1. Introduction
Fluorescence spectroscopy, one of the most informative and sensitive analytical techniques, has played and continues to play key roles in modern research. Indeed, unraveling the inner workings of biomolecules, cells and organisms relied on the development of fluorescence-based tools. As many of the players in these sophisticated interactions and exceedingly complex systems are not inherently emissive, researchers have relied on synthesizing fluorescent analogs of the building blocks found in biological macromolecules. These are the constituents of the cell surface and cell membrane, as well as proteins and nucleic acids. This review article is dedicated to emissive analogs of these relatively small molecules.
For organizational purposes, we have arbitrarily selected to approach these diverse families of biomolecules by imagining “a journey into the center of the cell”. Approaching the exterior of a cell, one first encounters oligosaccharides that decorate the cell surface and are involved in cell recognition and signaling. Next, we arrive at the cell membrane itself. This semi-permeable envelope sets the cell boundaries and regulates its traffic. Several types of building blocks assemble this membrane, most notably among them are the phospholipids. Upon entering the cell, the cytosol reveals a plethora of small and large molecules, including proteins, as well as soluble RNA molecules and RNA-rich ribosomes. Within the cytosol of eukaryotes and prokaryotes lies the nucleus or nucleoid, respectively. This membrane-enclosed control center contains most of the cells’ genetic material. DNA, the cellular blueprint, is permanently found in the nucleus, which also hosts diverse RNA molecules. Accordingly, we first discuss emissive carbohydrate derivatives. We then present fluorescent membrane constituents, followed by emissive amino acids. Our journey ends by focusing on emissive analogs of nucleosides and nucleotides, the building blocks of nucleic acids.
The common biomolecular building blocks, excluding a few amino acids, lack appreciably useful fluorescence properties. This implies that structural modifications are required to impart such photophysical features. Ideally, a designer probe should closely resemble its natural counterpart in size and shape without the loss of the original function (a feature we refer to as “isomorphicity”). This presents a fundamental predicament, as any modification attempting to alter the electronic nature of a molecule, typically by including aromatic residues or extending conjugation, will also alter its steric bulk and therefore the interactions with its surroundings.
Clearly not all biomolecular building blocks can or need to accommodate strict isomorphic design criteria. The heterocycles found in nucleosides already provide a platform that facilitates the extension of π-conjugation, which is also true for some aromatic amino acids. In contrast, employing fluorescence spectroscopy to membrane research requires very creative probe designs. Saccharides can be viewed as the most restrictive in this context, as no chemical modification is conceivable without a major structural disruption and likely loss of function. Such aliphatic biomolecules accommodate labeling only, where an established fluorophore is covalently conjugated to provide an emissive derivative. We therefore reserve the term probe to molecular designs that are expected to furnish useful modified biomolecules capable of reliable reporting. Understandably, fluorescent probes must meet the most stringent isomorphic design principles to ensure a biologically meaningful read-out. The isomorphic design principle is therefore a central theme of this review.
This article focuses on designing fluorescent probes for the four major families of macromolecular building blocks discussed above. Although not necessarily in chronological order, it spans roughly four decades of probe design with emphasis, when justified, on recent contributions. As the reader may imagine, this topic encapsulates a vast research field and cannot be comprehensively reviewed within the space limitation of Chemical Reviews. Nevertheless, we have attempted to summarize the most important and general contributions discussing fluorescent probes that were designed to shed light on biological processes and refer the reader to other resources.1 Although a few examples have found their way into the text, we do not generally address here the development of small molecule fluorophores and sensors that are not part of biomolecular assemblies. We open this article with a brief overview of the key features of fluorescence spectroscopy, where essential theoretical, experimental, and practical elements are discussed.
2. Fluorescence Spectroscopy Techniques in a Nutshell
2.1. Essentials and Benefits of Fluorescence Spectroscopy
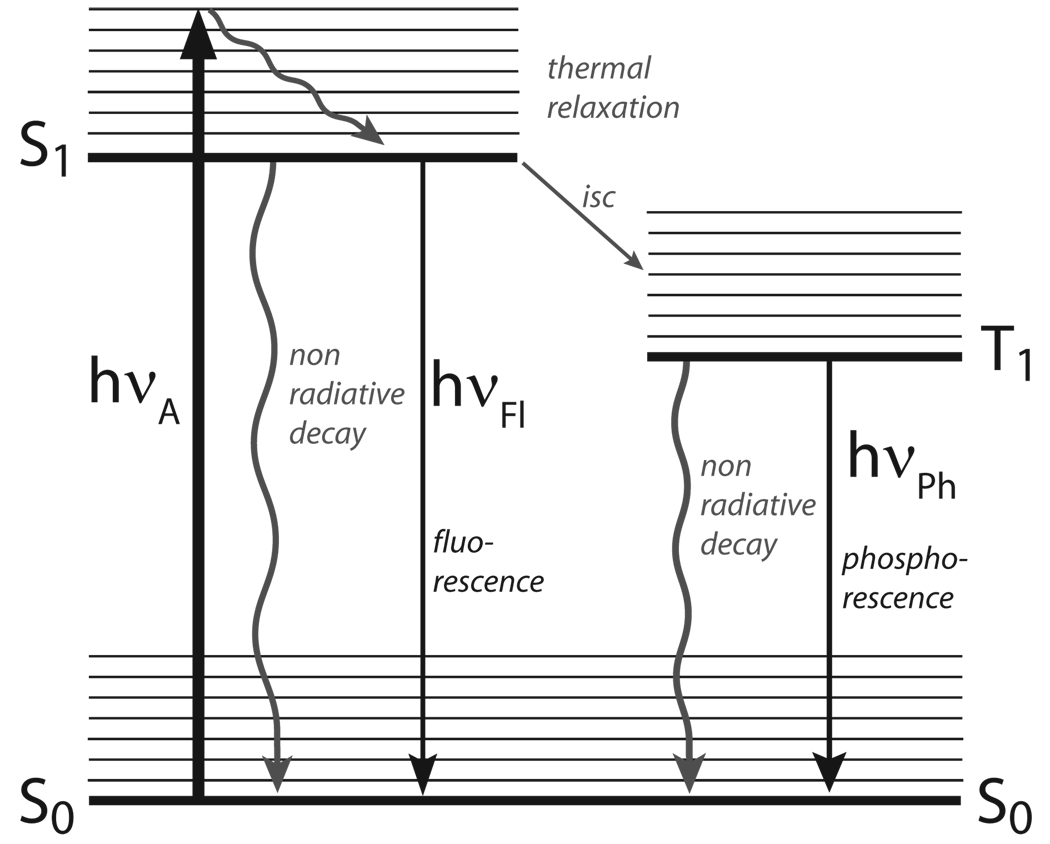
Any spectroscopy-based technique is associated with inherent sensitivity traits and time-scale features, which are dependent on the fundamental nature of the transitions involved. Optical excitation of a chromophore generates the Franck–Condon state extremely rapidly (within 10−15 sec). The efficiency of this process is related to the chromophore’s absorption cross-section (σ), which is proportional to its extinction coefficient (ε). Vibrational relaxation (within 10−12−10−10 sec) quickly populates the lowest vibronic state of the chromophore’s excited state (Figure 2.1, Jablonski diagram). This relaxation process, generating the emissive state, accounts for the lower emission energy of a chromophore compared to its excitation energy (Stokes shift). Typical organic chromophores reside in their excited state for a period of 0.5–20 × 10−9 seconds. The excited state lifetime reflects the sum of the various radiative and non-radiative processes the excited chromophore undergoes in decaying back to the ground state (τ0). The fraction responsible for emitting a photon, or the fluorescence lifetime (τ), reflects the emission quantum yield of the chromophores (Q=Φ= τ/τ0). In some studies, the brightness (ε × Φ) of a fluorophore is reported, which is the product of the molar absorptivity (ε) and the fluorescence quantum yield (Φ). This becomes useful when comparing the utility of two fluorophores with similar fluorescence quantum yields, but very different molar absorptivities.
Figure 2.1.
A simplified Jablonski diagram.
Fluorescence-based techniques are commonly appreciated for their versatility and sensitivity (up to a 1000-fold higher than absorption spectrophotometry). Creative probe design can provide chromophores with appropriate excitation and emission wavelengths, while minimizing interference by other emissive cellular constituents. Selective excitation coupled to the sensitivity of many chromophores to various environmental parameters (pH, polarity, viscosity, presence of quenchers, etc.), make molecular fluorescence an extremely effective tool for in vitro biophysical and biochemical analyses, as well as in vivo cellular imaging capable of providing spatial and temporal information.2,3
Before discussing the chromophoric biomolecular building blocks themselves, we first survey the most common techniques and tools used in fluorescence spectroscopy. For additional theoretical and technical details, the interested reader is referred to Valeur’s ‘Molecular Fluorescence’,4 Turro’s ‘Modern Molecular Photochemistry of Organic Molecules’,5 and to Lakowicz’s comprehensive monograph entitled the ‘Principles of Fluorescence Spectroscopy’.2
2.2. Steady-State Fluorescence Spectroscopy
The simplest and most frequently used technique is steady-state fluorescence spectroscopy. Upon excitation of a chromophore (typically at its absorption maximum) with a light source providing a constant photon flow, an emission spectrum is recorded, revealing the energy maximum and intensity of emission. At low concentrations (absorbance < 10−2), the emission intensity is typically proportional to the concentration of the chromophore (with approximately 1% deviation from linearity). While the emission maximum is an intrinsic characteristic of a chromophore, it is frequently sensitive to environmental perturbations.
Fluorophores with emission maxima that display sensitivity to polarity can be used to estimate the properties of the chromophore’s microenvironment. If the dipole moment of the excited state is greater than that of the ground state, rearrangement of solvent molecules can lower the energy of the excited state prior to emission, resulting in a red shift of the emission maximum.6,7 This phenomenon has been employed, for example, to investigate the local polarity in membranes,8 proteins9,10 and DNA.11 Dielectric constants (ε), reflecting a bulk property, were initially used to express polarity as orientational polarizability, Δf.6,7 With the development of microscopic solvent polarity parameters (such as Reichardt’s ET(30) scale), polarity could be quantified at the molecular level.12 This is of significance for the study of confined cavities in biomolecules, where the local polarity is likely to differ dramatically from the aqueous bulk polarity. Indeed, microscopic polarity parameters, show a better linear correlation with Stokes shifts (νabs−νem), when compared to dielectric constants or orientational polarizability values (Figure 2.2).13
Figure 2.2.
Correlation of solvent polarity and Stokes shift of PRODAN.
2.3. Fluorescence Quenching and Resonance Energy Transfer
Two common processes that cause loss of emission intensity are collisional and static quenching. The former process is described by the Stern–Volmer equation14 and a modification thereof, the Lehrer equation.15 Dynamic quenching is characterized by a linear correlation between the quenching effect and the quencher concentration. Deviation from linearity typically implies the involvement of static quenching, where a sphere of effective quenching exists, or a non-fluorescent ground state complex is formed, as formulated by the Perrin Model.16 Quenching experiments have been used, for example, to study conformational and dynamic properties of proteins,17,18 microdomains in membranes,19 and RNA folding dynamics.20 Despite their relative simplicity, the interpretation of quenching experiments could be complex.18
A more sophisticated, yet related, phenomenon involves resonance energy transfer (RET), a non-radiative transfer of excitation energy between distinct chromophores, typically referred to as donors and acceptors. Different mechanisms can facilitate energy transfer. The Dexter mechanism (or electron exchange) operates at short ranges and requires an intermolecular orbital overlap. The Förster mechanism, a Coulombic or dipolar interaction, operates at larger distances and is facilitated when the emission band of the donor overlaps with the absorption band of the acceptor.2 Förster (commonly, albeit somewhat inaccurately, substituted for fluorescence) resonance energy transfer, FRET, therefore, results in a quenched donor emission and a concomitant increase in the lower energy emission of the acceptor. The strong dependence of the energy transfer rate on donor–acceptor distance (kET ∼ r−6) contributes to the utility of this phenomenon and facilitates the calculation of distances between interacting donors and acceptors.21
Resonance energy transfer experiments have been extensively used in biophysics and biology, where the participating partners are labeled with highly emissive and typically large donors and acceptors. Scattered and relatively recent examples include the study of protein folding, protein–protein interactions and cellular signaling events in living cells.17,22–24 FRET has also been used to elucidate folding and dynamics of RNA,25 as well as the sequence dependent structure, stability and dynamics of nucleosomes.26 Membrane researchers have used FRET to study, for example, microdomain formation19 and trans-membrane peptides in surface supported bilayers.27
2.4. Time Resolved Fluorescence Spectroscopy
Steady state measurements are instrumental in detecting changes in fluorescence intensity, as well as emission and excitation maxima. Steady-state spectra give, however, an average emission profile of all excited fluorophores present in the sample. This technique, therefore, cannot distinguish between individual fluorophores found in a heterogeneous population, such as those associated with different conformational states. Time-resolved measurements, yielding excited state lifetimes, provide insight into the excited state dynamics and the decay pathways of the excited chromophore. In this fashion, it is possible, for example, to extract information on different excited species in a single sample based on differences in their fluorescence lifetime. Time resolved quenching experiments can distinguish between collisional (lifetime is affected) or static (lifetime is unaffected) quenching. As steady-state, time resolved fluorescence spectroscopy also gives an averaged profile of the excited chromophores in a sample. With deconvolution, however, it is possible to resolve more than one decay pathway, each of which representing an average across a population. Moreover, in contrast to steady state analysis, time resolved fluorescence spectroscopy is concentration independent.
2.5. Fluorescence Anisotropy
Within the short time-window, after excitation but before emission, the excited fluorophore undergoes Brownian motion. Its tumbling rate is affected by temperature, solvent viscosity, its size and bound species. This can be investigated with polarized fluorescence spectroscopy, also called fluorescence anisotropy. Polarization (P) is defined as the difference between intensities of parallel (I∥) and perpendicular (I┴) polarized emission divided by the sum of the two, and is interchangeable with anisotropy [r = (I∥−I┴)/(I∥+2I┴)]. In a practical setup, optical polarizers for excitation and emission are used. Vertically polarized light is used for excitation, while the emission is detected once after vertical and once after horizontal polarization. A low molecular weight fluorophore by itself typically shows complete depolarization, since its rotational correlation time is normally much shorter than its excited state lifetime.2 When attached to a larger (bio)molecule or when the viscosity of the medium is increased, its Brownian molecular rotation is slowed down. As a result, the excited state remains partially aligned and its emission polarized. This principle has been widely used to follow biomolecular binding events. Note, that depending on the size and correlation times of the partners involved, fluorophores of different excited state lifetimes are needed for accurate polarization measurements, with very large biomolecular complexes requiring probes with extended lifetimes (up to 10−6 sec).
Fluorescence anisotropy has been widely used in membrane studies with a particular emphasis on properties like fluidity and microviscosity,28 but also to determine aqueous bulk-membrane partition coefficients of fluorophores.29 Protein dynamics,30 and protein–protein interactions31 and protein–nucleic acids interactions32,33 have been studied with fluorescence anisotropy as well.
2.6. Fluorescence Microscopy and Single Molecule Spectroscopy
The sensitivity of fluorescence-based techniques, coupled to advances in instrumentation, has dramatically revolutionized cellular visualization techniques. Technical developments encompass total internal reflection, confocal, two- or multi-photon fluorescence microscopy.34–38 Single molecule spectroscopy has proven very useful, and combinations of these techniques have been extensively used in the study on membranes, proteins and nucleic acids.17,39–42 Although beyond the scope of this article, many of these studies have benefited greatly from the discovery and heterologous expression of the green fluorescent protein (GFP).43 The development and use of fluorescent proteins is discussed in paragraph 5.2 of this review.
2.7. In Vivo Fluorescence-Based Imaging
Non-fluorescence-based imaging techniques, including magnetic resonance imaging (MRI), X-ray, positron-emission tomotography (PET) and ultrasound are invaluable for modern medicine. They are, however, expensive, could suffer from poor resolution and contrast, and do not necessarily respond to specific physiological changes.44 These limitations have triggered interest in optical-based techniques. Probes that absorb and emit in the ultra-violet and visible range of the electromagnetic spectrum, the main focus of this review, are ill-equipped for in vivo fluorescence-based imaging techniques, due to the absorption and light scattering of these frequencies by living tissues. Near-infrared (NIR) wavelengths (700–1000 nm), however, propagate efficiently through centimeters of living tissue due to minimized, absorption by water, lipids, as well as oxy- and deoxyhemoglobin.45–47
Progress in fluorescence-based imaging techniques has benefited from both technological advances and new probe development. For example, differences in fluorescence lifetimes have been exploited to distinguish probe emission from the emission of tissue components.48,49 From a probe design perspective, it is of great importance for the probe to have a low energy excitation wavelength in conjunction with a large Stokes shift. Examples of fluorescent probes suitable for in vivo (and ex vivo) fluorescence studies are diverse50–52and include modified amino acids53,54 and nucleosides,55–57 as well as high molecular weight entities such as nanoparticles, dendrimers, and quantum dots.54,58,59 An infrared-fluorescent protein has recently been engineered by Tsien from bacterial phytochromes.60 The low excitation-energy employed to excite fluorescent NIR probes is typically harmless and therefore provides the prospect for whole-body fluorescence tomography.61 This evolving field of NIR fluorescent probes, targeting strategies, and their application for in vivo imaging has been described in recent reviews.44,47,61–64
3. Fluorescent Analogs of Carbohydrates
3.1. Function of Carbohydrates in Biological Systems
Monosaccharides, Cn(H2O)n, are well appreciated for their roles in metabolism and energy storage. These essential building blocks make up the cell wall of plants, bacteria and insects. Perhaps of more importance for this review, monosaccharides are the building blocks of complex oligosaccharides, also referred to as glycans. Abundant on cell surfaces and typically covalently linked to other biomolecules (e.g., proteins, lipids, etc.), glycans play essential roles in signaling, as well as in cell–cell and cell–pathogen recognition.65–68 Oligosaccharides also serve numerous intracellular functions and impact protein folding and trafficking.65,69 These highly significant biological roles are encoded in the fundamental chemistry of their building blocks.
A glimpse into the complex chemistry of carbohydrates is provided in Figure 3.1. A monosaccharide in solution can exist in a cyclic or acyclic form. Cyclization to a hemiacetal (or hemi-ketal) can generate either a 5-membered ring (furanose) or a 6-membered ring (pyranose). In addition, the newly formed chiral anomeric center can form the α- or β-anomers (Figure 3.1).65 Furthermore, monosaccharides can be chemically strung by forming acetals (or ketals), named glycosidic bonds, where a hydroxyl group from one monosaccharide reacts with the anomeric center of another. Disaccharides, trisaccharides and higher oligosaccharides are enzymaticaly fabricated and conjugated. Due to the large number of possible regioisomers, stereochemical combinations and branching, as well as heterogeneity and additional chemical modification (e.g., sulfation), the chemical and structural diversity of oligosaccharides and glycans is vast.65
Figure 3.1.
Cyclization of the acyclic form of d-glucose shown in the open, pyranose, and furanose forms. Hemiacetal formation produces both the α and β anomers (i.e., C-1 epimers).
While fluorescent analogs of biopolymers, such as peptides and oligonucleotides, can be constructed and exploited, the situation is much more complex in the context of carbohydrates. It is apparent from the brief description of their chemistry, any modification of the carbohydrate skeleton is likely to impede its biological activity. Genuine emissive and biologically acceptable analogs of monosaccharide building blocks cannot be actually conceived. This section concisely discusses, therefore, methodologies for fluorescence-based saccharide sensing, oligosaccharide mapping and cell-surface glycan labeling.
3.2. Sensors for Saccharides
Lectins are naturally occurring carbohydrate-binding proteins, with Concanavalin A (Con A) being one of the archetypal examples.70 Con A, extracted from jack beans, shows no appreciable fluorescence. Saturating its four binding sites with fluorescein-labeled high molecular weight dextran facilitates the evaluation of carbohydrate binding via competition experiments, where dextran displacement by competing saccharides results in increased emission.71 This methodology was later improved by labeling Con-A with rhodamine to facilitate FRET-based analysis. When the fluorescein-labeled dextran was competed off, energy transfer from fluorescein (the donor) to rhodamine (the acceptor) ceased.72
The biological significance of carbohydrates prompted the development of numerous synthetic saccharide sensors. Early work focused on the use of functionalized macrocycles, including decorated porphyrins.73–76 Such non-covalent, supramolecular, optical sensors for saccharides have been reviewed.77
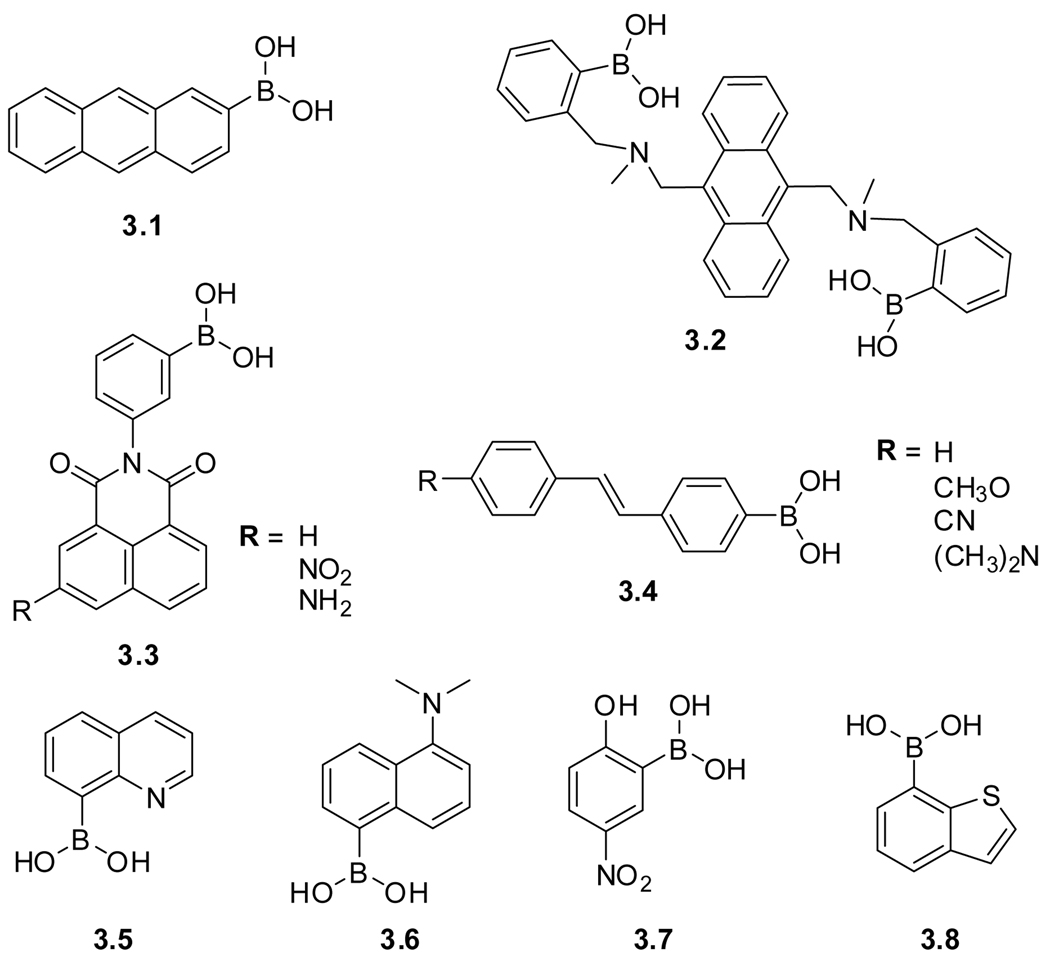
The high affinity of boronic acids to diols has been exploited for the fabrication of numerous carbohydrate receptors and sensors. Boronic acids form 5- or 6-membered cyclic esters with 1,2 or 1,3 diols, respectively. Early receptors devised by Czarnik relied on photoinduced electron transfer (PET) processes to impact the fluorescence of a known fluorophore, such as anthracene (3.1), by attaching the boronic acids to the aromatic ring (Figure 3.2).78 Changes in fluorescence upon binding carbohydrates were modest and pH dependent. Incorporation of an anthracene-based chelating tertiary amine (3.2), as designed by Shinkai, demonstrated improved performance and resulted in fluorescence enhancement upon saccharide binding.79–81 These design principles have been refined and advanced, resulting in a multitude of colorimetric and fluorometric sensors for carbohydrates. Examples include N-phenylnaphthalimide sensors (3.3),82–84 stilbenes (3.4),81 as well as boronic acids derived from quinoline (3.5),85 naphthalene (3.6),86 nitrophenol (3.7),87 and benzothiophene (3.8).88 Their structures and properties are discussed in a number of overview articles.86,89–94
Figure 3.2.
Structures of boronic acid–based saccharide sensors.
3.3. Fluorescent Labeling of Reducing Saccharides
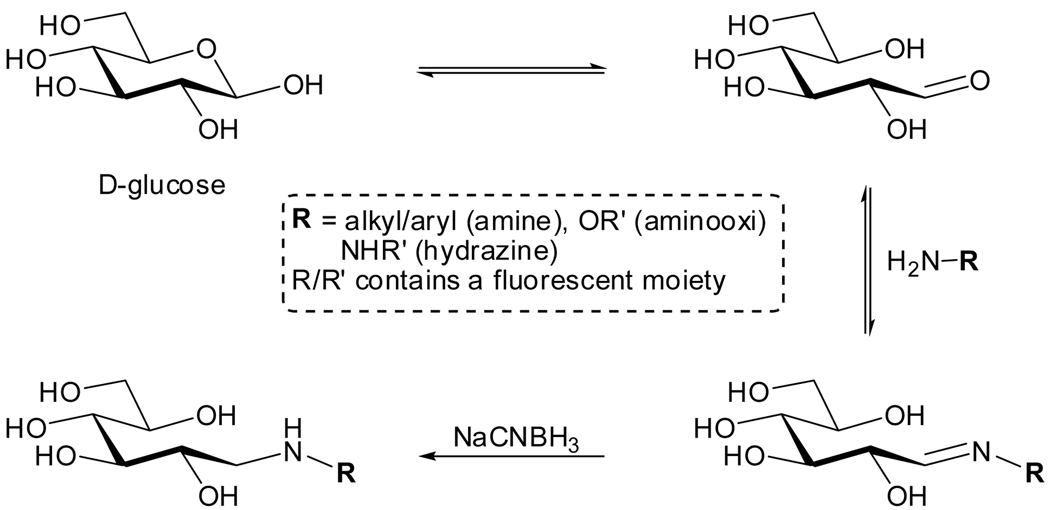
Carbohydrates, in contrast to other important biomolecular building blocks including certain amino acids, nucleosides and even naturally occurring fatty acids, possess no conjugated π-system at all. This obviously eliminates any useful absorption and emission features. As pointed out above, significant structural modification is therefore required to confer useful photophysical properties upon saccharides. Fortunately, reducing carbohydrates, being hemiacetals or hemiketals, are chemically unique as they contain a masked carbonyl moiety (see Figures 3.1 and 3.3). As such, they are susceptible to condensation reactions with primary amines to form Schiff-bases, a reversible reaction in an aqueous environment. Under reducing conditions (e.g., in the presence of NaCNBH3), known as ‘reductive amination’, the condensation becomes irreversable (Figure 3.3).95,96 This unique feature has been exploited for labeling purposes by reacting reducing sugars with fluorescent amines, hydrazines and aminooxi derivatives.97–99 If no reducing ends are present, periodate-mediated oxidation of vicinal diols, naturally present in oligosaccharides, can be used to introduce reactive aldehydes. This approach has been applied to whole cells.100,101
Figure 3.3.
Labeling of reducing carbohydrates with amine–containing fluorophores.
3.4. Metabolic Saccharide Engineering: Exploiting the Sialic Acid Pathway
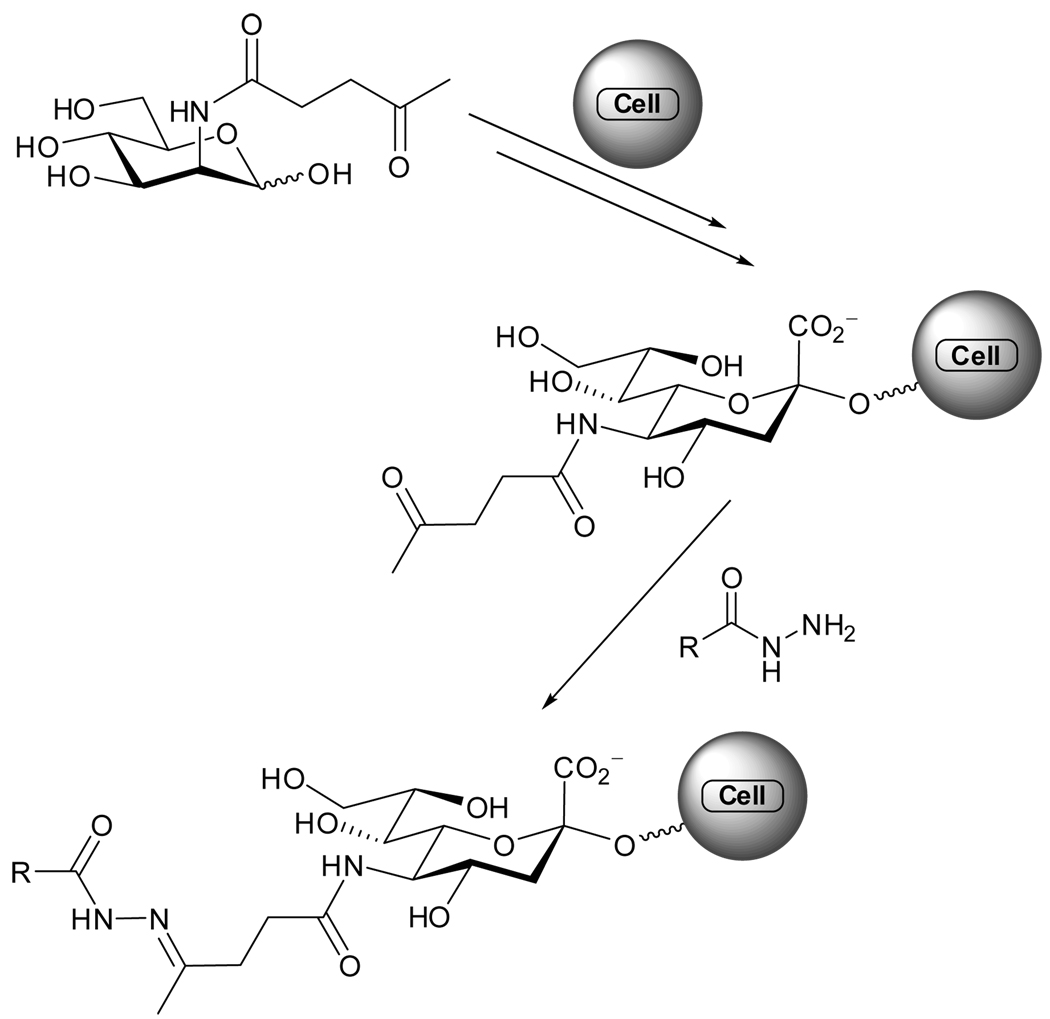
The tolerance of the sialic acid biosynthesis pathway to unnatural N-acyl substitutions, discovered in 1992,102 facilitates cell-surface expression of modified oligosaccharides containing bioorthogonal groups (e.g., reactive ketones, azides), which can be further functionalized.103 This pathway was used to decorate cell-surfaces with membrane-anchored glycoproteins comprised of a ketone functionality by exposing cells to media enriched with N-levulinoyl-d-mannosamine (ManLev).104,105 The newly introduced ketone can participate in a chemoselective cell-surface coupling to hydrazides, forming an acyl hydrazone, which can carry additional tags or labels (Figure 3.4).104–106 It is worth noting that that hydrazone or oxime formation is a reversible condensation reaction in aqueous media, with its kinetics being dependent on concentration and pH.107 A methodology for favoring imine formation at low concentrations, using aniline catalysis, has been developed108–110 and applied to cells as well.111
Figure 3.4.
ManLev, its expression on the cell-surface and subsequent acylhydrazone formation.
The use of this biosynthetic pathway has been expanded in recent years to incorporate additional functional groups, particularly azides. This bioorthogonal entity, upon Staudinger reduction to the corresponding amine, can be engaged in condensation reactions, named Staudinger ligations.112 Additionally, copper-mediated and copper free ‘click chemistry’ has been used to decorate cells of live zebrafish.113 To further advance the scope of click chemistry, the sialic acid pathway has been utilized to express ethynyl functionalized glycans on cell surfaces in live mice.114 Click chemistry could then be used to label and stain cells with a desired marker for fluorescence microscopy analysis.113,114
4. Fluorescent Analogs of Phospholipids and Fatty Acids
4.1. Biological Membranes
The lipid bilayer, discovered in 1925 by Grendel and Gorter,115 is a key component of all biological membranes, and thereby, vital for sustaining cellular integrity and function. Formation of this fluid double layer structure,116 a complex supramolecular architetcture, is enabled by the special properties of amphipathic lipids. These structural building blocks constitute 50% of the mass of most animal cell membranes.117
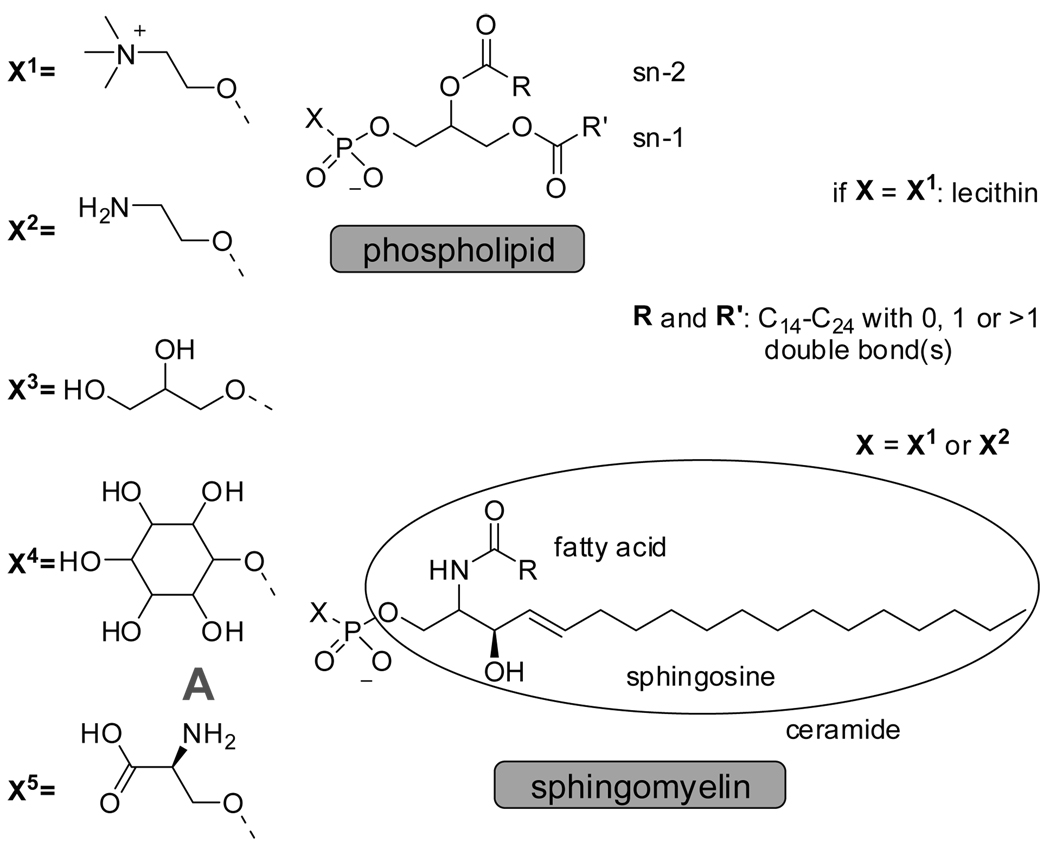
Phospholipids span a range of sizes (MW 300–1200 Da) and are characterized by a polar (hydrophilic) head-group, connected to a phosphate functionalized glycerol unit, which in turn links two apolar (hydrophobic) tails (Figure 4.1).118 More than 50% of all phospholipids are sphingomyelin and lecithin and their ratios vary widely per cell type and per species for the same cell type, and is subject to change with age.119 Both phospholipids have a similar polar head-group, a choline for lecithin and a choline or aminoethanol for sphingomyelin, but differ significantly in their apolar moiety. Lecithin is comprised of two esters that connect the lipophilic fatty acid part to the polar head-group. The ceramide unit in sphingomyelin has an acylated sphingosine moiety (Figure 4.1). In addition, the average length of the hydrocarbon chains in lecithin is shorter with a higher degree of unsaturation compared to sphingomyelin. This structural distinction gives rise to a difference in the net dipole and ability to form hydrogen bonds, which ultimately impacts the the constitution and dynamics of lipid bilayers.120–123
Figure 4.1.
General structures of glycerophospholipids, sphingomyelin and examples of natural head groups.
The lamellar phase or lipid bilayer with a head to head distance of 35 and 43 Å for dipalmitoylphosphatidylcholine (DPPC) vesicles,124–126 has been firmly established as the fundamental structural motif of all cellular membranes,117 although pure lipids have been shown to organize into other assemblies in aqueous environments including planar bilayers, hexagonal, or cubic phases (see Figure 4.2 for examples).127 Membranes are not homogeneous. The formation and function of lipid assemblies within the homogeneous fluid bulk of the lipid bilayer,128–130 referred to as superlattices129 or lipid rafts,131 has been described. The superlattice model proposes a regular, rather than random, distribution of membrane components, formed by favorable lipid packing, where steric and columbic interactions between phosphatidylcholine (PC), sphingomyelin (SM), and phosphatidylethanolamine (PE) building blocks are optimized.129 Rafts are characterized by asymmetry with respect to the composition of their exoplasmic and cytoplasmic leaflets. The former is enriched with sphingomyelin and glycosphingolipids, and the latter mainly consist of glycerolipids.131 Regardless of the two theories, the concept of phase-separated microdomains adds a new level of complexity to the already sophisticated role membranes play in biology.
Figure 4.2.
Phospholipid architectures in aqueous media.
Besides affecting the cell’s membrane constitution, the type and ratio of its building blocks also determines its interaction with extra cellular entities. The plasma membrane exterior of most mammalian cells, for instance, is characterized by the presence of zwitterionic phospholipids such as phosphatidylcholine and sphingomyelin,132 while bacterial cells contain a high fraction of anionic phospholipids and related anionic amphiphiles on the outer surface.133 This surface charge difference enhances the selectivity of positively charged antimicrobial agents to bacterial over mammalian cells.134 Importantly, lipids are not merely structural elements of membranes, but are involved in many important metabolic pathways and diseases. Sphingomyelin and glycerolipids can act as signaling molecules involved in differentiation, proliferation, and apoptosis (programmed cell death).135–137 For its latter role in cancer cells, ceramide has been called the “tumor suppressor lipid”.138
While learning about living cells is the ultimate goal, their heterogeneity and complex constitution make them less suitable for fundamental biophysical and biochemical studies. Instead, model membrane systems based on phospholipid bilayers and detergent-based micelles are commonly employed. Recent reviews discuss artificial membranes and giant unilamellar vesicles and their applications.37,139 The application of membrane model systems comes with the predicament that they are comprised of an ideal two phase system, each physically and chemically uniform, while in equilibrium with its monomeric building blocks.140,141 Biological include membranes, however, are much more complex by nature since their make up includes a divers constituents.
This section discuses the plethora of fluorescent probes, labels and methodologies used in membrane research. While fluorescent analogs of phospholipids and sphingolipids are commercially available, the abundant literature in this field highlights the active development of custom made probes to meet specific requirements.142,143 Diverse approaches have been employed, including the use of non-covalent probes, as well as modification of distinct domains of the common building blocks. Since the position of the probe dictates, by and large, its function, this section is organized according to this criterion.
4.2. Non-Covalent Fluorescent Membrane Probes
The term non-covalent is somewhat ambiguous in this context since membranes themselves are non-covalent architectures. For organization purposes, however, we distinguish between probes that are covalently linked to a membrane building block and probes that are lipophilic dyes that show no immediate structural likeliness to phospholipids. Figure 4.3 depicts prototypical examples of the latter and Table 1 lists their key photophysical parameters.
Figure 4.3.
Non-covalent membrane probes that reside in the cell membrane interior.
Table 1.
Spectroscopic Properties of Selected ‘Non-Covalent’ Probesa
| # | name | solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 4.1 | DPHb | EtOH | - | - | 0.24 | 2.2 |
| hexane | 352, 370 | 430 | 0.64 | 15.7 | ||
| 4.2 | M-9-A | MeOH | 361 (7.1) | 461 | 0.071 | - |
| hexane | - | 447 | - | 12.1 | ||
| 4.3 | Perylenec | EtOH | 252, 408 (63.1), 434 | - | - | - |
| dodecane | - | - | 0.89 | 4.9 | ||
| 4.4 | Pyrenec,d | EtOH | 241 (79.4), 272, 334 | 376 | 0.65 | 410 |
| 4.5 | ANSe | Water | 340 | 555 | 0.003 | 0.42 |
| dioxane | - | 472 | 0.57 | 11.8 | ||
| 4.7 | DCVJ | MeOH | 455 (62) | - | 0.0022 | - |
| glycerol | 469 | 508 | - | - | ||
| 4.8 | FCVJ | ethylene glycol | 483 | 503 | - | - |
λ, ε, and τ are given in nm, 103 M−1cm−1, and ns respectively
ε is given only for the most intense λabs.170
λem, Φfl and τ are from Hermetter.142
λabs is extracted from a graph, λem is highly solvent polarity sensitive, several values for λem and τ have been reported, some of which are contradicting.154
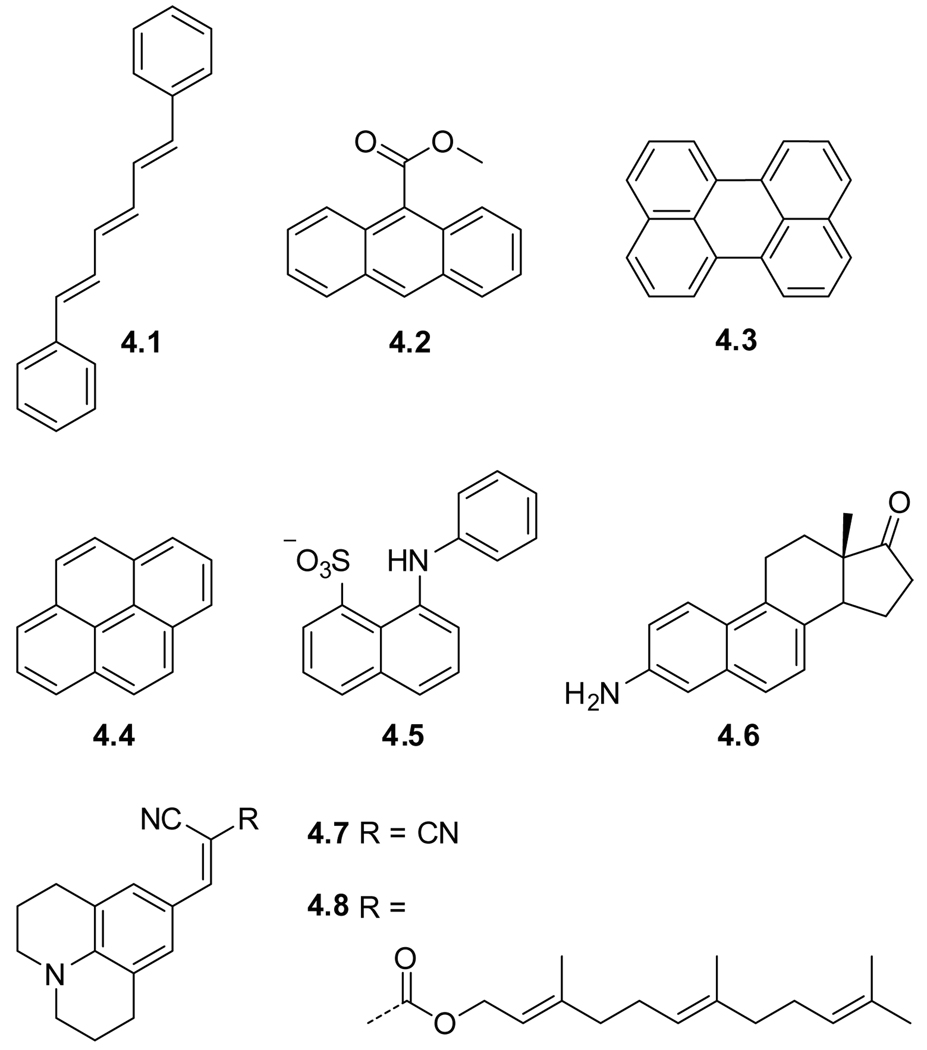
An example of an extensively studied non-covalent probe is diphenylhexatriene (DPH) (4.1),120,144,145 which resides in the non-polar regions of the cell membrane.146 DPH has been used in numerous studies concerned with molecular order and motion (also termed fluidity) within liposome bilayers.28,147 Whereas the extended structure of DPH shows, albeit minimal, elements of similarity to lipid building blocks, it is clear that methyl-9-anthroate (M-9-A) (4.2) is, from a design perspective, nothing more than a lipophilic fluorophore. It is not as abundantly used as its counterparts attached to various positions of the alkyl chain of a lipid (vide infra). Together with anthranoyl labeled lipids, M-9-A has been used to study phase transitions of dipalmitoyl phosphatidylcholine,148 and to explore microviscosity barriers around the double bond in unsaturated phosphatidylcholines comprised bilayers.149
Other popular examples of lipophilic fluorophores used in countless membrane studies are perylene (4.3) and the smaller pyrene (4.4). Both are characterized by high emission quantum yields and long fluorescence lifetimes. At low concentrations pyrene emits in the violet. At higher concentrations, easily reached in membranes, pyrene eximers, emitting in the green, are formed.150 Polarity studies with 1-ethylpyrene within liposomes has indicated a much higher polarity in the hydrocarbon core of liposomes than expected (ε = 10.4–12.3 vs.1.9 and 80.2 for hexane and water, respectively).8 The surface residing probe 1-anilino-8-napthalene sulfonate (ANS) (4.5) has been used to probe dynamic behavior in model membranes,151 as well as sulfate dependent uptake processes in ascites tumor cells,152 and membrane fluidizing effects of Paclitaxel (Taxol) with fluorescence anisotropy measurements.153 Since ANS has been found to perturb membranes, its popularity has declined.154,155 Aminodesoxyequilenin (EQ, 4.6), a non-covalent probe resembling a steroidal skeleton, was used to study dynamics in model membranes.151,156
A membrane probe very different in design from the probes mentioned above is the intensely studied 4-(dicyanovinyl)julolidine (DCVJ, 4.7).157,158 This probe belongs to a family of chromophores coined molecular rotors, which are characterized by a twisted intramolecular charge transfer excited singlet state. The typical low quantum yield of these probes in non-viscous environments is ascribed to rotational relaxation, a dominating non-radiative decay pathway. Increasing the viscosity, however, impedes rotation around the single bond joining the two π-systems. The resulting structural rigidification causes a stark increase in the fluorescence quantum yield.159–163 This property was utilized in membrane–fluidity and microviscosity studies with DCVJ (4.7).163–165 DCVJ was also found to bind to proteins166 facilitating its cellular uptake resulting in fluorescence from the cytoplasm, organelle membranes, and nucleolus.164 To enhance localization in the membrane, a hydrophopic farnesyl chain has been connected to the julolidine core (FCVJ, 4.8). Even better control over the positioning of the probe was obtained by connecting the core chromphore to the head-group and the tail end of a phospholipid (Sections 4.3 and 4.4).164,167
The main advantage of employing non-covalent fluorophores as probes is the minimal design and synthesis required. The location of a lipophilic probe at the membrane-water interface or deeper in the lipophilic inner domain in aqueous micellar suspensions is, however, ambiguous and might lead to multiple interpretations.171–173 In addition, micelles and bilayers are able to compartmentalize lipophilic molecules, thereby jeopardizing proper readout.174 These challenges could explain the limited use of some of the probes described above. Better certainty of the probe’s localization is obtained by attaching it to a membrane building block. The following sections discuss such covalently modified phospholipids and their analogs, where the probe can be placed near the polar head-groups, at the end of the chain or within the hydrophobic chain.
4.3. Polar Head-Group Labeling
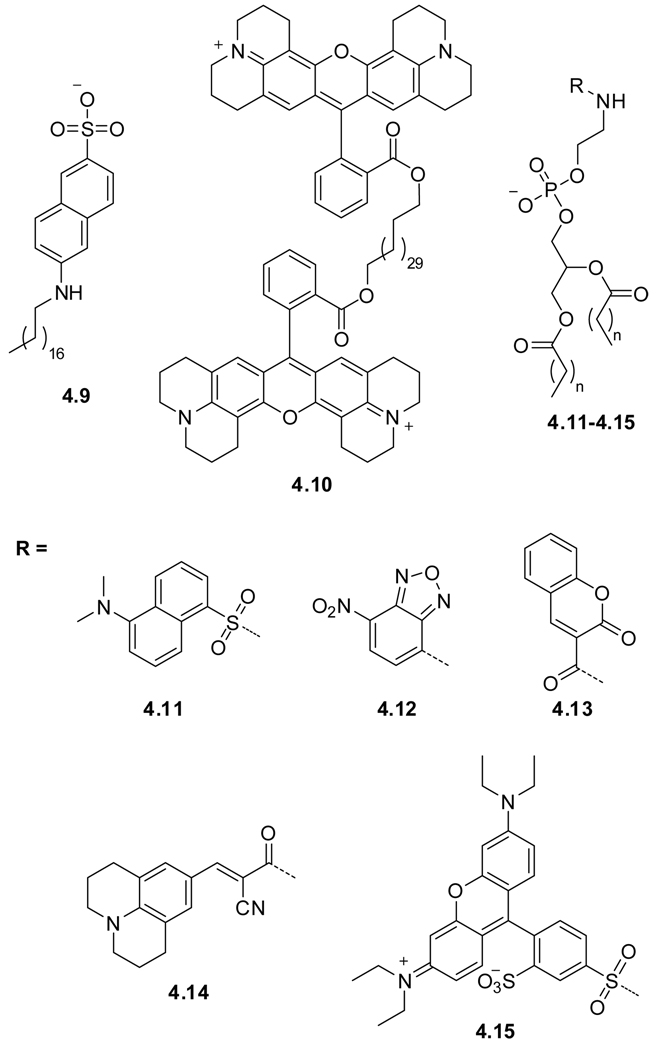
To explore the outer cell surface, the polar head groups can be part of a charged fluorophore or be labeled with a known fluorophore (Figure 4.4). Such membrane-spanning bolaamphiphile fixates the fluorophore at the water–lipid interface. Two fundamental designs have been explored: (a) Labeling the head group with a fluorophore or replacing the head group with a charged fluorophore, and (b) utilizing a long hydrocarbon to connect two fluorescent residues. The two distinct approaches are exemplified with octadecyl naphtylamine sulfonate ONS (4.9),175 and the bis rhodamine 101 labeled diacid, Rh-101 (4.10),176,177 respectively. The latter design requires the probe to span the head to head distance of a typical bilayer, ranging between 35 and 43 Å for dipalmitoylphosphatidylcholine (DPPC) vesicles.124,125,178 Figure 4.4 provides typical examples, and Table 2 summarizes the spectroscopic properties of the corresponding fluorophores.
Figure 4.4.
Head–group labeled mamebrane probes.
Table 2.
Spectroscopic Properties of Selected ‘Head-Group’ Probesa
| # | name | solvent | λmax (ε) | λem | Φ |
|---|---|---|---|---|---|
| 4.9 | ONS | MeOH | 349 (0.95) | 427b | - |
| 4.10 | Rh-101 | EtOH | 577 (90) | 601c | 0.9 |
| 4.11 | DPE | MeOH | 346 (3.6)d | 514e | - |
| 4.12 | NBD-PEf | MeOH | 463 (21) | 536 | - |
| 4.13 | Coumarin | - | - | - | - |
| 4.14 | Head-CVJ | MeOH | 320, 396, 470 | 490 | - |
| 4.15 | Rh-B | MeOH | 560 (75) | 581 | - |
Although fatty acids functionalized with fluorescent probes have been reported, their phospholipids-based counterparts, many of which are commercially available, enjoy greater popularity. An early example is a dansyl labeled phosphatidyl ethanolamine (DPE) (4.11).175 This and related probes have been used to study the structure, dynamics and local polarity of biological membranes.175,179 A commonly used probe is the commercially available nitrobenzoxadiazole labeled phospholipid, NBD-PE (4.12).150 NBD is characterized by high quantum yields in apolar media, but is non-emissive in aqueous media. Its emission maximum is polarity and pH sensitive. Moreover, NBD undergoes self-quenching at higher concentrations and has therefore been used in phase separation studies.180 An example of a specific outer cell surface application is the head-group labeled phospholipid (4.13), containing coumarin as a fluorophore, which has been used as an on/off fluorescence sensor for the detection of OH radicals.181 The 4-(cyanovinyl)julolidine functionalized phospholipid Head-CVJ (4.14) is comprised of a molecular rotor moiety (Section 4.2). Molecular rotors show a strong viscosity dependent quantum yield. Since the probe is located on the membrane perimeter in this case, no response to membrane viscosity changes has been observed.167 A study describing lipid bilayer organization and its perturbation employed the commercial rhodamine B furnished phospholipid, Rh-B (4.15).176
4.4. Chain-End and On-Chain Labeling
Introducing a probe at the very end of a lipophilic chain places it in the interior of the membrane with reasonable certainty. Two major design principles, ‘chain-end’ and ‘on-chain’, have been employed. A different impact on membrane stability is exerted, with the ‘chain-end’ approach appearing to be less perturbing compared to ‘on-chain’ placement. The former might suffer, however, from looping back of the chromophore, which could lead to ambiguity regarding its positioning within the bilayer.182
Polyaromatic hydrocarbons have been the chromophores of choice due to their apolar nature and rigid structure, ensuring a sufficient emission quantum yield. Not surprisingly, modifications of the ‘non-covalent’ probes discussed above with an apolar chain and polar head group generates many of these probes. Figure 4.5 depicts key examples, and Table 3 lists their primary spectroscopic characteristics.
Figure 4.5.
Examples of ‘chain-end’ (4.13–4.21) and ‘on-chain’ (4.22) labeling.
Table 3.
Spectroscopic Properties of Selected ‘Chain-End’ and ‘On-Chain Probesa
| # | name | solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 4.16 | TMA-DPH | MeOH | 354 (53)b | 440b | - | 0.27 |
| 4.17 | Dansyl-FAc | MeOH | 335 (4.0) | 518 | 0.23 | - |
| toluene | 336 (3.8) | 475 | 0.40 | - | ||
| 4.18 | BODIPY-FA | MeOH | 506 (>90) | 512 | 0.94 | - |
| 4.19 | DPH-PCd | MeOH | 354 (81) | 428 | - | - |
| 4.20 | Anthr-PC | EtOH | 378 (4.8) | ~385, 410, 430 | - | - |
| 4.21 | Pyrene-PC | MeOH | 342 (37)d | 376d | 0.65e | 410e |
| 4.22 | NBD-PC | EtOH | 340, 460 (21) | 525 | 0.39f | - |
| 4.23 | Fluorene-PCg | DMPC | 290, 308, 316 | 319 | - | 3.0 |
| 4.24 | Cor-PCh | DMPC | 308, 344 | 448 | - | 98.2 |
| 4.25 | Tail-CVJ | MeOH | 320, 396, 470 | 490 | - | - |
| 4.26 | 12-AS | MeOH | 362 (7.8) | 458 | 0.071 | 1.6 |
| hexane | - | 446 | - | 10.5 |
λ, ε, and τ are given in nm, 103 M−1cm−1, and ns respectively
Data from Thomas et al.183
Reported data is for 5-(dimethylamino)-N-methylnaphthalene-1-sulfonamide.13
Data from www.invitrogen.com.150 Monomer and eximers emission in bilayers are 400 and 470 nm respectively.184
In EtOH, data from Hermetter et al.142
Data from Chattopadhyay.180
Only a spectroscopic study in DMPC vesicles is reported, λabs and λem given are extracted from graphs showing more complexity; only the most contributing τ is given.
Only a spectroscopic study in DMPC vesicles is reported, the fluorescence spectrum is complex, and only the wavelength of the most intense fluorescence peak is given.
Modifying DPH with a trimethylammonium head group to give TMA-DPH (4.16) facilitates a more accurate positioning within the bilayer.185 Examples of functionalized fatty acids include dansyl-FA (4.17),179 and BODIPY-FA (4.18). In contrast to the minimal use of dansyl-FA (4.17), studies using BODIPY-FA are very abundant.186,187 The BODIPY fluorophore has high molar absorptivity (> 90,000 M−1cm−1) at a long emission wavelength (> 500 nm) and shows a concentration dependent eximer emission.150
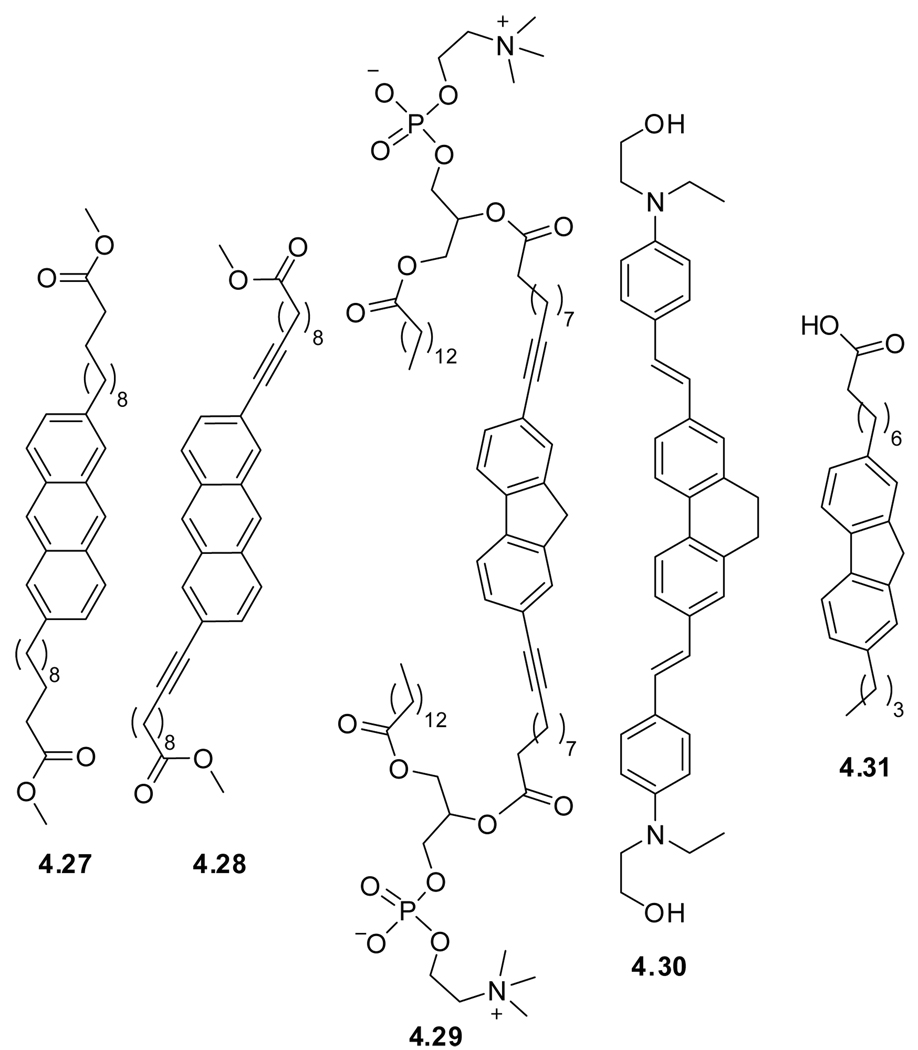
A more common practice is the modification of phosphatidyl choline with fluorophores to mimic the naturally occurring membrane building blocks. Examples include: DPH-PC (4.19),188 Anthr-PC (4.20),189–191 Pyrene-PC (4.21),8,184,192 and NBD-PC (4.22).193 Due to its polarity sensitive emission maximum and high quantum yields, NBD labeled probes are often used to assess location within membranes.180 Unfortunately, a ‘chain-end’ NBD labeled phospholipid can loop back, making its location within the membrane uncertrain.180 More recent examples include the use of fluorene-PC (4.23),194 and a coronene adduct of phosphatidyl choline, (Cor-PC) (4.24).195 Other fluorescent chain-end modified PCs are commercially available.
Similarly to the chain-end labeling, ‘on-chain’ fluorophores must be accommodated by the highly apolar environment of the inner membrane. Tail-CVJ (4.25) represents an example of a phospholipid functionalized with a molecular rotor. The viscosity dependent quantum yield of the chromophore was used to probe changes in membrane viscosity.167 A common fluorophore for ‘on-chain’ labeling of fatty acids is anthracene. Examples include 12-(9-anthoyloxy) stearic acid (12-AS) (4.26),175 and 9-(9-anthroyloxy) stearic acid,141,148 where the number preceding the parenthesis indicates the position of the fluorophore on the chain. Despite the covalent attachment, the linker typically permits ample rotational freedom in the highly accommodating fluid lipid phase,196 thereby complicating spectroscopic analysis.191 The orientation and motion of various probes, including chain-end and on-chain anthracene labeled fatty acids, has been studied with fluorescence polarization, and has demonstrated sensitivity to structural changes induced by cholesterol addition, lipid type or temperature.151
4.5. In-Chain Labeling
Making the dangling ‘on-chain’ and ‘chain-end’ fluorophore part of the fatty acid chain, as in the ‘in-chain’ labeling strategy, minimizes probe-induced membrane perturbation. Fluorophores related to the ones discussed above can be employed as long as they accommodate functionalization on either side. Symmetrical modification tends to minimize membrane disruption and chain length selection ultimately impacting the depth of the probe within the bilayer. Figure 4.6 presents selected examples, and Table 4 provides primary spectroscopic characteristics for the chromophores most commonly used in this catagory.
Figure 4.6.
Examples of ‘in-chain’ labeled chromophores.
Table 4.
Spectroscopic Properties of Selected ‘In-Chain’ Probesa
| # | Name | solvent | λmax (ε)b | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 4.27 | BA-Anthr-FEc | CHCl3 | 334, 350, 364 (5.1), 384 | 396, 418, 442 | 0.19 | 3.9 |
| 4.28 | BA-exAnthr-FEc | CHCl3 | 342, 358, 378 (13.0), 399 | 408, 430, 457 | 0.28 | 3.1 |
| 4.29 | BA-exFluorene-PCd | DMPC | 308,329 | 334 | - | 1.3 |
| 4.30 | C8A-Fl-C4 | MeOH | 270 (38.0), 297, 309 | 319 | 0.65 | - |
λ, ε, and τ are given in nm, 103 M−1cm−1, and ns respectively
Only ε values for the most intense peak are given.
Data extracted from graphs.197
Only a spectroscopic study in dimyristoylphosphatidylcholine (DMPC) vesicles is reported, λabs and λem are extracted from graphs, and only the most contributing τ is given.
Known fluorophores that have been incorporated into symmetrical bolaamphiphiles are anthracene (BA-Anthr-FE, 4.27),197 ethynyl-extended anthracene (BA-exAnthr-FE, 4.28),197 ethynyl extended fluorene (BA-exFluorene-PC, 4.29),194 and vinyl extended dihydrophenanthrene (exdhPhenanthrene, 4.30).198 Extending the conjugation of the central polyaromatics tends to impart favorable photphysical features upon the chromophore (e.g., higher emission quantum yield), in addition to the structural rigidification imposed. The membrane spanning bolaamphiphile design is of specific interest, since the polar head-groups serve as anchors, thereby limiting longitudinal and transverse maneuverability of the probes, resulting in a higher accuracy of the probes’ positioning.199–201 A somewhat unique example is the asymmetrically substituted fluorene fatty acid (C8A-FL-C4, 4.31).202
4.6. Polyene Fatty Acids
Polyenes are linear hydrocarbon chains characterized by conjugated multiple double or triple bonds. These minimally perturbing chromophores are a valuable substitution for saturated alkyl chains, which possess no useful emissive qualities. In addition, the high degree of unsaturation introduces rigidity, virtually preventing looping or folding of the probe. If the chain length matches the membrane width (referred to as ‘biomimetic membrane-spanning’)124 and is equipped with polar groups on either side, a bolaamphiphile is obtained, which is accurately positioned in a transverse location.
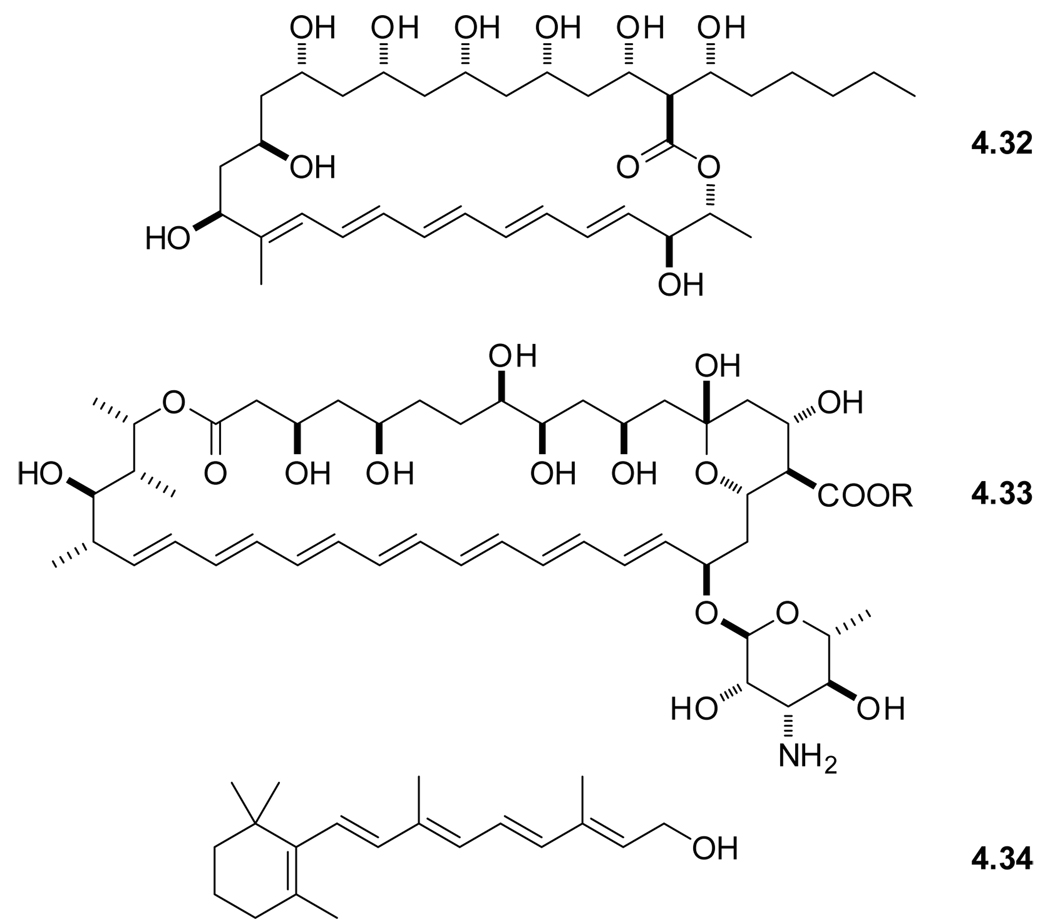
Early membrane studies with polyenes made use of rather large and structurally complex natural products. Examples include the macrolide polyene antibiotics filipin (4.32) and amphotericin (4.33),203 which are known to cause cell lyses (Figure 4.7).204 Examples of linear naturally occurring polyenes include retinol (4.34),205 retinal and other carotenoids.206 Table 5 summarizes the photophysical characteristics of the key chromophores discussed in this section. Note, the spectroscopy of polyenes, being “classical” chromophores, dates back to the 1930’s,207–211 and is discussed in later reviews as well.28,212
Figure 4.7.
Naturally occurring polyenes.
Table 5.
Spectroscopic Properties of Selected Polyenesa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 4.34b | Retinol | MeOH | - | - | 0.007 | 1.5 |
| cyclohexane | 325 | 520 | 0.020 | 5.0 | ||
| 4.35c | cis-PnA | MeOH | 318.6, 303.8 (79)b | ‘432’d | 0.017 | 1.3 |
| decane | 320,8, 305.8 (74) | 432 | 0.054 | 5.2 | ||
| 4.36e | trans-PnA | MeOH | 313.0, 298.6 (92) | ‘422’d | 0.031 | <1 |
| decane | 315.3, 300.7 (88) | 422 | 0.009 | 3.1 | ||
| 4.37d | trans-PA | EtOH | 344.7 (103) | 468 | 0.075 | 10.7 |
| dioxane | 348.9 (81) | 468 | 0.14 | 13.7 | ||
| 4.38e | trans-PdA | CHCl3 | 353 (92), 335 (95), 320 (60) | 474 | 0.14g | - |
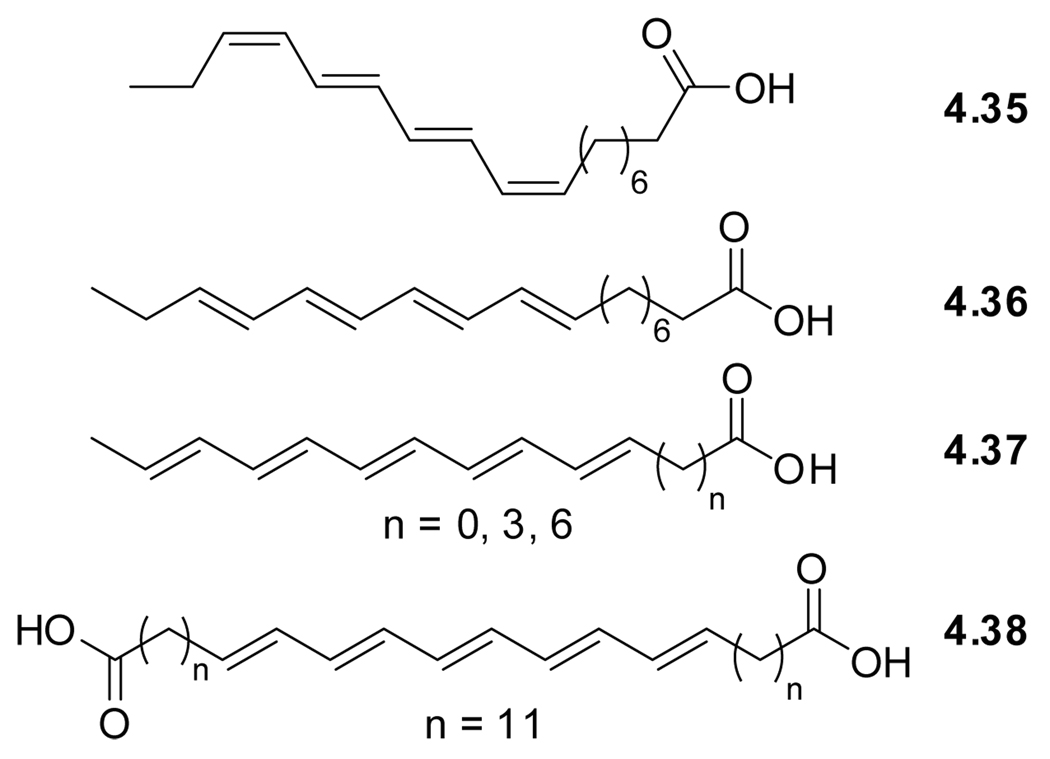
Designer polyenes originate form another naturally occurring polyene, α-parinaric acid (4.35), isolated for the first time from Parinari laurinum in 1933, to be identified two decades later as the (Z),(E),(E),(Z)-isomer (Figure 4.8).216 This compound clearly resembles membrane lipids. Treatment of the natural occurring α-parinaric acid (cis-PnA, 4.35) with iodine gave β-parinaric acid (trans-PnA, 4.36) with all double bonds in the (E)-configuration.217 Trans-PnA (4.36), spectroscopically characterized in 1977,213 got considerable attention for its use as a fluorescent probe in the research on synthetic phospholipid membranes in the late 70’s.218
Figure 4.8.
Structures of naturally occurring α-parinaric acid (4.31) and synthetic all trans-PnA (4.32), trans-PA (4.33), and all trans-PdA (4.34).
Extending the conjugation by a single double bond gives all trans-penteanoic acid (trans-PA, 4.37).219 This polyene was used for studying protein–lipid interactions by functioning as a FRET acceptor for tryptophan emission.214 More recently, polyene lipids have been used as probes in live cells, highlighting their superior properties compared to membrane perturbing NBD and BODIPY tags.220 To minimize mobility, implementation of the bolaamphiphile design principle gave all trans-penteanoic diacid (trans-PdA, 4.38). This probe has been used in polarized two-photon fluorescence microscopy to allow direct observation of the emission transition moment orientation of the probes in lipid bilayers.125
Modern synthetic methods, in particular Pd-mediated sp2−sp2 cross coupling reactions facilitate the synthesis of such polyenes.221,222 This is frequently replaced by an alternative approach comprised of sp−sp2 transition-metal-mediated cross-coupling reactions to give an ene-yne, followed by partial reduction of the alkyne.223 A more classical approach involves consecutive Wittig and Wadsworth-Horner-Emmons reactions.219,220 Regardless of the synthetic approach, the final polyene must be isomerized to the all-(E) isomer, typically by the use of iodine.217,224
In spite of their relatively simple and short π-system, polyenes are characterized by high molar extinction coefficients and multiple emission maxima. Both are typically polarity independent.213 Both cis-PnA (4.35) and trans-PnA (4.36) display solvent independent fluorescence maxima around 425 nm with a solvent dependent fluorescence lifetime ranging from 4 to 11 ns. Emission quantum yields vary from 0.020 (chloroform) to 0.054 (decane) for 4.35 and 0.010 (chloroform) to 0.031 (decane) for 4.36.212,213 As expected, the extended all trans-penteanoic acid (trans-PA, 4.37) exhibits additional lower energy transitions and a fluorescence maximum around 470 nm.219 This illustrates the tuneability of the spectroscopic properties of these isomorphic fluorescent membrane analogs.222 It is worth noting that the addition of small amounts of polyunsaturated fatty acids can stabilize artificial phospholipid membranes, whereas larger amounts can cause destabilization.225
4.7. Applications
Fluorescent probes have greatly contributed to our understanding of the properties and function of biological membranes. While categorizing the plethora of membrane studies has been previously attempted,226–228 capturing over 40 years of membrane research using fluorescent spectroscopy is a clearly impossible. The brief discussion below and Table 6 summarize key studies involving the probes presented in this section.
Table 6.
Fluorescent Membrane Probes and Selected Applications
| # | Name | Application | Description/Remarks | |
|---|---|---|---|---|
| Non-covalent | 4.1 | DPH | B147,246,247 | Study of membrane fluidity |
| B1144 | Comparison of normal lymphocytes vs. malignant lymphoma cells | |||
| B4144 | Comparison of normal lymphocytes vs. malignant lymphoma cells | |||
| B4145,231 | In artificial phospholipid membranes | |||
| C146 | In model lipid bilayer membrane systems | |||
| B, E248 | Lipid mobility influence on prothrombinase complex activity | |||
| E230 | Effect of propoxycaine·HCl on the properties of neuronal membranes | |||
| F249 | Effect of H2O2 on Saccharomyces cerevisiae membrane permeability | |||
| 4.2 | M-9-A | B148 | Properties and membrane locations of fluidity probes | |
| B1149 | Influence of unsaturated acyl chains on membrane microviscosity | |||
| 4.3 | Perylene | B1250,251 | Cholesterol influence on membrane microviscosity and order | |
| B4251 | Cholesterol influence on membrane microviscosity and order | |||
| 4.4 | Pyrene | A8 | Heteroexcimers in single bilayer liposomes with 1-ethylpyrene | |
| A, B2, B4252 | Organization and dynamics of hippocampal membranes | |||
| 4.5 | ANS | B153 | Membrane fluidizing effect of taxol | |
| D151 | Dynamic fluorescent probes’ behavior in a model lipid bilayer | |||
| F&E253 | E.coli membrane disruption by granulysin derived G15 | |||
| 4.7 | DCVJ | B165 | Study of dynamical properties of lipid membranes | |
| B1254 | Temperature-dependent viscosity changes and phase transition study | |||
| B1163 | Microviscosity measurements of phospholipid-bilayers | |||
| B1255 | Shear-stress induced viscosity changes in membranes | |||
| Head-group | 4.9 | ONS | A175 | Polarity studies in phosphatidyl choline bilayers |
| D151 | Dynamic fluorescent probes’ behavior in a model lipid bilayer | |||
| 4.10 | Rh-101 | D178 | Membrane spanning probe behavior in model membrane | |
| 4.11 | DPE | A175 | Polarity studies in phosphatidyl choline bilayers | |
| A, B4256 | Local polarity estimation at the polar head region in lipid vesicles | |||
| E257 | Influence of mono- and divalent cations on hemolysis | |||
| E258 | Influence of bee venom and cytolysin A-III on hemolysis | |||
| 4.12 | NBD-PE | B2, B4259 | Organization and dynamics of bovine hippocampal membranes | |
| D260 | Probe location in model membranes | |||
| E261 | Lipid interactions with human antiphospholipid antibody | |||
| F262 | Haemolytic effect of merulinic acid on biomembranes | |||
| 4.13 | Coumarin | G181 | Hydroxyl radical sensing on the membrane outer surface | |
| 4.15 | Rh-B | D176 | Probe influence on bilayer organization | |
| Chain-end & on- chain | 4.16 | TMA-DPH | B263 | Order–disorder transitions in complexes of glycerophosphocholines |
| B4264 | Influence of cholesterol and ergosterol on membrane dynamics | |||
| C186 | Localization of DPH and its derivatives within membranes | |||
| E265 | Interactions of TAT-PTD peptide with model lipid membranes | |||
| F266 | Effect of lactose permease on the anisotropy of liposomes | |||
| F262 | Haemolytic effect of merulinic acid on biomembranes | |||
| 4.17 | Dansyl-FA | C179 | Membrane location of dansyl and related probes | |
| 4.18 | BODIPY-FA | C186 | Localization of probe within membranes | |
| E187 | Study of probe binding to fatty acid-binding proteins | |||
| G267 | Characterization of DNA/lipid complexes by FRET | |||
| 4.19 | DPH-PC | B268 | Hydration and order in lipid bilayers | |
| B4269,270 | Lateral distribution of cholesterol and superlattice domain formation | |||
| B5271 | Superlattice domains in phosphatidylcholine bilayers | |||
| G267 | Characterization of DNA/lipid complexes by FRET | |||
| 4.20 | Anthra-PC | B191 | Study of phospholipid molecular motion in the gel phase | |
| B2190 | The study of lateral diffusion of lipids in membranes | |||
| D189 | Probe behavior in egg phosphatidylcholine liposomes | |||
| F272 | Membrane penetration and localization of adriamycin | |||
| 4.21 | Pyrene-PC | B184 | Lateral organization of phospholipids in synthetic membranes | |
| B4273 | Cholesterol’s influence on the interdigitation in phosphatidylethanols | |||
| B5274–276 | Studies on regularity in lipid distribution | |||
| E277 | Protein catalyzed import of phosphatidylcholine | |||
| F278 | Free fatty acids’ influence on membrane permeability | |||
| 4.22 | NBD-PC | B, B2,C279 | Location and dynamics of NBD-labeled phosphatidylcholine | |
| B5280 | Role of ceramides in the maintenance of membrane microdomains | |||
| E281 | Membrane fluidizing effect of annexin V | |||
| F282 | Defining lipid transport pathways in animal cells | |||
| F278 | Free fatty acids’ influence on membrane permeability | |||
| 4.23 | Fluorene-PC | B3283 | Temperature influence on probe behavior in model membranes | |
| D283 | Probe behavior in model membranes | |||
| 4.24 | Cor-PC | B195 | Investigation of submicrosecomd lipid fluctuations | |
| 4.26 | 12-AS | A, C175 | Polarity/localiztion studies in phosphatidyl choline bilayers | |
| B, C141,148 | Properties and membrane locations of fluidity probes | |||
| E284 | Viral membrane protein association with lipid bilayer | |||
| In-chain | 4.27 | BA-Anthr-FE | D197 | Probe orientation in vesicles |
| 4.28 | BA-exAnthr-FE | D197 | Probe orientation in vesicles | |
| 4.29 | BA-Fluorene-PC | B3283 | Probe behavior in model membranes | |
| D283 | Probe behavior in model membranes | |||
| 4.30 | exdhPhenanthrene | G198 | Membrane imaging | |
| 4.31 | C8A-Fl-C4 | C182,202 | Depth analysis in membranes | |
| Polyenes | 4.32 | Filipin | B203 | Filipin III interaction with vesicles and membranes |
| C285 | Probe localization with spin-label | |||
| 4.33 | Amphotericin | B203 | Amphotericin B interaction with vesicles and membranes | |
| 4.34 | Retinol | B205 | Absorption and fluorescence of Retinol in membranes | |
| 4.36 | trans-PnA | B286 | Study on lipid clustering in bilayers | |
| B287 | Phase transition studies | |||
| D213,218 | Probe characterization and behavior in membranes | |||
| E288 | Probing of binding domains in neutrophil elastase | |||
| E289 | Study of the probe binding to bovine serum albumin | |||
| 4.37 | (trans-PA) | B, D, E214 | Fluidity, probe behavior and FRET studies with gramicidin | |
| G220 | Fluorescence microscopy on cells with pentaene comprised sphingomyelin |
|||
| 4.38 | (trans-PdA) | B, D215 | Dynamics of bolaamphiphilic fluorescent polyenes in lipid bilayers | |
A. Membrane polarity
Suitable environmentally sensitive probes located in biomolecular cavities can be used to approximate local polarity by changes in their fluorescence quantum yield (hyperchromic or hypochromic effects) and/or emission maxima (hypsochromic or bathochromic shifts). The correlation between the spectroscopic characteristics of polarity sensitive probes and empirical polarity parameters and scales has recently been discussed.13
B. Fluidity
Fluidity gradient, or membrane lipid dynamics, is a fundamental physical characteristic of biomembranes encompassing the concepts of packing, average orientation, motion and lateral movement of phospholipid chains.148,191 These features can influence the bilayer permeability and optimal activity of membrane bound proteins.194 It is worth noting that the term ‘fluidity’ and what it encompasses remains under debate.28,229,230 A number of studies fall into this category: B1. Microviscosity. Measuring the rotational freedom of a probe with fluorescence polarization could facilitate the determination of its local viscosity. B2. Lateral diffusion. The fluid mosaic nature of membranes116 suggests high rates of lateral diffusion of lipids and proteins. This parameter is considered to be the most important in the description of membrane mobility.230 B3. Influence of temperature. The ‘main’ or chain-melting transition temperature describes the transition from a highly ordered quasi two-dimensional crystalline solid to a quasi two-dimensional liquid and is a reflection of membrane lipid composition.128 B4. Effect of cholesterol. Due to their flat and rigid molecular structure, sterols induce conformational ordering in neighboring aliphatic lipid chains. Cholesterol, being the most familiar sterol in animals, controls many aspects of membrane structure. It influences acyl chain dynamics,231 and function,128 and is involved in inhibition of membrane ion release.232 Moreover, cholesterol can facilitate phase segregation, generating microdomains.128 B5. Microdomains. Membrane microdomains are comprised of long saturated alkyl chains of sphingolipids. Their formation is dependent (lipid raft) or independent (superlattice) of local cholesterol concentration. These microdomains are thought to be involved in specific proteins attachment, membrane transport and intercellular signaling.129,131
C. Depth
Depth analysis is concerned with membrane penetration and localization of, for example, membrane-bound proteins, peptides,233 or cholesterol231,234,235 as well as the topology of phospholipids.182,186 The depth of the probe within the membrane is related to the polarity of its microenvironment. Depth analysis typically relies on the comparison of emission maxima and fluorescence lifetimes of the probe in pure solvents of different polarity to that observed when incorporated into membranes.175 The emission maximum, however, is not only related to the depth, but also reflects probe-specific interactions with its surrounding and probe induced polarity perturbation.2,236 Moreover, an isotropic solvent does not resemble an organized, yet dynamic, architecture like a bilayer.237 Others used dipole–dipole (Förster) energy transfer for depth analysis studies,146,238–241 which have proven to be rather complex. Additional approaches used spin labels141,242 or brominated probes243–245 in fluorescence quenching experiments.
D. Probe behavior
These fundamental studies are concerned with the orientation and mobility of a fluorescent probe and its locally-provoked perturbation upon incorporation into lipid bilayers.151
E. Protein–lipid interactions
Membrane proteins can be located using FRET experiments between tryptophan and an appropriate acceptor (e.g., pentaenoic acid).214 While membrane protein function can be influenced by membrane permeable drugs, it is not always clear if the observed effect is due to a specific drug–protein interaction, or a drug-induced change in local lipid composition.230
F. Membrane permeability
These studies are concerned with transport across the cell membrane and cellular-uptake.
G. Miscellaneous
This category encompasses studies that do not fit in the categories listed above. A brief description is given in the last column.
5. Fluorescent Analogs of Amino Acids
5.1. The Chemistry and Biology of Proteins and Peptides
In the grand scheme of biological macromolecules, recapitulated in the Central Dogma of Biology, proteins appear last, but are responsible for the majority of cellular functions. Diversity in structure and function is encoded in their sequence, a linear string of twenty different α-amino acids, their fundamental building blocks, which are linked through amide (also called peptide) bonds.290,291 Protein recognition, function, cell localization and fate, in addition to being primarily dependent on their three-dimensional fold, are also susceptible to environmental factors (e.g., polarity, ionic strength, etc.), and posttranslational modifications (e.g., glycosylation, phosphorylation, acetylation, etc.). The central role proteins play in modern biology has stimulated extensive exploration of their biochemistry and biophysics. Not surprisingly, fluorescence spectroscopy has proven extremely instrumental in shedding light on their intricacies.
This section provides an overview of amino acid analogs that display favorable spectroscopic properties. A number of review articles have discussed non-canonical amino acids292 and their fluorescent counterparts in particular.293,294 Compared to fluorescent analogs of phospholipids and nucleosides, most α-amino acid-based probes show limited diversity in their design. The acute dependency of protein function on its correct fold is likely to constrain the structural modifications that can be tolerated, thus prohibiting radical structural redesign of the fundamental building blocks. In this section, the probes have been organized based on their structural features. Their basic spectroscopic properties and diverse applications have been tabulated to facilitate comparison. The modified amino acid overview section is preceded by a brief discussion of fluorescent proteins and inherently fluorescent native amino acids, illustrating that Nature has set the bar relatively high when it comes to the generation of useful fluorophores.
5.2. Fluorescent Proteins
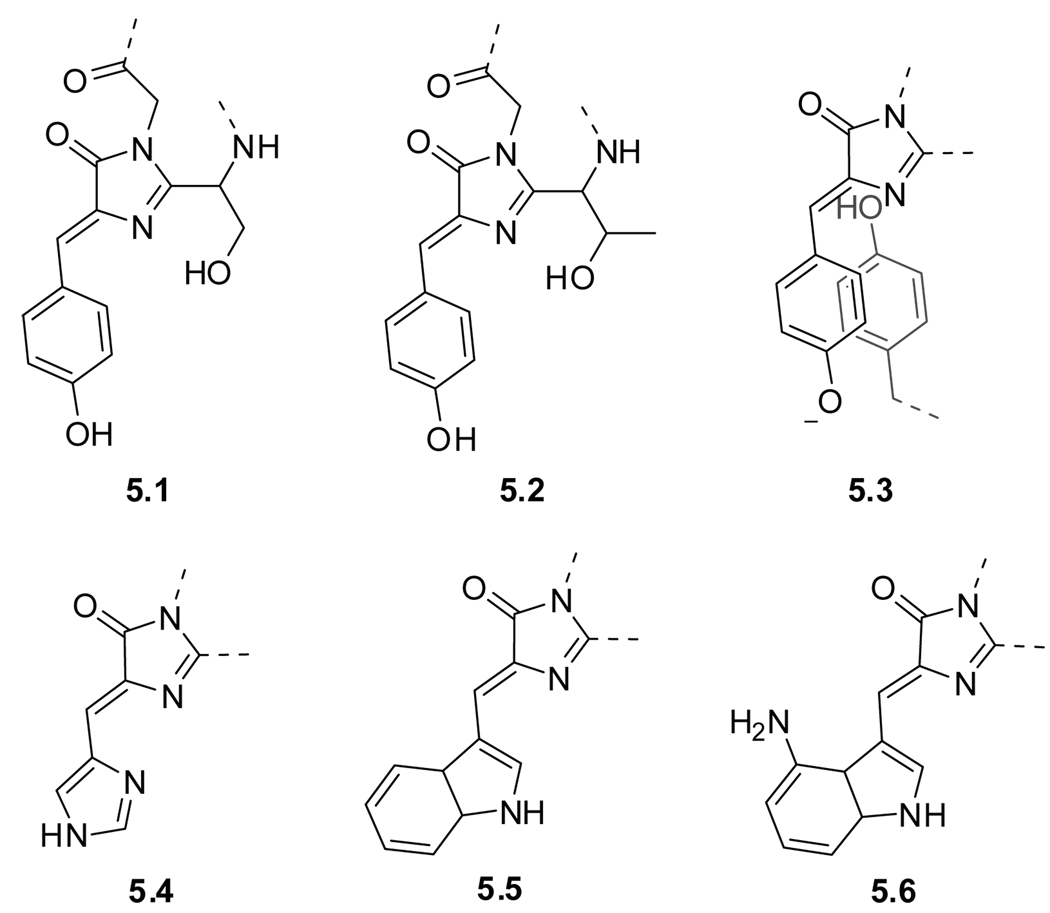
One cannot discuss fluorescent amino acids without addressing fluorescent proteins, best exemplified by the Green Fluorescent Protein (GFP). This “spontaneously generated” and highly emissive chromophore has become one of the most useful tools in modern biology and was instrumental in enabling live-cell imaging.43,295 The isolation of GFP from the jellyfish Aequorea was first reported in 1962,296 and was soon followed by characterization of its remarkable spectral properties.297 The actual GFP fluorophore component is p-hydroxybenzylideneimidazolinone (5.1) formed, in case of the wild type, by condensation of a three residue sequence, Ser–Tyr–Gly (Figure 5.1).
Figure 5.1.
Fluorophores found in fluorescent proteins. Wildtype GFP (5.1) and the S65T point mutation EGFP (5.2), topaz (5.3), P4-3 (5.4), ECFP (5.5), and GdFP (5.6).
About thirty years after its discovery, Tsien et al. published the first major improvement with a single point mutation (S65T) resulting in an emissive protein with enhanced quantum yield and better photostability compared to GFP (EGFP, 5.2).298 Color mutants developed later clearly show how the chemical structure of the chromophore impacts its spectroscopic properties (Figure 5.1 and Table 7).43,299 Examples of engineered fluorescent proteins include topaz (5.3), a yellow fluorescent protein (YFP), whose emissive properties are attributed to a deprotonated tyrosine involved in π-π stacking. 43,299 Substitution of the phenol ring by an imidazole changes the emission to blue (P4-3, 5.4), while substitution with an indole moiety gives an enhanced cyan fluorescent protein (ECFP, 5.5). Moreover, modification of the indole ring at the 4-position with an amine group results in GdFP (5.6), possessing a ‘golden’ emission.300 Advances in visibly fluorescent proteins and their applications have been discussed in various reviews.43,299,301–305
Table 7.
Spectroscopic Properties of Selected Fluorescent Proteinsa
| # | Name | λmax (ε) | λem | Φ |
|---|---|---|---|---|
| 5.1 | GFP Wild type | 396 (27.5) | 504 | 0.79 |
| 5.2 | EGFP | 489 (55) | 510 | 0.64 |
| 5.3 | YFP, Topaz | 514 (94.5) | 527 | 0.6 |
| 5.4 | P4-3 | 382 (22.3) | 446 | 0.3 |
| 5.5 | ECFP | 452 | 505 | - |
| 5.6 | GdFP | 466 (23.4) | 574 | - |
Although a great tool in molecular and cell biology, the use of fluorescent proteins in intact animals is limited due to poor tissue penetration of visible light. This hurdle can be overcome by imaging with far-red and near infrared probes (Section 2.7).44,62 The low excitation-energy employed is non-invasive and provides the prospect for whole-body scale studies.306 The development of (near) infrared-fluorescent proteins (IFP’s) is, therefore, an active area of exploration. A recent example is the engineering of tetrapyrrolic biliverdin-containing Deinococcus radiodurans resulting in an IFP characterized by an excitation maximum of 684 nm (ε > 90 M−1 cm−1) and concomitant emission maximum of 708 nm with a quantum yield of 0.07.60
While GFP and its variants have found unprecedented utility in modern cell biology as intracellular labels,307,308 it is worth noting that their size (∼28 kD or ∼230 amino acids) could alter the location, stability and functionality of their specific fusion partners.295
In this context, it is worthwhile to briefly discuss an elegant exogenous labeling procedure for recombinant proteins. The protocol, developed by Tsien, facilitates genetically targeted labeling with a low molecular weight fluorophore in living cells.309 Fusing the protein of interest to a string of amino acids that contains four cysteine residues in an X3Cys2X2Cys2X3 motif, facilitates in vivo labeling by exposing the cells to the permeable and non-emissive 4’,5’-bis(1,3,2-dithioarsolan-2-yl)fluorescein (named FLASH-EDT2 for fluorescein arsenical helix binder bis-EDT adduct).309 Upon ligand displacement with the uniquely spaced cys residues, a highly emissive peptide–fluorophore complex is formed (Φ=0.49). This in situ and versatile labeling technique increases the mass of the protein of interest only slightly, compared to GFP fusion, and has become popular in the last decade.310–312
The fluorophore in fluorescent proteins is formed by a complex intramolecular reaction involving the peptide backbone, which is consequently compromised.303 Nevertheless, from a fluorophore design perspective, it shows that small fluorophores can be chemically modified to obtain and tune desirable spectroscopic qualities.313–316 Indeed, synthetic analogs, inspired by the GFP fluorophore have been reported.317 In designing fluorescent amino acids analogs, however, the backbone is typically left intact to ensure proper incorporation and folding.
5.3. Naturally Occurring Fluorescent Amino Acids
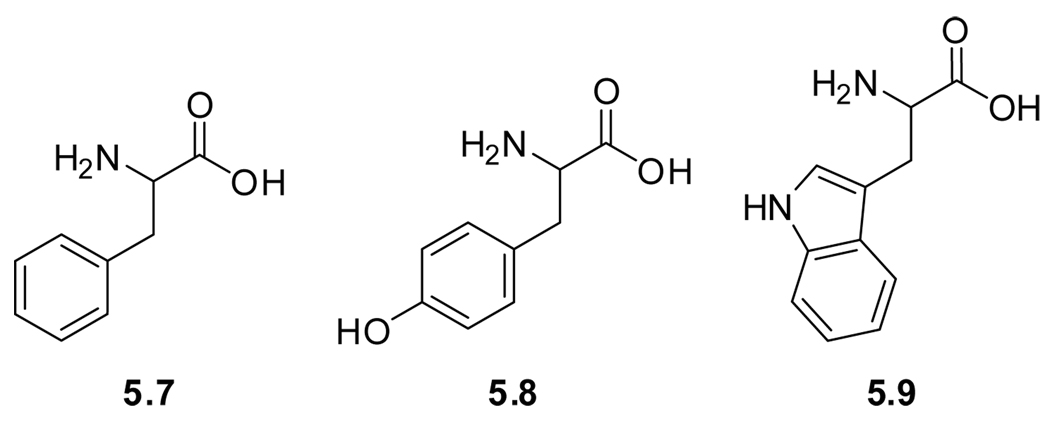
Due to their aromatic side chains, the native amino acids phenylalanine (5.7), tyrosine (5.8), and tryptophan (5.9) possess favorable spectroscopic properties and have been frequently employed as “built in” fluorescent probes (Figure 5.2 and Table 8). The combination of its low quantum yield and low molar extinction coefficient makes phenylalanine detectable only in proteins that are deficient in tryptophan or tyrosine. Tyrosine, however, does possess a reasonable quantum yield (Table 8). While it lacks significant sensitivity to its environmental polarity, its photophysics is pH dependent due to its acidic side chain (pKa ∼10). Deprotonation of the phenolic hydroxyl group results in a bathochromic shift of the emission maximum from 310 to 340 nm.
Figure 5.2.
Naturally occurring fluorescent amino acids.
Table 8.
Spectroscopic Properties of Emissive Native Amino Acidsa
Tryptophan is by far a more favorable probe than phenylalanine or tyrosine, as it benefits from higher brightness (ε × ΦF).319 Due to the large dipole moment of its excited state, tryptophan’s fluorescence quantum yield and emission maximum are highly sensitive to polarity.322 As an apolar amino acid, tryptophan is most often located in the hydrophobic interior of a protein,323 where it emits at 309 nm.324 Changes in tertiary structures, induced, for instance, by unfolding can expose tryptophan to more polar aqueous environments, with concomitant red shift of its emission maximum to 355 nm.323 The wide range of quantum yields displayed by tryptophan is attributed to the diverse surroundings the chromophoric indole ring can experience. In addition, diverse quenchers, including disulfide bonds, protonated histidines and peptide bonds, as well as metal ions, heme groups, and coenzymes, can all affect the excited state of tryptophan. For this reason, unfolding of a tryptophan containing protein typically results in a consecutive red shift of the absorption maximum and significant alteration of the fluorescence quantum yield. These sensitive spectroscopic properties of tryptophan have been widely used to explore protein dynamics, folding and ligand binding, as discussed in a number of review articles.321,323,325,326
Despite tryptophan’s inherent favorable photophysical properties and its relative low abundance in proteins, the presence of multiple residues in different environments within a single protein can complicate the resulting spectroscopy. This might necessitate site directed mutagenesis of all but one tryptophan residue with tyrosine or phenylalanine to mitigate tryptophan emission while minimizing structural perturbations. Another approach involves the introduction of a non-natural amino acid with distinct spectroscopic characteristics. Such modification, however, can potentially perturb protein folding, and hence function. Even the substitution of all three tryptophan residues in barstar by 4-aminotryptophan, a relatively small analog, can result in protein destabilization and compromised function.327 This illustrates the challenges facing protein chemists who attempt to design benign yet spectroscopically useful modified amino acids as discussed below.
5.4. Side-chain Modified Amino Acids
5.4.1. Tryptophan Mimics
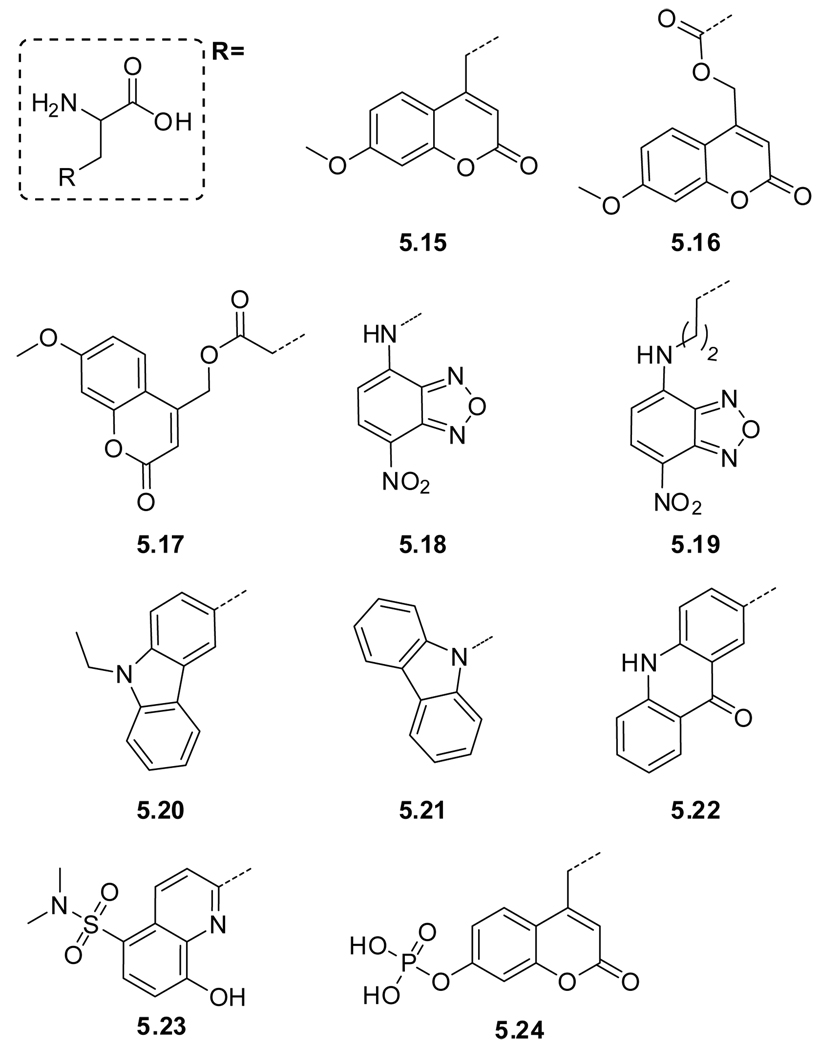
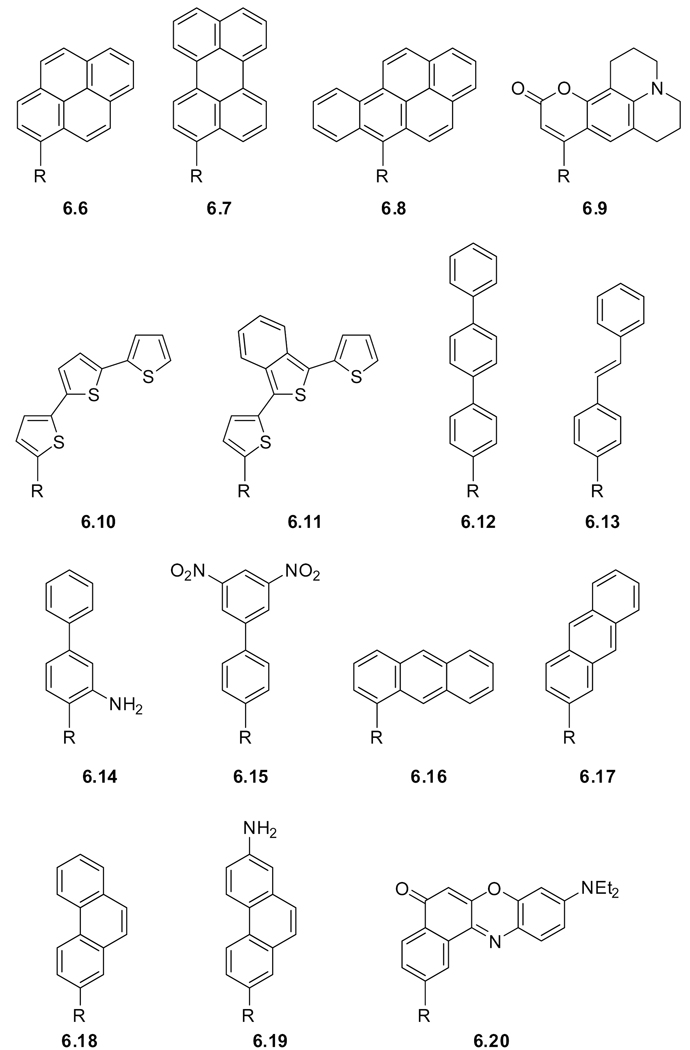
Even though intrinsic probes (native amino acids) facilitate the biophysical study of proteins without the need for chemical modification, extrinsic probes (modified amino acids) have been employed due to their distinct spectroscopic parameters. To minimize potential perturbation upon incorporation of modified fluorescent amino acids, mimicking the size and polarity of tryptophan is a logical approach. Examples of such structures are shown in Figure 5.3 and include the blue emitting azulene (azuAla, 5.10).328 The spectroscopic properties of two other tryptophan derivatives, 5-hydroxytryptophan (5OHTrp, 5.11)329 and 7-azatryptophan (7azaTrp, 5.12)329,330 have been conveniently compared to tryptophan and other tryptophan mimics (Table 9).331 Both 5OHTrp (5.11) and 7azaTrp (5.12) display a 20 nm bathochromic shift of their absorption maximum relative to tryptophan, facilitating selective excitation.332 Two other examples, benzofuranyl alanine (BfAla, 5.13)333 and benzothiophenyl (BtAla, 5.14),334 only differ from tryptophan in their ring heteroatom.335–337 Tirrell and coworkers have incorporated BtAla (5.14) and other tryptophan mimics to modify the spectral properties of fluorescent proteins.338 These and other tryptophan mimics have been discussed in review articles.293,294,327,339
Figure 5.3.
Tryptophan mimics.
Table 9.
Basic Spectroscopic Properties of Selected Modified Amino Acidsa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ | |
|---|---|---|---|---|---|---|---|
| Trp mimics | 5.10 | azuAla | K3PO4 buffer | 276, 339 | 381 | 0.031b | - |
| 5.11 | 5OHTrpc | Water | 279, 297 | 336 | 0.27 | 3.46 | |
| 5.12 | 7azaTrpc | water | 291 | 391 | 0.01 | 1.24 | |
| MeOH | 297 | 366 | 0.01 | - | |||
| 5.14 | BtAlad | EtOH | 228, 297 | 0.019 | 0.28 | ||
| Heterocycles | 5.15 | mchAlae | buffer | 325 (1.4) | - | 0.36 | - |
| 5.16 | Asp(OMc)e | " | " | " | " | " | |
| 5.17 | Glu(OMc)e | " | " | " | " | " | |
| 5.18 | NBDAlaf | EtOH | 264, 330, 462 (19.7) | 532 | 0.38 | - | |
| 5.19 | NBDLysf | " | " | " | " | " | |
| 5.20 | EtcbzAlag | EtOH | 340 | 437 | - | 7.8 | |
| 5.21 | cbzAlag | " | " | " | - | " | |
| 5.22 | acroAla | Water | 388 (5.6), 407 | 420 | 0.95 | - | |
| THF | 378, 395 (6.3) | 422 | 0.21 | - | |||
| 5.23 | Sox | In peptide −Zn | - | 500 | <0.005 | - | |
| In peptide +Zn | 360 (6.2) | 500 | 0.16 | - | |||
| 5.24 | pCAP | In peptide | - | - | - | - | |
| CAP | In peptide | 334 | 460 | pCAP × 104 | - | ||
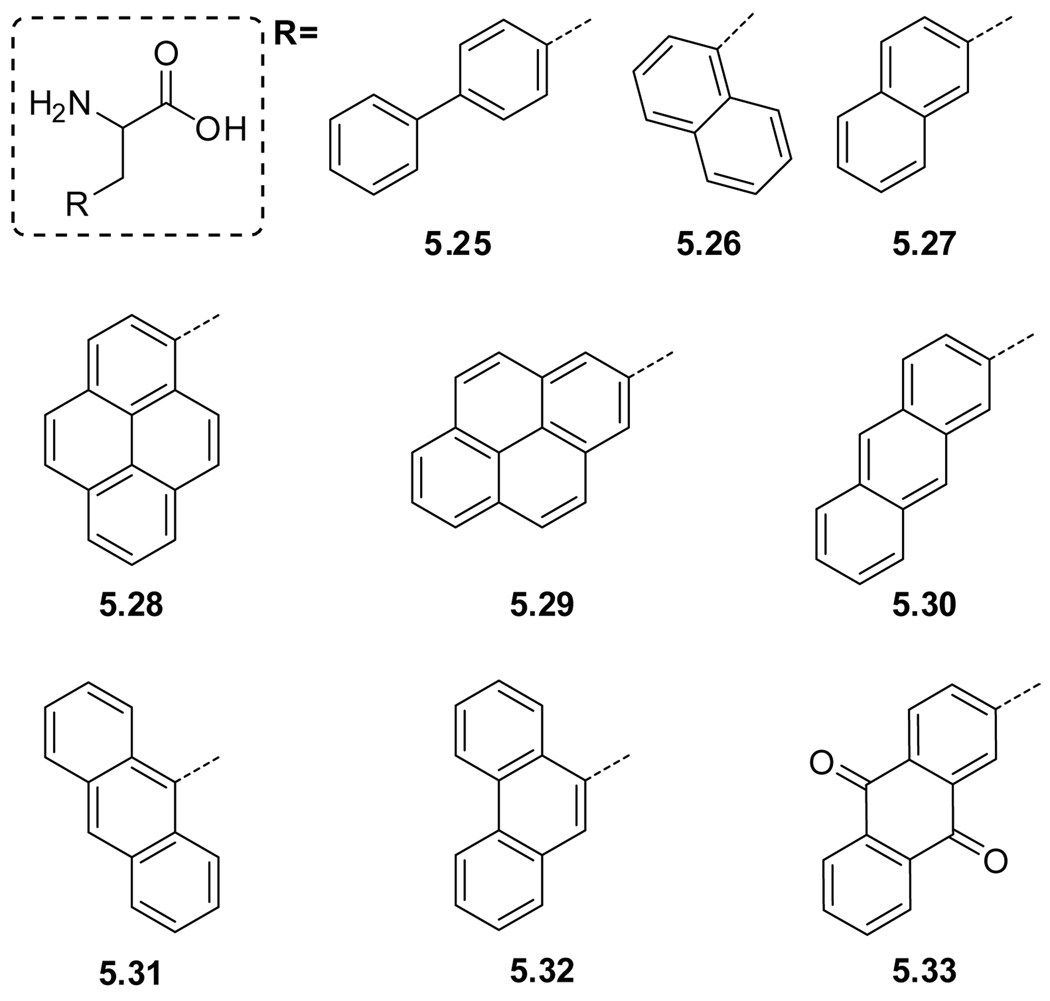
| Hydrocarbons | 5.25 | pbpAlah | (CH3O)3PO | 256 (2.2) | 302, 319 | 0.14 | - |
| 5.26 | 1napAlah | (CH3O)3PO | 272, 283 (0.8), 295 | 326 | - | - | |
| 5.27 | 2napAla | (CH3O)3PO | - | 340 | - | - | |
| 5.28 | 1pyrAlai | EtOH | 241 (79.4), 272, 334 | 376 | 0.65 | 410 | |
| 5.29 | 2pyrAlai | " | " | " | " | " | |
| 5.30 | 9antAlaj | EtOH | 252 (199.5), 338, 357, 376 | 398 | 0.30 | ||
| 5.31 | 2antAla | Water | 342 (5.2) | 384 | 0.11 | - | |
| 5.32 | 9phantAlak | EtOH | 250 (50.1), 293, 330, 346 | - | - | ||
| 5.33 | anthrAlal | EtOH | 253 (50.1), 326, 405 | - | - | - | |
| Dansyl | 5.34 | 51dansylAlam | MeOH | 335 (4.0) | 518 | 0.23 | - |
| dioxane | 335 (4.1) | 479 | 0.54 | - | |||
| 5.35 | 52dansylLysm | MeOH | 335 (4.0) | 518 | 0.23 | - | |
| dioxane | 335 (4.1) | 479 | 0.54 | - | |||
| Imides | 5.38 | 4DAPAn | water | 408 | 562 | - | - |
| dioxane | 378 | 457 | 0.62 | 14.9 | |||
| 5.39 | 6DMNAo | water | 388 | 592 | 0.002 | - | |
| dioxane | 372 | 498 | 0.22 | - | |||
| 5.40 | 4DMNAp | MeOH | 422 | 524 | 0.01 | 0.2 | |
| dioxane | 403 | 500 | 0.76 | 9.2 | |||
| 5.41 | Aladanq | water | 364 (14.5) | 531 | - | 2.1 | |
| cyclohexane | 342 | 401 | - | 1.6 | |||
| 5.42 | azoAla | water | 350 | 450 | - | - | |
Wavelength maxima are in nm, ε in 104 M−1cm−1 and τ in ns. ε and λem are only given for the most intense peaks. For a number of probes, no spectroscopic data could be found. In such cases, the relevant parameter of the actual fluorophore is given.
Azulene in EtOH.376
More solvents are given, τ is the mean lifetime.331
Values for benzothiophene.377
Values for 7-methoxycoumarine-4-acetic acid.
Values are for 7-benzylamino-4-nitrobenz-2-oxa-1,3-diazole.378
Values for N-ethylcarbazole.318
Values for the modification in a central position of oligo-glycine.
Values for phenanthrene.381
Values for anthraquinone.382
Data reported is for 5-(dimethylamino)-N-methylnaphthalene-1-sulfonamide.13
Values for 4-(N,N-dimethylamino)-phthalimide.383
Values for model compound 6DMN-GlyOMe.
Values for dimethylaminonaphthalimide.384
Values for PRODAN.385
5.4.2. Side Chain Modification With Heterocyclic Chromophores
Attaching an established fluorophore to a side chain of a non-emissive amino acid such as Ala is a rational approach for the generation of fluorescent amino acids (Figure 5.4). Examples include 7-methoxy-coumarine labeled alanine (mchAla, 5.15), aspartic acid (Asp(OMc), 5.16), 340and glutamic acid (Glu(OMc), 5.17). 340 Other examples include NBD-labeled alanine (NBDAla, 5.18),341 NBD-labeled lysine (NBDLys, 5.19),342,343 carbazole labeled alanines 3-(9-ethylcarbazolylalanine (EtcbzAla, 5.20),344 and 9-carbazolylalanine (cbzAla, 5.21).345 A recent addition is the 2-acrydonylalanine (acroAla, 5.22) which is reported to possess high fotodurability.346,347 Interestingly, most of these probes have found limited use in exploring natural systems, but have been used in studying synthetic photoactive polypeptides (Tables 9 and 10).348,349
Figure 5.4.
Examples of amino acids containing heterocyclic chromophores.
Table 10.
Applications of Selected Fluorescent Amino Acids
| # | Name | Applications | Brief description | |
|---|---|---|---|---|
| Trp mimics | 5.10 | azuAla | D328 | Incorporation by solid phase assisted peptide synthesis |
| 5.11 | 5OHTrp | D1329 | Incorporation in β-galactosidase | |
| D1332 | Incorporation in mannitol transporter (EIImtl), a membrane protein | |||
| F339 | DNA–protein binding | |||
| 5.12 | 7azaTrp | D1329 | Incorporation in β-galactosidase | |
| D1332 | Incorporation in mannitol transporter (EIImtl), a membrane protein | |||
| F339 | DNA–protein binding | |||
| 5.13 | BfAla | D396,397 | Site specific in vivo incorporation | |
| H336 | Used as a competitive inhibitor for indoleamine 2,3-dioxygenase | |||
| 5.14 | BtAla | A398 | Substitutions in the 3-position of cyclic β-casomorphin analogs | |
| D338 | Incorporation in cyan fluorescent protein (CFP6) | |||
| H336 | Used as a competitive inhibitor for indoleamine 2,3-dioxygenase | |||
| Heterocycles | 5.15 | mchAlae | D340 | Incorporation in streptavidin |
| 5.16 | Asp(OMc)e | D340 | Incorporation in streptavidin | |
| 5.17 | Glu(OMc)e | D340 | Incorporation in streptavidin | |
| 5.18 | NBDAla | A341 | Probing structure and function of the Tachykinin Neurokinin-2 Receptor |
|
| D370 | Incorporation into a hexapeptide | |||
| D394 | Review | |||
| 5.19 | NBDLys | D345 | Incorporation in streptavidin | |
| 5.20 | NEtcarbazole | G349 | Study of photoinduced electron transfer in synthetic poly peptides | |
| 5.21 | carbazole | D345 | Incorporation in streptavidin | |
| 5.23 | Sox | H350 | Chemosensor scaffold for divalent zinc | |
| H351–354 | Protein kinase activity (Sox and Sox derivatives) | |||
| 5.24 | pCAP | H355–357 | Tyrosine phosphatase activity | |
| Hydrocarbons | 5.25 | pbpAla | D345 | Incorporation in streptavidin |
| 5.26 | 1napAla | D399 | In vivo incorporation using Elongation Factor Tu mutants | |
| G348 | Review | |||
| G358 | Singlet energy transfer | |||
| H400 | Pressor and antidiuretic peptides | |||
| H363 | Photoenergy trapping in vesicles | |||
| 5.27 | 2napAla | D342 | Streptavidin | |
| D399 | In vivo incorporation using Elongation Factor Tu mutants | |||
| G359 | CD spectroscopy on synthetic polypeptides | |||
| G348 | Review | |||
| H400 | Pressor and antidiuretic peptides | |||
| H363 | Photoenergy trapping in vesicles | |||
| 5.28 | 1pyrAla | D399 | In vivo incorporation using Elongation Factor Tu mutants | |
| F401 | Enkephalin–opiate receptor interaction | |||
| F402 | Peptide–antibody interaction | |||
| G349 | PET in synthetic poly peptides | |||
| G360 | Eximers formation | |||
| G403 | Helix–helix interaction | |||
| 5.30 | 9antAla | D399 | In vivo incorporation using Elongation Factor Tu mutants | |
| G348 | Review | |||
| H363,364 | Photoenergy trapping in vesicles | |||
| 5.31 | 2antAla | D340,345 | Incorporation in streptavidin | |
| 5.32 | 9phantAla | H363 | Photoenergy trapping in vesicles | |
| 5.33 | anthrAla | D399 | In vivo incorporation using Elongation Factor Tu mutants | |
| D361 | λ-Cro repressor protein | |||
| G348 | Review | |||
| G365,404 | Synthesis and conformation of poly(L-2-anthraquinonylalanine) | |||
| H361 | Duplex DNA photocleavage | |||
| Dansy | 5.34 | 51dansylAla | B366 | Monitoring of the unfolding of human superoxide dismutase |
| C1370 | Incorporation into a hexapeptide | |||
| D1329 | β-Galactosidase | |||
| D366 | Superoxide dismutase | |||
| F405,406 | Peptide–protein binding | |||
| G348 | Review | |||
| Imides | 5.38 | 4DAPA | Cl370 | Incorporation ito a hexapeptide |
| F368 | Peptide–protein | |||
| 5.39 | 6DMNA | C1370 | Incorporation into a hexapeptide | |
| F369 | Peptide-protein binding | |||
| 5.40 | 4DMNA | C1370 | Incorporation into a hexapeptide | |
| 5.41 | Aladan | C1370 | Incorporation into a hexapeptide | |
| C29 | Petide-protein binding | |||
| F372 | Investigation of phosphorylation–dependent protein associations | |||
| 5.42 | azoAla | A374,407 | Studies on a series of azopolypeptides | |
| E373 | Photoswitching of an NAD+–mediated enzyme reaction | |||
In contrast to the aforementioned fluorophores, some probes undergo chemical modification in the probing process. Sox (5.23), a quinoline derivative functionalized amino acid developed by the Imperiali group, shows a considerable fluorescence enhancement upon chelation of divalent zinc, a process termed “chelation-enhanced fluorescence” (CHEF).350 This property has been utilized in the study of protein kinase activity. Although Sox alone is capable of chelating Mg2+ with concomitant fluorescent enhancement, phosphorylation of a nearby serine, threonine, or tyrosine in a β–turn sequence, results in a ∼10-fold enhancement of the binding affinity and thus a strong increase in the fluorescence signal.351,352 Improved Sox-based probes appeared shortly thereafter.353,354 Another example of probing based on chemical modification is the dephosphorylation of pCAP (5.24), a phosphorylated coumarin derivative functionalized amino acid.355 Enzyme mediated dephosphorylation leads to augmented fluorescence intensity which has been used in protein tyrosine phosphatase studies.356,357
5.4.3. Labeling With Aromatic Hydrocarbons
Aromatic hydrocarbons are often highly emissive and can therefore be attached to an amino acid side chain to provide fluorescent building blocks (Figure 5.5). Examples include p-biphenyl labeled alanine (pbpAla, 5.25),358 1-naphthyl (1napAla, 5.26), 358 and 2-naphthyl labeled alanine (2napAla, 5.27),359 as well as 1-pyrenyl (1pyrAla, 5.28)360 and 2-pyrenyl labeled alanine (2pyrAla, 5.29).345 The anthracene functionalized alanine 2-anthryl (2antAla, 5.30), 345,361 has very comparable spectroscopic properties in polar and apolar environments, which makes it useful as a fluorescent tag, but hampers its use as a reporting probe.362 Other examples include 9-anthryl modified alanine (9antAla, 5.31),363,364 9-phenanthryl labeled alanine (9phantAla, 5.32),364 and anthraquinone based alanine (anthrAla, 5.33).365 Basic spectroscopic properties of various fluorescent amino acid analogs can be found in Table 9.
Figure 5.5.
Amino acids labeled with emissive heterocarbons.
5.4.4. Dansyl Modified Amino Acids
Due to its spectroscopic qualities, the dansyl fluorophore has been used as a probe with most biomolecules, including amino acids (Figure 5.6). The dansyl modified alanine, 51dansylAla (5.34) is probably the most studied (Figure 5.6).329,366 Dansyl modified lysine (51dansylLys, 5.35) has been used as well.367 Their basic spectroscopic properties can be found in Table 9. Two phenylalanine-based designs, 62dansylPhe (5.36) and 52dansylPhe (5.37), have been proposed,362 but have not, to our knowledge, been explored.
Figure 5.6.
Examples of dansyl decorated amino acids.
5.4.5. Diaminopropionic Acid Derivatives
Push-pull aromatic hydrocarbons have been linked to the peptide backbone through an imide or amide derived 2,3-diaminopropanoic acid, maintaining the α-amino acid core (Figure 5.7). Imperiali and coworkers have developed three probes, 4DAPA (5.38), 368 6DMNA (5.39),369 and 4DMNA (5.40),370 based on this design principle. PRODAN-based Aladan (5.41) represents another example of this design (Figure 5.7).9,371,372
Figure 5.7.
Polarity sensitive phthalimide analogs.
These charge transfer dyes impart upon the resulting amino acid their characteristic dependency on environmental polarity, which is manifested in their absorption and emission maxima (Table 9). This useful spectroscopic property, in combination with knowledge of the probe’s location after incorporation into a peptide or protein, makes these probes effective for investigating binding events.368,369,371 The Imperiali group has recently reported a comparative study of the responsiveness of 4DAPA (5.38), 6DMNA (5.39), 4DMNA (5.40), NBD (5.18), 51dansylAla (5.34), and aladan (5.41) when incorporated in the fourth position of a six-residue sequence.370 While each probe had its own favorable properties, 4DMNA (5.40) was found to be of particular interest, due to its chemical stability and minimal structural perturbation upon incorporation.370 Similarly, Aladan (5.41) has been employed to estimate the local polarity in proteins.9 Despite its desirable spectral characteristics, it has been shown to be destabilizing.10
5.4.6. Modification With a Photoswitch
A chromophore that undergoes a reversible light induced structural change (cis-trans isomerization), for instance p-phenylazophenylalanine (azoAla, 5.42), can be used to control enzymatic activity373 and polypeptide conformation (Figure 5.8, Table 9).374 Interestingly, introduction of a dimethylamino group gives non-fluorescent dabcyl-diaminopropionic acid (5.43),375 which has been used as a fluorescent acceptor with 7azaAla (5.12, Figure 5.3) in FRET studies.
Figure 5.8.
Photo–switchable probes 5.40 and 5.41.
5.5. Incorporation of Modified Amino Acids
Incorporating modified amino acids into peptides or small proteins can be done by solid phase peptide synthesis, using the appropriately protected modified building blocks. Longer peptides or proteins can be obtained by means of native chemical ligation of two peptides, 386 independently synthesized.
Strategies utilizing the translational machinery have also been developed for the incorporation of modified amino acids into proteins.387,388 These include in vitro translation reactions in cell-free extracts, or alternatively, in vivo expression of modified proteins. Note that these techniques require unique codon–anticodon interactions (achieved using, for example, Amber codon suppression,389,390 orthogonal nucleobases,391,392 or extended 4- and 5-base codons340,343,362,393). Additionally, the corresponding tRNAs have to be synthetically charged (in the case of in vitro translation) or be a substrate for a specific synthetase (for in vivo applications). Despite their complexity, these techniques have found extensive applications in modern chemical biology.292,394,395
5.6. Applications
The fluorescent probes discussed in this section were, in most cases, designed for specific applications. Table 10 below is not all encompassing, but correlates chromophore design, properties and applications. Listed below are the main areas where these probes have found applications.
A. Structure, conformation and function
Protein function relies on its structure and proper conformation. Probes in this category are used to study the conformational behavior or to investigate the influence of the modified amino acids on the protein’s native function.
B. Folding/unfolding
Unfolding (i.e., denaturation) increases exposure to bulk water. Fluorophores with spectroscopic characteristics that are dependent on polarity (i.e., solvatochromic probes) are ideal for such purposes. Tryptophan is the classical example.
C. Electrostatics/polarity
Electrostatics plays a key role in virtually all aspects of protein structure and activity and is of particular relevance for proteins whose function involves charge stabilization.9 The sensitivity of Coulombic interactions to polarity are analogous to the susceptibility of solvatochromic probes to changes in polarity. Such chromophores can be either used to estimate local polarity or to study binding events.
D. Incorporation
This application lists the in vitro or in vivo corporation of emissive probes into peptides and proteins. The incorporation of a large number of non-natural amino acids, tabulated below, has been discussed in a couple of review articles.362,394
E. Photoswitching
A photoswitchable chromophore is employed to photocontrol a biological function.
F. Binding events
Since binding sites typically become less exposed to the aqueous bulk upon ligand binding, polarity-sensitive probes can be used to monitor such processes.
G. Synthetic polypeptides
Synthetic peptides have found numerous applications as protein models in biophysical studies and in material sciences. Well defined secondary structures (e.g., α-helix) provide useful scaffold for placing chromophores and exploring their interactions.348
H. Miscellaneous
Studies that do not fit in any of the categories listed above are included here. A brief description is provided in the last column.
6. Fluorescent Nucleoside Analogs
6.1. Introduction
As we complete our “journey into the center of the cell” we approach nucleosides, nucleotides and oligonucleotides, molecules of utmost importance in cell biology. While originally regarded as molecules of heredity, our contemporary view of the flow of biochemical information from deoxyribonucleic acid (DNA) to ribonucleic acid (RNA) and then to proteins suggests much more complex roles for nucleic acids.408 Briefly, DNA sequences coding for specific genes are transcribed in the cell nucleus to yield heterogeneous nuclear RNAs. The resulting primary RNA transcripts are processed, yielding mature RNAs, which are exported to the cytoplasm where ribosome-based protein synthesis takes place. The transport, localization, stability and translation efficiency of individual messenger RNA molecules are all well regulated. Additionally, recent developments suggest significant roles for non-coding RNA sequences in cellular regulatory processes, adding to the multifaceted and intricate roles of these biomolecules in the cell.409–412
Nucleic acids are phosphodietser-based biopolymers composed of several different building blocks in no particular or repeating linear sequence. The minimal building blocks, or monomers, are nucleotides, which can be viewed as phosphorylated nucleosides (Figure 6.1). Nucleosides, in turn, are composed of a five-carbon monosaccharide (d-ribose) linked to nitrogenous heterocyclic rings (pyrimidines and purines). While a handful of rare naturally occurring nucleosides are emissive,413–416 and despite very early studies suggesting that nucleobases and nucleic acids are fluorescent,417 the purines and pyrimidines commonly found in nucleic acids are practically non-emissive in neutral aqueous conditions. Accurate measurements reveal exceedingly low fluorescence quantum yields for the natural nucleobases (ΦF = 0.5×10−4 − 3×10−4) associated with sub-picosecond excited state lifetimes (Table 11).418 Quite expectedly, mother Nature has selected the building blocks for its precious genetic material to rapidly decay back to their ground state upon photochemical excitation.419 This property has presented, however, a major challenge to the biophysical community interested in exploring nucleic acids, which stimulated an extensive search for emissive nucleoside analogs, as discussed in this section.
Figure 6.1.
The naturally occurring ribo- and deoxyribo-nucleosides. Note: in RNA, the bases A, T, G, C and U are connected to d-ribose at the 1’-position, where the sugar moiety in DNA is 2’-deoxy-d -ribose.
Table 11.
Spectroscopic Properties of Native Nucleosides and Nucleotides in Watera
| # | Name | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|
| 6.1 | A | 260 (14.9) | 310 | 5 × 10−5 | 0.53 |
| AMP | 259 (15.4) | 312 | 5 × 10−5 | 0.52 | |
| 6.2 | T | 267 (9.7) | 327 | 10 × 10−5 | 0.70 |
| TMP | 267 (10.2) | 330 | 12 × 10−5 | 0.98 | |
| 6.3 | G | 253 (13.6) | - | - | 0.69 |
| GMP | 252 (13.7) | 340 | 0.8 × 10−5 | 0.86 | |
| 6.4 | C | 271 (9.1) | 324 | 7 × 10−5 | 0.76 |
| CMP | 271 (9.1) | 330 | 12 × 10−5 | 0.95 |
Unlike the majority of biomolecular building blocks discussed in previous sections, the pyrimidines and purines present a fertile ground for synthetic organic chemists. These aromatic heterocycles are receptive to diverse modifications, where minimal structural and electronic perturbations can, in certain cases, dramatically alter their photophysical characteristics. Early work was inspired by naturally occurring emissive heterocycles, such as the wyeosine bases, a family of tricyclic guanine derivatives. Leonard’s pioneering work, where an etheno bridge was constructed across the H-bonding face of the purines and pyrimidines, furnished a series of emissive nucleobase analogs, with ethenoadenosine (εA) becoming one of the most useful early emissive nucleosides.420 The advance of solid-phase oligonucleotide synthesis, facilitating the incorporation of modified nucleoside into oligomers, further propelled nucleoside chemists to explore new analogs. As presented in this section, the contemporary landscape of fluorescent nucleoside analogs is vast. We attempt to provide the reader with an up to date and systematically organized view of this rapidly evolving field. The diverse applications, summarized in table 20, illustrate the breadth of this growing field and the great utility of judiciously implemented fluorescent nucleosides. Although beyond the scope of this review, fluorescent nucleoside mimics and surrogates have also been explored.421–424
Table 20.
Fluorescent Nucleosides and Some of Their Typical Applications
| # | Probe name | Applicationref | Remarks | |
|---|---|---|---|---|
| Chromophoric base analogs | 6.9 | Coumarin | Time resolved Stokes shift: | |
| C428 | Helix interior | |||
| C446 | Sequence dependence | |||
| C447,448 | Counter ions | |||
| C450 | Structural changes/probe position | |||
| C444,445,451 | Probe position | |||
| B449 | Endonuclease APE1 | |||
| 6.14 | M | B654 | Denaturation | |
| 6.15 | O | B654 | Denaturation | |
| 6.18 | Nile red nucleoside | E431 | Influence of β-cyclodextrin on emission maximum |
|
| Pteridines | 6.19 | 3-MI | B456,457 | HIV-1 Intergrase activity |
| B/C456,655 | DNA-HU interactions | |||
| B/C456 | Interaction of DNA with RNA polymerase | |||
| D656 | Aminoglycoside/RNA binding | |||
| B456,643 | Detection of complementary strand | |||
| C456 | Alkyl transferase | |||
| E456,643 | Cellular uptake of oligonucleotides | |||
| 6.20 | 6-MI | B657 | RecA-DNA interactions | |
| B/C456 | Interaction of DNA with RNA polymerase | |||
| D656 | Monitoring Aminoglycoside/RNA binding | |||
| 6.22 | 6-MAP | C456,658 | Investigating A-tracts | |
| Expanded nucleobases | 6.23 | Etheno-A | B657 | RecA-DNA interactions |
| 6.24 | Benzo-A | C470 | Base pairing/stacking effects | |
| 6.25 | BgQ | B476,477 | Triplex formation | |
| D659 | Tat–TAR binding | |||
| 6.26 | Cf | B476,477 | Triplex formation | |
| D660 | Bleomycin analogs cleavage of DNA | |||
| 6.27 | tC | B483,485 | Klenow fragment DNA polymerase | |
| B661 | Structural measurements on DNA using FRET | |||
| 6.28 | tCo | B483 | Klenow fragment DNA polymerase | |
| B661 | Structural measurements on DNA using FRET | |||
| 6.29 | Çf | A662 | Discrimination of all 4 bases | |
| 6.30 | 8-oxoG-Clamp | C488–490 | Detection of 8-oxoG (free nucleoside and incorporated into an oligonucleotide) |
|
| 6.31 | BPP | A492 | A vs. G detection | |
| 6.32 | NPP | A493,663 | A vs. G detection | |
| 6.33 | MDA | A494 | T vs. C detection | |
| 6.34 | MDI | A494 | T vs. C detection | |
| 6.35 | dChpp | A664 | A vs. G detection | |
| 6.39 | dCppi | A665 | A vs. G detection | |
| Extended nucleobases | 6.51 | PYU | A666 | A vs. C detection |
| B667 | RNA poly(A) tracts | |||
| C668 | Detection of T•Hg•T base pairs | |||
| 6.52 | PYC | A666 | G vs. T detectiona | |
| B669 | B to Z transition | |||
| 6.53 | DMAP-PYU | B514 | Dual emission | |
| 6.54 | 5-(1-pyrenyl)-dU | B670 | Multiple consecutive modified nucleosides | |
| 6.55 | 8-(1-pyrenyl)-dG | A/B422 | Mismatch vs. perfect complement Detection/Charge transfer studies |
|
| 6.57 | UP | B671 | B to Z transition | |
| B672 | Molecular beacons | |||
| B673 | Probe base pairing interactions | |||
| C510,674,675 | Charge transfer studies | |||
| C676 | HIV TAR RNA base conformations | |||
| B677 | Hybridization detection | |||
| A678 | Mismatch vs perfect complement detection | |||
| B679 | Multiple consecutive modified nucleosides | |||
| A680 | A vs. C/G/T detection | |||
| C681 | Ultrafast structural dynamics | |||
| B682 | Duplex stability | |||
| A683 | Mismatch detection | |||
| E684 | White-light-emitting DNA | |||
| 6.58 | CP | B685 | Molecular beacon/Thermal denaturation | |
| 6.59 | AP | B671 | B to Z transition | |
| B672,686 | Molecular beacons | |||
| B673 | Probe base pairing interactions | |||
| B678,688 | G-quadruplex transition | |||
| A680 | A vs. C/G/T detection | |||
| A689 | Homoadenine signalling system for SNP typing | |||
| B690 | Photophysics of modified poly-A oligonucleotides |
|||
| 6.60 | GP | B685 | Molecular beacon/Thermal denaturation | |
| B669 | B to Z transition | |||
| 6.61 | APY | B691 | RNA hybridization | |
| 6.62 | UPE | C675,692 | Charge transfer studies | |
| 6.66 | 5-(perylen-3-ylethynyl)dU | B682 | Duplex stability | |
| A683 | Mismatch detection | |||
| 6.67 | dApymcm | A and B693 | Mismatch vs. perfect complement detection Hanging vs. blunt ends detection |
|
| 6.68 | MMeU | B497 | Hybridization detection | |
| 6.69 | PHU | B497 | Hybridization detection | |
| 6.70 | MeOPhU | B497 | Hybridization detection | |
| 6.71 | PYA | A694 | C vs. T detection | |
| 6.74 | DNCU | B/C695 | Polarity inside DNA-binding protein (KF) | |
| 6.75 | PDNU | A499 | Mismatch vs. perfect complement detection | |
| 6.76 | PDNC | A499 | Mismatch vs. perfect complement detection | |
| 6.77 | PDNA | A499 | Mismatch vs. perfect complement detection | |
| 6.78 | PDNG | A499 | Mismatch vs. perfect complement detection | |
| 6.79 | danC | B/C500 | Groove polarity (minor and major of A/B DNA) |
|
| B/C696 | Groove polarity (minor and major of Z-DNA) | |||
| 6.80 | danG | B/C500 | Groove polarity (minor and major of A/B DNA) |
|
| B/C696 | Groove polarity (minor and major of Z-DNA) | |||
| 6.81 | 5-aminodansyl-dU | B/C501,697,698 | Groove polarity (major/minor interactions and sequence dependence) |
|
| 6.82 | 2-Anthracenecarboxamide-dU | A699 | Mismatch vs. perfect complement detection | |
| 6.83 | 9-Anthracenecarboxamide-dU | A699 | Mismatch vs. perfect complement detection | |
| 6.84 | NeT | A/B700 | A vs. G detection/Hybridization detection | |
| 6.85 | AeT | A/B700 | A vs. G detection/Hybridization detection | |
| 6.86 | AeeT | A/B700 | A vs. G detection/Hybridization detection | |
| 6.87 | UFL | A502 | Mismatch vs. perfect complement detection | |
| 6.96.c | 5-[3(phen)ethynyl]dU | A531 | Discrimination of all 4 bases | |
| 6.98.g | 5-[5-Ru(bpy)3ethynyl] dU-TP | A538 | Luminescent and electrochemical detection | |
| 6.104.g | 5-[5-Ru(bpy)3ethynyl] dC-TP | A538 | Luminescent and electrochemical detection | |
| 6.111.g | 7-[7-deaza-5-Ru(bpy)3 ethynyl]dG-TP |
A538 | Luminescent and electrochemical detection | |
| 6.116.g | 7-[7-deaza-5-Ru(bpy)3 ethynyl]dA-TP |
A538 | Luminescent and electrochemical detection | |
| Isomorphic nucleobases | 6.123 | 2-AP | B701 | Molecular beacon |
| B702 | Duplex denaturation | |||
| B703 | Helicase activity | |||
| B704 | Hammerhead ribozyme | |||
| B705 | DNA polymerase fidelity in vitro | |||
| B657 | RecA-DNA interactions | |||
| B706 | HIV-I DIS RNA structure | |||
| B/D707 | G-quadruplex structure and ligand binding | |||
| C708 | Local conformation via CD | |||
| C702,709 | Base flipping | |||
| C710–712 | Abasic site structure/dynamics | |||
| C713 | Strength of BP interactions | |||
| D656,714–717 | Aminoglycoside/RNA binding | |||
| B/C718–720 | Interaction of DNA with RNA polymerase | |||
| 6.126 | 8-vinyl-6-aminopurine | A721 | short/long excited state lifetimes | |
| 6.127 | 2-amino-6-(2-thienyl)purine | B571 | Detection of fluorescently labeled complementary strand |
|
| B569 | Denaturation of RNA (hairpin and tRNA) | |||
| 6.128 | 2-amino-6-(2-thiazolyl)purine | B571 | Detection of fluorescently labeled complementary strand |
|
| 6.129 | m5K | B657,722 | ssDNA–Escherichia coli RecA binding | |
| 6.130 | pC | A609 | Mismatch vs. perfect complement detection | |
| B20 | Duplex denaturation | |||
| B701 | Molecular beacon | |||
| B608 | Hybridization detection | |||
| C723 | Local conformation via CD | |||
| C/D724 | Metal ion biosensors | |||
| B725 | DNA–reverse transcriptase binding | |||
| B607,726 | T-7 RNA polymerase | |||
| C727 | Base flipping | |||
| B728 | tRNA conformations | |||
| 6.131 | MMepC | B608 | Hybridization detection | |
| 6.132 | PhpC | A609 | Mismatch vs. perfect complement detection | |
| 6.133 | PyIpC | B729 | Molecular beacon | |
| 6.134 | PyIIpC | B729 | Molecular beacon | |
| 6.135 | 5-(fur-2-yl)dU | C589 | Abasic site detection | |
| B/C11 | Major groove polarity | |||
| 6.136 | 5-(fur-2-yl)U | D595 | Tat/TAR binding | |
| D591 | aminoglycoside/A-site binding | |||
| 6.141 | 5-(fur-2-yl)dC | C596 | Detection of 8-oxoG and its transverse mutation product |
|
| 6.161 | Thieno[3,4-d]-U | A597 | C mismatch detection | |
| B/C599 | Activity of toxic ribosome-inactivating protiens | |||
| 6.162 | BTU | B548 | Hybridization detection | |
| 6.168 | 5-MeOQuinazolinedione | D600 | FRET enabled detection of aminglycoside- RNA binding |
|
Amide moiety is vital to probe sensitivity.
6.2. Chromophoric Base Analogs
Replacing the natural nucleobases with established fluorophores, typically polycyclic aromatic hydrocarbons (PAH), yields an unusual family of chromophoric base analogs that lacks the Watson-Crick (W-C) hydrogen bonding face (Figure 6.2). Many of these fluorescent nucleobases have isolated absorption bands (≥ 345 nm) that facilitate selective excitation in the presence of the natural nucleobases and high emission quantum efficiencies approaching unity (Table 12).427
Figure 6.2.
Selected examples of chromophoric base analogs, where R = 2′-deoxyribose.
Table 12.
Spectroscopic Properties of Selected Chromophoric Base Analogsa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 6.6 | Pyreneb,f | MeOH | 241, 345 (39.0) | 375, 395 | 0.12 | - |
| 6.7 | Peryleneb,f | MeOH | 440 (39.2) | 433, 472 | 0.88 | - |
| 6.8 | Benzopyreneb,f | MeOH | 394 (28.2) | 408 | 0.98 | - |
| 6.9 | Coumarinb,c | Bufferd | 400 | 515 | - | 7.4 |
| 6.10 | Terthiophenee,f | MeOH | 358 (31.4) | 432 | 0.059 | - |
| 6.11 | Benzoterthiophenee,f | MeOH | 437 (18.3) | 536 | 0.67 | - |
| 6.12 | Terphenyle,f | MeOH | 285 (40.1) | 345 | 0.42 | - |
| 6.13 | stilbenee,f | MeOH | 301 (21.1) | 356 | 0.055 | - |
| 6.16 | 1-anthraceneg | N/A | - | - | - | - |
| 6.17 | 2-anthraceneg | N/A | - | - | - | - |
| 6.18 | Phen | - | - | - | - | - |
| 6.19 | PhenNH2 | - | - | - | - | - |
| 6.20 | Nile red nucleosideh | MeOH | 557 (3.38) | 632 | 0.09 | - |
Kool and co-workers have utilized such PAH analogs for the investigation of enzyme–substrate recognition, demonstrating that size and shape are important factors in these template directed events.432 When linked via phosphodiester bonds to form oligomeric structures resembling DNA, these oligodeoxyfluorosides yield unique water soluble fluorophores, where the photophysical properties are dictated by the composition and sequence of the individual chromophores. The complex electronic interactions between the stacked chromophores lead to fluorophores that typically display large Stokes shifts and a wide range of emission wavelengths and quantum yields.429,433–439 A coumarin 102 containing nucleoside (figure 6.2),440 having photophysical properties similar to its parent chromophore (Table 12),441–443 was designed to pair with an abasic site in DNA. It was used to explore environmental and dynamics features of DNA oligonucleotides.428,444–452 A phenanthrenyl nucleoside, recently reported by Leumann, was used to explore electron transfer in DNA.453,454
6.3. Pteridines
Pteridines are naturally occurring, highly emissive heterocycles whose structures are related to that of the purines (Figure 6.3. Table 13). Their intense (Φ = 0.39–0.88) and visible fluorescence (∼430 nm), characterized by a relatively long excited state lifetime (τ = 3.8–6.5 ns), results from an isolated absorption band above 300 nm. The development of the pteridines as fluorescent nucleoside analogs was initiated and advanced almost exclusively by Hawkins and co-workers.455,456
Figure 6.3.
Selected examples of pteridines (R = 2′-deoxyribose or ribose).
Table 13.
Spectroscopic Properties of Pteridine Nucleoside Analogs in Buffera
| # | Name | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|
| 6.21 | 3-MI | 348 254 (3.69), 350 (4.13)b |
430 - |
0.88 - |
6.5 - |
| 6.22 | 6-MI | 340 (11.0) | 431 | 0.70 | 6.4 |
| 6.23 | DMAP | 330 | 430 | 0.48 | 4.8 |
| 6.24 | 6-MAP | 310 | 430 | 0.39 | 3.8 |
| 329 (8.51)c | - | - | - |
All four pteridine analogs (Figure 6.3), namely the G analogs (3-MI457 and 6-MI) and A analogs (DMAP and 6-MAP), retain their overall absorption and emission characteristics upon incorporation into oligonucleotides. Significant sequence-dependent quenching has been observed, however, with purines being more effective quenchers than pyrimidines.459 Incorporation of these modified nucleosides, except for 6-MI, typically results in sequence-dependant destabilizing effects similar to that of a single base pair mismatch.460 Nevertheless, these fluorescent nucleosides have found numerous applications and remain very useful due to their high quantum efficiency, well-documented quenching effects and commercial availability (Section 6.7).
6.4. Nucleosides Containing Expanded Nucleobases
Extending the conjugation of the natural nucleobases by fusing additional aromatic rings onto the pyrimidine and purine nuclei generates diverse expanded nucleobases (Figures 6.4 and 6.5). Most retain their W-C hydrogen bonding face (εA being an exception), although their large surface area could structurally perturb the resulting oligonucleotides. Having an extended, aromatic surface typically results in favorable photophysical properties, with red shifted absorption bands compared to their natural counterparts, emission bands near or in the visible range, and rather high emission quantum efficiencies, ranging from 0.2–0.82 (Table 14 and 15).
Figure 6.4.
Examples of expanded nucleobase analogs (R= 2’-deoxyribose, 2’-OMe ribose or ribose and R2=3’,5’-O-TBDMS-2’-deoxyribose).
Figure 6.5.
Expanded nucleobase analogs (R = 2′-deoxyribose).
Table 14.
Spectroscopic Properties of Expanded Nucleoside Analogsa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 6.25 | Etheno-A | Bufferc | 258 (5.0), 265 (6.0), 275 (6.0), 294 (3.1) | 415 | 0.6 | 20 |
| 6.27 | Benzo-Ab | Bufferc | 340, 356 | 358, 379, 395 | 0.44 | - |
| 6.28 | BgQb | Bufferc | 260 (49.8), 292 (16.0), 360 (14.4) | 434 | 0.82 | - |
| 6.29 | Cfb | Bufferc | 370 | 456 | 0.62 | - |
| 6.30 | tC | Bufferd | 375 (4.0) | 500 | 0.17 | 3.7 |
| 6.33 | G-clamp and derivatives | CHCl3 | ~365e | ~450 | - | - |
Table 15.
Spectroscopic Properties of Size Expanded Nucleosidesa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 6.34 | BPPe | Bufferb | 347 | 390 | 0.04 | - |
| 6.35 | NPPe | Bufferb | 365 | 395 | 0.26 | - |
| 6.36 | MDA | Bufferb | 327 | 397, 427 | 0.118 | - |
| 6.37 | MDI | Bufferb | 315 | 442 | 0.117 | - |
| 6.38 | dChpp | Bufferb | 300 | 360 | 0.12 | |
| 6.39 | dChpd | Bufferb | 300 | 360 | - | - |
| 6.40 | dCmpp | Bufferb | 300 | 360 | - | - |
| 6.41 | dCppp | Bufferc | 369 (4.76) | 490 | 0.105 | - |
| 6.42 | dCppi | Bufferc | 374 (4.157) | 513 | 0.006 | - |
| 6.43 | 3-fluoro-dCPPI | Bufferc | 371 (6.284) | 505 | 0.019 | - |
| 6.44 | 3-cyano-dCPPI | Bufferc | 369 (6.998) | 487 | 0.061 | - |
| 6.45 | 3-methoxy-dCPPI | Bufferc | 375 (5.321) | 511 | 0.005 | - |
| 6.46 | 3-methlythio-dCPPI | Bufferc | 372 (4.11) | 511 | 0.002 | - |
| 6.47 | 3-N,N-dimethylamino-dCPPI | Bufferd | 366 (9.05) | 486 | 0.068 | - |
| 6.48 | 3-methylsulfonyl-dCPPI | Bufferc | 367 (7.38) | 484 | 0.078 | - |
| 6.49 | 3-N,N,N-trimethylammoniumiodido-dCPPI | Bufferc | 368 (8.468) | 483 | 0.096 | - |
λ, ε, and τ are given in nm, 103 M−1 cm−1, and ns respectively. All spectra taken in aqueous buffer
pH = 7.0,
pH = 7.4, and
pH = 3.0.
Data from Wilson and Kool.427
Leonard and co-workers first investigated etheno-A (εA, 6.25)462,463 and benzo-A (6.27)464 in the early 1970’s following an initial report showing that adenine and cytosine could be cyclized to produce nucleobases with red shifted absorptions bands.465 While the fused structure of εA, reminiscent of the naturally occurring fluorescent nucleoside wyosine,466 masks the hydrogen bonding face, it also improves the photophysical properties. This is most notably with a red shifted absorption (294 nm) and an intense emission band in the visible (415 nm, Φ = 0.56), which is associated with a rather large stokes shift (9,917 cm−1) and a relatively long lifetime for a small organic chromophore (τ = 20 ns).463,467 εATP, identified as a fluorescent replacement for ATP, is recognized as a substrate by AMP/ATP binding enzymes.463 Seela and co-workers have investigated a 7-deaza analog of εA, which displays a larger Stokes shift and similar quantum efficiency. The 7-deaza εA derivative, however, shows higher stability over a larger range of pHs and can be used to monitor oligonucleotide denaturation.468
Benzo-A (6.27) retains the hydrogen bonding face of adenine. The extended heterocycle is responsible, however, for the significantly improved photophysical properties compared to adenosine. A structural and photophysical comparison of εA and benzo-A reveals the intricacies shown by modified nucleosides. Fusing a benzene ring into the purine core, as in benzo-A (6.27), results in a tremendous red shift of the absorption bands, leading to a rather small stokes shift (∼40 nm) for emission and lower quantum efficiency in comparison to εA. These nucleoside analogs have found unique applications in recent years, beyond the realm of fluorescence-based applications, particularly in exploring size-expanded DNAs.469–475
Two naptho-expanded nucleosides, BgQ (6.28) and Cf (6.29), are relatively recent additions to this class of fluorescent nucleosides (Figure 6.5).476,477 Both analogs display a strong emission band in the visible range (Φ = 0.82, 0.62, respectively), resulting from a red shifted absorption band (360 and 370 nm, respectively).476,477 Due to their desirable photophysical properties and large surface area, BgQ and Cf were employed for the study of double and triple-stranded oligonucleotides (see Section 6.7).
A cytidine analog, tC (6.30), originally synthesized for antisense applications by Matteucci,478 forms W-C like base pairs with guanosine,479 and somewhat surprisingly, does not suffer a dramatic reduction in quantum efficiency upon in corporation into PNA480 or DNA,481 unlike most other fluorescent nucleosides. Like many expanded analogs, tC emits in the visible (500 nm) with a somewhat low quantum efficiency (Φ = 0.17) for this class of nucleobases.480,482–484 Most recently, Millar and Tahmassebi have utilized tC along with a non-fluorescent quencher (TEMPO) to demonstrate the utility of a fluorescent nucleoside/quencher combination.485
Sasaki and co-workers have utilized an emissive expanded base analog (G-clamp), first introduced by Matteucci.486,487 The photophysical properties of G-clamp and derivatives are similar to that of tC, with an absorption maximum around 365 nm and a corresponding emission maximum of 450 nm.488,489 The ability of the protected G-clamp nucleoside (8-oxoG-Clamp) and its derivatives to detect the presence of 8-oxodG have been explored (see Section 6.7).488–490
In 2003, Saito and co-workers introduced Base-Discriminating Fluorescent (BDF) nucleosides, designed primarily for single nucleotide polymorphism (SNP) analysis.491 Such emissive nucleoside analogs can be divided into two main categories: (a) nucleobases with pendent fluorophores (extended nucleobases – see section 6.4) and (b) ring-expanded nucleobases. Benzopyridopyrimidine (BPP, 6.31) is a cytidine analog that forms stable pairs with both A (wobble bp) and G (W-C bp).492 BPP displays an isolated absorption band (347 nm), but its low quantum efficiency (Φ = 0.04) prompted the synthesis of naphthopyridopyrimidine (NPP, 6.35) (Figure 6.5). While showing similar absorption and emission wavelengths as BPP, NPP displays a substantially higher emission quantum efficiency (Φ = 0.26) (Table 15).493 With an acceptable purine discriminating fluorescent nucleoside in hand, Saito and co-workers designed adenosine (MDA, 6.36) and inosine (MDI, 6.37) analogs as pyrimidine discriminating fluorescent nucleosides (Section 6.7).494 Both MDA and MDI have rather large Stokes shifts in comparison to BPP/NPP, resulting from a blue shifted absorption and red shifted emission, although their quantum efficiency remains rather modest (Φ = 0.12).494
Sekine and co-workers have investigated cyclized dC analogs, which maintain the H bonding face of the parent nucleoside, but extend the heterocycle surface by linking the 4 and 5 positions on the pyrimidine core. Early derivatives included dChpp 6.38, dChpd 6.39 and dCmpp 6.40 whose absorption band around 300 nm resulted in an emission near the visible range (375 nm). Extending these bicyclic systems into a tricyclic system (dCppp, 6.41) resulted in a large red shift in both absorption (369 nm) and emission (490 nm).495 The photophysical properties of the further expanded dCppi system (6.42), a family of dC analogs that can be viewed as having a fused indole ring, can be tuned by altering the remote 3 position of the heterocycle.496 These analogs all display very large Stokes shifts (∼ 7,000 cm−1), which grow with increasing polarity from toluene to methanol, and then decrease again with further increase in polarity. A detailed investigation of the solvatochromatic effects of these nucleoside analogs revealed complex trends in the sensitivity of absorption, emission and quantum yields to solvent polarity, suggesting susceptibility to a multitude of factors.496
6.5. Nucleosides Containing Extended Nucleobases
Extended fluorescent nucleoside analogs are distinguished by fluorescent moieties that are linked or conjugated to the natural nucleobases, either via flexible or rigid linkers (Figure 6.6 and Figure 6.7, respectively). Connecting known chromophores via electronically non-conjugating linkers yields nucleoside analogs with photophysical features that are normally very similar to that of the parent fluorophore. Extending the purines and pyrimidines by electronically conjugating them to additional aromatic moieties typically generates a new chromophore with unique, and somewhat unpredictable, photophysical characteristics.
Figure 6.6.
Selected examples of extended base analogs (R1 = 2′-deoxyribose and R2 = 2′-deoxyuridine or 2′,3′-dideoxyuridine).
Figure 6.7.
Examples of extended nucleobase analogs.
Seela and co-workers have diligently investigated the impact of extending the conjugation of 7-deaza-adenosine and 8-aza-7-deaza-adenosine on the photophysical characteristics by attaching functionalized alkenes and alkynes to the 7-position.504,505 Although the absorption spectra of the parent compounds, 7-deaza-adenosine and 8-aza-7-deaza-adenosine, are slightly red shifted in comparison to adenosine (270 nm and 270 nm vs. 260 nm, respectively), they are not emissive, unlike the alkene- and alkyne-conjugated analogs (Figure 6.6).505
Unusual 2-substituted adenosine analogs, 6.52 and 6.53, have been recently explored by Baranger and co-workers (Figure 6.6).506 These probes show dramatic photophysical changes when compared to adenosine, all due to the non-conjugated phenylalkyl substituents.507 While the free nucleosides display very low emission quantum efficiencies and rather long lifetimes (Table 16), incorporation into RNA hairpins results in significant increase in quantum efficiency and slight shortening of the excited state lifetimes. It is worth noting that such enhancement of quantum efficiency upon incorporation into oligonucleotides is very rarely seen in emissive nucleoside analogs, let alone those with such non-conjugated systems.
Table 16.
Spectroscopic Properties of Conjugated Nucleoside Analogsa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 6.50 | 8-aza-7-deaza-7-phenylethynyl | Waterb | 294 | 360 | 0.08 | 0.55 |
| 6.51 | 7-deaza-7-phenylethynyl | Waterb | 296 | 412 | 0.02 | 0.69 |
| 6.52 | 2-phenylpropyl dA | Water | 215, 262 | 385 | 0.011 | 6.22 |
| 6.53 | 2-phenylbutyl dA | Water | 215, 262 | 396 | 0.007 | 7.13 |
| 6.54 | PYU | Bufferc | 341 | 397 | 0.2 | - |
| 6.55 | PYC | Bufferc | 329 | 393 | 0.15 | - |
| 6.56 | DMAP-PYU | Bufferc | 303 | 440 LE, 540 ICT | 0.01 | - |
| 6.57 | 5-(1-pyrenyl)-dU | MeOH | 342 (28.5) | 474 | 0.027 | - |
| 6.59 | 5-(1-pyrenoyl)-dU | MeOH | 357 (6.68) | 395 | 0.002 | - |
| 6.60 | 5-(1-ethynylpyrenyl)-dU (UP) | MeOH | 392 | 400, 424 | - | - |
| 6.66 | Ethynyl-FL | Bufferd | 320, 492 (23.9, 57.0) | 520 | 0.53 | - |
| 6.67 | Phenylethynyl-FL | Bufferd | 322, 492 (39.2, 63.3) | 516 | - | - |
| 6.68 | Ethynylphenylethynyl-FL | Bufferd | 330, 492 (55.0, 63.7) | 520 | - | - |
| 6.71 | MMeU | Water | 350e | 450e | - | - |
| 6.72 | PhU | Water | 320e | 400e | - | - |
| 6.73 | MeOPhU | Water | 330e | 450e | - | - |
| 6.75 | 5(DAN)-dUf | CH2Cl2 | 341 (1.15) | 507 | 0.032 | - |
| 6.76 | 5(DANethylyl-one)-dUf | CH2Cl2 | 410 (2.11) | 532 | 0.041 | - |
| 6.77 | DNCUf | CH2Cl2 | 332 (1.42) | 421 | 0.126 | - |
| 6.78 | PDNUg | Water | 380 | 524 | 0.033 | - |
| 6.82 | danCh | Water | 321 | 459 | - | - |
| 6.84 | 5-aminodansyl-dUi | Water | 330 | 523 | - | - |
| 6.90 | UFLj | CH3Cl | 325 | 405 | 0.139 | - |
| 6.91 | 5(difluoroBODIPY)phenyl-dU | MeOH | 496 (69.3) | 507 | 0.42 | - |
| 6.92 | 5(fluoromethoxyBODIPY)phenyl-dU | MeOH | 497 (69.1) | 508 | 0.42 | - |
| 6.93 | 5(dimethoxyBODIPY)phenyl-dU | MeOH | 498 (70.8) | 509 | 0.47 | - |
| 6.94 | 5(difluorodiethylBODIPY)phenyl-dU | MeOH | 522 (57.5) | 534 | 0.61 | - |
| 6.95 | 5 (fluoromethoxydiethylBODIPY)pheny l-dU |
MeOH | 523 (55.2) | 535 | 0.62 | - |
| 6.96 | 5(dimethoxydiethylBODIPY)phenyl- dU |
MeOH | 523 (53.6) | 535 | 0.59 | - |
λ, ε, and τ are given in nm, 103 M−1cm−1, and ns respectively. All spectra were collected in
doubly distilled water,
aqueous phosphate buffer (pH=7.0)427, or
aqueous phosphate buffer (pH=7.2).
Excitation and emission data are for single-stranded oligomers (13-mer), where the modified nucleoside is located in the center.497
Data from Saito and Okamoto et al.498
Okamoto et al.499
Majima et al.500
Barawkar and Ganesh501
Kim et al.502
Data from Ehrenschwender and Wagenknecht.503
Netzel and co-workers have linked pyrene to the 5-position of 2′-deoxyuridine directly and via an amide or ketone linkages (Figure 6.6).508,509 While primarily developed as tools to evaluate electron transfer processes in DNA, detailed analysis of their photophysical characteristics, including steady state and time-resolved studies provided evidence that such nucleosides can be responsive to changes in their microenvironment.508–510 Similarly, Berlin and co-workers linked pyrene via an ethynyl linkage to the 5-position of dU.511 All pyrene nucleosides share similar photophysical properties with red shifted absorption in comparison to the native nucleobases (342 – 392 nm) and a weak emission band (Φ = 0.002–0.027) near the visible range (395–474 nm). 5-(1-Ethynylpyrenyl)-dU has been utilized by numerous groups for a variety of applications (Section 6.7).510,512,513 The corresponding 8-substituted purines, which can be viewed as rather perturbing analogs, have also found utility (Section 6.7).512,513
In pursuit of fluorescent nucleosides capable of SNP detection, Saito and co-workers have bridged pyrene to 2′-deoxyuridine or cytosine via a propargylamide linker (Figure 6.6). Nucleosides 6.54 and 6.55 retain the hydrogen bonding face of U/C and the typical photophysical properties of pyrene, showing an isolated absorption band at ∼335 nm and a relatively intense emission band around ∼400 nm (Φ = ∼0.2, Table 16). Despite the distance between the pyrene and the pyrimidine core and the non-conjugating linker, these BDFs have been very successful probes for SNP analysis (Section 6.7).491 Modifying the pyrene moiety with a dimethylaminopyridine group (DMAP-PyU, 6.56) generates a probe with dual emission, resulting from either locally excited or charge transfer states populated via a rather energetic absorption band (Table 16).514
While diverse xanthene-type fluorophores (e.g., fluorescein) have been linked to dideoxynucleotides for sequencing applications, only recently has fluorescein been rigidly conjugated to dU and ddU by Burgess (Figure 6.6).515 Upon excitation at 320 nm, nucleoside 6.66, where the fluorophore is conjugated via an ethynyl linkage, emits at 520 nm (Table 16). Extending the rigid linker by a phenyl or a phenylethynyl moiety increases the extinction coefficient of the nucleoside without red shifting the excitation wavelength (Table 6.6). Preparation of the triphosphates for exploring polymerase-based incorporation reactions was accomplished by synthesizing 5-iodo-UTP, followed by cross-coupling reactions with ethynyl-linked fluorescein derivatives. Only the derivative with the longest linker (6.68), either in the deoxy or dideoxy form, showed acceptable levels of enzymatic incorporation, albeit lower than the commonly used rhodamine-based probe (6-TAMRA-ddTTP).515 In addition to the classically multi-color fluorescent nucleoside triphosphates made by flexibly conjugating established fluorophores to nucleobases (e.g., FAM, TAMRA), emissive nucleosides and fluorophore/quencher pairs have been developed for molecular beacon and sequencing applications.516–528
Having shown that the emission of 3- and 3,8-arylethynyl-extended 1,10-phenanthroline derivatives respond to polarity changes,529,530 Tor and coworkers have attached this moiety to the 5-position of dU, using Pd-mediated cross-coupling reactions.531 Nucleoside 6.99.c (dUphen) displays an absorption band at 333 nm, which was insensitive to solvent polarity. Excitation of dUphen results in emission ranging from 385 nm (dichloromethane) to 408 nm (water).531 The sensitivity of this emissive nucleoside to its environment has been used to explore its utility as a SNP probe upon incorporation into oligonucleotides (Section 6.7).531
Utilizing the phenanthroline-extended dU as a core structure, the corresponding polypyridine RuII and OsII containing nucleosides (6.102.h and 6.103.i) were prepared by cross-coupling the brominated metal-containing polypyridyl precursors [e.g., (bpy)2Ru(3-Br-1,10-phen)2+] with 5-ethynyldU.532–534 The electrochemical and photophysical properties of the resulting metal-containing nucleosides were investigated.532–534 The presence of coordinately saturated polypyridine complexes in these nucleosides results in typical visible MLCT absorption bands (∼ 460 nm). The RuII based nucleoside shows a moderately strong luminescence (Φ = 0.137 at 629 nm) and a rather long excited state lifetime (2.8×103 ns), while the OsII containing nucleoside displays a very weak luminescence (Φ = 0.0003 at 749 nm), which is associated with a very short excited state lifetime (78 ns). Incorporation of these nucleosides into oligonucleotides results in minimal duplex destabilization. This facilitated a thorough investigation of donor–acceptor interactions in systematically Ru/Os-modified oligonucleotides.533 It is worth noting that the diasteromerically-pure nucleosides were also synthesized and incorporated into oligonucleotides.534 While the photophysical characteristics of the diastereomerically-pure Δ-6.102.h and Λ-6.102.h nucleosides are essentially identical, analysis of time resolved data suggests the Δ-(bpy)2Ru(phen) metal center is better accommodated within the major groove of a DNA duplex.534
Hocek and co-workers have recently investigated the emissive properties of both pyrimidine and purine analogs with conjugated bipyridine, terpyridine and phenanthroline moieties.535–537 The chelators were attached through ethynyl and phenyl linkages to the 5 position on pyrimidines and the 7 position on 7-deazapurine. The non-metallated nucleoside analogs were prepared via cross-coupling reactions of the ethynyl/phenyl-modified polypyridyl arm with the unprotected halo-nucleosides. These conjugated chromophores show an isolated absorption in the 306–329 nm range, with corresponding emission bands between 389 and 451 nm (Table 17 and Figure 6.7: 6.114–6.125) R = a, b, d and e). Diverse metal-containing nucleoside and nucleotide triphosphates have also been prepared (Figure 6.7).536–538 Enzymatic incorporation of these metal-containing triphosphates (6.101.g, 6.107.g, 6.114.g and 6.119.g), by vent(exo-) and Pwo polymerases, produced modified oligonucleotides that were employed for SNP detection using luminescence (in case of RuII containing oligonucleotides) or electrochemical detection (for OsII containing oligonucleotides) (Section 6.7).536–538 Ru complexes have also been connected through a propargylamide linker to the 5-position of dU, yielding nucleosides with photophysical properties similar to the ones listed above.539,540 Tuning the redox potential of Ru and Os-containing nucleotides has been discussed.541
Table 17.
Spectroscopic Properties of Conjugated Base Analogsa
| # | Name | Solvent | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|---|
| 6.97.a | 5-[6(bpy)ethynyl]dU | MeOH | 316 (31.0) | 389 | <0.01 | - |
| 6.98.b | 5-[5(bpy)ethynyl]dU | MeOH | 327 (39.0) | 421 | 0.16 | - |
| 6.99.c | 5-[3(phen)ethynyl]dU | H2O | 248 (19.0), 260 (18.0), 278 (20.0), 296 (18.0), 330 (20.0), 345 (18.0) |
408 | 0.16 | - |
| 6.100.e | 5-[4(tpy)ethynyl]dU | MeOH | 282 (44.0) | 408 | 0.01 | - |
| 6.101.g | 5-[5-Ru(bpy)3ethynyl]dU-TP | Bufferb | - | 645 | 0.0156 | - |
| 6.102.h | 5-[3(phen)Ru(bpy)2-ethynyl]dU | CH3CN | 242 (40.0), 256 (39.0), 286 (69.0), 352 (26.0), 450 (13.0) |
629 | 0.137 | 2.8×103 |
| 6.103.i | 5-[3(phen)Os(bpy)2-ethynyl]dU | CH3CN | 242 (38.0), 254 (36.0), 290 (64.0), 358 (26.0), 436 (11.0), 484 (11.0) |
749 | 0.0003 | 78 |
| 6.104.a | 5-[6(bpy)ethynyl]dC | MeOH | 326 (21.0) | 397 | <0.01 | - |
| 6.105.b | 5-[5(bpy)ethynyl]dC | MeOH | 329 (54.0) | 435 | 0.11 | - |
| 6.106.e | 5-[4(tpy)ethynyl]dC | MeOH | 283 (37.0) | 445 | 0.02 | - |
| 6.107.g | 5-[5-Ru(bpy)3ethynyl]dC-TP | Buffera | - | 646 | 0.0132 | - |
| 6.108.a | 5-[6(bpy)phenyl]dU | MeOH | 309 (36.0) | 399 | <0.01 | - |
| 6.109.b | 5-[5(bpy)phenyl]dU | MeOH | 315 (53.0) | 437 | 0.29 | - |
| 6.110.e | 5-[4(tpy)phenyl]dU | MeOH | 285 (34.0) | 427 | 0.08 | |
| 6.111.a | 5-[6(bpy)phenyl]dC | MeOH | 286 (39.0) | 389 | <0.01 | |
| 6.112.b | 5-[5(bpy)phenyl]dC | MeOH | 306 (37.0) | 451 | 0.18 | |
| 6.113.e | 5-[4(tpy)phenyl]dC | MeOH | 286 (12.0) | 444 | 0.04 | |
| 6.114.g | 7-[7-deaza-5-Ru(bpy)3- ethynyl]dG-TP |
Bufferb | - | 635 | 0.0111 | - |
| 6.115.a | 7-[7-deaza-6(bpy)ethynyl]dA | CHCl3 | 282 (28.0), 321 (24.0) | 406 | - | - |
| 6.116.b | 7-[7-deaza-5(bpy)ethynyl]dA | CHCl3 | 285 (35.0), 336 (25.0) | 427 | - | - |
| 6.117.f | 7-[7-deaza-6-Ru(bpy)3- ethynyl]dA |
CH3CN | 288 (82.0), 488 (14.0) | 639 | 0.00021 | - |
|
6.118.g [6.119.g] |
7-[7-deaza-5-Ru(bpy)3- ethynyl]dA [TP analog] |
CH3CN [Bufferb] |
254 (36.0), 287 (98.0), 384 (26.0) [−] |
665 [642] |
0.0289 [0.0187] |
- [−] |
| 6.120.a | 7-[7-deaza-6(bpy)phenyl]dA | CHCl3 | 291 (31.0), 325 (30.0) | 395 | - | - |
| 6.121.d | 7-[7-deaza-2(phen)phenyl]dA | CHCl3 | 283 (51.0), 322 (45.0) | 424 | - | - |
| 6.122.e | 7-[7-deaza-4(tpy)phenyl]dA | CHCl3 | 253 (47.0), 284 (54.0) | 425 | - | - |
| 6.123.f | 7-[7-deaza-6-Ru(bpy)3- phenyl]dA |
CH3CN | 245 (33.0), 289 (73.0), 450 (12.0) |
667 | 0.00019 | - |
| 6.124.j | 7-[7-deaza-2(phen)Ru(bpy)2- phenyl]dA |
CH3CN | 287 (71.0), 448 (10.0) | 648 | 0.00043 | - |
| 6.125.k | 7-[7-deaza- 4(tpy)Ru(tpy)phenyl]dA |
CH3CN | 273 (51.0), 308 (64.0), 485 (23.0) |
633 | 0.00024 | - |
λ, ε, and τ are given in nm, 103 M−1cm−1, and ns respectively
Buffer = 3.3 mM Tris-Cl, pH=8.5.
6.6. Isomorphic Nucleobases
Isomorphic nucleobase analogs are heterocycles that closely resemble the corresponding natural nucleobases with respect to their overall dimensions, hydrogen bonding patterns, and ability to form isostructural W-C base pairs (Figures 6.8 and 6.9). A clear advantage of these analogs is their strong similarity to the native nucleosides and minimally perturbing nature, when compared to the diverse analogs discussed above. Since favorable photophysical characteristics (e.g., red shifted absorption and high emission quantum efficiencies) are typically associated with significant structural perturbation and extended conjugation, isomorphic fluorescent nucleosides are the most challenging to design.
Figure 6.8.
Examples of isomorphic nucleobase analogs, where R = 2′-deoxyribose or ribose.
Figure 6.9.
Examples of isomorphic nucleobase analogs.
2-aminopurine (2-AP, 6.126), one of the first and most widely utilized fluorescent nucleosides, is a constitutional isomer of adenine with substantially enhanced photophysical features (Figure 6.8, Table 18). Since the initial publication in 1969 describing its fluorescence properties as a nucleoside or within oligonucleotides,550 2-AP has been reported in more than 1,600 contributions. The seminal paper by Reich and Stryer suggests 2-AP to be an ideal emissive nucleoside analog. Its ability to form W-C like base pairs with dT/U, high quantum efficiency (Φ = 0.68 in water), isolated absorption band (303 nm), minimal sensitivity to pH changes, and importantly, sensitivity to environmental polarity, all contribute to its great utility.550 Specifically, 2-AP’s emission, and to a lesser extent its absorption, undergo a bathochromic shift with increasing solvent polarity.551 Interestingly, 2,6-diaminopurine and formycin, two related emissive adenosine analogs (Figure 6.8), display substantially lower quantum efficiencies (0.01 and 0.06, respectively, Table 18).550 Other variations of 2-AP, including 7-deaza and 8-aza-7-deaza, have been studied by Seela and co-workers.552 While these analogs display larger Stokes shifts, their emission quantum efficiencies are lower in comparison to 2-AP (0.47 and 0.53 vs. 0.68, respectively).552
Table 18.
Spectroscopic Properties of Isomorphic Nucleoside Analogs in Watera
| # | Name | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|
| 6.126 | 2-aminopurine | 303 (6.8) | 370 | 0.68 | 7.0 |
| 6.127 | 2,6-diaminopurine | 280 (10.0b) | 350 | 0.01 | - |
| 6.128 | formycin | 295 (0.8c) | 340 | 0.06 | <1 |
| 6.129 | 8-vinyl-6-aminopurined | 290 (12.6) | 382 | 0.66 | 4.7 |
| 6.130 | 2-amino-6-(2-thienyl)purinee | 297 (9.9), 348 (14.0) | 434 | 0.41 | - |
| 6.131 | 2-amino-6-(2-thiazolyl)purinee | 297 (8.8), 359 (9.6) | 461 | 0.46 | - |
| 6.132 | 5-methyl-2-pyrimidinone (m5K) | 280 | 400 | - | 4.09f |
| 6.133 | Pyrrolo-dC (pC)e | 350 (5.9)d | 460 | 0.2 | - |
| 6.138 | 5-(fur-2-yl)dU | 316 (11.0) | 431 | 0.03 | 1.0 |
| 6.139 | 5-(fur-2-yl)U | 254 (12.3), 316 | 440 | 0.035 | - |
| 6.140 | 5-(thiophen-2-yl)dU | 314 (9.0) | 434 | 0.01 | - |
| 6.141 | 5-(thiophen-2-yl)U | 315 (8.7) | 439 | 0.024 | - |
| 6.142 | 5-(oxazol-2-yl)dU | 296 (10.0) | 400 | <0.01 | - |
| 6.143 | 5-(thiazol-2-yl)dU | 316 (11.5) | 404 | <0.01 | - |
| 6.144 | 5-(fur-2-yl)dC | 310 (5.0) | 443 | 0.02 | - |
| 6.145 | 8-(fur-2-yl)A | 304 (18.0) | 374 | 0.69 | - |
| 6.146 | 8-(fur-2-yl)G | 294 (16.0) | 378 | 0.57 | - |
| 6.147 | 2’-deoxyisoinosineg | 242 (2.9), 314 (4.6), | 382 | - | - |
| 6.148 | phenyl-UDP-Glc | 278 | 403 | - | - |
| 6.149 | 4-methoxyphenyl-UDP-Glc | 278 | 444 | - | - |
| 6.150 | 4-chlorophenyl-UDP-Glc | 281 | 398 | - | - |
| 6.151 | 2-furyl-UDP-Glc | 314 | 437 | - | - |
| 6.152 | 8-(benzyltriazol-4-yl)Ah | 290 (16.0) | 344 | 0.64 | - |
| 6.153 | 8-(phenylethyltriazol-4-yl)Ah | 289 (21.0) | 342 | 0.63 | - |
| 6.154 | 8-(pyridin-4-ylmethyltriazol-4-yl)Ah | 289 (16.0) | 346 | 0.49 | - |
| 6.155 | 8-(isopentyltriazol-4-yl)Ah | 289 (18.0) | 343 | 0.62 | - |
| 6.156 | 8-(pentyltriazol-4-yl)Ah | 289 (17.0) | 342 | 0.62 | - |
| 6.157 | 8-(3-aminophenyltriazol-4-yl)Ah | 296 (24.0) | 402 | 0.38 | - |
| 6.158 | 8-(4-methoxyphenyltriazol-4-yl)Ah | 294 (23.0) | 370 | 0.03 | - |
| 6.159 | 8-(4-tolyltriazol-4-yl)Ah | 294 (21.0) | 368 | 0.05 | - |
| 6.160 | 8-(4-chlorophenyltriazol-4-yl)Ah | 294 (21.0) | 396 | 0.05 | - |
| 6.161 | N-Thieno[3,2-d]-dRi | 293 | 350 | 0.02 | - |
| 6.162 | N-Thieno[3,2-d]-R | 292 | 351 | 0.058 | - |
| 6.163 | 4-Thieno [3,2-d]-dR | 294 | 351 | 0.037 | - |
| 6.164 | Thieno[3,4-d]-U | 304 (3.65) | 412 | 0.48 | - |
| 6.165 | BTUj | 333, 344 | 383 | 0.48 | - |
| 6.166 | 5-aza-7-deazapurine-2’-deoxyribosidek | 250 | 410,500 | - | - |
| 6.167 | Quinazolinedione | 307l (2.34) 306m (2.07) |
370 354 |
0.31 - |
- - |
| 6.168 | 5-MeOQuinazolinedione | 320l (6.27) 314m (6.13) |
395 362 |
0.16 - |
- - |
| 6.169 | 3-MeOQuinazolinedione | 298l (6.33) 297m (5.00), 306m (4.82) |
356 335 |
0.08 - |
- - |
λ, ε, and τ are given in nm, 103 M−1cm−1, and ns respectively.
For ε282542
For ε318543 Spectra were collected in buffer
pH=7.5 (HEPES) and
pH=7.0.
For singly modified oligonucleotides; stated to be similar to the free nucleoside.544
Data from Seela and Chen.545
Data from Grotli et al.546
Data from Seaman.547
Data was collected in MeOH.548
Data was collected in MeCN.549
Data was collected in phosphate buffer (pH=7.0),
Data collected in dioxane.
While 2-AP pairing with T/U does not disturb the secondary structure of either A- or B-form DNA/RNA,553 it can also pair with cytosine in various forms depending upon the pH.554–556 When incorporated into oligonucleotides, 2-AP’s emission is significantly quenched. This phenomenon, which has been exploited in numerous assays (see section 6.7), is sequence dependent. Adding to its complex photophysics, energy transfer processes have been documented for 2-AP containing duplexes.557 A is the most efficient donor among the native nucleobases, while energy transfer from C/T or G is very inefficient, except in one particular case, when 2-AP is found at the end of a G pentamer.558–561 Numerous theoretical and experimental approaches have probed the electronic structure of 2-AP and the origin of its unique photophysical characteristics.562–566
Substituting the hydrogen at the 8 position of adenine with a vinyl moiety results in remarkable photophysical changes compared to the parent nucleobase.567,568 Upon excitation of 8-vinyl-2′-deoxyadenosine (8vdA, 6.129) at its absorption maximum (290 nm), an intense emission is observed at 382 nm. 8vdA, thus, exhibits a significantly larger Stokes shift (8300 cm−1) compared to 2-AP (5970 cm−1) with a comparable quantum yield (Φ = 0.66).567 The emission of 8vdA was shown to be responsive to changes in temperature and solvent, while insensitive to pH changes (between 5–10), displaying desirable properties as a probe (see Section 6.7). Incorporation into oligonucleotides showed sequence dependent, albeit minimal, disruption to duplex stability.567 Much like 2-AP, the intense emission of 8vdA is quenched upon incorporation into oligonucleotides, albeit to a lesser extent.567
Hirao and co-workers have demonstrated a site-specific fluorescent labeling of RNA via an unnatural base pair, in which both components are fluorescent nucleobases analogs.569–571 The two purine analogs, 2-amino-6-(2-thienyl)purine and 2-amino-6-(2-thiazolyl)purine, are isomorphic fluorescent nucleosides that can be viewed as 2-AP derivatives. The incorporation of a thiophene or thiazole ring to the 6-position results in a red shifted absorption band in comparison to 2-AP (∼355 nm) and displays a strong emission (Φ ≈ 0.4) in the visible range (∼450 nm).571 The pyrimidine pairing partner was an extended nucleobase analog where known chromophores (FAM, TAMRA and Dansyl) were attached via a linker to the 5-position of a 2-pyrimidinone core.570 This unnatural base pair facilitates enzymatic labeling of RNA for various applications (Section 6.7). In this context, it is useful to comment on additional novel base pairs, including the well-studied isoG–isoC system, as they facilitate the incorporation of various emissive analogs (Figure 6.8).572–579 Some of these derivatives, developed primarily to expand the genetic alphabet, including 5-aza-7-deazapurine-2’-deoxyriboside549, are emissive.
5-methyl-2-pyrimidione (s or m5K, 6.132) has been explored as a T analog (Figure 6.8).580,581 Its synthesis and incorporation into oligonucleotides were initially reported in the late 1980’s.582,583 Early photophysical studies described its isolated absorption (280 nm), and time resolved data were used to probe its stacking ability in a single stranded oligonucleotide (Table 18).544 The recent interest in d5 as a non-natural base for enzymatic incorporation584–586 or as a base in triple helix motifs,587,588 and not as a fluorescent probe, stems likely from its inability to form an adequate W-C base pair with adenine.
Tor and co-workers have developed a series of isomorphic fluorescent T/C analogs by conjugating aromatic five membered heterocycles at the pyrimidine’s 5-position (6.138–6.144, Figure 6.8).589–591 The absorption spectra of each modified nucleoside reveals, in addition to the typical pyrimidine transitions, a lower energy absorption band (∼310 nm) that remains practically unchanged upon changing solvent polarity. In contrast, the emission profile of the conjugated nucleosides is much more sensitive to the chromophore’s microenvironment, resulting in both bathochromic and hyperchromic shifts upon increasing solvent polarity.589–591 These nucleosides have very large Stokes shifts (8,400–9,700 cm−1) for such small organic chromophores, while their quantum efficiency is relatively low (Φ = 0.01–0.035).589–591 The responsive furan and thiophene analogs (6.138, 6.140, respectively), having the most desirable properties in terms of emission wavelength, quantum yield and sensitivity to microenvironmental polarity, were selected as probes (Section 6.7). These nucleosides can be incorporated into oligonucleotides using either solid-phase or enzymatic syntheses,592–594 causing no destabilization of the resulting oligonucleotides.589–591,595 Their simple synthesis and useful properties have facilitated diverse applications, including the detection of abasic sites and the monitoring of RNA–ligand interactions (Section 6.7).11,589,591,595,596
Incorporating a furan ring at the 8-position of dG and dA resulted in highly emissive nucleosides with remarkably different properties when compared to their pyrimidine cousins.590 The purine analogs (6.145 and 6.146) lack a separate absorption band, but instead, display one major red shifted transition around ∼300 nm, which is largely unaffected by changes in solvent polarity.590 In contrast to the corresponding modified pyrimidine analogs, the substituted purines display a very strong emission centered around 375 nm (Φ = 0.69 and 0.57 for 6.145 and 6.148, respectively),590 which displays limited susceptibility to changes in solvent polarity.
Tor and co-workers have also investigated fused thiophene derivatives (6.161–6.164), where the fusion position of the thiophene ring shows a striking effect on the photophysical properties of the resulting analogs.597,598 The [3,2] isomeric nucleosides, prepared as both a pyrimidine and purine analog (N-nucleoside 6.161/162 and C-nucleoside 6.163, respectively), show an absorption at ∼ 290 nm with a corresponding weak emission centered at ∼350 nm (Φ = 0.02 – 0.058).598 In contrast, the isomeric [3,4] analog (6.164), while displaying a similar absorption band (304 nm), gives rise to a strong and red shifted visible emission (412 nm, Φ = 0.48).597 The most important property of these fused analogs is the sensitivity of their photophysical parameters to polarity. When incorporated into oligonucleotides and hybridized to perfect complements, the highly responsive analog 6.164 displayed significant emission quenching. In contrast, substantial fluorescence enhancement was observed when the modified oligonucleotide was hybridized to complementary oligonucleotides that contain an abasic site opposite the reporter. This key observation inspired the development of a new fluorescence-based approach for monitoring the depurination activity of toxic Ribosome Inactivating Proteins (see Section 6.8).599
A series of expanded isomorphic U analogs based on a quinazoline-2,4(1H,3H)-dione core (6.167) has recently been introduced by Tor and coworkers (Figure 6.8, Table 18).600 Among the analogs 6.167–6.169, the 5-methoxyquinazoline-2,4(1H,3H)-dione derivative (6.168, Φ = 0.16) was found to be an ideal FRET donor for chromophores derived from 7-diethylaminecoumarine-3-carboxylic acid (with a critical Förster radius R0 of 27 Å). The pair was used to devise a robust analysis and discovery platform for antibiotics targeting the bacterial rRNA A-site, by placing the new emissive U surrogate into the RNA construct and labeling the aminoglycosides with the coumarine chromophore.600
PyrroloC (pC, 6.133), an emissive C analog, was originally discovered following ammonolysis of furo[2,3-d]pyrimidinone, a side product in the Pd-mediated Sonogashira coupling reactions of terminal alkynes with 5-iodo-U.601–603 Initial investigations were focused on the biological activity of the furo and pyrrolo nucleosides analogs.604,605 Once the fluorescent properties of pC were recognized, its potential utility as a fluorescent probe became clear.20,606 PyrroloC’s low energy absorption (350 nm) is considerably isolated from that of the native nucleobases. Its visible emission (460 nm) is reasonably intense (estimated as Φ = 0.2), 607 although it appears to be significantly quenched upon incorporation into single stranded oligonucleotides and further quenched upon duplex formation.20,607 These favorable photophysical properties, along with its minimally perturbing structure, have resulted in a variety of applications (Section 6.7). Modified pC analogs have also been developed and implemented in recent years.608–611
Wagner and co-workers have recently developed a series of emissive 5-substituted UDP glucose analogs, whose design was inspired by Tor and co-workers’ original reports.612 Their method relies on the conversion of the U nucleus to an emissive analog at a late stage of the synthesis, thereby facilitating the preparation of a number of analogs for diverse applications (Figure 6.8). A range of absorption (278–314 nm) and emission (403–444 nm) maxima can be obtained by varying the 5-Aryl moiety (Table 18). The fluorescent properties of small isomorphic analogs, such as those containing the 2-furyl moiety are unaffected by substituents attached to the 5′ hydroxyl (Table 18, 6.139 vs. 6.151). These isomorphic analogs are currently being used to develop assays for monitoring glycosyltransferases activity.612
Lastly, we discuss a series of nucleobase analogs that, although have not been incorporated into oligonucleotides, show intriguing properties. Castellano and co-workers have used the purine nucleus as a scaffold for the placement of diverse donor and acceptor groups, generating “push-pull” purines (Figure 6.9).613,614 The basic design consists of attaching amino, methylamino and dimethylamino donor moieties to the 2 and 6 positions, while placing cyano, methyl ester and carboxyamide acceptor groups at the 8 position. These substituted purines show certain desirable photophysical properties when compared to their acceptor-free analogs, including red shifted absorption maximum (20–50nm), enhanced quantum efficiency (approaching unity) and solvatochromatic effects (Table 19). While such analogs might be too perturbing for incorporation into nucleic acids, they may find utility in material sciences and biosensing applications.613,614
Table 19.
Spectroscopic Properties of Isomorphic Nucleoside Analogs in Dichloromethanea
| # | Name | λmax (ε) | λem | Φ | τ |
|---|---|---|---|---|---|
| 6.170 | 2-amino | 304 (7.9) | 357 | 0.20 | - |
| 6.171 | 2-methylamino | 317 (6.3) | 386 | 0.25 | - |
| 6.172 | 2-dimethylamino | 330 (6.3) | 393 | 0.76 | - |
| 6.173 | 2-amino-6-O-benzyl | 281 (10.0) | 360 | 0.033 | - |
| 6.174 | 2-amino-6-dimethylamino | 286 (12.6) | 351 | 0.013 | - |
| 6.175 | 2-dimethylamino-6-amino | 299 (7.9) | 360 | 0.12 | - |
| 6.176 | 2,6-tetramethylamino | 297 (12.6) | 392 | 0.033 | - |
| 6.177 | 2-amino-8-carbonitrile | 326 (1.6) | 371 | 0.20 | 0.5 |
| 6.178 | 2-methylamino-8-carbonitrile | 341 (6.3) | 383 | 0.58 | - |
| 6.179 | 2-dimethylamino-8-carbonitrile | 361 (15.8) | 429 | 0.90 | 4.25 |
| 6.180 | 2-amino-6-O-benzyl-8-carbonitrile | 311 (20.0) | 355 | 0.81 | 3.1 |
| 6.181 | 2-amino-6-dimethylamino-8-carbonitrile | 324 (15.8) | 375 | 0.30 | 1.6 |
| 6.182 | 2-dimethylamino-6-amino-8-carbonitrile | 336 (12.6) | 387 | >0.95 | 3.2 |
| 6.183 | 2,6-tetramethylamino-8-carbonitrile | 348 (25.1) | 388 | 0.20 | - |
| 6.184 | 2-amino-8-carboxylate | 328 (12.6) | 379 | 0.42 | 1.8 |
| 6.185 | 2-methylamino-8- carboxylate | 345 (1.6) | 403 | 0.65 | - |
| 6.186 | 2-dimethylamino-8- carboxylate | 362 (12.6) | 433 | 0.81 | 3.37 |
| 6.187 | 2-amino-6-O-benzyl-8- carboxylate | 315 (20.0) | 371 | >0.95 | 2.5 |
| 6.188 | 2-amino-6-dimethylamino-8- carboxylate | 330 (15.8) | 393 | >0.95 | 3.1 |
| 6.189 | 2-dimethylamino-6-amino-8- carboxylate | 338 (12.6) | 409 | >0.95 | 3.3 |
| 6.190 | 2,6-tetramethylamino-8- carboxylate | 351 (20.0) | 409 | 0.90 | 2.67 |
| 6.191 | 2,6-tetramethylamino-8- butylcarboxamide | 338 (15.8) | 402 | 0.87 | 2.27 |
| 6.192 | 2,6-tetramethylamino-8-butylcarboxamide | 343 (20.0) | 407 | 0.91 | - |
λ, ε, and τ are given in nm, 103 M−1 cm−1, and ns respectively.
6.7. Incorporation of Modified Nucleosides into Oligonucleotides
A number of approaches, including solid-phase assisted synthesis, enzymatic incorporation, and post synthetic modification are available to arrive at fluorescent oligonucleotides.615–617 The first two approaches are most relevant to the fluorescent nucleoside probes discussed herein.
Most popular and versatile is the incorporation of modified nucleosides into oligonucleotides using solid-phase assisted synthesis, which facilitates modification of oligonucleotides at virtually any position in any sequence. The predominantly employed techniques rely on a 3’-phosphoramidite group for the introduction of the phosphate group in both DNA and RNA oligonucleotides.618–620 For DNA, the standard 5’-dimethoxytrityl (DMTr) group and acyl or N,N-dimethylformamidine621 protection of the exocyclic amines is typically utilized. RNA oligonucleotide synthesis requires additional protection of the 2’-hydroxyl group. Two “modern” approaches are available: (i), the 5'-O-DMTr-2'-O-[(trisisopropylsilyl)-oxy]methyl (2'-O-TOM) protecting scheme developed by Pitsch,622 and (ii) the 5'-O-Silyl-2'-O-orthoester (2’-ACE) protecting group scheme, introduced by Scaringe and Caruthers.623,624 The cycle employed in the standard solid-phase phosphoramidite chemistry is shown in Figure 6.10. Key steps include: (1) acidic removal of the 5’-DMT protecting group of the solid-supported nucleoside, (2): mild acidic activation of the phosphoramidite (e.g., using 1H-tetrazole), (3) coupling of the available 5’-hydroxyl to the activated phosphoramidite, (4) oxidation of the trivalent phosphorous to the pentavalent phosphorus, and (5) capping of unreacted hydroxyl groups. This cycle continues until termination and removal of the full-length oligonucleotide from the solid support, typically under basic/nucleophilic conditions (e.g., ammonium hydroxide).
Figure 6.10.
Common cycle for solid-phase assisted phosphoramidite oligonucleotide synthesis.
Alternatively, enzymatic incorporation of fluorescent nucleotides can, in certain cases, be utilized. Unlike the almost universal solid-phase assisted synthesis, enzymatic incorporation has to be evaluated on a case by case as DNA and RNA polymerases of different origin display diverse tolerance levels to unnatural nucleotides.483,594 Conversion of the modified nucleosides into the corresponding triphosphate is, of course, necessary for implementation of this approach.575,591,593,625–627 Fortunately, the synthesis of many triphosphates does not require any protection of the exocyclic groups and is performed in a one-pot/two steps procedure following established methods.628–630 Additionally, enzymatic incorporation of fluorescent nucleotides has been achieved through non-natural base-pairs,569–575,631–635 the use of terminal transferases636,637 or a reactive amino group on the recently incorporated nucleoside able to undergo conjugation with an appropriately selected tag.638,639
6.8. Applications of Fluorescent Nucleosides
Fluorescent nucleosides have greatly contributed to our still growing understanding of nucleic acid folding, structure, recognition and function. While numerous review and overview articles have previously appeared,427,455,456,467,491,640–645 capturing over 40 years of fluorescent nucleoside research is clearly a daunting and almost impossible task. We have therefore classified below the main areas where fluorescent nucleoside analogs have found utility. Table 20 lists the various nucleoside analogs developed over the years and their main applications. It is worth noting that “classical” emissive nucleosides, such as 2-AP (6.123), reported four decades ago by Reich and Stryer, are still finding vast utility in contemporary biophysics, while others, such as benzo-A (6.123), originally reported by Leonard, are finding new applications distinctly different from their original use.469,470
A. Single Nucleotide Polymorphism (SNP) Detection
The detection of single base substitutions located either within or outside a gene has attracted attention due to their relevance to human health and ultimately for the development of personalized medicine.516,646–650 To identify SNPs, emissive oligonucleotides, complementary to the domain of interest, are hybridized to their target DNA. The fluorescent nucleoside probes are typically placed across from the base of interest, yielding, under ideal circumstances, markedly different signals depending upon their pairing partner. Saito and co-workers have named such fluorescent nucleosides, Base Discriminating Fluoroscent (BDF) nucleosides.494,651
B. Nucleic acid structure and function
Nucleic acids can be found in different forms, aggregated states and polymorphs, which could be related to a specific cellular function.652,653 Appropriately selected fluorescent nucleosides, strategically placed within the nucleic acid strands, can photophysically signal hybridization, folding and conformational changes. Additionally, fluorescent nucleoside analogs have been used to fabricate assays that monitor enzymes operating on nucleic acids (e.g., polymerases) or nucleic acid-based enzymes (e.g., ribozymes).
C. Nucleic acid microenvironment
Nucleic acids experience a variety of reversible and irreversible perturbations, which may include, nucleobase damage, depurination/depyrimidination events or base flipping. Fluorescent nucleoside analogs that are sensitive to their local microenvironment have become powerful tools for investigating these perturbations. In addition, emissive fluorescent nucleosides have been employed to assess the polarity of nucleic acid grooves.
D. Ligand binding
Responsive fluorescent nucleoside analogs find utility as reporters in diverse DNA and RNA discovery or biophysical assays, particularly for monitoring ligand binding (both high and low molecular weight). Special attention is given to isomorphic/isosteric nucleosides, which are unlikely to perturb the native fold and recognition patterns characteristics of the target of interest.
E. Miscellaneous
Applications that do not fall into the above categories are grouped here. When possible, a footnote is added to the table to list the specific utilization of the probe.
7. Epilogue
Biochemists and biophysicists have long relied on fluorescence-based techniques to explore the fundamental structural, folding, recognition and reactivity characteristics of biomolecules, as well as their cellular localization and dynamics. While this review encompasses fluorescent analogs of all major biomolecular building blocks (lipids, monosaccharides, amino acids and nucleosides), it is apparent that distinct approaches have to be implemented for each category. This ultimately reflects the fundamental burden of their chemical structure and the tolerance level of the relevant biological context. In an ideal situation, an emissive analog of any naturally occurring biomolecular building block should closely resemble its natural counterpart and retain the original function (analogs we refer to as isosteric or isomorphic). As most parent, naturally-occurring molecules (excluding a few amino acids) do not display appreciably useful fluorescence properties, structural alteration is necessary to impart such features. This predicament ultimately leads to fluorescent analogs of diverse range in utility and applications, some having a rather limited scope.
As apparent from this review, not all applications require the strict imposition of isomorphic design criteria. Furthermore, the different chemical nature of the distinct families of biomolecular building blocks inherently controls the possible structural and electronic changes. As evident from the size of the last section discussing fluorescent nucleoside analogs, the heterocyclic nucleobases provide a fertile platform for modifications that easily alter the photophysical characteristics. This also holds true for certain aromatic amino acids. In contrast, turning phospholipids or monosaccharides into emissive analogs require rather creative sometimes drastic modifications, with saccharides being viewed as the most limiting in this respect.
Inspiration for emissive analogs had come, in many cases, from Nature. In other cases, rational approaches have been implemented, the simplest being the conjugation of established fluorophores to the biomolecular core. Viewing the data summarized here and the challenge of designer fluorophores from a fundamental physical organic chemistry perspective, it is apparent that predicting the emissive properties of small organic molecules based on their structure is, at this stage, unrealistic. Probe design and implementation remain, for the most part, an empirical task. Very few chromophores have enjoyed a systematic and thorough exploration of their properties by experimentalists, as well as theoreticians. Even 2-AP, one of the most commonly used and investigated isomorphic fluorescent nucleoside, does not always function optimally within oligonucleotides. This highlights certain fundamental challenges in this field, as the photophysical properties of any emissive biomolecular building block are further impacted when embedded within macromolecules by rigidification, de-solvation, and excited-state processes involving neighboring chromophores.
We do not end, however, on a pessimistic note. Rather, we view these challenges as stimulating and past accomplishments as a celebration of creativity. We note that this rather extensive review reflects multiple decennia of development. The numerous publications are testimony to the power of fluorescent spectroscopy in unraveling the intricacies of biological macromolecular structures themselves and their interaction with their complex environment. The great number of recent articles cited indicates that the field is blooming, and many more advancements are to be expected. The future of this colorful field is clearly bright! New ingenious probes, reflecting seemingly endless creativity, will always be embraced by chemists, biologists and biophysicists.
Acknowledgments
We are grateful to the National Institutes of Health (particularly to NIGMS, grant number GM069773) for generous support. We thank Yun Xie, Lisa McCoy, and Dr. Beth Wilson for carefully proofreading the manuscript.
Biographies

Renatus W. Sinkeldam earned his Ph.D. in 2006 under the supervision of Professor E. W. Meijer and Dr J. A. J. M. Vekemans at Eindhoven University of Technology in The Netherlands, for his work on the design, synthesis, and spectroscopic analysis of small organic dyes and helical foldamers. He is currently a postdoctoral fellow in the group of Professor Yitzhak Tor at the University of California, San Diego. His work focuses on isomorphic fluorescent nucleosides and their incorporation into DNA oligonucleotides. His main research interests include the design, synthesis, and spectroscopic evaluation of molecular probes that are responsive to environmental parameters, and their application in supramolecular and biological molecules.

Nicholas J. Greco, having earned his B.S. in Chemistry from Stonehill College (Easton, MA) in 2002, joined the laboratory of Professor Yitzhak Tor at the University of California, San Diego. In 2008, he earned his Ph.D. for his work on the design, synthesis and incorporation of novel fluorescent nucleosides. After a postdoctoral stay at Boston College in the laboratory of Professor Larry W. McLaughlin, he moved to Western Connecticut State University where he is currently an Assistant Professor of Chemistry. His main research interests include novel nucleosides and their effect on the structure and function of biological systems.

Yitzhak Tor carried out his doctorate work at the Weizmann Institute of Science earning his Ph.D. in 1990. After a postdoctoral stay at the California Institute of Technology, he took his first faculty position at the University of Chicago. In 1994, he moved to the University of California, San Diego, where he is currently a Professor of Chemistry and Biochemistry and a Traylor Scholar in Organic Chemistry. His research interests include chemistry and biology of nucleic acids, the development of novel antiviral and antibacterial agents, the mechanisms and applications of low MW cellular delivery agents and the discovery and implementation of fluorescent nucleoside analogs.
References
- 1.Sameiro M, Goncalves T. Chem. Rev. 2009;109:190. doi: 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]
- 2.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd ed. New York: Springer; 2006. [Google Scholar]
- 3.Bacia K, Schwille P. Methods. 2003;29:74. doi: 10.1016/s1046-2023(02)00291-8. [DOI] [PubMed] [Google Scholar]
- 4.Valeur B. Molecular Fluorescence, Principles and Applications. Weinheim: Wiley-VCH Verlag GmbH; 2002. [Google Scholar]
- 5.Turro NJ. Modern Molecular Photochemistry of Organic Molecules. New York: University Science Books; 2009. [Google Scholar]
- 6.Lippert EZ. Elektrochem. 1957;61:962. [Google Scholar]
- 7.Mataga N, Kaifu Y, Koizumi M. Bull. Chem. Soc. Jpn. 1956;29:465. [Google Scholar]
- 8.Waka Y, Mataga N, Tanaka F. Photochem. Photobiol. 1980;32:335. doi: 10.1111/j.1751-1097.1983.tb04507.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BE, McAnaney TB, Park ES, Jan YN, Boxer SG, Jan LY. Science. 2002;296:1700. doi: 10.1126/science.1069346. [DOI] [PubMed] [Google Scholar]
- 10.Sundd M, Robertson AD. Nat. Struct. Biol. 2002;9:500. doi: 10.1038/nsb0702-500. [DOI] [PubMed] [Google Scholar]
- 11.Sinkeldam RW, Greco NJ, Tor Y. ChemBioChem. 2008;9:706. doi: 10.1002/cbic.200700714. [DOI] [PubMed] [Google Scholar]
- 12.Reichardt C. Chem. Rev. 1994;94:2319. [Google Scholar]
- 13.Sinkeldam RW, Tor Y. Org. Biomol. Chem. 2007;5:2523. doi: 10.1039/b707719j. [DOI] [PubMed] [Google Scholar]
- 14.Stern O, Volmer M. Phys. Z. 1919;20:183. [Google Scholar]
- 15.Lehrer SS. Biochemistry. 1971;10:3254. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- 16.Perrin F. C.R. Hebd. Seances Acad. Sci. 1924;178:1978. [Google Scholar]
- 17.Michalet X, Weiss S, Jager M. Chem. Rev. 2006;106:1785. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matyus L, Szollosi J, Jenei A. J. Photochem. Photobiol. B. 2006;83:223. doi: 10.1016/j.jphotobiol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Silvius JR, Nabi IR. Mol. Membr. Biol. 2006;23:5. doi: 10.1080/09687860500473002. [DOI] [PubMed] [Google Scholar]
- 20.Tinsley RA, Walter NG. RNA. 2006;12:522. doi: 10.1261/rna.2165806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Förster T. Ann. Phys. 1948;437:55. [Google Scholar]
- 22.Schuler B, Eaton WA. Curr. Opin. Struct. Biol. 2008;18:16. doi: 10.1016/j.sbi.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziv G, Haran G. J. Am. Chem. Soc. 2009;131:2942. doi: 10.1021/ja808305u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prinz A, Reither G, Diskar M, Schultz C. Proteomics. 2008;8:1179. doi: 10.1002/pmic.200700802. [DOI] [PubMed] [Google Scholar]
- 25.Li PTX, Vieregg J, Tinoco I. Annu. Rev. Biochem. 2008;77:77. doi: 10.1146/annurev.biochem.77.061206.174353. [DOI] [PubMed] [Google Scholar]
- 26.Kelbauskas L, Woodbury N, Lohr D. Biochem. Cell Biol. 2009;87:323. doi: 10.1139/O08-126. [DOI] [PubMed] [Google Scholar]
- 27.Merzlyakov M, Li E, Hristova K. Biointerphases. 2008;3:FA80. doi: 10.1116/1.2912096. [DOI] [PubMed] [Google Scholar]
- 28.Lentz BR. Chem. Phys. Lipids. 1993;64:99. doi: 10.1016/0009-3084(93)90060-g. [DOI] [PubMed] [Google Scholar]
- 29.Santos NC, Prieto M, Castanho M. Biochim. Biophys. Acta, Biomembr. 2003;1612:123. doi: 10.1016/s0005-2736(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 30.Bucci E, Steiner RF. Biophys. Chem. 1988;30:199. doi: 10.1016/0301-4622(88)85017-8. [DOI] [PubMed] [Google Scholar]
- 31.Yan YL, Marriott G. Curr. Opin. Chem. Biol. 2003;7:635. doi: 10.1016/j.cbpa.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 32.LiCata VJ, Wowor AJ. Biophysical Tools for Biologists: Vol 1 in Vitro Techniques. 2008;Vol. 84:243. [Google Scholar]
- 33.Anderson BJ, Larkin C, Guja K, Schildbach JF. Methods Enzymol. Vol. 450. New York: Academic Press; 2008. p. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molitoris BA, Sandoval RM. Adv. Drug Delivery Rev. 2006;58:809. doi: 10.1016/j.addr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Benninger RKP, Hao M, Piston DW. Rev. Physiol., Biochem. Pharmacol. 2008;160:71. doi: 10.1007/112_2008_801. [DOI] [PubMed] [Google Scholar]
- 36.Kapanidis AN, Strick T. Trends Biochem. Sci. 2009;34:234. doi: 10.1016/j.tibs.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Bagatolli LA. Biochim. Biophys. Acta, Biomembr. 2006;1758:1541. doi: 10.1016/j.bbamem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Diaspro A, Chirico G, Collini MQ. Rev. Biophys. 2005;38:97. doi: 10.1017/S0033583505004129. [DOI] [PubMed] [Google Scholar]
- 39.Huang B, Bates M, Zhuang X. Annu. Rev. Biochem. 2009;78:993. doi: 10.1146/annurev.biochem.77.061906.092014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borgia A, Williams PM, Clarke J. Annu. Rev. Biochem. 2008;77:101. doi: 10.1146/annurev.biochem.77.060706.093102. [DOI] [PubMed] [Google Scholar]
- 41.Pljevaljcic G, Millar DP. Methods Enzymol. Vol. 450. New York: Academic Press; 2008. p. 233. [DOI] [PubMed] [Google Scholar]
- 42.Yang H. Curr. Opin. Chem. Biol. 2009;14 doi:10.1016/j.cbpa.2009.10.015. [Google Scholar]
- 43.Tsien RY. Annu. Rev. Biochem. 1998;67:509. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 44.Ballou B, Ernst LA, Waggoner AS. Curr. Med. Chem. 2005;12:795. doi: 10.2174/0929867053507324. [DOI] [PubMed] [Google Scholar]
- 45.Jobsis FF. Science. 1977;198:1264. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 46.Chance B. In: Advances in Optical Biopsy and Optical Mammography. Alfano RR, editor. Vol. 838. New York: New York Academy of Sciences; 1998. p. 29. [Google Scholar]
- 47.Frangioni JV. Curr. Opin. Chem. Biol. 2003;7:626. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Goiffon RJ, Akers WJ, Berezin MY, Lee H, Achilefu S. J. Biomed. Opt. 2009;14:020501. doi: 10.1117/1.3095800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akers W, Lesage F, Holten D, Achilefu S. Mol. Imaging. 2007;6:237. [PubMed] [Google Scholar]
- 50.Berezin MY, Akers WJ, Guo K, Fischer GM, Daltrozzo E, Zumbusch A, Achilefu S. Biophys. J. 2009;97:L22. doi: 10.1016/j.bpj.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berezin MY, Lee H, Akers W, Achilefu S. Biophys. J. 2007;93:2892. doi: 10.1529/biophysj.107.111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki E, Kojima H, Nishimatsu H, Urano Y, Kikuchi K, Hirata Y, Nagano T. J. Am. Chem. Soc. 2005;127:3684. doi: 10.1021/ja042967z. [DOI] [PubMed] [Google Scholar]
- 53.Kimura RH, Cheng Z, Gambhir SS, Cochran JR. Cancer Res. 2009;69:2435. doi: 10.1158/0008-5472.CAN-08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K, Lee M, Park H, Kim JH, Kim S, Chung H, Choi K, Kim IS, Seong BL, Kwon IC. J. Am. Chem. Soc. 2006;128:3490. doi: 10.1021/ja057712f. [DOI] [PubMed] [Google Scholar]
- 55.Williams DC, Soper SA. Anal. Chem. 1995;67:3427. doi: 10.1021/ac00115a010. [DOI] [PubMed] [Google Scholar]
- 56.McWhorter S, Soper SA. Electrophoresis. 2000;21:1267. doi: 10.1002/(SICI)1522-2683(20000401)21:7<1267::AID-ELPS1267>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 57.Kricka LJ, Fortina P. Clinical Chemistry. 2009;55:670. doi: 10.1373/clinchem.2008.116152. [DOI] [PubMed] [Google Scholar]
- 58.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Almutairi A, Guillaudeu SJ, Berezin MY, Achilefu S, Frechet JMJ. J. Am. Chem. Soc. 2008;130:444. doi: 10.1021/ja078147e. [DOI] [PubMed] [Google Scholar]
- 60.Shu XK, Royant A, Lin MZ, Aguilera TA, Lev-Ram V, Steinbach PA, Tsien RY. Science. 2009;324:804. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ntziachristos V. Annu. Rev. Biomed. Eng. 2006;8:1. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 62.Rao JH, Dragulescu-Andrasi A, Yao HQ. Curr. Opin. Biotechnol. 2007;18:17. doi: 10.1016/j.copbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Amiot CL, Xu SP, Liang S, Pan LY, Zhao JXJ. Sensors. 2008;8:3082. doi: 10.3390/s8053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hilderbrand SA, Weissleder R. Curr. Opin. Chem. Biol. 2009;14 doi: 10.1016/j.cbpa.2009.09.029. doi:10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. second ed. New York: Cold Spring Harbor Laboratory Press: Cold Spring Harbor; 2009. [PubMed] [Google Scholar]
- 66.Marth JD, Grewal PK. Nat. Rev. Immunol. 2008;8:874. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lowe JB, Marth JD. Annu. Rev. Biochem. 2003;72:643. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 68.Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 69.Dube DH, Prescher JA, Quang CN, Bertozzi CR. Proc. Nat. Acad. Sci. U.S.A. 2006;103:4819. doi: 10.1073/pnas.0506855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reeke GN, Becker JW, Edelman GM. J. Biol. Chem. 1975;250:1525. [PubMed] [Google Scholar]
- 71.Mansouri S, Schultz JS. Bio-Technology. 1984;2:885. [Google Scholar]
- 72.Meadows D, Schultz JS. Talanta. 1988;35:145. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 73.Ladomenou K, Bonar-Law RP. Chem. Commun. 2002;18:2108. doi: 10.1039/b206646g. [DOI] [PubMed] [Google Scholar]
- 74.Kim YH, Hong JI. Angew. Chem. Int. Ed. 2002;41:2947. doi: 10.1002/1521-3773(20020816)41:16<2947::AID-ANIE2947>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 75.Rusin O, Lang K, Kral V. Chem. Eur. J. 2002;8:655. doi: 10.1002/1521-3765(20020201)8:3<655::AID-CHEM655>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 76.Kral V, Rusin O, Schmidtchen FP. Org. Lett. 2001;3:873. doi: 10.1021/ol007069w. [DOI] [PubMed] [Google Scholar]
- 77.Bell JW, Hext NM. Chem. Soc. Rev. 2004;33:589. doi: 10.1039/b207182g. [DOI] [PubMed] [Google Scholar]
- 78.Yoon J, Czarnik AW. J. Am. Chem. Soc. 1992;114:5874. [Google Scholar]
- 79.James TD, Sandanayake K, Shinkai S. Angew. Chem. Int. Ed. 1994;33:2207. [Google Scholar]
- 80.James TD, Sandanayake K, Iguchi R, Shinkai S. J. Am. Chem. Soc. 1995;117:8982. [Google Scholar]
- 81.DiCesare N, Lakowicz JR. J. Phys. Chem. A. 2001;105:6834. doi: 10.1021/jp010076x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adhikiri DP, Heagy MD. Tetrahedron Lett. 1999;40:7893. [Google Scholar]
- 83.Cao HS, Diaz DI, DiCesare N, Lakowicz JR, Heagy MD. Org. Lett. 2002;4:1503. doi: 10.1021/ol025723x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao H, McGill T, Heagy MD. J. Org. Chem. 2004;69:2959. doi: 10.1021/jo035760h. [DOI] [PubMed] [Google Scholar]
- 85.Yang WQ, Yan J, Springsteen G, Deeter S, Wang BH. Bioorg. Med. Chem. Lett. 2003;13:1019. doi: 10.1016/s0960-894x(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 86.Gao X, Zhang Y, Wang B. New. J. Chem. 2005;29:579. [Google Scholar]
- 87.Ni WJ, Fang H, Springsteen G, Wang BH. J. Org. Chem. 2004;69:1999. doi: 10.1021/jo0350357. [DOI] [PubMed] [Google Scholar]
- 88.Akay S, Yang WQ, Wang JF, Lin L, Wang BH. Chem. Biol. Drug Des. 2007;70:279. doi: 10.1111/j.1747-0285.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 89.James TD, Sandanayake K, Shinkai S. Angew. Chem. Int. Ed. 1996;35:1910. [Google Scholar]
- 90.Wang W, Gao XM, Wang BH. Curr. Org. Chem. 2002;6:1285. [Google Scholar]
- 91.Striegler S. Curr. Org. Chem. 2003;7:81. [Google Scholar]
- 92.Cao HS, Heagy MD. J. Fluorescence. 2004;14:569. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 93.Jelinek R, Kolusheva S. Chem. Rev. 2004;104:5987. doi: 10.1021/cr0300284. [DOI] [PubMed] [Google Scholar]
- 94.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJS. Biosens. Bioelectron. 2005;20:2555. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Abraham G, Low PS. Biochim. Biophys. Acta. 1980;597:285. doi: 10.1016/0005-2736(80)90106-6. [DOI] [PubMed] [Google Scholar]
- 96.Ingham KC, Brew SA. Biochim. Biophys. Acta. 1981;670:181. doi: 10.1016/0005-2795(81)90007-6. [DOI] [PubMed] [Google Scholar]
- 97.Anumula KR, Dhume ST. Glycobiology. 1998;8:685. doi: 10.1093/glycob/8.7.685. [DOI] [PubMed] [Google Scholar]
- 98.Whitham KM, Hadley JL, Morris HG, Andrew SM, Nieduszynski IA, Brown GM. Glycobiology. 1999;9:285. doi: 10.1093/glycob/9.3.285. [DOI] [PubMed] [Google Scholar]
- 99.He LP, Sato K, Abo M, Okubo A, Yamazaki S. Anal. Biochem. 2003;314:128. doi: 10.1016/s0003-2697(02)00633-4. [DOI] [PubMed] [Google Scholar]
- 100.Gahmberg CG, Andersson LC. J. Biol. Chem. 1977;252:5888. [PubMed] [Google Scholar]
- 101.De Bank PA, Kellam B, Kendall DA, Shakesheff KM. Biotechnol. Bioeng. 2003;81:800. doi: 10.1002/bit.10525. [DOI] [PubMed] [Google Scholar]
- 102.Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. J. Biol. Chem. 1992;267:16934. [PubMed] [Google Scholar]
- 103.Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Glycobiology. 2001;11:11R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- 104.Mahal LK, Yarema KJ, Bertozzi CR. Science. 1997;276:1125. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 105.Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. J. Biol. Chem. 1998;273:31168. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- 106.Lee JH, Baker TJ, Mahal LK, Zabner J, Bertozzi CR, Wiemer DF, Welsh MJ. J. Biol. Chem. 1999;274:21878. doi: 10.1074/jbc.274.31.21878. [DOI] [PubMed] [Google Scholar]
- 107.Dube DH, Bertozzi CR. Curr. Opin. Chem. Biol. 2003;7:616. doi: 10.1016/j.cbpa.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Dirksen A, Dirksen S, Hackeng TM, Dawson PE. J. Am. Chem. Soc. 2006;128:15602. doi: 10.1021/ja067189k. [DOI] [PubMed] [Google Scholar]
- 109.Dirksen A, Dawson PE. Bioconjugate Chem. 2008;19:2543. doi: 10.1021/bc800310p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dirksen A, Hackeng TM, Dawson PE. Angew. Chem. Int. Ed. 2006;45:7581. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- 111.Zeng Y, Ramya TNC, Dirksen A, Dawson PE, Paulson JC. Nat. Methods. 2009;6:207. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 113.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang PV, Chen X, Smyrniotis C, Xenakis A, Hu T, Bertozzi CR, Wu P. Angew. Chem. Int. Ed. 2009;48:4030. doi: 10.1002/anie.200806319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gorter E, Grendel F. J. Exp. Med. 1925;41:439. doi: 10.1084/jem.41.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singer SJ, Nicolson GL. Science. 1972;175:720. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 117.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Fourth Edition ed. New York: Garland Science; 2002. [Google Scholar]
- 118.Schiller J, Muller M, Fuchs B, Arnold K, Huster D. Curr. Anal. Chem. 2007;3:283. [Google Scholar]
- 119.Rouser G, Yamamoto A. Lipids. 1968;3:284. doi: 10.1007/BF02531202. [DOI] [PubMed] [Google Scholar]
- 120.Lentz BR, Barenholz Y, Thompson TE. Biochemistry. 1976;15:4521. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- 121.Sundaralingam M. Ann. N. Y. Acad. Sci. 1972;195:324. [PubMed] [Google Scholar]
- 122.Epand RM. Biophys. J. 2003;84:3102. doi: 10.1016/S0006-3495(03)70035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steinbauer B, Mehnert T, Beyer K. Biophys. J. 2003;85:1013. doi: 10.1016/S0006-3495(03)74540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagle JF, Tristram-Nagle S. Curr. Opin. Struct. Biol. 2000;10:474. doi: 10.1016/s0959-440x(00)00117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quesada E, Acuna AU, Amat-Guerri F. Angew. Chem. Int. Ed. 2001;40:2095. [PubMed] [Google Scholar]
- 126.Villarreal MR. (LadyofHats), Phospholipids aqueous solution structures. http://commons.wikimedia.org/wiki/File:Phospholipids_aqueous_solution_structures.svg, 11/24/2009.
- 127.Epand RM. Biochim. Biophys. Acta, Rev. Biomembr. 1998;1376:353. doi: 10.1016/s0304-4157(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 128.Simons K, Vaz WLC. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 129.Somerharju P, Virtanen JA, Cheng KH. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 1999;1440:32. doi: 10.1016/s1388-1981(99)00106-7. [DOI] [PubMed] [Google Scholar]
- 130.Binder WH, Barragan V, Menger FM. Angew. Chem. Int. Ed. 2003;42:5802. doi: 10.1002/anie.200300586. [DOI] [PubMed] [Google Scholar]
- 131.Simons K, Ikonen E. Nature. 1997;387:569. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 132.Boon JM, Smith BD. Med. Res. Rev. 2002;22:251. doi: 10.1002/med.10009. [DOI] [PubMed] [Google Scholar]
- 133.Ratledge C, Wilkinson SC. Microbial Lipids. Vol. 1. London: Academic Press; 1988. p. 3. [Google Scholar]
- 134.Epand RM, Epand RF. Biochim. Biophys. Acta, Biomembr. 2009;1788:289. doi: 10.1016/j.bbamem.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 135.Kolesnick RN, Kronke M. Annu. Rev. Physiol. 1998;60:643. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 136.Jarvis WD, Grant S. Curr. Opin. Oncology. 1998;10:552. doi: 10.1097/00001622-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 137.Pettus BJ, Chalfant CE, Hannun YA. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2002;1585 doi: 10.1016/s1388-1981(02)00331-1. PII S1388. [DOI] [PubMed] [Google Scholar]
- 138.Hannun YA. In: Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury 2 Parts A & B. Honn KV, Nigam S, Marnett LJ, editors. Vol. 400. New York: PLENUM PRESS; 1998. p. 305. [Google Scholar]
- 139.Chan YHM, Boxer SG. Curr. Opin. Chem. Biol. 2007;11:581. doi: 10.1016/j.cbpa.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barrow GM. Physical Chemistry. Tokyo: McGraw-Hill kogakusha; 1973. [Google Scholar]
- 141.Blatt E, Sawyer WH. Biochim. Biophys. Acta. 1985;822:43. doi: 10.1016/0304-4157(85)90003-6. [DOI] [PubMed] [Google Scholar]
- 142.Rasmussen JAM, Hermetter A. Prog. Lipid Res. 2008;47:436. doi: 10.1016/j.plipres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 143.Cairo CW, Key JA, Sadek CM. Curr. Opin. Chem. Biol. 2009;14 doi: 10.1016/j.cbpa.2009.09.032. doi:10.1016/j.cbpa.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 144.Shinitzk M, Inbar M. J. Mol. Biol. 1974;85:603. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- 145.Andrich MP, Vanderkooi JM. Biochemistry. 1976;15:1257. doi: 10.1021/bi00651a013. [DOI] [PubMed] [Google Scholar]
- 146.Davenport L, Dale RE, Bisby RH, Cundall RB. Biochemistry. 1985;24:4097. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- 147.Tricerri MA, Garda HA, Brenner RR. Chem. Phys. Lipids. 1994;71:61. doi: 10.1016/0009-3084(94)02303-4. [DOI] [PubMed] [Google Scholar]
- 148.Thulborn KR, Sawyer WH. Biochim. Biophys. Acta. 1978;511:125. doi: 10.1016/0005-2736(78)90308-5. [DOI] [PubMed] [Google Scholar]
- 149.Thulborn KR, Treloar FE, Sawyer WH. Biochem. Biophys. Res. Commun. 1978;81:42. doi: 10.1016/0006-291x(78)91628-5. [DOI] [PubMed] [Google Scholar]
- 150.Invitrogen Corporation. Molecular - Probes The Handbook. http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook.html, 11/24/2009.
- 151.Badley RA, Schneider H, Martin WG. Biochemistry. 1973;12:268. doi: 10.1021/bi00726a015. [DOI] [PubMed] [Google Scholar]
- 152.Levinson C, Villereal ML. J. Cell. Physiol. 1975;86:143. doi: 10.1002/jcp.1040860116. [DOI] [PubMed] [Google Scholar]
- 153.Dhanikula AB, Panchagnula R. Lipids. 2008;43:569. doi: 10.1007/s11745-008-3178-1. [DOI] [PubMed] [Google Scholar]
- 154.Slavik J. Biochim. Biophys. Acta. 1982;694:1. doi: 10.1016/0304-4157(82)90012-0. [DOI] [PubMed] [Google Scholar]
- 155.Gutowicz J, Krawczyk A. Chem. Phys. Lipids. 1986;39:357. doi: 10.1016/0009-3084(86)90117-9. [DOI] [PubMed] [Google Scholar]
- 156.Kellner BMJ, Cadenhead DA. Biochim. Biophys. Acta. 1978;513:301. doi: 10.1016/0005-2736(78)90200-6. [DOI] [PubMed] [Google Scholar]
- 157.Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. Bioorg. Chem. 2005;33:415. doi: 10.1016/j.bioorg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 158.Haidekker MA, Theodorakis EA. Org. Biomol. Chem. 2007;5:1669. doi: 10.1039/b618415d. [DOI] [PubMed] [Google Scholar]
- 159.Oster G, Nishijima Y. J. Am. Chem. Soc. 1956;78:1581. [Google Scholar]
- 160.Loutfy RO, Law KY. J. Phys. Chem. 1980;84:2803. [Google Scholar]
- 161.Loutfy RO, Arnold BA. J. Phys. Chem. 1982;86:4205. [Google Scholar]
- 162.Abdelmottaleb MSA, Loutfy RO, Lapouyade R. J. Photochem. Photobiol., A. 1989;48:87. [Google Scholar]
- 163.Kung CE, Reed JK. Biochemistry. 1986;25:6114. [Google Scholar]
- 164.Haidekker MA, Ling TT, Anglo M, Stevens HY, Frangos JA, Theodorakis EA. Chem. Biol. 2001;8:123. doi: 10.1016/s1074-5521(00)90061-9. [DOI] [PubMed] [Google Scholar]
- 165.Hartel S, Tykhonova S, Haas M, Diehl HA. J. Fluorescence. 2002;12:465. [Google Scholar]
- 166.Akers WJ, Cupps JM, Haidekker MA. Biorheology. 2005;42:335. [PubMed] [Google Scholar]
- 167.Haidekker MA, Brady T, Wen K, Okada C, Stevens HY, Snell JM, Frangos JA, Theodorakis EA. Bioorg. Med. Chem. 2002;10:3627. doi: 10.1016/s0968-0896(02)00240-7. [DOI] [PubMed] [Google Scholar]
- 168.Bondarev SL, Bachilo SM. J. Photochem. Photobiol., A. 1991;59:273. [Google Scholar]
- 169.Cehelnik ED, Cundall RB, Lockwood JR, Palmer TF. J. Phys. Chem. 1975;79:1369. [Google Scholar]
- 170.Hirayama K. Handbook of Ultraviolet and Visible Absorption Spectra of Organic Compounds. New York: Springer - Verlag; 1967. [Google Scholar]
- 171.Eriksson JC, Gillberg G. Acta Chem. Scand. 1966;20:2019. [Google Scholar]
- 172.Rehfeld SJ. J. Phys. Chem. 1971;75:3905. [Google Scholar]
- 173.Fendler JH, Patterson LK. J. Phys. Chem. 1971;75:3907. [Google Scholar]
- 174.Fendler JH. J. Phys. Chem. 1980;84:1485. [Google Scholar]
- 175.Waggoner AS, Stryer L. Proc. Nat. Acad. Sci. U.S.A. 1970;67:579. doi: 10.1073/pnas.67.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lapinski MM, Blanchard G. J. Chem. Phys. Lipids. 2007;150:12. doi: 10.1016/j.chemphyslip.2007.06.210. [DOI] [PubMed] [Google Scholar]
- 177.Avanti Polar Lipids inc. Fluorescent Lipids. http://www.avantilipids.com/index.php?option=com_content&view=article&id=11&Itemid=17, 11/24/2009.
- 178.Karolin J, Bogen ST, Johansson LBÅ, Molotkovsky JG. J. Fluorescence. 1995;5:279. doi: 10.1007/BF00723899. [DOI] [PubMed] [Google Scholar]
- 179.Asuncion-Punzalan E, Kachel K, London E. Biochemistry. 1998;37:4603. doi: 10.1021/bi9726234. [DOI] [PubMed] [Google Scholar]
- 180.Chattopadhyay A. Chem. Phys. Lipids. 1990;53:1. doi: 10.1016/0009-3084(90)90128-e. [DOI] [PubMed] [Google Scholar]
- 181.Soh N, Makihara K, Ariyoshi T, Seto D, Maki T, Nakajima H, Nakano K, Imato T. Anal. Sci. 2008;24:293. doi: 10.2116/analsci.24.293. [DOI] [PubMed] [Google Scholar]
- 182.Lala AK. Chem. Phys. Lipids. 2002;116:177. doi: 10.1016/s0009-3084(02)00026-9. [DOI] [PubMed] [Google Scholar]
- 183.Cundall RB, Johnson I, Jones MW, Thomas EW, Munro IH. Chem. Phys. Lett. 1979;64:39. [Google Scholar]
- 184.Jones ME, Lentz BR. Biochemistry. 1986;25:567. doi: 10.1021/bi00351a009. [DOI] [PubMed] [Google Scholar]
- 185.Prendergast FG, Haugland RP, Callahan PJ. Biochemistry. 1981;20:7333. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- 186.Kaiser RD, London E. Biochemistry. 1998;37:8180. doi: 10.1021/bi980064a. [DOI] [PubMed] [Google Scholar]
- 187.Thumser AE, Storch J. Mol. Cell. Biochem. 2007;299:67. doi: 10.1007/s11010-005-9041-2. [DOI] [PubMed] [Google Scholar]
- 188.Morgan CG, Thomas EW, Moras TS, Yianni YP. Biochim. Biophys. Acta. 1982;692:196. doi: 10.1016/0005-2736(82)90521-1. [DOI] [PubMed] [Google Scholar]
- 189.De Bony J, Tocanne JF. Chem. Phys. Lipids. 1983;32:105. [Google Scholar]
- 190.De Bony J, Tocanne JF. Eur. J. Biochem. 1984;143:373. doi: 10.1111/j.1432-1033.1984.tb08383.x. [DOI] [PubMed] [Google Scholar]
- 191.Vincent M, Gallay J, Debony J, Tocanne JF. Eur. J. Biochem. 1985;150:341. doi: 10.1111/j.1432-1033.1985.tb09026.x. [DOI] [PubMed] [Google Scholar]
- 192.Encinas MV, Lissi EA, Alvarez J. Photochem. Photobiol. 1994;59:30. [Google Scholar]
- 193.Monti JA, Christian ST, Shaw WA, Finley WH. Life Sci. 1977;21:345. doi: 10.1016/0024-3205(77)90515-x. [DOI] [PubMed] [Google Scholar]
- 194.Starck JP, Nakatani Y, Ourisson G. Tetrahedron. 1995;51:2629. [Google Scholar]
- 195.Davenport L, Shen B, Joseph TW, Straher MP. Chem. Phys. Lipids. 2001;109:145. doi: 10.1016/s0009-3084(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 196.Vincent M, Gallay J. Biochemistry. 1984;23:6514. [Google Scholar]
- 197.Quesada E, Ardhammar M, Norden B, Miesch M, Duportail G, Bonzi-Coulibaly Y, Nakatani Y, Ourisson G. Helv. Chim. Acta. 2000;83:2464. [Google Scholar]
- 198.Ventelon L, Charier S, Moreaux L, Mertz J, Blanchard-Desce M. Angew. Chem. Int. Ed. 2001;40:2098. [PubMed] [Google Scholar]
- 199.Yamamoto M, Warnock WA, Milon A, Nakatani Y, Ourisson G. Angew. Chem. Int. Ed. 1993;32:259. [Google Scholar]
- 200.Delfino JM, Schreiber SL, Richards FM. J. Am. Chem. Soc. 1993;115:3458. [Google Scholar]
- 201.Gaffney BJ, Willingham GL, Schepp RS. Biochemistry. 1983;22:881. doi: 10.1021/bi00273a027. [DOI] [PubMed] [Google Scholar]
- 202.Lala AK, Koppaka V. Biochemistry. 1992;31:5586. doi: 10.1021/bi00139a023. [DOI] [PubMed] [Google Scholar]
- 203.Bittman R, Chen WC, Anderson OR. Biochemistry. 1974;13:1364. doi: 10.1021/bi00704a009. [DOI] [PubMed] [Google Scholar]
- 204.Lampen JO. Am. J. Clin. Path. 1969;52:138. doi: 10.1093/ajcp/52.2.138. [DOI] [PubMed] [Google Scholar]
- 205.Radda GK, Smith DS. FEBS Lett. 1970;9:287. doi: 10.1016/0014-5793(70)80379-9. [DOI] [PubMed] [Google Scholar]
- 206.Chance B. Biomembranes. 1975;7:33. doi: 10.1007/978-1-4684-7668-2_3. [DOI] [PubMed] [Google Scholar]
- 207.Hausser KW, Kuhn R, Kuhn E. Z. PHYSIK. Chem. 1935;B29:417. [Google Scholar]
- 208.Hausser KW, Kuhn R, Smakula A. Z. PHYSIK. Chem. 1935;B29:384. [Google Scholar]
- 209.Hausser KW, Kuhn R, Smakula A, Hoffer M. Z. PHYSIK. Chem. 1935;B29:371. [Google Scholar]
- 210.Hausser KW, Kuhn R, Smakula A, Kreuchen KH. Z. PHYSIK. Chem. 1935;B29:363. [Google Scholar]
- 211.Platt JR. J. Chem. Phys. 1956;25:80. [Google Scholar]
- 212.Hudson B, Kohler B. Annu. Rev. Phys. Chem. 1974;25:437. [Google Scholar]
- 213.Sklar LA, Hudson BS, Petersen M, Diamond J. Biochemistry. 1977;16:813. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- 214.Mateo CR, Souto AA, Amat-Guerri F, Acuna AU. Biophys. J. 1996;71:2177. doi: 10.1016/S0006-3495(96)79419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Acuna AU, Amat-Guerri F, Quesada E, Velez M. Biophys. Chem. 2006;122:27. doi: 10.1016/j.bpc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 216.Eckey EWM, L P. Vegetable Fats and Oils. New York: Reinhold; 1954. [Google Scholar]
- 217.Kaufmann HP, Sud RK. Chem. Ber. Recl. 1959;92:2797. [Google Scholar]
- 218.Sklar LA, Hudson BS, Simoni RD. Biochemistry. 1977;16:819. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- 219.Souto AA, Acuna AU, Amat-Guerri F. Tetrahedron Lett. 1994;35:5907. [Google Scholar]
- 220.Kuerschner L, Ejsing CS, Ekroos K, Shevchenko A, Anderson KI, Thiele C. Nat. Methods. 2005;2:39. doi: 10.1038/nmeth728. [DOI] [PubMed] [Google Scholar]
- 221.Stille JK. Angew. Chem. Int. Ed. 1986;25:508. [Google Scholar]
- 222.Quesada E, Acuna AU, Amat-Guerri F. Eur. J. Org. Chem. 2003;1308 [Google Scholar]
- 223.Boland W, Pantke S. J. Prakt. Chem. 1994;336:714. [Google Scholar]
- 224.Quesada E, Delgado J, Hornillos V, Acuna AU, Amat-Guerri F. Eur. J. Org. Chem. 2007;2285 [Google Scholar]
- 225.Hac-Wydro K, Wydro P. Chem. Phys. Lipids. 2007;150:66. doi: 10.1016/j.chemphyslip.2007.06.213. [DOI] [PubMed] [Google Scholar]
- 226.Azzi AQ. Rev. Biophys. 1975;8:237. doi: 10.1017/s0033583500001803. [DOI] [PubMed] [Google Scholar]
- 227.Radda GK, Vanderkooi J. Biochim. Biophys. Acta. 1972;265:509. [Google Scholar]
- 228.Brand L, Gohlke JR. Annu. Rev. Biochem. 1972;41:843. doi: 10.1146/annurev.bi.41.070172.004211. [DOI] [PubMed] [Google Scholar]
- 229.Hulbert AJ. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2008;150:196. doi: 10.1016/j.cbpa.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 230.Lee JH, Kim DI, Mun H, Lee SK, Park JS, Kim JH, Park YH, Jeon YC, Yoon UC, Bae MK, Jang HO, Wood WG, Yun I. Chem. Phys. Lipids. 2008;154:19. doi: 10.1016/j.chemphyslip.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 231.Kutchai H, Chandler LH, Zavoico GB. Biochim. Biophys. Acta. 1983;736:137. doi: 10.1016/0005-2736(83)90277-8. [DOI] [PubMed] [Google Scholar]
- 232.Bangham AD, Standish MM, Watkins JC, Weissman G. Protoplasma. 1967;63:183. [PubMed] [Google Scholar]
- 233.Uemura A, Kimura S, Imanishi Y. Biochim. Biophys. Acta. 1983;729:28. doi: 10.1016/0005-2736(83)90452-2. [DOI] [PubMed] [Google Scholar]
- 234.Blatt E, Sawyer WH, Ghiggino KP. Aus. J. Chem. 1983;36:1079. [Google Scholar]
- 235.Vincent M, Deforesta B, Gallay J, Alfsen A. Biochem. Biophys. Res. Commun. 1982;107:914. doi: 10.1016/0006-291x(82)90610-6. [DOI] [PubMed] [Google Scholar]
- 236.Jones JD, Gierasch LM. Biophys. J. 1994;67:1534. doi: 10.1016/S0006-3495(94)80627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Loew LM, Simpson L, Hassner A, Alexanian V. J. Am. Chem. Soc. 1979;101:5439. [Google Scholar]
- 238.Hasselbacher CA, Preuss DK, Dewey TG. Biochemistry. 1986;25:668. [Google Scholar]
- 239.Baird B, Holowka D. Biochemistry. 1985;24:6252. doi: 10.1021/bi00343a032. [DOI] [PubMed] [Google Scholar]
- 240.Kleinfeld AM. Biochemistry. 1985;24:1874. doi: 10.1021/bi00329a011. [DOI] [PubMed] [Google Scholar]
- 241.Dewey TG, Hammes GG. Biophys. J. 1980;32:1023. doi: 10.1016/S0006-3495(80)85033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chattopadhyay A, London E. Biochemistry. 1987;26:39. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- 243.Kao YJ, Soutar AK, Hong KY, Pownall HJ, Smith LC. Biochemistry. 1978;17:2689. doi: 10.1021/bi00606a036. [DOI] [PubMed] [Google Scholar]
- 244.Markello T, Zlotnick A, Everett J, Tennyson J, Holloway PW. Biochemistry. 1985;24:2895. doi: 10.1021/bi00333a012. [DOI] [PubMed] [Google Scholar]
- 245.Jain MK, Maliwal BP. Biochim. Biophys. Acta. 1985;814:135. doi: 10.1016/0005-2736(85)90428-6. [DOI] [PubMed] [Google Scholar]
- 246.Shinitzk M, Barenholz Y. J. Biol. Chem. 1974;249:2652. [PubMed] [Google Scholar]
- 247.Kawato S, Kinosita K, Ikegami A. Biochemistry. 1977;16:2319. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- 248.Higgins DL, Callahan PJ, Prendergast FG, Nesheim ME, Mann KG. J. Biol. Chem. 1985;260:3604. [PubMed] [Google Scholar]
- 249.Folmer V, Pedroso N, Matias AC, Lopes S, Antunes F, Cyrne L, Marinho HS. Biochim. Biophys. Acta, Biomembr. 2008;1778:1141. doi: 10.1016/j.bbamem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 250.Rudy B, Gitler C. Biochim. Biophys. Acta. 1972;288:231. doi: 10.1016/0005-2736(72)90242-8. [DOI] [PubMed] [Google Scholar]
- 251.Cogan U, Shinitzk M, Weber G, Nishida T. Biochemistry. 1973;12:521. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- 252.Saxena R, Shrivastava S, Chattopadhyay A. J. Phys. Chem. B. 2008;112:12134. doi: 10.1021/jp804353m. [DOI] [PubMed] [Google Scholar]
- 253.Ramamoorthy A, Thennarasu S, Tan AM, Lee DK, Clayberger C, Krensky AM. Biochim. Biophys. Acta, Biomembr. 2006;1758:154. doi: 10.1016/j.bbamem.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 254.Lukac S. J. Am. Chem. Soc. 1984;106:4386. [Google Scholar]
- 255.Haidekker MA, L'Heureux N, Frangos JA. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1401. doi: 10.1152/ajpheart.2000.278.4.H1401. [DOI] [PubMed] [Google Scholar]
- 256.Bernik DL, Negri RM. J. Colloid Interface Sci. 1998;203:97. [Google Scholar]
- 257.Liu JW, Blumenthal KM. Biochim. Biophys. Acta. 1988;937:153. doi: 10.1016/0005-2736(88)90237-4. [DOI] [PubMed] [Google Scholar]
- 258.Liu J, Blumenthal KM. J. Biol. Chem. 1988;263:6619. [PubMed] [Google Scholar]
- 259.Mukherjee S, Kalipatnapu S, Pucadyil TJ, Chattopadhyay A. Mol. Membr. Biol. 2006;23:430. doi: 10.1080/09687860600803223. [DOI] [PubMed] [Google Scholar]
- 260.Chattopadhyay A, London E. Biochim. Biophys. Acta. 1988;938:24. doi: 10.1016/0005-2736(88)90118-6. [DOI] [PubMed] [Google Scholar]
- 261.Tang DX, Borchman D, Harris N, Pierangeli S. Biochim. Biophys. Acta, Biomembr. 1998;1372:45. doi: 10.1016/s0005-2736(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 262.Stasiuk M, Jaromin A, Kozubek A. Biochim. Biophys. Acta, Biomembr. 2004;1667:215. doi: 10.1016/j.bbamem.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 263.Prendergast FG, Lu J, Wei GJ, Bloomfield VA. Biochemistry. 1982;21:6963. doi: 10.1021/bi00269a053. [DOI] [PubMed] [Google Scholar]
- 264.Shrivastava S, Chattopadhyay A. Biochem. Biophys. Res. Commun. 2007;356:705. doi: 10.1016/j.bbrc.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 265.Tiriveedhi V, Butko P. Biochemistry. 2007;46:3888. doi: 10.1021/bi602527t. [DOI] [PubMed] [Google Scholar]
- 266.Merino-Montero S, Montero MT, Hernandez-Borrell J. Biophys. Chem. 2006;119:101. doi: 10.1016/j.bpc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 267.Madeira C, Loura LMS, Aires-Barros MR, Fedorov A, Prieto M. Biophys. J. 2003;85:3106. doi: 10.1016/S0006-3495(03)74729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ho C, Slater SJ, Stubbs CD. Biochemistry. 1995;34:6188. doi: 10.1021/bi00018a023. [DOI] [PubMed] [Google Scholar]
- 269.Cannon B, Lewis A, Metze J, Thiagarajan V, Vaughn MW, Somerharju P, Virtanen J, Huang JY, Cheng KH. J. Phys. Chem. B. 2006;110:6339. doi: 10.1021/jp0558371. [DOI] [PubMed] [Google Scholar]
- 270.Parker A, Miles K, Cheng KH, Huang J. Biophys. J. 2004;86:1532. doi: 10.1016/S0006-3495(04)74221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cannon B, Heath G, Huang JY, Somerharju P, Virtanen JA, Cheng KH. Biophys. J. 2003;84:3777. doi: 10.1016/S0006-3495(03)75106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Dupoucezanne L, Sautereau AM, Tocanne JF. Eur. J. Biochem. 1989;181:695. doi: 10.1111/j.1432-1033.1989.tb14779.x. [DOI] [PubMed] [Google Scholar]
- 273.Bondar OP, Rowe ES. Biochim. Biophys. Acta, Biomembr. 1998;1370:207. doi: 10.1016/s0005-2736(97)00264-2. [DOI] [PubMed] [Google Scholar]
- 274.Somerharju PJ, Virtanen JA, Eklund KK, Vainio P, Kinnunen PKJ. Biochemistry. 1985;24:2773. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- 275.Tang D, Chong PLG. Biophys. J. 1992;63:903. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chong PLG, Tang D, Sugar IP. Biophys. J. 1994;66:2029. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nicolay K, Hovius R, Bron R, Wirtz K, Dekruijff B. Biochim. Biophys. Acta. 1990;1025:49. doi: 10.1016/0005-2736(90)90189-u. [DOI] [PubMed] [Google Scholar]
- 278.Langner M, Hui SW. Biochim. Biophys. Acta, Biomembr. 2000;1463:439. doi: 10.1016/s0005-2736(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 279.Loura LMS, Ramalho JPP. Biochim. Biophys. Acta, Biomembr. 2007;1768:467. doi: 10.1016/j.bbamem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 280.Staneva G, Momchilova A, Wolf C, Quinn PJ, Koumanov K. Biochim. Biophys. Acta, Biomembr. 2009;1788:666. doi: 10.1016/j.bbamem.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 281.Saurel O, Cezanne L, Milon A, Tocanne JF, Demange P. Biochemistry. 1998;37:1403. doi: 10.1021/bi971484n. [DOI] [PubMed] [Google Scholar]
- 282.Pagano RE, Sleight RG. Science. 1985;229:1051. doi: 10.1126/science.4035344. [DOI] [PubMed] [Google Scholar]
- 283.Starck JP, Nakatani Y, Ourisson G, Cowley DJ, Duportail G. New. J. Chem. 1996;20:1293. [Google Scholar]
- 284.Lenard J, Wong CY, Compans RW. Biochim. Biophys. Acta. 1974;332:341. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Castanho M, Prieto M. Biophys. J. 1995;69:155. doi: 10.1016/S0006-3495(95)79886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mateo CR, Brochon JC, Lillo MP, Acuna AU. Biophys. J. 1993;65:2237. doi: 10.1016/S0006-3495(93)81257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tecoma ES, Sklar LA, Simoni RD, Hudson BS. Biochemistry. 1977;16:829. doi: 10.1021/bi00624a003. [DOI] [PubMed] [Google Scholar]
- 288.Tyagi SC, Simon SR. J. Biol. Chem. 1991;266:15185. [PubMed] [Google Scholar]
- 289.Sklar LA, Hudson BS, Simoni RD. Biochemistry. 1977;16:5100. doi: 10.1021/bi00642a024. [DOI] [PubMed] [Google Scholar]
- 290.Wiltschi B, Budisa N. In: Probes and Tags to Study Biomolecular Function. Miller LW, editor. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2008. p. 139. [Google Scholar]
- 291.Miller LW, editor. Probes and Tags to Study Biomolecular Function for Proteins, RNA, and Membranes. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2008. [Google Scholar]
- 292.Connor RE, Tirrell DA. J. Macromol. Sci., Polym. Rev. 2007;47:9. [Google Scholar]
- 293.Twine SM, Szabo AG. Biophotonics Pt A. 2003;360:104. doi: 10.1016/s0076-6879(03)60108-4. [DOI] [PubMed] [Google Scholar]
- 294.Katritzky AR, Narindoshvili T. Org. Biomol. Chem. 2009;7:627. doi: 10.1039/b818908k. [DOI] [PubMed] [Google Scholar]
- 295.Jakobs S, Andresen M, Wurm CA. In: Probes and Tags to Study Biomolecular Function. Miller LW, editor. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2008. p. 73. [Google Scholar]
- 296.Shimomura O, Johnson FH, Saiga Y. J. Cell. Comp. Physiol. 1962;59:223. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 297.Johnson FH, Gershman LC, Waters JR, Reynolds GT, Saiga Y, Shimomura O. J. Cell. Comp. Physiol. 1962;60:85. [Google Scholar]
- 298.Heim R, Cubitt AB, Tsien RY. Nature. 1995;373:663. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 299.Cubitt AB, Woollenweber LA, Heim R. Methods Cell BIOL. 1999;58:19. doi: 10.1016/s0091-679x(08)61946-9. [DOI] [PubMed] [Google Scholar]
- 300.Bae JH, Rubini M, Jung G, Wiegand G, Seifert MHJ, Azim MK, Kim JS, Zumbusch A, Holak TA, Moroder L, Huber R, Budisa N. J. Mol. Biol. 2003;328:1071. doi: 10.1016/s0022-2836(03)00364-4. [DOI] [PubMed] [Google Scholar]
- 301.Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Trends Biochem. Sci. 1995;20:448. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- 302.Wachter RM. Photochem. Photobiol. 2006;82:339. doi: 10.1562/2005-10-02-IR-708. [DOI] [PubMed] [Google Scholar]
- 303.Wachter RM. Acc. Chem. Res. 2007;40:120. doi: 10.1021/ar040086r. [DOI] [PubMed] [Google Scholar]
- 304.Ibraheem A, Campbell RE. Curr. Opin. Chem. Biol. 2009;14 doi: 10.1016/j.cbpa.2009.09.033. doi:10.1016/j.cbpa.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 305.Shaner NC, Steinbach PA, Tsien RY. Nat. Methods. 2005;2:905. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 306.Pakhomov AA, Martynov VI. Chem. Biol. 2008;15:755. doi: 10.1016/j.chembiol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 307.Verkhusha VV, Lukyanov KA. Nat. Biotechnol. 2004;22:289. doi: 10.1038/nbt943. [DOI] [PubMed] [Google Scholar]
- 308.Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. Nat. Chem. Biol. 2007;3:423. doi: 10.1038/nchembio.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Griffin BA, Adams SR, Tsien RY. Science. 1998;281:269. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 310.Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. J. Am. Chem. Soc. 2002;124:6063. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 311.Martin BR, Giepmans BNG, Adams SR, Tsien RY. Nat. Biotechnol. 2005;23:1308. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- 312.Luedtke NW, Dexter RJ, Fried DB, Schepartz A. Nat. Chem. Biol. 2007;3:779. doi: 10.1038/nchembio.2007.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Follenius-Wund A, Bourotte M, Schmitt M, Iyice F, Lami H, Bourguignon JJ, Haiech J, Pigault C. Biophys. J. 2003;85:1839. doi: 10.1016/S0006-3495(03)74612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Chen KY, Cheng YM, Lai CH, Hsu CC, Ho ML, Lee GH, Chou PT. J. Am. Chem. Soc. 2007;129:4534. doi: 10.1021/ja070880i. [DOI] [PubMed] [Google Scholar]
- 315.Pruger B, Bach T. Synthesis. 2007:1103. [Google Scholar]
- 316.Stafforst T, Diederichsen U. Eur. J. Org. Chem. 2007:899. [Google Scholar]
- 317.Wu LX, Burgess K. J. Am. Chem. Soc. 2008;130:4089. doi: 10.1021/ja710388h. [DOI] [PubMed] [Google Scholar]
- 318.Gokel GW. Dean's Handbook of Organic Chemistry. 2nd Edition ed. New York: McGraw-Hill; 2004. [Google Scholar]
- 319.Ross JA, Jameson DM. Photochem. Photobiol. Sci. 2008;7:1301. doi: 10.1039/b804450n. [DOI] [PubMed] [Google Scholar]
- 320.Munishkina LA, Fink AL. Biochim. Biophys. Acta, Biomembr. 2007;1768:1862. doi: 10.1016/j.bbamem.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 321.Eftink M, Shastry MCR, Brand L, Johnson ML. Methods Enzymol. Vol. 278. New York: Academic Press; 1997. p. 258. [Google Scholar]
- 322.Pierce DW, Boxer SG. Biophys. J. 1995;68:1583. doi: 10.1016/S0006-3495(95)80331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Royer CA. Chem. Rev. 2006;106:1769. doi: 10.1021/cr0404390. [DOI] [PubMed] [Google Scholar]
- 324.Szabo AG, Stepanik TM, Wayner DM, Young NM. Biophys. J. 1983;41:233. doi: 10.1016/S0006-3495(83)84433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Eftink M, Brand L, Johnson ML. Methods Enzymol. Vol. 278. New York: Academic Press; 1997. p. 221. [Google Scholar]
- 326.Engelborghs Y. J. Fluorescence. 2003;13:9. [Google Scholar]
- 327.Budisa N, Pal PP. Biol. Chem. 2004;385:893. doi: 10.1515/BC.2004.117. [DOI] [PubMed] [Google Scholar]
- 328.Loidl G, Musiol HJ, Budisa N, Huber R, Poirot S, Fourmy D, Moroder L. J. Pept. Sci. 2000;6:139. doi: 10.1002/(SICI)1099-1387(200003)6:3<139::AID-PSC240>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 329.Steward LE, Collins CS, Gilmore MA, Carlson JE, Ross JBA, Chamberlin AR. J. Am. Chem. Soc. 1997;119:6. [Google Scholar]
- 330.De Filippis V, De Boni S, De Dea E, Dalzoppo D, Grandi C, Fontana A. Protein Sci. 2004;13:1489. doi: 10.1110/ps.03542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lotte K, Plessow R, Brockhinke A. Photochem. Photobiol. Sci. 2004;3:348. doi: 10.1039/b312436c. [DOI] [PubMed] [Google Scholar]
- 332.Broos J, Gabellieri E, Biemans-Oldehinkel E, Strambini GB. Protein Sci. 2003;12:1991. doi: 10.1110/ps.03142003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Erlenmeyer H, Grubenmann W. Helv. Chim. Acta. 1947;30:297. [PubMed] [Google Scholar]
- 334.Chapman NB, Scrowsto Rm, Westwood R. J. Chem. Soc. 1969:1855. doi: 10.1039/j39690001855. [DOI] [PubMed] [Google Scholar]
- 335.Rajh HM, Uitzetter JH, Westerhuis LW, Vandendries CL, Tesser GI. Int. J. Pept. Protein Res. 1979;14:68. doi: 10.1111/j.1399-3011.1979.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 336.Cady SG, Sono M. Arch. Biochem. Biophys. 1991;291:326. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 337.Podea PV, Tosa ML, Palzs C, Irimie FD. Tetrahedron: Asymmetry. 2008;19:500. [Google Scholar]
- 338.Kwon I, Tirrell DA. J. Am. Chem. Soc. 2007;129:10431. doi: 10.1021/ja071773r. [DOI] [PubMed] [Google Scholar]
- 339.Ross JBA, Szabo AG, Hogue CWV. Methods Enzymol. Vol. 278. New York: Academic Press; 1997. p. 151. [Google Scholar]
- 340.Murakami H, Hohsaka T, Ashizuka Y, Hashimoto K, Sisido M. Biomacromolecules. 2000;1:118. doi: 10.1021/bm990012g. [DOI] [PubMed] [Google Scholar]
- 341.Turcatti G, Nemeth K, Edgerton MD, Meseth U, Talabot F, Peitsch M, Knowles J, Vogel H, Chollet A. J. Biol. Chem. 1996;271:19991. doi: 10.1074/jbc.271.33.19991. [DOI] [PubMed] [Google Scholar]
- 342.Hohsaka T, Ashizuka Y, Sasaki H, Murakami H, Sisido M. J. Am. Chem. Soc. 1999;121:12194. [Google Scholar]
- 343.Hohsaka T, Sisido M. Nucleic Acids Symp. Ser. 2000:99. doi: 10.1093/nass/44.1.99. [DOI] [PubMed] [Google Scholar]
- 344.Taku K, Sasaki H, Kimura S, Imanishi Y. Amino Acids. 1994;7:311. doi: 10.1007/BF00807706. [DOI] [PubMed] [Google Scholar]
- 345.Hohsaka T, Kajihara D, Ashizuka Y, Murakami H, Sisido M. J. Am. Chem. Soc. 1999;121:34. [Google Scholar]
- 346.Szymanska A, Wegner K, Lankiewicz L. Helv. Chim. Acta. 2003;86:3326. [Google Scholar]
- 347.Hamada H, Kameshima N, Szymanska A, Wegner K, Lankiewicz L, Shinohara H, Taki M, Sisido M. Bioorg. Med. Chem. 2005;13:3379. doi: 10.1016/j.bmc.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 348.Sisido M. Prog. Polym. Sci. 1992;17:699. [Google Scholar]
- 349.Jones G, Vullev VI. Org. Lett. 2002;4:4001. doi: 10.1021/ol026656+. [DOI] [PubMed] [Google Scholar]
- 350.Shults MD, Pearce DA, Imperiali B. J. Am. Chem. Soc. 2003;125:10591. doi: 10.1021/ja0355980. [DOI] [PubMed] [Google Scholar]
- 351.Shults MD, Imperiali B. J. Am. Chem. Soc. 2003;125:14248. doi: 10.1021/ja0380502. [DOI] [PubMed] [Google Scholar]
- 352.Shults MD, Carrico-Moniz D, Imperiali B. Anal. Biochem. 2006;352:198. doi: 10.1016/j.ab.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 353.Lukovic E, Gonzalez-Vera JA, Imperiali B. J. Am. Chem. Soc. 2008;130:12821. doi: 10.1021/ja8046188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Gonzalez-Vera JA, Lukovic E, Imperiali B. J. Org. Chem. 2009;74:7309. doi: 10.1021/jo901369k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Mitra S, Barrios AM. Bioorg. Med. Chem. Lett. 2005;15:5142. doi: 10.1016/j.bmcl.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 356.Mitra S, Barrios AM. Anal. Biochem. 2007;370:249. doi: 10.1016/j.ab.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 357.Mitra S, Barrios AM. ChemBioChem. 2008;9:1216. doi: 10.1002/cbic.200800046. [DOI] [PubMed] [Google Scholar]
- 358.Kuragaki M, Sisido M. J. Phys. Chem. 1996;100:16019. [Google Scholar]
- 359.Sisido M, Egusa S, Imanishi Y. J. Am. Chem. Soc. 1983;105:4077. [Google Scholar]
- 360.Egusa S, Sisido M, Imanishi Y. Chem. Lett. 1983:1307. [Google Scholar]
- 361.Sasaki H, Ikeda K, Suzuki M, Ninomiya K, Sisido M. Peptide Science. 2004;76:21. doi: 10.1002/bip.10564. [DOI] [PubMed] [Google Scholar]
- 362.Sisido M, Hohsaka T. Appl. Microbiol. Biotechnol. 2001;57:274. doi: 10.1007/s002530100791. [DOI] [PubMed] [Google Scholar]
- 363.Sasaki H, Sisido M, Imanishi Y. Langmuir. 1991;7:1944. [Google Scholar]
- 364.Sasaki H, Sisido M, Imanishi Y. Langmuir. 1991;7:1949. [Google Scholar]
- 365.Matsubara T, Shinohara H, Sisido M. Macromolecules. 1997;30:2651. [Google Scholar]
- 366.Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. Proc. Nat. Acad. Sci. U.S.A. 2006;103:9785. doi: 10.1073/pnas.0603965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Cornish VW, Benson DR, Altenbach CA, Hideg K, Hubbell WL, Schultz PG. Proc. Nat. Acad. Sci. U.S.A. 1994;91:2910. doi: 10.1073/pnas.91.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Vazquez ME, Rothman DM, Imperiali B. Org. Biomol. Chem. 2004;2:1965. doi: 10.1039/b408001g. [DOI] [PubMed] [Google Scholar]
- 369.Vazquez ME, Blanco JB, Imperiali B. J. Am. Chem. Soc. 2005;127:1300. doi: 10.1021/ja0449168. [DOI] [PubMed] [Google Scholar]
- 370.Loving G, Imperiali B. J. Am. Chem. Soc. 2008;130:13630. doi: 10.1021/ja804754y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Nitz M, Mezo AR, Ali MH, Imperiali B. Chem. Commun. 2002;17:1912. doi: 10.1039/b205224e. [DOI] [PubMed] [Google Scholar]
- 372.Vazquez ME, Nitz M, Stehn J, Yaffe MB, Imperiali B. J. Am. Chem. Soc. 2003;125:10150. doi: 10.1021/ja0351847. [DOI] [PubMed] [Google Scholar]
- 373.Hohsaka T, Kawashima K, Sisido M. J. Am. Chem. Soc. 1994;116:413. [Google Scholar]
- 374.Goodman M, Kossoy A. J. Am. Chem. Soc. 1966;88:5010. doi: 10.1021/ja00968a056. [DOI] [PubMed] [Google Scholar]
- 375.Anderson RD, Zhou J, Hecht SM. J. Am. Chem. Soc. 2002;124:9674. doi: 10.1021/ja0205939. [DOI] [PubMed] [Google Scholar]
- 376.Birks JB. Chem. Phys. Lett. 1972;17:370. [Google Scholar]
- 377.de Melo JS, Rodrigues LM, Serpa C, Arnaut LG, Ferreira I, Queiroz M. Photochem. Photobiol. 2003;77:121. doi: 10.1562/0031-8655(2003)077<0121:papot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 378.Kenner RA, Aboderin AA. Biochemistry. 1971;10:4433. doi: 10.1021/bi00800a013. [DOI] [PubMed] [Google Scholar]
- 379.Speight JG. Lange’s Handbook of Chemistry. 16th Edition ed. New York: McGraw-Hill; 2005. [Google Scholar]
- 380.Badger GM. J. Chem. Soc. 1952;1175 [Google Scholar]
- 381.Mayneord WV, Roe EMF. Proc. R. Soc. London, Ser. A. 1935;152A:299. [Google Scholar]
- 382.Morton RA, Earlam WT. J. Chem. Soc. 1941;Part 1:159. [Google Scholar]
- 383.Soujanya T, Fessenden RW, Samanta A. J. Phys. Chem. 1996;100:3507. [Google Scholar]
- 384.Saha S, Samanta A. J. Phys. Chem. A. 2002;106:4763. [Google Scholar]
- 385.Weber G, Farris FJ. Biochemistry. 1979;18:3075. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- 386.Dawson PE, Muir TW, Clarklewis I, Kent SBH. Science. 1994;266:776. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 387.Noren CJ, Anthonycahill SJ, Griffith MC, Schultz PG. Science. 1989;244:182. doi: 10.1126/science.2649980. [DOI] [PubMed] [Google Scholar]
- 388.Bain JD, Glabe CG, Dix TA, Chamberlin AR, Diala ES. J. Am. Chem. Soc. 1989;111:8013. [Google Scholar]
- 389.Monahan SL, Lester HA, Dougherty DA. Chem. Biol. 2003;10:573. doi: 10.1016/s1074-5521(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 390.Cropp TA, Schultz PG. Trends in Genetics. 2004;20:625. doi: 10.1016/j.tig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 391.Zhang ZW, Alfonta L, Tian F, Bursulaya B, Uryu S, King DS, Schultz PG. Proc. Nat. Acad. Sci. U.S.A. 2004;101:8882. doi: 10.1073/pnas.0307029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Liu WS, Brock A, Chen S, Chen SB, Schultz PG. Nat. Methods. 2007;4:239. doi: 10.1038/nmeth1016. [DOI] [PubMed] [Google Scholar]
- 393.Hohsaka T, Ashizuka Y, Taira H, Murakami H, Sisido M. Biochemistry. 2001;40:11060. doi: 10.1021/bi0108204. [DOI] [PubMed] [Google Scholar]
- 394.Hohsaka T, Sisido M. Curr. Opin. Chem. Biol. 2002;6:809. doi: 10.1016/s1367-5931(02)00376-9. [DOI] [PubMed] [Google Scholar]
- 395.Cornish VW, Mendel D, Schultz PG. Angew. Chem. Int. Ed. 1995;34:621. [Google Scholar]
- 396.Behrens C, Nielsen JN, Fan XJ, Doisy X, Kim KH, Praetorius-Ibba M, Nielsen PE, Ibba M. Tetrahedron. 2000;56:9443. [Google Scholar]
- 397.Bentin T, Hamzavi R, Salomonsson J, Roy H, Ibba M, Nielsen PE. J. Biol. Chem. 2004;279:19839. doi: 10.1074/jbc.M401278200. [DOI] [PubMed] [Google Scholar]
- 398.Schmidt R, Wilkes BC, Chung NN, Lemieux C, Schiller PW. Int. J. Pept. Protein Res. 1996;48:411. [PubMed] [Google Scholar]
- 399.Doi Y, Ohtsuki T, Shimizu Y, Ueda T, Sisido M. J. Am. Chem. Soc. 2007;129:14458. doi: 10.1021/ja075557u. [DOI] [PubMed] [Google Scholar]
- 400.Lammek B, Czaja M, Derdowska I, Rekowski P, Trzeciak HI, Sikora P, Szkrobka W, Stojko R, Kupryszewski G. J. Pept. Res. 1997;49:261. doi: 10.1111/j.1399-3011.1997.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 401.Mihara H, Lee S, Shimohigashi Y, Aoyagi H, Kato T, Izumiya N, Costa T. Int. J. Pept. Protein Res. 1987;30:605. doi: 10.1111/j.1399-3011.1987.tb03371.x. [DOI] [PubMed] [Google Scholar]
- 402.Imaizumi M, Harada M, Sisido M. J. Phys. Chem. 1995;99:3810. [Google Scholar]
- 403.Mihara H, Nishino N, Fujimoto T. Chem. Lett. 1992:1809. [Google Scholar]
- 404.Shinohara H, Matsubara T, Sisido M. Macromolecules. 1997;30:2657. [Google Scholar]
- 405.Torok K, Cowley DJ, Brandmeier BD, Howell S, Aitken A, Trentham DR. Biochemistry. 1998;37:6188. doi: 10.1021/bi972773e. [DOI] [PubMed] [Google Scholar]
- 406.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Nature. 1999;399:159. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 407.Sisido M, Ishikawa Y, Itoh K, Tazuke S. Macromolecules. 1991;24:3993. [Google Scholar]
- 408.Watson JD. Molecular biology of the gene. 6th ed. Cold Spring Harbor: Pearson/Benjamin Cummings; Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 409.Bartel DP. Cell. 2009;136:215. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Eddy SR. Nat. Rev. Genet. 2001;2:919. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 411.Mattick JS, Makunin IV. Hum Mol Genet. 2006;15:R17. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 412.Montange RK, Batey RT. Ann Rev Biophys. 2008;37:117. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 413.Munninger KO, Chang SH. Biochem. Biophys. Res. Commun. 1972;46:1837. doi: 10.1016/0006-291x(72)90059-9. [DOI] [PubMed] [Google Scholar]
- 414.Maelicke A, Vonderha F, Cramer F. Biopolymers. 1973;12:27. doi: 10.1002/bip.1973.360120104. [DOI] [PubMed] [Google Scholar]
- 415.Paszyc S, Rafalska M. Nucleic Acids Res. 1979;6:385. doi: 10.1093/nar/6.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.McCloskey JA, Crain PF, Edmonds CG, Gupta R, Hashizume T, Phillipson DW, Stetter KO. Nucleic Acids Res. 1987;15:683. doi: 10.1093/nar/15.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Stimson SMM, Reuter SMA. J. Am. Chem. Soc. 1941;63:697. [Google Scholar]
- 418.Peon J, Zewail AH. Chem. Phys. Lett. 2001;348:255. [Google Scholar]
- 419.Serrano-Andres L, Merchan M. J. Photochem. Photobiol., C. 2009;10:21. [Google Scholar]
- 420.Leonard NJ, Tolman GL. Ann. N. Y. Acad. Sci. 1975;255:43. doi: 10.1111/j.1749-6632.1975.tb29212.x. [DOI] [PubMed] [Google Scholar]
- 421.Miller GP, Silverman AP, Kool ET. Bioorg. Med. Chem. 2008;16:56. doi: 10.1016/j.bmc.2007.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Wanninger-Weiss C, Valis L, Wagenknecht HA. Bioorg. Med. Chem. 2008;16:100. doi: 10.1016/j.bmc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 423.Samain F, Malinovskii VL, Langenegger SM, Häner R. Bioorg. Med. Chem. 2008;16:27. doi: 10.1016/j.bmc.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 424.Bethge L, Jarikote DV, Seitz O. Bioorg. Med. Chem. 2008;16:114. doi: 10.1016/j.bmc.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 425.Sprecher CA, Johnson WC. Biopolymers. 1977;16:2243. doi: 10.1002/bip.1977.360161012. [DOI] [PubMed] [Google Scholar]
- 426.Callis PR. Annu. Rev. Phys. Chem. 1983;34:329. [Google Scholar]
- 427.Wilson JN, Kool ET. Org. Biomol. Chem. 2006;4:4265. doi: 10.1039/b612284c. [DOI] [PubMed] [Google Scholar]
- 428.Brauns EB, Madaras ML, Coleman RS, Murphy CJ, Berg MA. J. Am. Chem. Soc. 1999;121:11644. [Google Scholar]
- 429.Strässler C, Davis NE, Kool ET. Helv. Chim. Acta. 1999;82:2160. doi: 10.1002/(sici)1522-2675(19991215)82:12<2160::aid-hlca2160>3.0.co;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Coleman RS, Mortensen MA. Tetrahedron Lett. 2003;44:1215. [Google Scholar]
- 431.Okamoto A, Tainaka K, Fujiwara Y. J. Org. Chem. 2006;71:3592. doi: 10.1021/jo060168o. [DOI] [PubMed] [Google Scholar]
- 432.Matray TJ, Kool ET. Nature. 1999;399:704. doi: 10.1038/21453. [DOI] [PubMed] [Google Scholar]
- 433.Gao JM, Strassler C, Tahmassebi D, Kool ET. J. Am. Chem. Soc. 2002;124:11590. doi: 10.1021/ja027197a. [DOI] [PubMed] [Google Scholar]
- 434.Gao JM, Watanabe S, Kool ET. J. Am. Chem. Soc. 2004;126:12748. doi: 10.1021/ja046910o. [DOI] [PubMed] [Google Scholar]
- 435.Ren RXF, Chaudhuri NC, Paris PL, Rumney S, Kool ET. J.Am. Chem. Soc. 1996;118:7671. doi: 10.1021/ja9612763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Wilson JN, Gao JM, Kool ET. Tetrahedron. 2007;63:3427. doi: 10.1016/j.tet.2006.07.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Teo YN, Kool ET. Bioconjugate Chem. 2009;20:2371. doi: 10.1021/bc9003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Teo YN, Wilson JN, Kool ET. Chem. Eur. J. 2009;15:11551. doi: 10.1002/chem.200901607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Teo YN, Wilson JN, Kool ET. J. Am. Chem. Soc. 2009;131:3923. doi: 10.1021/ja805502k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Coleman RS, Madaras ML. J. Org. Chem. 1998;63:5700. [Google Scholar]
- 441.Kitamura N, Fukagawa T, Kohtani S, Kitoh S, Kunimoto KK, Nakagaki RJ. Photochem. Photobiol., A. 2007;188:378. [Google Scholar]
- 442.Horng ML, Gardecki JA, Papazyan A, Maroncelli M. J. Phys. Chem. 1995;99:17311. [Google Scholar]
- 443.Moog RS, Davis WW, Ostrowski SG, Wilson GL. Chem. Phys. Lett. 1999;299:265. [Google Scholar]
- 444.Andreatta D, Perez Lustres JL, Kovalenko SA, Ernsting NP, Murphy CJ, Coleman RS, Berg MA. J. Am. Chem. Soc. 2005;127:7270. doi: 10.1021/ja044177v. [DOI] [PubMed] [Google Scholar]
- 445.Andreatta D, Sen S, Perez Lustres JL, Kovalenko SA, Ernsting NP, Murphy CJ, Coleman RS, Berg MA. J. Am. Chem. Soc. 2006;128:6885. doi: 10.1021/ja0582105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Brauns EB, Madaras ML, Coleman RS, Murphy CJ, Berg MA. Phys. Rev. Lett. 2002;88:158101. doi: 10.1103/PhysRevLett.88.158101. [DOI] [PubMed] [Google Scholar]
- 447.Gearheart LA, Somoza MM, Rivers WE, Murphy CJ, Coleman RS, Berg MA. J. Am. Chem. Soc. 2003;125:11812. doi: 10.1021/ja0363617. [DOI] [PubMed] [Google Scholar]
- 448.Sen S, Gearheart LA, Rivers E, Liu H, Coleman RS, Murphy CJ, Berg MA. J. Phys. Chem. B. 2006;110:13248. doi: 10.1021/jp056327+. [DOI] [PubMed] [Google Scholar]
- 449.Sen S, Paraggio NA, Gearheart LA, Connor EE, Issa A, Coleman RS, Wilson DM, 3rd, Wyatt MD, Berg MA. Biophys. J. 2005;89:4129. doi: 10.1529/biophysj.105.062695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Somoza MM, Andreatta D, Murphy CJ, Coleman RS, Berg MA. Nucleic Acids Res. 2004;32:2494. doi: 10.1093/nar/gkh577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Coleman RS, Berg MA, Murphy CJ. Tetrahedron. 2007;63:3450. [Google Scholar]
- 452.Berg MA, Coleman RS, Murphy CJ. Phys. Chem. Chem. Phys. 2008;10:1229. doi: 10.1039/b715272h. [DOI] [PubMed] [Google Scholar]
- 453.Grigorenko NA, Leumann CJ. Chem. Eur. J. 2009;15:639. doi: 10.1002/chem.200801135. [DOI] [PubMed] [Google Scholar]
- 454.Grigorenko NA, Leumann CJ. Chem. Commun. 2008;42:5417. doi: 10.1039/b810751c. [DOI] [PubMed] [Google Scholar]
- 455.Hawkins ME, Ludwig Brand, Michael LJ. Methods Enzymol. Vol. Volume 450. Academic Press; 2008. p. 201. [Google Scholar]
- 456.Hawkins ME. Cell. Biochem. Biophys. 2001;34:257. doi: 10.1385/CBB:34:2:257. [DOI] [PubMed] [Google Scholar]
- 457.Hawkins ME, Pfleiderer W, Mazumder A, Pommier YG, Falls FM. Nucleic Acids Res. 1995;23:2872. doi: 10.1093/nar/23.15.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Stanley RJ, Hou ZJ, Yang AP, Hawkins ME. J. Phys. Chem. B. 2005;109:3690. doi: 10.1021/jp0455982. [DOI] [PubMed] [Google Scholar]
- 459.Driscoll SL, Hawkins ME, Balis FM, Pfleiderer W, Laws WR. Biophys. J. 1997;73:3277. doi: 10.1016/S0006-3495(97)78352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Hawkins ME, Pfleiderer W, Balis FM, Porter D, Knutson JR. Anal. Biochem. 1997;244:86. doi: 10.1006/abio.1996.9879. [DOI] [PubMed] [Google Scholar]
- 461.Godde F, Aupeix K, Moreau S, Toulmé J-J. Antisense Nucleic Acid Drug Dev. 1998;8:469. doi: 10.1089/oli.1.1998.8.469. [DOI] [PubMed] [Google Scholar]
- 462.Secrist JA, Weber G, Leonard NJ, Barrio JR. Biochemistry. 1972;11:3499. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- 463.Secrist JA, Barrio JR, Leonard NJ. Science. 1972;175:646. doi: 10.1126/science.175.4022.646. [DOI] [PubMed] [Google Scholar]
- 464.Scopes DIC, Barrio JR, Leonard NJ. Science. 1977;195:296. doi: 10.1126/science.188137. [DOI] [PubMed] [Google Scholar]
- 465.Kochetko NK, Shibaev VN, Kost AA. Tetrahedron Lett. 1971;12:1993. [Google Scholar]
- 466.RajBhandary UL, Chang SH, Stuart A, Faulkner RD, Hoskinson RM, Khorana HG. Proc. Nat. Acad. Sci. U.S.A. 1967;57:751. doi: 10.1073/pnas.57.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Rist MJ, Marino JP. Curr. Org. Chem. 2002;6:775. [Google Scholar]
- 468.Seela F, Schweinberger E, Xu KY, Sirivolu VR, Rosemeyer H, Becker EM. Tetrahedron. 2007;63:3471. [Google Scholar]
- 469.Liu H, Gao J, Maynard L, Saito YD, Kool ET. J. Am. Chem. Soc. 2004;126:1102. doi: 10.1021/ja038384r. [DOI] [PubMed] [Google Scholar]
- 470.Gao J, Liu H, Kool ET. J. Am. Chem. Soc. 2004;126:11826. doi: 10.1021/ja048499a. [DOI] [PubMed] [Google Scholar]
- 471.Lee AH, Kool ET. J. Org. Chem. 2005;70:132. doi: 10.1021/jo0483973. [DOI] [PubMed] [Google Scholar]
- 472.Lee AH, Kool ET. J. Am. Chem. Soc. 2006;128:9219. doi: 10.1021/ja0619004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Liu H, Gao J, Kool ET. J. Am. Chem. Soc. 2005;127:1396. doi: 10.1021/ja046305l. [DOI] [PubMed] [Google Scholar]
- 474.Liu H, Gao J, Kool ET. J. Org. Chem. 2005;70:639. doi: 10.1021/jo048357z. [DOI] [PubMed] [Google Scholar]
- 475.Lu H, He K, Kool ET. Angew. Chem. Int. Ed. 2004;43:5834. doi: 10.1002/anie.200461036. [DOI] [PubMed] [Google Scholar]
- 476.Godde F, Toulme JJ, Moreau S. Nucleic Acids Res. 2000;28:2977. doi: 10.1093/nar/28.15.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Godde F, Toulmé JJ, Moreau S. Biochemistry. 1998;37:13765. doi: 10.1021/bi9811967. [DOI] [PubMed] [Google Scholar]
- 478.Lin KY, Jones RJ, Matteucci M. J. Am. Chem. Soc. 1995;117:3873. [Google Scholar]
- 479.Engman KC, Sandin P, Osborne S, Brown T, Billeter M, Lincoln P, Nordén B, Albinsson B, Wilhelmsson LM. Nucleic Acids Res. 2004;32:5087. doi: 10.1093/nar/gkh844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Wilhelmsson LM, Holmén A, Lincoln P, Nielsen PE, Nordén B. J. Am. Chem. Soc. 2001;123:2434. doi: 10.1021/ja0025797. [DOI] [PubMed] [Google Scholar]
- 481.Sandin P, Wilhelmsson LM, Lincoln P, Powers VE, Brown T, Albinsson B. Nucleic Acids Res. 2005;33:5019. doi: 10.1093/nar/gki790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Wilhelmsson LM, Sandin P, Holmen A, Albinsson B, Lincoln P, Norden B. J. Phys. Chem. B. 2003;107:9094. [Google Scholar]
- 483.Sandin P, Stengel G, Ljungdahl T, Borjesson K, Macao B, Wilhelmsson LM. Nucleic Acids Res. 2009;37:3924. doi: 10.1093/nar/gkp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Porterfield W, Tahmassebi DC. Bioorg. Med. Chem. Lett. 2009;19:111. doi: 10.1016/j.bmcl.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 485.Tahmassebi DC, Millar DP. Biochem. Biophys. Res. Commun. 2009;380:277. doi: 10.1016/j.bbrc.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Lin KY, Matteucci MD. J. Am. Chem. Soc. 1998;120:8531. [Google Scholar]
- 487.Flanagan WM, Wolf JJ, Olson P, Grant D, Lin K-Y, Wagner RW, Matteucci MD. Proc. Nat. Acad. Sci. U.S.A. 1999;96:3513. doi: 10.1073/pnas.96.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Nakagawa O, Ono S, Li Z, Tsujimoto A, Sasaki S. Angew. Chem. Int. Ed. 2007;46:4500. doi: 10.1002/anie.200700671. [DOI] [PubMed] [Google Scholar]
- 489.Nakagawa O, Ono S, Tsujimoto A, Li Z, Sasaki S. Nucleosides Nucleotides Nucleic Acids. 2007;26:645. doi: 10.1080/15257770701490498. [DOI] [PubMed] [Google Scholar]
- 490.Nasr T, Li Z, Nakagawa O, Taniguchi Y, Ono S, Sasaki S. Bioorg. Med. Chem. Lett. 2009;19:727. doi: 10.1016/j.bmcl.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 491.Okamoto A, Saito Y, Saito I. J. Photochem. Photobiol., C. 2005;6:108. [Google Scholar]
- 492.Okamoto A, Tainaka K, Saito I. J. Am. Chem. Soc. 2003;125:4972. doi: 10.1021/ja034090u. [DOI] [PubMed] [Google Scholar]
- 493.Okamoto A, Tainaka K, Saito I. Tetrahedron Lett. 2003;44:6871. [Google Scholar]
- 494.Okamoto A, Tanaka K, Fukuta T, Saito I. J. Am. Chem. Soc. 2003;125:9296. doi: 10.1021/ja035408l. [DOI] [PubMed] [Google Scholar]
- 495.Miyata K, Mineo R, Tamamushi R, Mizuta M, Ohkubo A, Taguchi H, Seio K, Santa T, Sekine M. J. Org. Chem. 2007;72:102. doi: 10.1021/jo0617767. [DOI] [PubMed] [Google Scholar]
- 496.Mizuta M, Seio K, Miyata K, Sekine M. J. Org. Chem. 2007;72:5046. doi: 10.1021/jo070206j. [DOI] [PubMed] [Google Scholar]
- 497.Hudson RHE, Ghorbani-Choghamarani A. Org. Biomol. Chem. 2007;5:1845. doi: 10.1039/b705805e. [DOI] [PubMed] [Google Scholar]
- 498.Okamoto A, Tainaka K, Unzai T, Saito I. Tetrahedron. 2007;63:3465. [Google Scholar]
- 499.Tainaka K, Tanaka K, Ikeda S, Nishiza K, Unzai T, Fujiwara Y, Saito I, Okamoto A. J. Am. Chem. Soc. 2007;129:4776. doi: 10.1021/ja069156a. [DOI] [PubMed] [Google Scholar]
- 500.Kimura T, Kawai K, Majima T. Org. Lett. 2005;7:5829. doi: 10.1021/ol052473m. [DOI] [PubMed] [Google Scholar]
- 501.Barawkar DA, Ganesh KN. Nucleic Acids Res. 1995;23:159. doi: 10.1093/nar/23.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Ryu JH, Seo YJ, Hwang GT, Lee JY, Kim BH. Tetrahedron. 2007;63:3538. [Google Scholar]
- 503.Ehrenschwender T, Wagenknecht H-A. Synthesis. 2008;2008:3657. [Google Scholar]
- 504.Seela F, Zulauf M. Chem. Eur. J. 1998;4:1781. [Google Scholar]
- 505.Seela F, Zulauf M, Sauer M, Deimel M. Helv. Chim. Acta. 2000;83:910. [Google Scholar]
- 506.Zhao Y, Baranger AM. J. Am. Chem. Soc. 2003;125:2480. doi: 10.1021/ja021267w. [DOI] [PubMed] [Google Scholar]
- 507.Zhao Y, Knee JL, Baranger AM. Bioorg. Chem. 2008;36:271. doi: 10.1016/j.bioorg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Kerr CE, Mitchell CD, Headrick J, Eaton BE, Netzel TL. J. Phys. Chem. B. 2000;104:1637. [Google Scholar]
- 509.Netzel TL, Zhao M, Nafisi K, Headrick J, Sigman MS, Eaton BE. J. Am. Chem. Soc. 1995;117:9119. [Google Scholar]
- 510.Netzel TL. Tetrahedron. 2007;63:3491. [Google Scholar]
- 511.Korshun VA, Prokhorenko IA, Gontarev SV, Skorobogatyi MV, Balakin KV, Manasova EV, Malakhov AD, Berlin YA. Nucleosides, Nucleotides Nucleic Acids. 1997;16:1461. [Google Scholar]
- 512.Venkatesan N, Seo YJ, Bang EK, Park SM, Lee YS, Kim BH. Bull. Korean Chem. Soc. 2006;27:613. [Google Scholar]
- 513.Wagenknecht HA. Ann. N. Y. Acad. Sci. 2008;1130:122. doi: 10.1196/annals.1430.001. [DOI] [PubMed] [Google Scholar]
- 514.Okamoto A, Tainaka K, Nishiza K, Saito I. J. Am. Chem. Soc. 2005;127:13128. doi: 10.1021/ja053609e. [DOI] [PubMed] [Google Scholar]
- 515.Thoresen LH, Jiao GS, Haaland WC, Metzker ML, Burgess K. Chem. Eur. J. 2003;9:4603. doi: 10.1002/chem.200304944. [DOI] [PubMed] [Google Scholar]
- 516.Whitcombe D, Theaker J, Guy SP, Brown T, Little S. Nat. Biotechnol. 1999;17:804. doi: 10.1038/11751. [DOI] [PubMed] [Google Scholar]
- 517.Seela F, Feiling E, Gross J, Hillenkamp F, Ramzaeva N, Rosemeyer H, Zulauf M. J. Biotechnol. 2001;86:269. doi: 10.1016/s0168-1656(00)00418-1. [DOI] [PubMed] [Google Scholar]
- 518.McKeen CM, Brown LJ, Nicol JTG, Mellor JM, Brown T. Org. Biomol. Chem. 2003;1:2267. doi: 10.1039/b301859h. [DOI] [PubMed] [Google Scholar]
- 519.May JP, Brown LJ, van Delft I, Thelwell N, Harley K, Brown T. Org. Biomol. Chem. 2005;3:2534. doi: 10.1039/b504759e. [DOI] [PubMed] [Google Scholar]
- 520.Ranasinghe RT, Brown T. Chem. Commun. 2005;44:5487. doi: 10.1039/b509522k. [DOI] [PubMed] [Google Scholar]
- 521.Ranasinghe RT, Rusling DA, Powers VEC, Fox KR, Brown T. Chem. Commun. 2005;20:2555. doi: 10.1039/b502325d. [DOI] [PubMed] [Google Scholar]
- 522.Rusling DA, Powers VEC, Ranasinghe RT, Wang Y, Osborne SD, Brown T, Fox KR. Nucleic Acids Res. 2005;33:3025. doi: 10.1093/nar/gki625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Marti AA, Li XX, Jockusch S, Stevens N, Li ZM, Raveendra B, Kalachikov S, Morozova I, Russo JJ, Akins DL, Ju JY, Turro NJ. Tetrahedron. 2007;63:3591. doi: 10.1016/j.tet.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ikeda S, Okamoto A. Chem. Asian J. 2008;3:958. doi: 10.1002/asia.200800014. [DOI] [PubMed] [Google Scholar]
- 525.Kubota T, Ikeda S, Okamoto A. Bull. Chem. Soc. Jpn. 2009;82:110. [Google Scholar]
- 526.Kubota T, Ikeda S, Yanagisawa H, Yuki M, Okamoto A. Bioconjugate Chem. 2009;20:1256. doi: 10.1021/bc900120a. [DOI] [PubMed] [Google Scholar]
- 527.Ikeda S, Kubota T, Yuki M, Okamoto A. Angew. Chem. Int. Ed. 2009;48:6480. doi: 10.1002/anie.200902000. [DOI] [PubMed] [Google Scholar]
- 528.Ikeda S, Yuki M, Yanagisawa H, Okamoto A. Tetrahedron Lett. 2009;50:7191. [Google Scholar]
- 529.Tzalis D, Tor Y. Tetrahedron Lett. 1995;36:6017. [Google Scholar]
- 530.Joshi HS, Jamshidi R, Tor Y. Angew. Chem. Int. Ed. 1999;38:2722. doi: 10.1002/(sici)1521-3773(19990917)38:18<2721::aid-anie2721>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 531.Hurley DJ, Seaman SE, Mazura JC, Tor Y. Org. Lett. 2002;4:2305. doi: 10.1021/ol026043x. [DOI] [PubMed] [Google Scholar]
- 532.Hurley DJ, Tor Y. J. Am. Chem. Soc. 1998;120:2194. [Google Scholar]
- 533.Hurley DJ, Tor Y. J. Am. Chem. Soc. 2002;124:13231. doi: 10.1021/ja020172r. [DOI] [PubMed] [Google Scholar]
- 534.Hurley DJ, Tor Y. J. Am. Chem. Soc. 2002;124:3749. doi: 10.1021/ja0123103. [DOI] [PubMed] [Google Scholar]
- 535.Kalachova L, Pohl R, Hocek M. Synthesis. 2009:105. [Google Scholar]
- 536.Vrabel M, Pohl R, Klepetarova B, Votruba I, Hocek M. Org. Biomol. Chem. 2007;5:2849. doi: 10.1039/b709245h. [DOI] [PubMed] [Google Scholar]
- 537.Vrabel M, Pohl R, Votruba I, Sajadi M, Kovalenko SA, Ernsting NP, Hocek M. Org. Biomol. Chem. 2008;6:2852. doi: 10.1039/b805632c. [DOI] [PubMed] [Google Scholar]
- 538.Vrabel M, Horakova P, Pivonkova H, Kalachova L, Cernocka H, Cahova H, Pohl R, Sebest P, Havran L, Hocek M, Fojta M. Chem. Eur. J. 2009;15:1144. doi: 10.1002/chem.200801538. [DOI] [PubMed] [Google Scholar]
- 539.Khan SI, Beilstein AE, Grinstaff MW. Inorg. Chem. 1999;38:418. doi: 10.1021/ic9806636. [DOI] [PubMed] [Google Scholar]
- 540.Khan SI, Beilstein AE, Smith GD, Sykora M, Grinstaff MW. Inorg. Chem. 1999;38:2411. [Google Scholar]
- 541.Weizman H, Tor Y. J. Am. Chem. Soc. 2002;124:1568. doi: 10.1021/ja017193q. [DOI] [PubMed] [Google Scholar]
- 542.Gilbert SD, Batey RT. Riboswitches methods and protocols - Monitoring RNA–Ligand Interactions Using Isothermal Titration Calorimetry. Totowa, NJ: Humana Press; 2009. [DOI] [PubMed] [Google Scholar]
- 543.Wierzchowski J, Shugar D. Photochem. Photobiol. 1982;35:445. [Google Scholar]
- 544.Wu P, Nordlund TM, Gildea B, McLaughlin LW. Biochemistry. 1990;29:6508. doi: 10.1021/bi00479a024. [DOI] [PubMed] [Google Scholar]
- 545.Seela F, Chen YM. Nucleic Acids Res. 1995;23:2499. doi: 10.1093/nar/23.13.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Dyrager C, Borjesson K, Diner P, Elf A, Albinsson B, Wilhelmsson LM, Grotli M. Eur. J. Org. Chem. 2009:1515. [Google Scholar]
- 547.Seaman SE. Nucleosides as Probes for DNA Structure and Recognition; Ph.D. San Diego, La Jolla: University of California; 2004. [Google Scholar]
- 548.Seo YJ, Bhuniya S, Tapadar S, Yi JW, Kim BH. Bull. Korean Chem. Soc. 2007;28:1923. [Google Scholar]
- 549.Rao P, Benner SA. J. Org. Chem. 2001;66:5012. doi: 10.1021/jo005743h. [DOI] [PubMed] [Google Scholar]
- 550.Ward DC, Reich E, Stryer L. J. Biol. Chem. 1969;244:1228. [PubMed] [Google Scholar]
- 551.Evans K, Xu D, Kim Y, Nordlund TM. J. Fluorescence. 1992;2:209. doi: 10.1007/BF00865278. [DOI] [PubMed] [Google Scholar]
- 552.Seela F, Becher G. Helv. Chim. Acta. 2000;83:928. [Google Scholar]
- 553.Nordlund TM, Andersson S, Nilsson L, Rigler R, Graslund A, Mclaughlin LW. Biochemistry. 1989;28:9095. doi: 10.1021/bi00449a021. [DOI] [PubMed] [Google Scholar]
- 554.Sowers LC, Shaw BR, Veigl ML, Sedwick WD. Mutat. Res. 1987;177:201. doi: 10.1016/0027-5107(87)90003-0. [DOI] [PubMed] [Google Scholar]
- 555.Sowers LC, Fazakerley GV, Eritja R, Kaplan BE, Goodman MF. Proc. Nat. Acad. Sci. U.S.A. 1986;83:5434. doi: 10.1073/pnas.83.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Sowers LC, Boulard Y, Fazakerley GV. Biochemistry. 2000;39:7613. doi: 10.1021/bi992388k. [DOI] [PubMed] [Google Scholar]
- 557.Nordlund TM, Xu DG, Evans KO. Biochemistry. 1993;32:12090. doi: 10.1021/bi00096a020. [DOI] [PubMed] [Google Scholar]
- 558.Nordlund TM. Photochem. Photobiol. 2007;83:625. doi: 10.1562/2006-04-05-IR-877. [DOI] [PubMed] [Google Scholar]
- 559.Davis SP, Matsumura M, Williams A, Nordlund TM. J Fluorescence. 2003;13:249. [Google Scholar]
- 560.Kawai M, Lee MJ, Evans KO, Nordlund TM. J. Fluorescence. 2001;11:23. [Google Scholar]
- 561.Xu DG, Nordlund TM. Biophys. J. 2000;78:1042. doi: 10.1016/S0006-3495(00)76663-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Serrano-Andres L, Merchan M, Borin AC. Proc. Nat. Acad. Sci. U.S.A. 2006;103:8691. doi: 10.1073/pnas.0602991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Mishra SK, Shukla MK, Mishra PC. Spectrochim. Acta, Part A. 2000;56A:1355. doi: 10.1016/s1386-1425(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 564.Mennucci B, Toniolo A, Tomasi J. J. Phys. Chem. A. 2001;105:4749. [Google Scholar]
- 565.Nir E, Kleinermanns K, Grace L, de Vries MS. J. Phys. Chem. A. 2001;105:5106. [Google Scholar]
- 566.Broo A. J. Phys. Chem. A. 1998;102:526. [Google Scholar]
- 567.Gaied NB, Glasser N, Ramalanjaona N, Beltz H, Wolff P, Marquet R, Burger A, Mely Y. Nucleic Acids Res. 2005;33:1031. doi: 10.1093/nar/gki253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Kenfack CA, Burger A, Mely Y. J. Phys. Chem. B. 2006;110:26327. doi: 10.1021/jp064388h. [DOI] [PubMed] [Google Scholar]
- 569.Kimoto M, Mitsui T, Harada Y, Sato A, Yokoyama S, Hirao I. Nucleic Acids Res. 2007;35:5360. doi: 10.1093/nar/gkm508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Kawai R, Kimoto M, Ikeda S, Mitsui T, Endo M, Yokoyama S, Hirao I. J. Am. Chem. Soc. 2005;127:17286. doi: 10.1021/ja0542946. [DOI] [PubMed] [Google Scholar]
- 571.Mitsui T, Kimoto M, Kawai R, Yokoyama S, Hirao I. Tetrahedron. 2007;63:3528. [Google Scholar]
- 572.Benner SA. Acc. Chem. Res. 2004;37:784. doi: 10.1021/ar040004z. [DOI] [PubMed] [Google Scholar]
- 573.Benner SA. Science. 2004;306:625. doi: 10.1126/science.1101104. [DOI] [PubMed] [Google Scholar]
- 574.Martinot TA, Benner SA. J. Org. Chem. 2004;69:3972. doi: 10.1021/jo0497959. [DOI] [PubMed] [Google Scholar]
- 575.Tor Y, Dervan PB. J. Am. Chem. Soc. 1993;115:4461. [Google Scholar]
- 576.Battersby TR, Albalos M, Friesenhahn M. J. Chem. Biol. 2007;14:525. doi: 10.1016/j.chembiol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 577.Shugar D, Kierdaszuk B. J. Biosci. 1985;8:657. [Google Scholar]
- 578.Yang Z, Hutter D, Sheng P, Sismour AM, Benner SA. Nucleic Acids Res. 2006;34:6095. doi: 10.1093/nar/gkl633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Marx A, Detmer I, Gaster J, Summerer D. Synthesis. 2004:1. [Google Scholar]
- 580.Kistler KA, Matsika S. Photochem. Photobiol. 2007;83:611. doi: 10.1562/2006-04-03-RA-866. [DOI] [PubMed] [Google Scholar]
- 581.Kistler KA, Matsika S. J. Phys. Chem. A. 2007;111:2650. doi: 10.1021/jp0663661. [DOI] [PubMed] [Google Scholar]
- 582.Connolly BA, Newman PC. Nucleic Acids Res. 1989;17:4957. doi: 10.1093/nar/17.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Gildea B, McLaughlin LW. Nucleic Acids Res. 1989;17:2261. doi: 10.1093/nar/17.6.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Rappaport HP. Biochem. J. 2004;381:709. doi: 10.1042/BJ20031776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Rappaport HP. Biochemistry. 1993;32:3047. doi: 10.1021/bi00063a016. [DOI] [PubMed] [Google Scholar]
- 586.Rappaport HP. Nucleic Acids Res. 1988;16:7253. doi: 10.1093/nar/16.15.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Buchini S, Leumann CJ. Eur. J. Org. Chem. 2006:3152. [Google Scholar]
- 588.Buchini S, Leumann CJ. Angew. Chem. Int. Ed. 2004;43:3925. doi: 10.1002/anie.200460159. [DOI] [PubMed] [Google Scholar]
- 589.Greco NJ, Tor Y. J Am Chem Soc. 2005;127:10784. doi: 10.1021/ja052000a. [DOI] [PubMed] [Google Scholar]
- 590.Greco NJ, Tor Y. Tetrahedron. 2007;63:3515. doi: 10.1016/j.tet.2007.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Srivatsan SG, Tor Y. J. Am. Chem. Soc. 2007;129:2044. doi: 10.1021/ja066455r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Greco NJ, Tor Y. Nat. Protoc. 2007;2:305. doi: 10.1038/nprot.2006.464. [DOI] [PubMed] [Google Scholar]
- 593.Srivatsan SG, Tor Y. Nat. Protoc. 2007;2:1547. doi: 10.1038/nprot.2007.222. [DOI] [PubMed] [Google Scholar]
- 594.Srivatsan SG, Tor Y. Chem-Asian J. 2009;4:419. doi: 10.1002/asia.200800370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Srivatsan SG, Tor Y. Tetrahedron. 2007;63:3601. doi: 10.1016/j.tet.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Greco NJ, Sinkeldam RW, Tor Y. Org. Lett. 2009;11:1115. doi: 10.1021/ol802656n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Srivatsan SG, Weizman H, Tor Y. Org. Biomol. Chem. 2008;6:1334. doi: 10.1039/b801054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Tor Y, Del Valle S, Jaramillo D, Srivatsan SG, Rios A, Weizman H. Tetrahedron. 2007;63:3608. [Google Scholar]
- 599.Srivatsan SG, Greco NJ, Tor Y. Angew. Chem. Int. Ed. 2008;47:6661. doi: 10.1002/anie.200802199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Xie Y, Dix AV, Tor Y. J. Am. Chem. Soc. 2009;131:17605. doi: 10.1021/ja905767g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Crisp GT, Flynn BL. J. Org. Chem. 1993;58:6614. [Google Scholar]
- 602.Robins MJ, Barr PJ. Tetrahedron Lett. 1981;22:421. [Google Scholar]
- 603.Robins MJ, Barr PJ. J. Org. Chem. 1983;48:1854. [Google Scholar]
- 604.McGuigan C, Yarnold CJ, Jones G, Velazquez S, Barucki H, Brancale A, Andrei G, Snoeck R, De Clercq E, Balzarini J. J. Med. Chem. 1999;42:4479. doi: 10.1021/jm990346o. [DOI] [PubMed] [Google Scholar]
- 605.Loakes D, Brown DM, Salisbury SA, McDougall MG, Neagu C, Nampalli S, Kumar S. Helv. Chim. Acta. 2003;86:1193. [Google Scholar]
- 606.Berry DA, Jung KY, Wise DS, Sercel AD, Pearson WH, Mackie H, Randolph JB, Somers RL. Tetrahedron Lett. 2004;45:2457. [Google Scholar]
- 607.Liu CH, Martin CT. J. Mol. Biol. 2001;308:465. doi: 10.1006/jmbi.2001.4601. [DOI] [PubMed] [Google Scholar]
- 608.Hudson RHE, Choghamarani AG. Nucleosides, Nucleotides and Nucleic Acids. 2007;26:533. doi: 10.1080/15257770701489839. [DOI] [PubMed] [Google Scholar]
- 609.Hudson RHE, Ghorbani-Choghamarani A. Synlett. 2007:870. [Google Scholar]
- 610.Esho N, Davies B, Lee J, Dembinski R. Chem. Commun. 2002;4:332. doi: 10.1039/b109501c. [DOI] [PubMed] [Google Scholar]
- 611.Seela F, Sirivolu VR. Org. Biomol. Chem. 2008;6:1674. doi: 10.1039/b719459e. [DOI] [PubMed] [Google Scholar]
- 612.Pesnot T, Wagner GK. Org. Biomol. Chem. 2008;6:2884. doi: 10.1039/b805216f. [DOI] [PubMed] [Google Scholar]
- 613.Butler RS, Cohn P, Tenzel P, Abboud KA, Castellano RK. J. Am. Chem. Soc. 2009;131:623. doi: 10.1021/ja806348z. [DOI] [PubMed] [Google Scholar]
- 614.Butler RS, Myers AK, Bellarmine P, Abboud KA, Castellano RK. J. Mater. Chem. 2007;17:1863. [Google Scholar]
- 615.Goodchild J. Bioconjugate Chem. 1990;1:165. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- 616.Verma S, Eckstein F. Annu. Rev. Biochem. 1998;67:99. doi: 10.1146/annurev.biochem.67.1.99. [DOI] [PubMed] [Google Scholar]
- 617.Chow CS, Mahto SK, Lamichhane TN. ACS Chem. Biol. 2008;3:30. doi: 10.1021/cb7002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Caruthers MH, Barone AD, Beaucage SL, Dodds DR, Fisher EF, McBride LJ, Matteucci M, Stabinsky Z, Tang JY. Methods Enzymol. 1987;Vol. 154:287. doi: 10.1016/0076-6879(87)54081-2. [DOI] [PubMed] [Google Scholar]
- 619.Beaucage SL, Iyer RP. Tetrahedron. 1992;48:2223. [Google Scholar]
- 620.Gait MJ. Oligonucleotide synthesis : a practical approach. Washington DC: Oxford University Press; 1984. [Google Scholar]
- 621.Froehler BC, Matteucci MD. Nucleic Acids Res. 1983;11:8031. doi: 10.1093/nar/11.22.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Pitsch S, Weiss PA, Jenny L, Stutz A, Wu XL. Helv. Chim. Acta. 2001;84:3773. [Google Scholar]
- 623.Scaringe SA, Wincott FE, Caruthers MH. J. Am. Chem. Soc. 1998;120:11820. [Google Scholar]
- 624.Scaringe SA. Methods. 2001;23:206. doi: 10.1006/meth.2000.1132. [DOI] [PubMed] [Google Scholar]
- 625.Da Costa CP, Fedor MJ, Scott LG. J. Am. Chem. Soc. 2007;129:3426. doi: 10.1021/ja067699e. [DOI] [PubMed] [Google Scholar]
- 626.Loakes D, Brown DM, Salisbury SA, McDougall MG, Neagu C, Nampalli S, Kumar S. Tetrahedron Lett. 2003;44:3387. [Google Scholar]
- 627.Borsenberger V, Kukwikila M, Howorka S. Org. Biomol. Chem. 2009;7:3826. doi: 10.1039/b906956a. [DOI] [PubMed] [Google Scholar]
- 628.Ludwig J. Acta Biochim. Biophys. Hung. 1981;16:131. [PubMed] [Google Scholar]
- 629.Burgess K, Cook D. Chem. Rev. 2000;100:2047. doi: 10.1021/cr990045m. [DOI] [PubMed] [Google Scholar]
- 630.Vaghefi M. Nucleoside Triphosphates and their Analogs: Chemistry, Biotechnology, and Biological Applications. Boca Raton: CRC Press, Taylor & Francis Group; 2005. [Google Scholar]
- 631.Hwang GT, Romesberg FE. Nucleic Acids Res. 2006;34:2037. doi: 10.1093/nar/gkl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Krueger AT, Kool ET. Chem. Biol. 2009;16:242. doi: 10.1016/j.chembiol.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Loakes D, Gallego J, Pinheiro VB, Kool ET, Holliger P. J. Am. Chem. Soc. 2009;131:14827. doi: 10.1021/ja9039696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Ogawa AK, Wu Y, McMinn DL, Liu J, Schultz PG, Romesberg FE. J. Am. Chem. Soc. 2000;122:3274. [Google Scholar]
- 635.Seo YJ, Matsuda S, Romesberg FE. J. Am. Chem. Soc. 2009;131:5046. doi: 10.1021/ja9006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Barone AD, Chen C, McGall GH, Rafii K, Buzby PR, Dimeo JJ. Nucleosides, Nucleotides Nucleic Acids. 2001;20:1141. doi: 10.1081/NCN-100002507. [DOI] [PubMed] [Google Scholar]
- 637.Vincent C, Tchen P, Cohen-Solal M, Kourilsky P. Nucleic Acids Res. 1982;10:6787. doi: 10.1093/nar/10.21.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Haralambidis J, Chai M, Tregear GW. Nucleic Acids Res. 1987;15:4857. doi: 10.1093/nar/15.12.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Cox WG, Singer VL. BioTechniques. 2004;36:114. doi: 10.2144/04361RR02. [DOI] [PubMed] [Google Scholar]
- 640.Asseline U. Curr. Org. Chem. 2006;10:491. [Google Scholar]
- 641.Cobb AJA. Org. Biomol. Chem. 2007;5:3260–3275. doi: 10.1039/b709797m. [DOI] [PubMed] [Google Scholar]
- 642.Cremo CR. Methods Enzymol. 2003;360:128. doi: 10.1016/s0076-6879(03)60109-6. [DOI] [PubMed] [Google Scholar]
- 643.Hawkins ME. Top. Fluoresc. Spectrosc. 2003;7:151. [Google Scholar]
- 644.Jameson DM, Eccleston JF. Methods Enzymol. 1997;278:363–390. doi: 10.1016/s0076-6879(97)78020-0. [DOI] [PubMed] [Google Scholar]
- 645.Dodd DW, Hudson RHE. Mini-Rev. Org. Chem. 2009;6:378. [Google Scholar]
- 646.Cheung VG, Gregg JP, Gogolin-Ewens KJ, Bandong J, Stanley CA, Baker L, Higgins MJ, Nowak NJ, Shows TB, Ewens WJ, Spielman RS. Nat. Genet. 1998;18:225. doi: 10.1038/ng0398-225. [DOI] [PubMed] [Google Scholar]
- 647.Howell WM, Jobs M, Gyllensten U, Brookes AJ. Nat. Biotechnol. 1999;17:87. doi: 10.1038/5270. [DOI] [PubMed] [Google Scholar]
- 648.Lyamichev V, Mast AL, Hall JG, Prudent JR, Kaiser MW, Takova T, Kwiatkowski RW, Sander TJ, de Arruda M, Arco DA, Neri BP, Brow MAD. Nat. Biotechnol. 1999;17:292. doi: 10.1038/7044. [DOI] [PubMed] [Google Scholar]
- 649.Pastinen T, Raitio M, Lindroos K, Tainola P, Peltonen L, Syvanen AC. Genome Res. 2000;10:1031. doi: 10.1101/gr.10.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Tyagi S, Bratu DP, Kramer FR. Nat. Biotechnol. 1998;16:49. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 651.Okamoto A, Tainaka K, Saito I. Chem. Lett. 2003;32:684. [Google Scholar]
- 652.Blackburn GM, Gait MJ. Nucleic acids in chemistry and biology. 2nd ed. Oxford, England; New York: Oxford University Press; 1996. [Google Scholar]
- 653.Bloomfield VA, Crothers DM, Tinoco I. Nucleic acids : structures, properties, and functions. Sausalito, Calif.: University Science Books; 2000. [Google Scholar]
- 654.Stoop M, Zahn A, Leumann CJ. Tetrahedron. 2007;63:3440. [Google Scholar]
- 655.Wojtuszewski K, Hawkins ME, Cole JL, Mukerji I. Biochemistry. 2001;40:2588. doi: 10.1021/bi002382r. [DOI] [PubMed] [Google Scholar]
- 656.Parsons J, Hermann T. Tetrahedron. 2007;63:3548. [Google Scholar]
- 657.Singleton SF, Roca AI, Lee AM, Xiao J. Tetrahedron. 2007;63:3553. doi: 10.1016/j.tet.2006.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Augustyn KE, Wojtuszewski K, Hawkins ME, Knutson JR, Mukerji I. Biochemistry. 2006;45:5039. doi: 10.1021/bi0518343. [DOI] [PubMed] [Google Scholar]
- 659.Arzumanov A, Godde F, Moreau S, Toulmè JJ, Weeds A, Gait MJ. Helv. Chim. Acta. 2000;83:1424. [Google Scholar]
- 660.Akiyama Y, Ma Q, Edgar E, Laikhter A, Hecht SM. Org. Lett. 2008;10:2127. doi: 10.1021/ol800445x. [DOI] [PubMed] [Google Scholar]
- 661.Borjesson K, Preus S, El-Sagheer AH, Brown T, Albinsson B, Wilhelmsson LM. J. Am. Chem. Soc. 2009;131:4288. doi: 10.1021/ja806944w. [DOI] [PubMed] [Google Scholar]
- 662.Cekan P, Sigurdsson ST. Chem. Commun. 2008;29:3393. doi: 10.1039/b801833b. [DOI] [PubMed] [Google Scholar]
- 663.Saito Y, Miyauchi Y, Okamoto A, Saito I. Tetrahedron Lett. 2004;45:7827. [Google Scholar]
- 664.Miyata K, Tamamushi R, Ohkubo A, Taguchi H, Seio K, Santa T, Sekine M. Org. Lett. 2006;8:1545. doi: 10.1021/ol053125n. [DOI] [PubMed] [Google Scholar]
- 665.Mizuta M, Seio K, Miyata K, Ohkubo A, Taguchi H, Sekine M. Nucleosides Nucleotides Nucleic Acids. 2007;26:1335. doi: 10.1080/15257770701533164. [DOI] [PubMed] [Google Scholar]
- 666.Okamoto A, Kanatani K, Saito I. J. Am. Chem. Soc. 2004;126:4820. doi: 10.1021/ja039625y. [DOI] [PubMed] [Google Scholar]
- 667.Tanaka K, Okamoto A. Bioorg. Med. Chem. 2008;16:400. doi: 10.1016/j.bmc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 668.Okamoto A, Ochi Y, Saito I. Bioorg. Med. Chem. Lett. 2005;15:4279. doi: 10.1016/j.bmcl.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 669.Okamoto A, Ochi Y, Saito I. Chem. Commun. 2005;9:1128. doi: 10.1039/b416965d. [DOI] [PubMed] [Google Scholar]
- 670.Mayer-Enthart E, Wagner C, Barbaric J, Wagenknecht HA. Tetrahedron. 2007;63:3434. [Google Scholar]
- 671.Seo YJ, Kim BH. Chem. Commun. 2006;2:150. doi: 10.1039/b514079j. [DOI] [PubMed] [Google Scholar]
- 672.Seo YJ, Hwang GT, Kim BH. Tetrahedron Lett. 2006;47:4037. [Google Scholar]
- 673.Hwang GT, Seo YJ, Kim BH. Tetrahedron Lett. 2005;46:1475. [Google Scholar]
- 674.Gaballah ST, Collier G, Netzel TL. J. Phys. Chem. B. 2005;109:12175. doi: 10.1021/jp044457x. [DOI] [PubMed] [Google Scholar]
- 675.Gaballah ST, Hussein YHA, Anderson N, Lian TQT, Netzel TL. J. Phys. Chem. A. 2005;109:10832. doi: 10.1021/jp053359o. [DOI] [PubMed] [Google Scholar]
- 676.Jeong HS, Kang S, Lee JY, Kim BH. Org. Biomol. Chem. 2009;7:921. doi: 10.1039/b816768k. [DOI] [PubMed] [Google Scholar]
- 677.Malakhov AD, Malakhova EV, Kuznitsova SV, Grechishnikova IV, Prokhorenko IA, Skorobogatyi MV, Korshun VA, Berlin YA. Bioorganicheskaya Khimiya. 2000;26:39. [PubMed] [Google Scholar]
- 678.Hwang GT, Seo YJ, Kim SJ, Kim BH. Tetrahedron Lett. 2004;45:3543. [Google Scholar]
- 679.Barbaric J, Wagenknecht HA. Org. Biomol. Chem. 2006;4:2088. doi: 10.1039/b605251g. [DOI] [PubMed] [Google Scholar]
- 680.Seo YJ, Ryu JH, Kim BH. Org. Lett. 2005;7:4931. doi: 10.1021/ol0518582. [DOI] [PubMed] [Google Scholar]
- 681.Trifonov A, Raytchev M, Buchvarov I, Rist M, Barbaric J, Wagenknecht HA, Fiebig T. J. Phys. Chem. B. 2005;109:19490. doi: 10.1021/jp052108c. [DOI] [PubMed] [Google Scholar]
- 682.Skorobogatyj MV, Pchelintseva AA, Ustinov AV, Korshun VA, Malakhov AD. Nucleosides Nucleotides Nucleic Acids. 2005;24:931. doi: 10.1081/ncn-200059283. [DOI] [PubMed] [Google Scholar]
- 683.Skorobogatyi MV, Kvach MV, Zhylinskaya MA, Yarmolinsky DG, Korshun VA, Shmanai VV. Nucleosides Nucleotides Nucleic Acids. 2007;26:767. doi: 10.1080/15257770701501187. [DOI] [PubMed] [Google Scholar]
- 684.Varghese R, Wagenknecht HA. Chem. Eur. J. 2009;15:9307. doi: 10.1002/chem.200901147. [DOI] [PubMed] [Google Scholar]
- 685.Wagner C, Rist M, Mayer-Enthart E, Wagenknecht HA. Org. Biomol. Chem. 2005;3:2062. doi: 10.1039/b504079e. [DOI] [PubMed] [Google Scholar]
- 686.Seo YJ, Bhuniya S, Kim BH. Chem. Commun. 2007;18:1804. doi: 10.1039/b618528b. [DOI] [PubMed] [Google Scholar]
- 687.Seo YJ, Lee IJ, Yi JW, Kim BH. Chem. Commun. 2007;27:2817. doi: 10.1039/b707278c. [DOI] [PubMed] [Google Scholar]
- 688.Seo YJ, Lee IJ, Kim BH. Bioorg. Med. Chem. Lett. 2008;18:3910. doi: 10.1016/j.bmcl.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 689.Seo YJ, Lee IJ, Kim BH. Mol. BioSyst. 2009;5:235. doi: 10.1039/b821543j. [DOI] [PubMed] [Google Scholar]
- 690.Seo YJ, Rhee H, Joo T, Kim BH. J. Am. Chem. Soc. 2007;129:5244. doi: 10.1021/ja069069i. [DOI] [PubMed] [Google Scholar]
- 691.Grunwald C, Kwon T, Piton N, Forster U, Wachtveitl J, Engels JW. Bioorg. Med. Chem. 2008;16:19. doi: 10.1016/j.bmc.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 692.Gaballah ST, Vaught JD, Eaton BE, Netzel TL. J. Phys. Chem. B. 2005;109:5927. doi: 10.1021/jp044968j. [DOI] [PubMed] [Google Scholar]
- 693.Seio K, Mizuta M, Tasaki K, Tamaki K, Ohkubo A, Sekine M. Bioorg. Med. Chem. 2008;16:8287. doi: 10.1016/j.bmc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 694.Saito Y, Miyauchi Y, Okamoto A, Saito I. Chem. Commun. 2004;15:1704. doi: 10.1039/b405832a. [DOI] [PubMed] [Google Scholar]
- 695.Okamoto A, Tainaka K, Saito I. Bioconjugate Chem. 2005;16:1105. doi: 10.1021/bc050077i. [DOI] [PubMed] [Google Scholar]
- 696.Kimura T, Kawai K, Majima T. Chem. Commun. 2006;14:1542. doi: 10.1039/b600026f. [DOI] [PubMed] [Google Scholar]
- 697.Barawkar DA, Ganesh KN. Biochem. Biophys. Res. Commun. 1994;203:53. doi: 10.1006/bbrc.1994.2147. [DOI] [PubMed] [Google Scholar]
- 698.Jadhav VR, Barawkar DA, Ganesh KN. J. Phys. Chem. B. 1999;103:7383. [Google Scholar]
- 699.Saito Y, Motegi K, Bag SS, Saito I. Bioorg. Med. Chem. 2008;16:107. doi: 10.1016/j.bmc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 700.Xiao Q, Ranasinghe RT, Tang AMP, Brown T. Tetrahedron. 2007;63:3483. [Google Scholar]
- 701.Marti AA, Jockusch S, Li ZM, Ju JY, Turro NJ. Nucleic Acids Res. 2006;34:e50. doi: 10.1093/nar/gkl134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Xu DG, Evans KO, Nordlund TM. Biochemistry. 1994;33:9592. doi: 10.1021/bi00198a027. [DOI] [PubMed] [Google Scholar]
- 703.Raney KD, Sowers LC, Millar DP, Benkovic SJ. Proc. Nat. Acad. Sci. U.S.A. 1994;91:6644. doi: 10.1073/pnas.91.14.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Kirk SR, Luedtke NW, Tor Y. Bioorg. Med. Chem. 2001;9:2295. doi: 10.1016/s0968-0896(01)00123-7. [DOI] [PubMed] [Google Scholar]
- 705.Goodman MF, Fygenson DK. Genetics. 1998;148:1475. doi: 10.1093/genetics/148.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Zhao C, Marino JP. Tetrahedron. 2007;63:3575. doi: 10.1016/j.tet.2006.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Kimura T, Kawai K, Fujitsuka M, Majima T. Tetrahedron. 2007;63:3585. [Google Scholar]
- 708.Johnson NP, Baase WA, von Hippel PH. Proc. Nat. Acad. Sci. U.S.A. 2004;101:3426. doi: 10.1073/pnas.0400591101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Nakano S-i, Uotani Y, Uenishi K, Fujii M, Sugimoto N. Nucl. Acids Res. 2005;33:7111. doi: 10.1093/nar/gki1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Rachofsky EL, Seibert E, Stivers JT, Osman R, Ross JBA. Biochemistry. 2001;40:957. doi: 10.1021/bi001665g. [DOI] [PubMed] [Google Scholar]
- 711.Stivers JT. Nucleic Acids Res. 1998;26:3837. doi: 10.1093/nar/26.16.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Sagher D, Strauss B. Nucleic Acids Res. 1985;13:4285. doi: 10.1093/nar/13.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Guest CR, Hochstrasser RA, Sowers LC, Millar DP. Biochemistry. 1991;30:3271. doi: 10.1021/bi00227a015. [DOI] [PubMed] [Google Scholar]
- 714.Barbieri CM, Kaul M, Pilch DS. Tetrahedron. 2007;63:3567. doi: 10.1016/j.tet.2006.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Tam VK, Kwong D, Tor Y. J. Am. Chem. Soc. 2007;129:3257. doi: 10.1021/ja0675797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Kaul M, Barbieri CM, Pilch DS. J. Am. Chem. Soc. 2004;126:3447. doi: 10.1021/ja030568i. [DOI] [PubMed] [Google Scholar]
- 717.Shandrick S, Zhao Q, Han Q, Ayida BK, Takahashi M, Winters GC, Simonsen KB, Vourloumis D, Hermann T. Angew. Chem. Int. Ed. 2004;43:3177. doi: 10.1002/anie.200454217. [DOI] [PubMed] [Google Scholar]
- 718.Fedoriw AM, Liu HY, Anderson VE, deHaseth PL. Biochemistry. 1998;37:11971. doi: 10.1021/bi980980o. [DOI] [PubMed] [Google Scholar]
- 719.Strainic MG, Sullivan JJ, Velevis A, deHaseth PL. Biochemistry. 1998;37:18074. doi: 10.1021/bi9813431. [DOI] [PubMed] [Google Scholar]
- 720.Sullivan JJ, Bjornson KP, Sowers LC, deHaseth PL. Biochemistry. 1997;36:8005. doi: 10.1021/bi970363k. [DOI] [PubMed] [Google Scholar]
- 721.Kenfack CA, Piemont E, Ben Gaied N, Burger A, Mely Y. J. Phys. Chem. B. 2008;112:9736. doi: 10.1021/jp8028243. [DOI] [PubMed] [Google Scholar]
- 722.Singleton SF, Shan F, Kanan MW, McIntosh CM, Stearman CJ, Helm JS, Webb KJ. Org. Lett. 2001;3:3919. doi: 10.1021/ol0167863. [DOI] [PubMed] [Google Scholar]
- 723.Johnson NP, Baase WA, von Hippel PH. Proc. Nat. Acad. Sci. U.S.A. 2005;102:7169. doi: 10.1073/pnas.0502359102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Chen P, He CA. J. Am. Chem. Soc. 2004;126:728. doi: 10.1021/ja0383975. [DOI] [PubMed] [Google Scholar]
- 725.Dash C, Rausch JW, Le Grice SF. J. Nucleic Acids Res. 2004;32:1539. doi: 10.1093/nar/gkh307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Liu CH, Martin CT. J. Biol. Chem. 2002;277:2725. doi: 10.1074/jbc.M108856200. [DOI] [PubMed] [Google Scholar]
- 727.Yang KS, Stanley RJ. Photochem. Photobiol. 2008;84:741. doi: 10.1111/j.1751-1097.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 728.Zhang CM, Liu CP, Christian T, Gamper H, Rozenski J, Pan DL, Randolph JB, Wickstrom E, Cooperman BS, Hou YM. RNA. 2008;14:2245. doi: 10.1261/rna.1158508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Saito Y, Shinohara Y, Bag SS, Takeuchi Y, Matsumoto K, Saito I. Tetrahedron. 2009;65:934. [Google Scholar]