Abstract
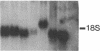
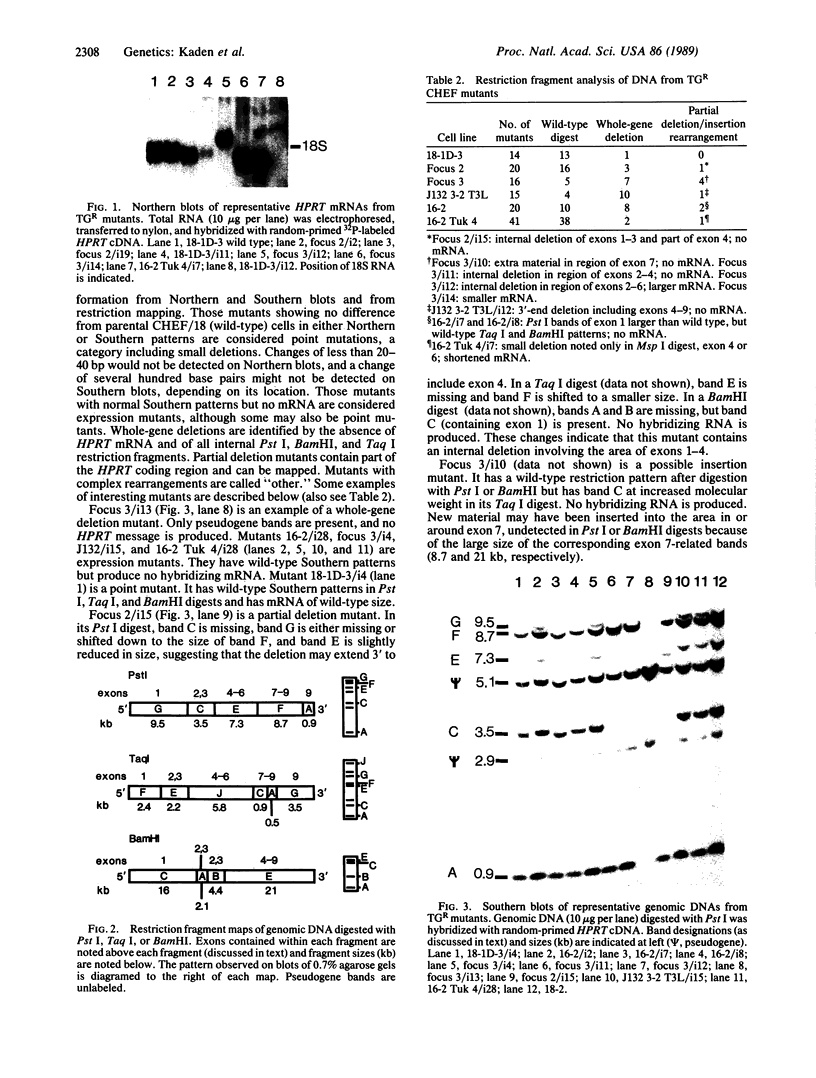
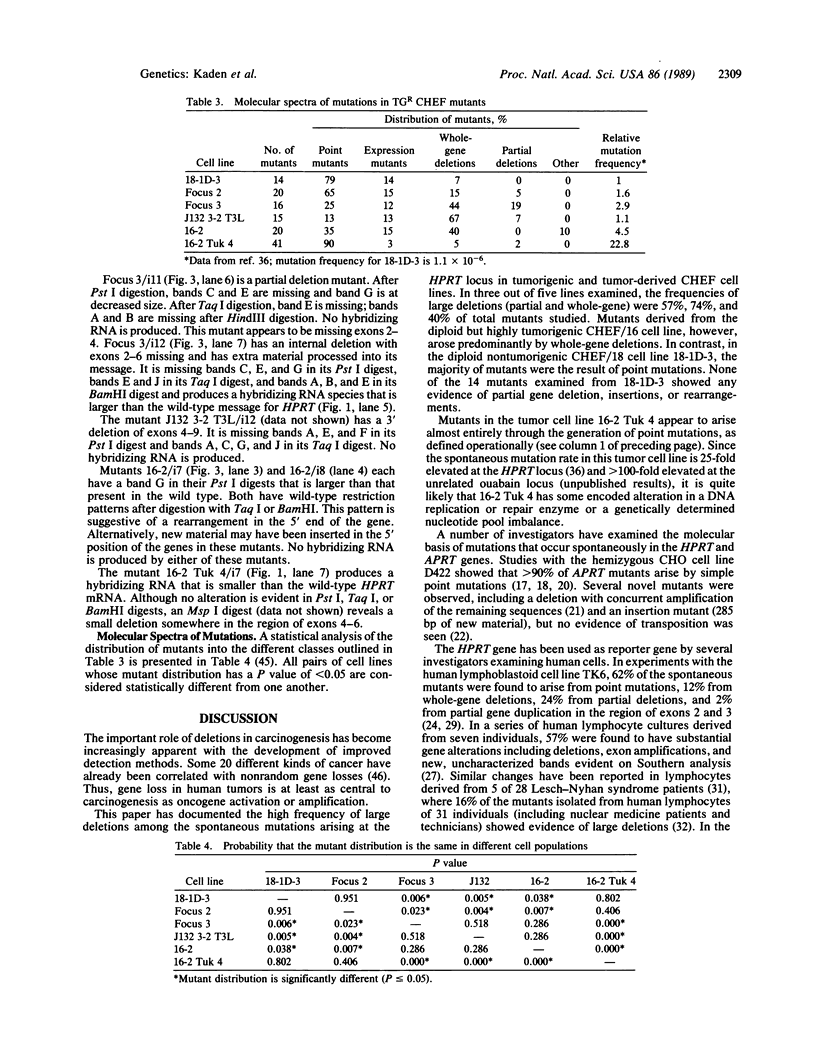
Spontaneous mutations arising at the HPRT locus were examined in 126 mutants recovered from a series of six CHEF-derived cell lines. Altered restriction fragment patterns were characterized by Southern blot hybridization, and gene expression by RNA blot hybridization. Point mutants and gene-expression mutants predominated in the control (nontumorigenic) 18-1D-3 cell line and in two tumor-derived lines, one of which (16-2 Tuk 4) displayed a mutator phenotype. In the other three lines, the majority of mutants had large partial or whole gene deletions. These results suggest that mutant enzymes in DNA replication or repair play an important role in neoplastic progression by causing extensive deletions in DNA, including excision of genes that encode tumor-suppressor functions, and deletion of regulatory sequences in protooncogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. T., Skopek T. R. Statistical test for the comparison of samples from mutational spectra. J Mol Biol. 1987 Apr 5;194(3):391–396. doi: 10.1016/0022-2836(87)90669-3. [DOI] [PubMed] [Google Scholar]
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Ashman C. R., Davidson R. L. Sequence analysis of spontaneous mutations in a shuttle vector gene integrated into mammalian chromosomal DNA. Proc Natl Acad Sci U S A. 1987 May;84(10):3354–3358. doi: 10.1073/pnas.84.10.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bradley W. E., Gareau J. L., Seifert A. M., Messing K. Molecular characterization of 15 rearrangements among 90 human in vivo somatic mutants shows that deletions predominate. Mol Cell Biol. 1987 Feb;7(2):956–960. doi: 10.1128/mcb.7.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand J., Konecki D. S., Caskey C. T. Expression of human and Chinese hamster hypoxanthine-guanine phosphoribosyltransferase cDNA recombinants in cultured Lesch-Nyhan and Chinese hamster fibroblasts. J Biol Chem. 1983 Aug 25;258(16):9593–9596. [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Fenwick R. G., Jr, Ledbetter D. H., Caskey C. T. Deletion and amplification of the HGPRT locus in Chinese hamster cells. Mol Cell Biol. 1983 Jun;3(6):1086–1096. doi: 10.1128/mcb.3.6.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennett I. N., Thilly W. G. Mapping large spontaneous deletion endpoints in the human HPRT gene. Mutat Res. 1988 Sep;201(1):149–160. doi: 10.1016/0027-5107(88)90121-2. [DOI] [PubMed] [Google Scholar]
- Grosovsky A. J., Drobetsky E. A., deJong P. J., Glickman B. W. Southern analysis of genomic alterations in gamma-ray-induced aprt- hamster cell mutants. Genetics. 1986 Jun;113(2):405–415. doi: 10.1093/genetics/113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. C., Gadi I. K., Kalvonjian S., Anisowicz A., Sager R. Plasmid-induced "hit-and-run" tumorigenesis in Chinese hamster embryo fibroblast (CHEF) cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2839–2843. doi: 10.1073/pnas.82.9.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Little J. B. Molecular and biochemical analyses of spontaneous and X-ray-induced mutants in human lymphoblastoid cells. Mutat Res. 1987 May;178(1):143–153. doi: 10.1016/0027-5107(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Goldthwait D. A., Samols D. Identification of Alu transposition in human lung carcinoma cells. Cell. 1988 Jul 15;54(2):153–159. doi: 10.1016/0092-8674(88)90547-8. [DOI] [PubMed] [Google Scholar]
- MCCLINTOCK B. Controlling elements and the gene. Cold Spring Harb Symp Quant Biol. 1956;21:197–216. doi: 10.1101/sqb.1956.021.01.017. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monnat R. J., Jr Molecular analysis of spontaneous hypoxanthine phosphoribosyltransferase mutations in thioguanine-resistant HL-60 human leukemia cells. Cancer Res. 1989 Jan 1;49(1):81–87. [PubMed] [Google Scholar]
- Nalbantoglu J., Goncalves O., Meuth M. Structure of mutant alleles at the aprt locus of Chinese hamster ovary cells. J Mol Biol. 1983 Jul 5;167(3):575–594. doi: 10.1016/s0022-2836(83)80099-0. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Hartley D., Phear G., Tear G., Meuth M. Spontaneous deletion formation at the aprt locus of hamster cells: the presence of short sequence homologies and dyad symmetries at deletion termini. EMBO J. 1986 Jun;5(6):1199–1204. doi: 10.1002/j.1460-2075.1986.tb04347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J., Miles C., Meuth M. Insertion of unique and repetitive DNA fragments into the aprt locus of hamster cells. J Mol Biol. 1988 Apr 5;200(3):449–459. doi: 10.1016/0022-2836(88)90535-9. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Phear G., Meuth M. DNA sequence analysis of spontaneous mutations at the aprt locus of hamster cells. Mol Cell Biol. 1987 Apr;7(4):1445–1449. doi: 10.1128/mcb.7.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Chakrabarti S., Joffee S., Seidman M. Mutagenesis of a shuttle vector plasmid in mammalian cells. Mol Cell Biol. 1984 Mar;4(3):435–441. doi: 10.1128/mcb.4.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Thacker J., Debenham P. G., Stretch A., Webb M. B. The use of a cloned bacterial gene to study mutation in mammalian cells. Mutat Res. 1983 Sep;111(1):9–23. doi: 10.1016/0027-5107(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Detection of deletion mutations in pSV2gpt-transformed cells. Mol Cell Biol. 1984 Jul;4(7):1411–1415. doi: 10.1128/mcb.4.7.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Evans H. J., Ray J. H., German J. Bloom's syndrome: evidence for an increased mutation frequency in vivo. Science. 1983 Aug 26;221(4613):851–853. doi: 10.1126/science.6879180. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Schultz R. A., Chang C. C., Wade M. H., Trosko J. E. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]
- Willis A. E., Weksberg R., Tomlinson S., Lindahl T. Structural alterations of DNA ligase I in Bloom syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8016–8020. doi: 10.1073/pnas.84.22.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandell D. W., Little J. B. Chromosome 14 marker appearance in a human B lymphoblastoid cell line of nonmalignant origin. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):231–239. doi: 10.1016/0165-4608(86)90078-6. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]
- de Jong P. J., Grosovsky A. J., Glickman B. W. Spectrum of spontaneous mutation at the APRT locus of Chinese hamster ovary cells: an analysis at the DNA sequence level. Proc Natl Acad Sci U S A. 1988 May;85(10):3499–3503. doi: 10.1073/pnas.85.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]