can require large volumes of expensive drug [5]. Implantable pumps require invasive surgical implantation, do not biodegrade in vivo, and require drug formulations that are stable in solution at 37°C [6]. As one example, adenosine augmentation therapy (AAT) represents a promising potential treatment for pharmacoresistant epilepsy. AAT is believed to act by correcting the brain’s seizure generating focal adenosine deficiency, providing neurochemically relevant epilepsy therapy [7, 8]. However, when administered systemically, adenosine induces severe systemic side effects, including suppression of cardiac function [9]. A system for site specific, implantable, sustained adenosine delivery is therefore desired both for its potential to enhance efficacy and to reduce side-effects. In previous studies, various techniques for site specific adenosine delivery have been shown to suppress seizures in kindled rodent animal models [10]. Studies of adenosine-releasing cell-based therapies demonstrated the potential of this approach [11-13]. The success of these studies has led to interest in development of implantable, biocompatible biomaterials for controlled site specific adenosine delivery.
A variety of biomedically relevant polymers has been studied towards sustained, controlled and related drug release goals (for reviews see [14-19]). Due to the unique properties suitable for implantable drug delivery applications, silk fibroin has been studied recently as a polymer vehicle for adenosine delivery [20, 21]. Silk is a biologically derived protein polymer that is biocompatible [22], biodegradable [23, 24], mechanically strong [22] and can be processed into a wide range of useful material formats [25-31]. Silk is attractive for sustained release applications because the degradation time frame can be controlled (from weeks to years) via regulation of beta sheet content during the processing steps with the protein [23, 24]. Further, the choice of silk for neurological implantation is supported by its frequent use as a suture material in the brain and nerve tissue [32].
Polymer film coatings on solid pharmaceutical dosage forms like pills are frequently used for controlled release applications, largely for oral delivery [33, 15]. Several natural and synthetic polymers have been studied as coating materials and have yielded different drug release profiles [33]. Moreover, desired release profiles adapted to a target drug delivery application can be obtained by varying the polymer coating formulation and processing parameters [15]. However, most current encapsulated reservoir research focuses on delivery of total drug load within short time frames (on the order of hours), corresponding to residence times in the gastro-intestinal organ as a result of oral delivery.
The advantages of silk-based implantable, sustained release systems, over traditional intravenous and oral delivery include stability of prolonged release, reduced systemic side-effects, improved cost-effectiveness, less drug waste due to efficient site-specific delivery and elimination of patient compliance problems [34]. However, the silk-based controlled release systems described here also exhibits crucial advantages over other polymer-coating based reservoir devices. Silk fibroin biodegrades to nontoxic products in vivo whereas many of the polymers studied for controlled release are nondegrading [34, 35] or require the use of organic solvents, which are undesirable for safety and environmental reasons [36, 37], as well as for their potential to damage encapsulated drugs [38]. To circumvent the use of organic solvents, either in dry powder form or as aqueous colloidal suspensions) are also employed for sustained release coatings. However, these particles must be heat annealed above the minimum film forming temperature (MFT) to form stable films. Several studies have using micronized polymer particles for coating observed a decrease in release rate relative to an increase in annealing time and temperature [39, 40], long term stability issues [36, 37], and incompletely cured coatings continuing to cure at 37°C in vivo, altering the release profile and potentially leading to dangerous dose dumping [41]. Two common biodegradable polymers, poly-(∊ -caprolactone) (PCL) and poly(lactide-co-glycolide) (PLGA) would avoid the need for surgical retrieval but required organic solvents for dip coatings and polyesters like PLGA are prone to formation of acidic hydrolysis products. Organic solvents and decreased local pH both represent potential sources of instability for acid sensitive, peptide and protein drugs [38, 40]. Other natural biodegradable polymers like alginate [42] and amylase [15] release drug loads too quickly, becoming highly permeable or disintegrating upon exposure to aqueous body fluids.
Implantation of silk polymers into kindled rat hippocampi demonstrated that silk implants loaded with adenosine could deliver therapeutically relevant drug loads. Moreover, polymers prepared in different doses were shown to induce a dose-dependent delay of seizure developments, validating the therapeutic potential of silk implants and the ability to alter the release of adenosine in vivo [20]. However, drug loading with respect to total implant volume in these systems was relatively small (around 14 μg of adenosine in an approximately 1 μL implant) so alternate systems were investigated as a route to longer duration, more zero order drug release for seizure suppression. Extrapolation of effective doses from previous studies [21] suggest that larger total drug loads will be needed for longer adenosine delivery time frames than 2 weeks, while the nature of implantation directly into the brain tissue suggests that total implant volume in the brain must be minimized. Therefore, in the present study, silk fibroin was used to coat reservoirs of solid adenosine to maximize drug loading, while offering control of release rate through manipulation of the silk coating. The objective of this study was to correlate release rate and profile to silk protein processing, such as coating thickness and crystallinity, in order to develop predictable release profiles using implantable, fully degradable systems for sustained, site-specific, controlled release of adenosine. We anticipate that this approach can be extended to other implant needs and other small molecule drugs.
2. Materials and Methods
2.1. Materials
Cocoons of Bombyx mori silkworm silk were purchased from Tajima Shoji Co., LTD (Sumiyoshicho, Naka-ku, Yokohama, Japan). All other chemicals including adenosine were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Silk fibroin solution preparation
Silk fibroin was prepared from the cocoons as we have previously described [29]. Briefly, cocoons were boiled for 40 minutes in a solution of 0.02 M Na2CO3 and rinsed, then dried at ambient conditions overnight. The dried fibroin was solubilized in a 9 M aqueous LiBr solution at 60°C for 4 hours, yielding a 20% (w/v) solution. LiBr was then removed from the solution by dialyzing the solution against distilled water for 2.5 days using Slide-a-Lyzer dialysis cassettes (MWCO 3,500, Pierce). Silk fibroin concentration was determined by evaporating water from a solution sample of known volume and massing using an analytical balance. The silk solution was concentrated by osmotic stress as previously described [28]. Briefly, silk fibroin aqueous solution (8% (w/v), 10 mL) was dialyzed against a 10-25% w/v PEG (10,000 g/mol) solution at ambient conditions, causing water molecules to move from the silk fibroin solution into the PEG solution through the dialysis membrane. A final concentration of silk in water of 8% (w/v) was diluted with distilled water to obtain the 4% (w/v) and 2% (w/v) solutions. All solutions were stored at 4-7°C before use.
2.3. Adenosine powder reservoir preparation and encapsulation
Press-fit adenosine reservoirs were prepared using an Econopress (Sigma-Aldrich, St. Louis, MO). One bolt was positioned in the press-fit chamber, approximately 100 mg of powdered adenosine was loaded into the chamber, then the second bolt was added and both bolts were manually tightened to compress the powder into solid powder reservoir form. Finished solid powder reservoirs for this study were approximately 10 mm in diameter, and 2 mm in height. These dimensions can be easily modified for future studies. Following a 15 minute incubation at −20°C, the solid adenosine tablet was removed from the press (incubation at cold temperatures served to minimize drug sticking to the bolt surface thereby yielding a more smooth even surface for coating, but had no noticeable effect on drug reservoir mechanical integrity). Reservoirs selected for study were all within ± 5 mg of 70 mg. Mannitol reservoirs were fabricated for controls. Mannitol is a commonly used chemically inert filler material for compressed tablets [43] and has been used as a placebo for orally administered adenosine tablet studies [44]. Total silk coating mass was determined by measuring the weight of the press-fit adenosine powder reservoirs with an analytical balance before and after coating.
Adenosine reservoirs were coated by dipping in aqueous silk solution at the desired protein concentration, drying for 30 min at 60°C, then immersing in methanol for 5 minutes to increase β sheet content for aqueous-insolubility. Following treatment, methanol was evaporated and the residue was resuspended in PBS and assayed for adenosine content. Adenosine and control/mannitol pills for the comparison of methanol treated versus untreated coatings were coated with 8% (w/v) silk fibroin solution and treated with a solution of 90% MeOH/10% H2O or left untreated. All other pills were treated with pure methanol. For the study of the silk coatings, 8% (w/v) aqueous silk fibroin solution was used and the process of dipping, drying and methanol treatment was repeated until the desired number of layers was achieved (1x, 2x, 4x or 8x). For the varied silk concentration study, the silk concentrations (in w/v) tested were 2%, 4%, 8%, 16% and 20%. For studies in which the silk concentration/coating thickness or number of silk coating layers were varied, n = 3. For the comparison of methanol versus untreated 8% (w/v) coatings study, n = 4.
2.4. Release studies in phosphate buffered saline (PBS) and Proteinase Type XIV
To determine adenosine release, silk coated powder reservoirs were immersed in 2 mL of Dulbecco’s PBS at 37°C. At desired time points, the buffer was removed and replaced with fresh buffer to approximate infinite sink conditions (i.e., modeling immediate clearance of adenosine released from systems implanted in vivo into the brain parenchyma [45]). The buffer removed periodically from the system was assayed for adenosine content using a modified fluorescence assay as previously described [20] and by measuring absorbance at 260 nm using a UV spectrophotometer. Percentage release was obtained by comparing adenosine mass to the total initial reservoir mass. Because silk-degrading proteases could be present at many potential in vivo implantation sites and degradation could impact release profile, release studies were also carried out in a proteinase type XIV solution to model potential in vivo proteolytic degradation of the silk coatings [23, 46]. Proteinase release studies also allowed comparative examination of purely diffusion-driven release (in PBS) with combined diffusion and degradation driven release (in proteinase). Proteinase release studies were performed using a 0.1 mg/mL proteinase type XIV solution in place of PBS.
At the end of the study when no additional adenosine was detected in the release buffer, the residual encapsulated reservoirs were degraded overnight in a 1.0 mg/mL proteinase K solution at 37°C and then assayed for any residual trapped adenosine that had not diffused from the reservoirs into the PBS.
2.5. Release kinetics
The release profiles were characterized by linear regression by determination of regression coefficients, R2, as zero order release is expected to correspond to constant drug release with respect to time. Values of t50 were taken as the time at which 50% of the drug was released from the tablet. The data for release of drug from encapsulated reservoirs was fit using Eq. (1) in order to assess possible release mechanisms. Mt/M∞ corresponds to the percent of adenosine released at time t relative to the total amount of drug in the tablet; K is a release constant and n is the release exponent indicating the type of drug release mechanism [47].
| [eq. 1] |
A fit to this equation is used to assess the relative importance of Fickian and zero order mechanisms in drug diffusion. Fickian transport is dominated by diffusion. Case II transport is dominated by polymer swelling and exhibits zero-order time independent release kinetics.
When n=0.5, pure diffusion for drug release is present, when n=1 swelling-controlled drug release or Case II transport is indicated. Other values for n suggest anomalous transport kinetics from combined mechanisms of pure diffusion and Case II transport. For linear fit, Eq. 1 is modified to Eq. 2. In this case, n is obtained from the slope of the plot of log(released%) versus log t.
| [eq. 2] |
2.6. Characterization
The surface and cross-sectional morphology of the silk encapsulated reservoirs was examined by scanning electron microscopy (SEM). Cross-sectional SEM images of the coated tablets were analyzed with Image J imaging software to determine the coating thickness. For each coating thickness determination, n = 3. Specimens were directly mounted and Au sputter-coated using a Ploaron SC502 Sputter Coater (Fison Instruments, UK). Samples were examined using a JEOL JSM 840A Scanning Electron Microscope (Peabody, MA) at 15 kV.
FTIR analysis was performed using a Bruker Equinox 55 FTIR spectrometer to confirm the structural changes for the coatings treated methanol or left untreated. Beta sheet structure was represented by curves with absorption bands in the frequency range of 1620–1630 cm−1 and 1695–1700 cm−1 [48].
3. Results and Discussion
3.1 Effect of Silk Fibroin Coating Solution Concentration and Number of Silk Coatings on Coating Thickness and Morphology
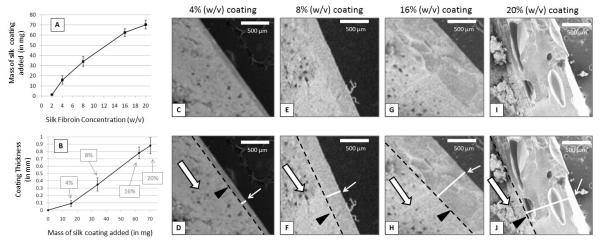
Examination of the surface morphology of 8% (w/v) silk coated adenosine reservoirs by scanning electron microscopy (SEM) revealed smooth, constant coating with no observed defects or cracks. The cross sections showed good packing of adenosine into the tablets and consistent, smooth silk coatings that exhibited constant thickness across the entire surface of the encapsulated reservoir. Sample SEM cross sections from a 4% (w/v) silk coated adenosine reservoirs (coating thickness = approximately 100 μm) are shown in Figure 1 to demonstrate the evenness of the silk coating. Examination of cross sections of silk fibroin coated adenosine reservoirs revealed increasing film thickness with increasing silk concentration and increasing number of coatings. Figure 2 shows sample SEM cross sections from reservoirs coated with 4% (w/v) (Figure 2C-D), 8% (w/v) (Figure 2E-F), 16% (w/v) (Figure 2G-H) and 20% (w/v) silk (Figure 2I-J). 2% (w/v) coatings were too thin to measure. The thickness of the film coating was determined by SEM and Image J software analysis and was found to increase with increasing silk concentration (reported in weight/volume percent) (Fig. 2A). The thickness of the film coating also increased with the mass of silk coating added (in milligrams) (Fig. 2B). The proportionality of coating thickness to mass of silk added suggests evenness of coating deposition and the proportionality of coating thickness relative to silk concentration suggests a controllable relationship exists between silk coating processing and final coating thickness.
Figure 1.

SEM images of cross-sections of encapsulated reservoirs coated with 4% (w/v) silk fibroin solution. Coating layer thickness is relatively consistent over the entire cross-section.
Figure 2.
(A) Average thickness of silk fibroin coating relative to silk fibroin solution concentration (n= 3, error bars represent standard deviation. (B) Average thickness of silk fibroin coating relative to mass of silk coating added (n= 3, error bars represent standard deviation). (C), (E), (G), and (I) SEM cross-sections of encapsulated reservoirs coated with 4% (w/v), 8% (w/v), 16% (w/v) and 20% (w/v) silk, respectively. (D), (F), (H) and (J) are the same SEM images as (C), (E), (G), and (I) but with additional markings to aid in distinguishing features present in the encapsulated reservoir cross-sections: black outlined empty white arrows indicate the adenosine reservoir, black triangles and black dotted lines indicate the interface between the silk coating and the adenosine reservoir. White lines accompanied by small white arrows mark the width of the coating film thickness. Scale bars are all 500 μm.
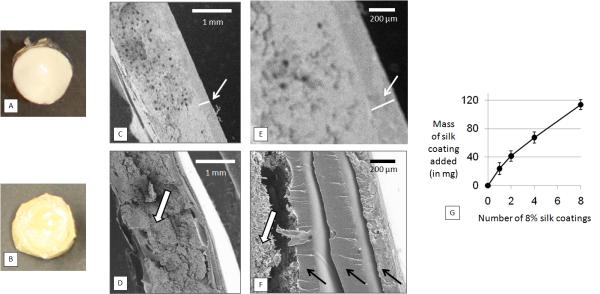
Figure 3 shows a comparison of encapsulated reservoirs coated with single and multiple coatings of 8% (w/v) silk. An unmagnified single coated reservoir and an unmagnified reservoir coated with eight 8% (w/v) silk layers are shown in Figures 3A and 3B, respectively. Figures 3C-3F show SEM images of cross-sections of a reservoir coated with a single silk layer (3C and 3D) and a reservoir coated with multiple silk layers (3E and 3F). Mass of coating relative to number of coatings applied is shown in Figure 3G (due to the inhomogeneity and variability of multiple dip coatings, thickness is more difficult to measure via SEM, making mass a more accurate reflection of the effects of the multi-layer coating process).
Figure 3.
Comparison of adenosine reservoirs coated with single 8% (w/v) silk coating (single-coating reservoirs) and an encapsulated reservoir coated with multiple 8% (w/v) coatings (multiple-coating reservoirs) (A) Reservoir coated with a single 8% (w/v) silk layer. (B) Reservoir coated with eight 8% (w/v) silk coatings (C) and (E) SEM images of cross section of single 8% (w/v) silk coated reservoir. (D) and (F) SEM cross section of multiple 8% (w/v) silk layer coated reservoir. (E) Graph of mass of coating added (in milligrams) relative to increasing number of 8% (w/v) silk coatings. Scale bars in (C) and (D) are 1 mm. Scale bars in (E) and (F) are 200 μm.
3.2. Effect of Silk Fibroin Coating Concentration/Film Thickness on Adenosine Release in PBS
Uncoated adenosine reservoirs dissolved immediately upon immersion in PBS and 100% of the drug load was recovered when the first sample was collected at 12 hours. Due to the insolubility of adenosine in methanol, no significant drug loss occurs during methanol treatment of the silk coatings to stabilize the beta sheet physical cross links. Less than 0.001 mg of adenosine out of an average total mass of approximately 70 mg (or 0.0014%) was recovered from the methanol residue. At the end of all release studies, when the encapsulated reservoirs had released their full adenosine drug load (i.e., adenosine was no longer detected in the release study buffer) the systems were degraded by protease and no residual adenosine was found.
The linearity of release increased and average release rate slowed with increasing silk concentration used in the coatings, up through 16% w/v silk (Figure 4). The only deviation from this trend was the 20% (w/v) silk coated reservoirs which showed more rapid release kinetics and decreased linearity compared to the 16% (w/v) release curve. Potential explanations for this discrepancy include cracking of thicker films during methanol treatment or uneven drying of the films due to the high silk concentration in the coating solution resulting in inhomogeneous films (see Figure 5). These phenomena have also been observed when thick (high concentration) silk films were cast on flat surfaces [29].
Figure 4.
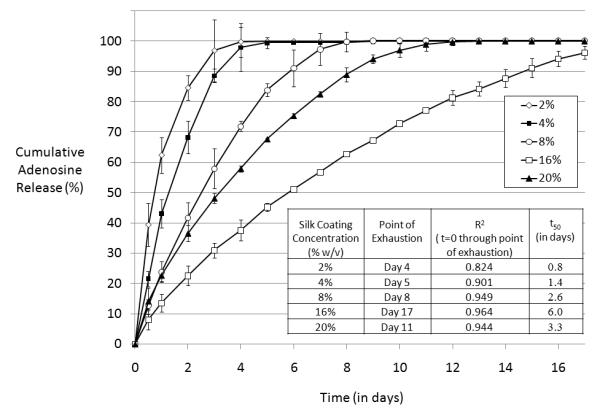
Cumulative adenosine release from single-coating encapsulated adenosine reservoirs coated with silk of varying concentration over time in PBS at 37° C. N=3, error bars represent standard deviations (where error bars aren’t shown they fall into background).
Figure 5.
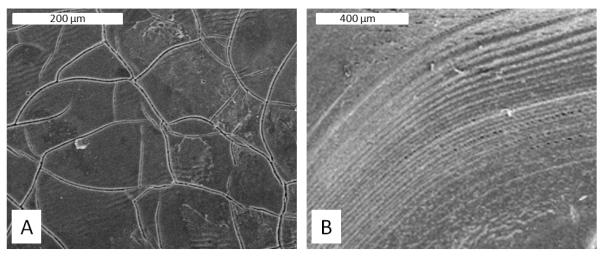
Microscopic surface defects observed on single coating 20% (w/v) silk encapsulated reservoir coating surfaces (A) Surface cracking, scale bar = 200 μm (B) Uneveness/rippling of the film surface towards the reservoir edge, scale bar = 400 μm.
Values of t50, or the time at which 50% of the total drug load was released from the reservoir, and regression coefficients (R2) are reported in the Table inset in Figure 4. The release profile closest to the zero-order target was observed in encapsulated reservoirs coated with the 16% w/v silk, which sustained release through day 17 with a regression coefficient (R2) of 0.96. Values for t50 for 2%, 4%, 8%, 16% and 20% (w/v) silk fibroin coating concentrations were 0.8 days, 1.4 days, 2.6 days, 6.0 days and 3.3 days, respectively. The n values of the log(release%) versus log(t) curves for 2%, 4%, 8%, 16% and 20% (w/v) silk fibroin coating concentrations were 0.351, 0.544, 0.707, 0.696 and 0.525, respectively. In this case the Siepmann-Peppas models suggest complexity of release mechanism (i.e., neither pure diffusion nor pure Case II release), so the results of the fit do not allow us to characterize release mechanism. However, the results of the fit do allow empirical observation of increased linearity at higher silk coating concentrations (i.e., as silk coating thickness increases, n increases, suggesting that the release profile from the reservoir becomes increasingly more linear with increasing film thickness).
3.3. Effect of Silk Fibroin Coating Concentration/Film Thickness on Adenosine Release in Proteinase XIV
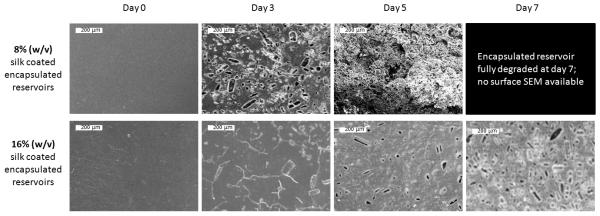
Gross morphology changes for encapsulated reservoirs coated with 8% (w/v) and 16% (w/v) silk coatings in PBS and 0.1 mg/mL proteinase type XIV solution at days 3, 5 and 7 are shown in Figure 6. Coatings on both 8% (w/v) and 16% (w/v) silk encapsulated reservoirs in PBS appeared unchanged over 7 days due to the absence of proteases in the release buffer. In proteinase type XIV solution the 16% (w/v) silk encapsulated reservoirs retained their shape through the first seven days and only began to show any visible signs of degradation at day 7. The 8% (w/v) silk encapsulated reservoirs showed translucence at day 3, partial degradation at day 5 and complete degradation by day 7. SEM images of the degrading encapsulated reservoir surfaces at day 0, day 3, day 5 and (in the case of 16% (w/v) silk coated reservoirs) day 7 are shown in Figure 7. Surface morphology changes rapidly for 8% (w/v) silk coated reservoirs. The progression of degradation of the 16% (w/v) silk coated reservoir surfaces can only be detected on the SEM images. The microscopic surface changes of 16% (w/v) encapsulated reservoirs are more subtle than changes seen in the 8% (w/v) silk coated reservoirs. For additional data of silk film proteolytic degradation, see Nuanchai et al., 2009 [49].
Figure 6.
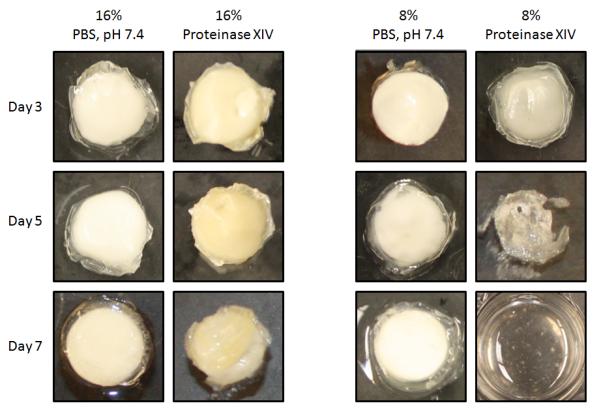
Gross morphological changes of single-coating silk encapsulated adenosine reservoirs in PBS and Proteinase type XIV solution over 7 days. The 8% (w/v) coated reservoirs in proteinase type XIV solution were beginning to become translucent at day 3 and showed extensive degradation by day 5. By day 7 in proteinase type XIV solution, 8% (w/v) encapsulated reservoirs were totally disintegrated. 16% (w/v) encapsulated reservoirs retained their shape through day 7.
Figure 7.
SEM of single-coating 8% (w/v) and 16% (w/v) silk encapsulated reservoir surfaces over the course of proteolytic degradation in 0.1 mg/mL proteinase type XIV at 37°C. Scale bars are all 200 μm.
Cumulative release profiles from the encapsulated adenosine reservoirs in proteinase type XIV are shown in Figure 8A. The shape of the cumulative release curve of the 16% silk coated reservoirs in the protease resembles the shape of the curve in PBS (R2Proteinase = 0.983, compared to R2PBS = 0.964), but the duration of release was shorter (t50, proteinase = 3.1 days compared to t50, PBS = 6.0 days). The release curves of 4% and 8% coated reservoirs were more similar in the proteinase than in PBS. Values for t50 for 4%, 8% and 16% (w/v) silk fibroin coating concentration samples in proteinase type XIV were 0.8 days, 1.0 days and 3.1 days, respectively. A side by side comparison of adenosine release through day 6 from 16% (w/v) silk encapsulated reservoirs immersed in PBS versus 0.1 mg/mL proteinase type XIV is shown in Figure 8B. Data is shown through day 6 because this time-frame corresponds to linear release for both systems, allowing for ease of comparison of average release rates: approx. 8.5% total adenosine load per day in PBS and approx. 15.9% total adenosine load per day in proteinase type XIV. In proteinase solution, adenosine release from silk encapsulated reservoirs (driven by diffusion and degradation) occurs at nearly double the rate observed in PBS (driven only by diffusion).
Figure 8.
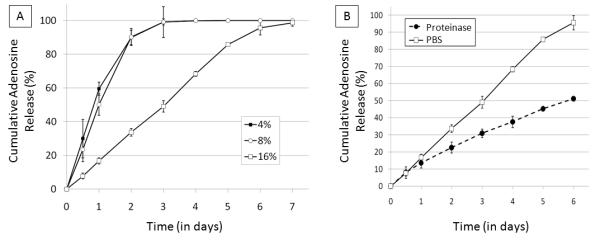
(A) Cumulative adenosine release from single-coating encapsulated adenosine reservoirs coated in silk of varying concentrations over time in 0.1 mg/mL proteinase type XIV solution at 37° C. N=3, error bars represent standard deviations (where error bars aren’t shown they fall into background) (B) Comparison of cumulative adenosine release curves of 16% (w/v) silk encapsulated reservoirs in PBS and in 0.1 mg/mL proteinase type XIV solution at 37°C through day 6. N=3, error bars represent standard deviations (where error bars aren’t shown they fall into background)
3.4. Effect of Number of Silk Fibroin Coatings on Release of Adenosine
Encapsulated reservoirs coated with 8 layers of 8% (w/v) silk fibroin exhibited a zero order release with a regression coefficient (R2) of 0.999 (Figure 9). Reservoirs coated with 4 layers of 8% (w/v) silk fibroin exhibited near zero-order release (R2=0.98). Reservoirs with 4 coatings released continuously for 13 days at an average release rate of 7.03 ± 2.83% of the total adenosine mass (average total adenosine mass = 71.5 mg) per day and encapsulated reservoirs with 8 coatings continued to release for 14 days at an average release of 6.53 ± 2.26% of the total reservoir mass per day. Values of t50 for 1x, 2x, 4x and 8x encapsulated reservoirs were 3.3 days, 4.3 days, 5.3 days and 7.1 days, respectively. Regression coefficients, release durations, values of t50 and average release rates are reported in the Table inset in Figure 9.
Figure 9.
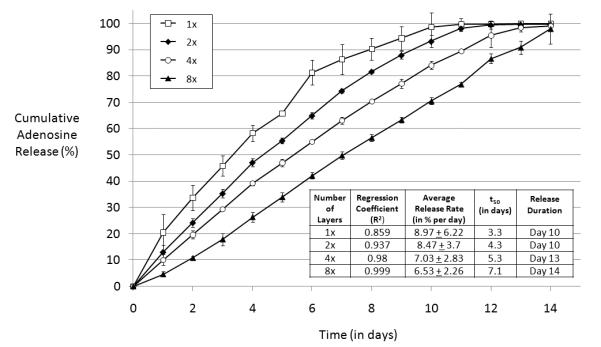
Cumulative adenosine release from multiple-coating encapsulated adenosine reservoir coated in varied number of 8% (w/v) silk coatings in PBS at 37° C. 1x = one coating, 2x = two coatings, 4x = four coatings, 8x = 8 coatings. N = 3, error bars represent standard deviations (where error bars aren’t shown they fall into background).
The n values of the log(release%) versus log(t) curves were 0.70 for 1 coating, 0.87 for 2 coatings, 0.89 for 4 coatings and 1.15 for 8 coatings. Encapsulated reservoirs coated with 8 layers of silk had an n>1, indicating zero-order release. The other reservoirs (1x, 2x and 4x) all had 0.5<n<1, suggesting a complex (i.e., neither pure diffusion nor pure Case II release) release mechanism [47]. As before, with increasing number of silk coatings and increasing thickness, n increases, suggesting that the release profile from the reservoir becomes increasingly more linear with increasing number of coatings.
3.5. Effect of Methanol Treatment of Silk Fibroin Coatings on Release of Adenosine
Methanol treatment of the 8% (w/v) silk reservoir coatings slowed release compared to the untreated 8% (w/v) silk coatings (Figure 10A). FTIR peak shifts confirmed that methanol treatment increased the crystallinity of the silk coating (Figure 10B). As seen in Figure 10B, silk coatings that received methanol treatment show an increase in the absorbance in the 1620-1630 cm−1 range (amide I region). The methanol treated coatings exhibit a characteristic peak at around 1625 cm−1 (indicative of β sheet conformation) while the untreated films only exhibit a peak at around 1645 cm−1. In the amide II region, methanol treated coatings also exhibit increased absorbance at 1515 cm−1 compared to untreated coatings, another indication that these coatings have increased β-sheet conformation. For a more in depth study on silk film morphology and beta sheet content see Lawrence et al., 2009 [50]. Values of t50 for pills coated with untreated silk films and methanol treated films were 1.8 days and 3.4 days, respectively.
Figure 10.
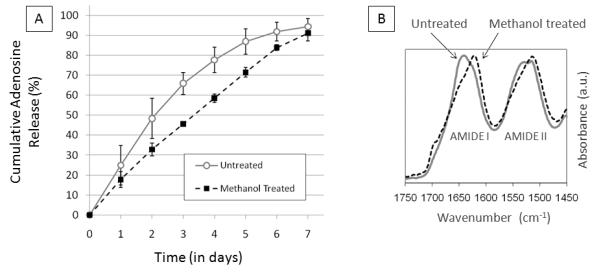
(A) Cumulative adenosine release from single-coating silk encapsulated adenosine reservoirs coated in 8% w/v silk treated with methanol or left untreated. N=4, error bars represent standard deviations (where error bars aren’t shown they fall into background) (B) FTIR spectra (amide I and amide II bands) of silk fibroin pill coatings comparing methanol treated silk coatings to untreated silk coatings.
In cases where drug release is slower than can be explained by simple diffusion, we speculate that adenosine release is delayed by the hydrophobic silk coating limiting penetration of water into the powder reservoir. As coating thickness, number of coatings and β-sheet content increase, the coating becomes increasingly less permeable to water and adenosine release correspondingly slows until zero-order release is observed. The introduction of proteinases in the release buffer degrades the silk coating, allowing greater penetration of water into the powder reservoir and speeding adenosine release compared to release in PBS.
Overall, increasing the thickness of the silk coating applied to the adenosine reservoir decreases burst, decreases average daily release rate and increases duration of release. However, 20% (w/v) silk coatings release adenosine more quickly than a 16% (w/v) coating or four 8% (w/v) layers, which we speculate is caused by the 20% (w/v) coating being too thick to be applied homogenously, cracking when methanol treated, or some combination of both these phenomena (see Figure 5). While the data shows that thickness impacts release profile, the resulting adenosine release from films of equivalent thickness with varying beta sheet content demonstrate that other silk material features can also affect the rate of diffusion through the silk coating. Encapsulated reservoirs coated with a single layer of 16% (w/v) silk and 4 individual layers of 8% (w/v) silk fibroin which had nearly the same mass of silk coating added (62.5±3.4 mg and 67.4±7.7 mg, respectively) had similar resulting adenosine release profiles. However, the use of multiple coatings increased the mass of silk that could be added to the reservoir without compromising zero-order release or consistency of daily release rate nearly two-fold (Figure 3). An average mass of 62.5±3.4 mg of silk coating was added to the adenosine reservoirs coated with 1 layer of 16% (w/v) silk, while an average of 113.6±4.2 mg of total silk was added to the adenosine reservoirs coated with 8 layers of 8% (w/v) silk. This suggests that while the concentration of the silk used for reservoir coating can only be increased up to around 16% (w/v) without compromising encapsulation layer homogeneity, multiple individual layers can be used to increase coating thickness.
These results suggest that silk fibroin coatings on solid reservoirs, such as in this case the press-fit pills, represents a promising system for controllable sustained, zero-order adenosine delivery. The choice of silk as the coating polymer ensures that the system will be safe for implantation in the brain, because the coating material is biocompatible [22], will degrade to non-toxic products in vivo [23, 24] and is an FDA approved biomaterial [23]. Silk has been shown to be less inflammatory than other commonly used biodegradable polymers like collagen and polylactic acid [48-51]. In the case of powder reservoirs coated with 8 layers of 8% (w/v) silk fibroin, a zero order release profile (R2 = 0.999, n>1) for adenosine was attained, and this release was sustained for 14 days (this was from a starting adenosine content of approx. 70 mg).
4. Conclusions
In this study we demonstrate the ability to achieve zero order release profiles, and an approximately constant and continuous release profile over extended time frames (up to 17 days in the present study with further time frames feasible based on the factors studied here). This represents a longer release duration and more linear profile than any previous silk-based small molecule drug delivery system (including any of our previous silk-based adenosine delivery systems) and presents preliminary data on a novel approach to silk-based controlled drug delivery. This fully degradable biomaterial system represents a promising epilepsy prevention therapy and is of interest to the field of therapeutic drug delivery in general. While many therapeutics to treat epilepsy could require decades of continuous administration to be effective, our previous studies suggested that transient adenosine delivery (if sustained for sufficient time-frames) might prevent the development of seizures as an indication of epilepsy [21]. Because the systems described in this study are capable of continuous adenosine delivery for a few weeks and then degrade in vivo [24] (requiring no retrieval surgery), a single one-shot implantation following traumatic brain injury might be sufficient to prevent the development of symptoms of epilepsy. Moreover, we observe relationships between the silk coating material features (crystallinity, morphology, thickness) and the resulting adenosine release profiles which suggest we will ultimately be able to achieve tight control of drug release from these systems and substantially extend duration of small-molecule drug release. We anticipate that a further increase in coating thickness (hundreds of individual coating layers could theoretically be added) will slow release rates and thereby extend delivery duration while maintaining zero-order release. Future work includes demonstrating delivery duration greater than 17 days and animal studies to demonstrate seizure suppression following implantation over these extended time frames. Also, release studies in chymotrypsin [51] or buffer with a protease cocktail more representative of the local brain environment (i.e., based on protease concentrations from the literature) would be of interest to better predict and/or control in vivo behavior of these types of implants.
Acknowledgements
This project was supported by grant R01NS058780 from the National Institute of Neurological Disorders and Stroke and by the Epilepsy Research Foundation through the generous support of the Arlene and Arnold Goldstein Family Foundation. We thank Anthony B. Barry, Wyeth BioPharma, for his technical assistance. The authors would like to thank Xiao Hu for his assistance with FTIR and Brian Aguilar for his help fabricating press-fit adenosine tablets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Acharya G, Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliver. Rev. 2006;58:387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [2].Langer R. Invited review: polymeric delivery systems for controlled drug release. Chem. Eng. Commun. 1980;6:1–48. [Google Scholar]
- [3].Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- [4].Dash AK, Cudworth GC., II Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- [5].Daugherty AL, Mrsny RJ. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliver. Rev. 2006;58:686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [6].Swarbrick J. Encyclopedia of Pharmaceutical Technology. third ed Informa HealthCare; New York: 2006. [Google Scholar]
- [7].Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends in Pharm. Sci. 2006;27:653–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- [8].Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog. Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dunwiddie TV. Adenosine and suppression of seizures. Adv. Neurol. 1999;79:1001–1010. [PubMed] [Google Scholar]
- [10].Boison D. Adenosine augmentation therapies (AATs) for epilepsy: Prospect of cell and gene therapies. Epilepsy Res. 2009;85:131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener. Dis. 2007;4:28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- [12].Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- [13].Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc. Natl. Acad. Sci. USA. 2001;98:7611–7616. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sokolosky-Papov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007;59:187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [15].Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends for controlled release coatings. J. Control. Release. 2008;125:1–15. doi: 10.1016/j.jconrel.2007.09.012. [DOI] [PubMed] [Google Scholar]
- [16].Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- [17].Sawyer AJ, Piepmeier JM, Saltzman WM. New methods for direct delivery of chemotherapy for treating brain tumors. Yale J. Bio. Med. 2006;79:141–152. [PMC free article] [PubMed] [Google Scholar]
- [18].Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2:201–214. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- [19].Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [20].Wilz A, Pritchard E, Li T, Lan J-Q, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Szybala C, Pritchard EM, Lusardi TA, Li T, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp. Neuro. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- [23].Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–3393. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- [24].Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang X, Kim HJ, Xu P, Matsumoto A, Kaplan DL. Biomaterial coatings by stepwise deposition of silk fibroin. Langmuir. 2005;22:11335–11341. doi: 10.1021/la051862m. [DOI] [PubMed] [Google Scholar]
- [26].Wang X, Hu X, Daley A, Rabotyagova O, Cebe P, Kaplan DL. Nanolayer biomaterial coatings of silk fibroin for controlled release. J. Control. Release. 2007;121:190–199. doi: 10.1016/j.jconrel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL. Silk microspheres for encapsulation and controlled release. J. Control Release. 2007;117:360–370. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- [28].Kim U-J, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and property of silk hydrogels. Biomacromolecules. 2004;5:786–792. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- [29].Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [30].Hofmann S, Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL. Silk fibroin as an organic polymer for controlled drug delivery. J. Control Release. 2006;111:219–227. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [31].Zhang X, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials. 2008;29:2217–2227. doi: 10.1016/j.biomaterials.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dehdashti AR, Muster M, Reverdin A, de Tribolet N, Ruefenacht DA. Preoperative silk suture embolization of cerebral and dural arteriovenous malformations. Neurosurg. Focus. 2001;11:e6. doi: 10.3171/foc.2001.11.5.7. [DOI] [PubMed] [Google Scholar]
- [33].Van Savage G, Rhodes CT. The sustained release coating of solid dosage forms: a historical review. Drug Dev. Ind. Pharm. 1995;21:93–118. [Google Scholar]
- [34].During MJ, Freese A, Sabel BA, Saltzman WM, Deutch A, Roth RH, Langer R. Controlled Release of Dopamine from a Polymeric Brain Implant: In Vivo Characterization. Ann. Neuro. 1989;25:351–356. doi: 10.1002/ana.410250406. [DOI] [PubMed] [Google Scholar]
- [35].Dahl TC, Sue IT. Mechanisms to control drug release from pellets coated with a silicone elastomer aqueous dispersion. Pharm. Res. 1992;9:398–405. doi: 10.1023/a:1015855305679. [DOI] [PubMed] [Google Scholar]
- [36].Siepmann F, Hoffmann A, Leclercq B, Carlin B, Siepmann J. How to adjust desired drug release patterns from ethylcellulose-coated dosage forms. J. Control. Release. 2007;119:182–189. doi: 10.1016/j.jconrel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [37].Lippold B, Sutter BK, Lippold BC. Parameters controlling drug release from pellets coated with aqueous ethyl cellulose dispersion. Int. J. Pharm. 1989;54:15–25. [Google Scholar]
- [38].Dorta MJ, Santoveña A, Llabrés M, Fariña JB. Potential applications of PLGA film-implants in modulating in vitro drugs release. Int. J. Pharm. 2002;248:149–156. doi: 10.1016/s0378-5173(02)00431-3. [DOI] [PubMed] [Google Scholar]
- [39].Pearnchob N, Bodmeier R. Dry polymer powder coating and comparison with conventional liquid-based coatings for Eudragit® RS, ethylcellulose and shellac. Eur. J. Pharm. Biopharm. 2003;56:363–369. doi: 10.1016/s0939-6411(03)00121-8. [DOI] [PubMed] [Google Scholar]
- [40].Vandamme TF, Mukendi J.-F. Ngombo. Controlled release of levamisole from poly-(∊- caprolactone) matrices: III. Effects of molecular weight and polymer coating on drug release. Int. J. Pharm. 1996;145:77–86. [Google Scholar]
- [41].Krajacic A, Tucker IG. Matrix formation in sustained release tablets: possible mechanism of dose dumping. Int. J. Pharm. 2003;251:67–78. doi: 10.1016/s0378-5173(02)00584-7. [DOI] [PubMed] [Google Scholar]
- [42].Abletshauser CB, Schneider R, Rupprecht H. Film coating of pellets with insoluble polymers obtained in situ crosslinking in the fluidized bed. J Conrol. Release. 1993;21:149–156. [Google Scholar]
- [43].Ward DR, Lathrop LB, Lynch MJ. Dissolution and compatibility considerations for the use of mannitol in solid dosage forms. J. Pharm. Sci. 1969;58:1464–1467. doi: 10.1002/jps.2600581208. [DOI] [PubMed] [Google Scholar]
- [44].Graven-Nielsen T, Jansson Y, Segerdahl M, Kristensen JD, Mense S, Arendt-Nielsen L, Sollevi A. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur. J. Pain. 2003;7:93–102. doi: 10.1016/s1090-3801(02)00069-1. [DOI] [PubMed] [Google Scholar]
- [45].Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local Delivery of Doxorubicin for the Treatment of Malignant Brain Tumors in Rats. Anticancer Res. 2005;25:3825–3831. [PMC free article] [PubMed] [Google Scholar]
- [46].Li M, Ogiso M, Minoura N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials. 2003;24:357–365. doi: 10.1016/s0142-9612(02)00326-5. [DOI] [PubMed] [Google Scholar]
- [47].Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987;5:23–36. [PubMed] [Google Scholar]
- [48].Ishida M, Asakura T, Yokoi M, Saito H. Solvent- and mechanical treatment-induced conformational transition of silk fibroins studied by high-resolution solid-state 13C NMR spectroscopy. Macromolecules. 1990;23:88–94. [Google Scholar]
- [49].Nuanchai K, Prasong S, Wilaiwan S. In vitro Degradation Behavior of Bombyx mori Silk Fibroin Films Exposure to Protease XXIII. Biotechnology. 2009;8:468–472. [Google Scholar]
- [50].Lawrence BD, Wharram S, Kluge JA, Leisk GG, Omenetto FG, Rosenblatt MI, Kaplan DL. Effect of Hydration on Silk Film Material Properties. Macromol. Biosci. 2009 doi: 10.1002/mabi.200900294. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tomomura A, Yamada H, Itagaki K, Fujimoto K, Katoh S. Rat brain expresses serum calcium-decreasing factor (caldecrin) Neurosci. Lett. 2002;317:17–20. doi: 10.1016/s0304-3940(01)02409-0. [DOI] [PubMed] [Google Scholar]