Abstract
Aim:
To investigate the co-culture of established intestinal epithelial cell lines and stromal cells in a series of collagen-based environments for production of tissue engineered intestinal epithelium for in vitro investigations.
Materials & methods:
Intestinal epithelial cells were co-cultured with fibroblasts on a range of supporting collagen matrices, including commercially available Promogran and on collagen-based gels.
Results:
Epithelial growth was achieved with one combination of vimentin-expressing stromal and cytokeratin-expressing intestinal epithelial cells grown on collagen gels supplemented with Matrigel, and held at an air-liquid interface.
Conclusions:
Collagen-based gels can support the co-culture of intestinal epithelial and stromal cells which results in the growth of an epithelium that has some morphological similarity to normal intestinal tissue.
Keywords: gut, epithelium, Rat-2, CRL-2102
Introduction
To begin to investigate the possibility of developing a tissue engineered model of intestinal tissue, we have investigated the co-culture of intestinal epithelial cell lines and stromal cells in a series of collagen-based environments, as a first step towards developing a tissue engineered intestinal construct for in vitro investigations.
With respect to producing intestinal tissue for future clinical use, two approaches have been used [1]. Firstly, to introduce a prosthetic scaffold into the damaged or diseased tissue, on which new tissue then grows in vivo. Secondly, to engineer intestinal tissue on an appropriate scaffold in vitro, which can then subsequently be introduced into the individual [2,3]. For this latter approach, cells are taken from the intestinal crypts (which contain multipotent cells) and then cultured [1]. This was first done in rats, where a method was developed that successfully established primary cultures of intestinal epithelial and associated mesenchymal cells, so called ‘intact organoids’ [4,5].
Our motivation for beginning this work is to try and develop tissue engineered intestinal tissue, which can then be used to maintain gastro-intestinal parasitic nematodes in vitro, to facilitate laboratory work on these species and to reduce the use of experimental animals. The future development of a physiologically relevant model of intestinal tissue would also have a range of other biomedical applications, such as drug uptake, metabolism and toxicity investigations. The work presented here is thus a first-step towards these applications.
Materials and Methods
Cell lines and culturing
The following cell lines were used: Rat-2, a cell line of fibroblast-like cells derived from rat embryo; IEC 6, a rat small intestinal epithelial cell line [6], IPI-21, a miniature male boar ileum epithelial cell line [7] and CRL-2102, a human epithelial cell line derived from colorectal adenocarcinoma. Rat-2, IEC 6 and IPI-21 were obtained from the European Collection of Cell Cultures; CRL-2102 was obtained from the American Type Culture Collection. Rat-2 was grown in culture medium consisting of Dulbecco's modification of Eagle's medium (DMEM) Glutamax (Invitrogen, UK) supplemented with 5% v/v fetal calf serum (FCS) (Invitrogen, UK); IEC 6 was grown in the same medium as for Rat-2 but additionally supplemented with insulin (Sigma, UK) to a final concentration of 1mg/mL; IPI-21 was grown in culture medium consisting of DMEM Glutamax supplemented with 10% v/v FCS; CRL-2102 was grown in DMEM supplemented with transferrin (Sigma, UK) to a final concentration of 10ng/mL and 10% v/v FCS. All culture media were additionally supplemented with penicillin, streptomycin and fungizone (all Sigma, UK) to final concentrations of 5,000U/mL, 5mg/mL and 250 mg/mL, respectively. All cell lines were maintained in adherent cultures on standard tissue culture plastic by sequential passage. Cells were harvested for passage or for experiments (below) with 0.05% w/v trypsin, 0.02% w/v EDTA solution (Invitrogen, UK). All cells used (below) were passaged less than ten times.
Immunohistochemistry
Monocultures of the cell lines and co-cultures of mixtures of two cell lines were grown on positively charged 75μm capillary gap microscope slides (Dako, UK) held inside 90mm diameter Petri dishes with 25mL of culture medium. In monocultures, cells were seeded at a density of ≤ 2.4 × 104 cells/mL and grown until the cells were confluent. At this point, the medium was removed and the slides were rinsed in phosphate buffered saline (PBS, pH 7.4, 0.1M), the PBS removed and the slides fixed in an excess of 10% v/v neutral buffered formalin. Cell lines IEC 6, IPI-21 and CRL-2102 were each separately co-cultured with Rat-2, seeded at a density of 6.4 × 103 cells/mL, at ratios of 1:1, 1:4, 1:10 and 1:20 (epithelial : Rat-2), and grown until the cells were confluent and then fixed, as above. Fixed mono- and co-cultures were subject to immunohistochemical labeling to determine expression of cytokeratin and vimentin. All immunohistochemistry was performed by a commercial laboratory (Animal Health Trust, Newmarket, UK). To do this, slides were re-hydrated through an alcohol gradient (100%, 90% and 70%) to water. The samples were pre-treated with heat in a microwave oven in 10% v/v citrate retrieval buffer (pH 9.0 for cytokeratin and pH 6.0 for vimentin) to unmask target antigenic epitopes. Samples were immunolabelled using an automated two-layered, indirect technique using the Dako REAL Detection Kit (Dako, UK). Vimentin was detected with mouse anti-pig vimentin; cytokeratin was detected with mouse anti-human cytokeratin (both Dako, UK). The second antibody was horseradish peroxidase conjugated goat anti-rabbit/mouse EnVision (Dako REAL, UK) which was visualised with 3,3′-diaminobenzidine. Slides were counterstained with haematoxylin and then de-hydrated through an alcohol gradient (70%, 90%, 100%), cleared in Histoclear (National Diagnostics, UK) and mounted under DPX (Raymond A. Lamb, UK).
3D co-culture on Promogran collagen supports
Cell lines IEC 6, IPI-21 and CRL-2102 were each separately co-cultured with Rat-2 in equal mixtures on Promogran (Johnson and Johnson, UK) collagen matrices. To do this, Promogran circles were cut to fill the bottom of wells of 24-well (c.17mm diameter) culture dishes and these circles pre-wetted with a small volume (≤ 500μL) of culture medium appropriate to the epithelial cell line being used. When the Promogran had been successfully wetted, mixtures of the cells (3 × 105 per cell line) were added to the wells in a small volume (≤ 1mL), left for 30 minutes, and then additional medium added as appropriate. In these experiments the culture medium used was that appropriate to the epithelial cell line (IEC 6, IPI-21 and CRL-2102) used in the co-culture; the medium was changed as required. In some experiments, after one day of culture, the Promogran disk was brought to the air-liquid interface. The rationale for this was that it would promote epithelial cell differentiation, as has been observed for non-intestinal epithelia [e.g. 8]. This was done with the use of a disk of polypropylene (thickness 2mm, hole diameter 3mm, 33% open area) (RA Components Ltd., Northamptonshire) onto which the Promogran disk was placed; this polypropylene disk (and the Promogran) then floated on the medium. Control cultures consisting of 3 × 105 Rat-2, IEC 6, IPI-21 or CRL-2102 cells only on Promogran were also prepared. In some experiments ratios other than 1 : 1 of epithelial cells : Rat-2 were used. At the end of these experiments the media were removed and the Promogran disks rinsed in PBS, the PBS removed and the Promogran disks fixed in an excess of 10% v/v neutral buffered formalin. These fixed samples were processed for standard histological analysis and the sections stained with haematoxylin and eosin. Sections of these samples were also immunolabelled for vimentin and cytokeratin. To do this, sections were placed on positively charged 75μm capillary gap microscope slides (Dako, UK), held overnight at 56°C, cleared in Histoclear and then processed as described above for the immuohistochemical labelling.
3D co-culture on collagen gels
Rat-2 cells were embedded in collagen gels, over which epithelial cells were laid. To do this, a 5 mg/mL collagen solution (rat tail type I) (Sigma, UK) was added to a 2.9-times concentrated modified Eagles' medium (MEM) containing antibiotics, and the pH adjusted to neutral (as judged by the color change of the medium) with the addition of a small volume of sodium hydroxide. Rat-2 cells were suspended in FCS and this was added to the mixture of collagen and medium to achieve a final concentration of 2.7mg/mL collagen, 13.6% v/v FCS in 0.9-times MEM containing 1 × 106 cells/mL. 600μL of this mixture was placed into individual wells of a 24-well culture dish and maintained at 37°C until the gel had solidified. Cells of the appropriate epithelial cell line (IEC 6, IPI-21 or CRL-2102) were prepared and 6 × 105 of each epithelial cell line were added to each well in their respective culture medium, the cultures left for 20 minutes, to allow the epithelial cells to settle onto the collagen surface, after which additional medium was added to each well. After one day of culture, the collagen gels were released from the edge of the wells. This was done by placing a micropipette tip between the edge of the collagen gel and the wall of the well and moving this through the circumference of the well. For all cultures the medium was changed when required. At the end of the experiment the medium was removed and the cultures rinsed in PBS, the PBS removed and the cultures fixed in an excess of 10% v/v neutral buffered formalin. These fixed samples were processed for standard histological analysis and stained with haematoxylin and eosin.
3D co-culture on collagen-Matrigel gels
These cultures consisted of a thin, basal collagen gel, overlaid with a mixed collagen-Matrigel gel in which Rat-2 cells were embedded, and over which epithelial cells were laid. This method was in part derived from methods described previously [8]. To do this, a 5 mg/mL collagen solution (rat tail type I) (Sigma, UK) was added to 2.9-times concentrated MEM medium, and the pH adjusted to neutral, as above, and mixed with FCS to make a solution with a final concentration of 0.75mg/mL collagen, 10% v/v FCS in 2.2-times MEM. 350μL of this solution was placed into individual wells of a 24-well culture dish and maintained at 37°C until the gel had solidified. To prepare the second layer, Rat-2 cells were suspended in FCS and added, as above, to a mixture of collagen, concentrated MEM and Matrigel (BD Biosciences, UK), to achieve a solution with a final concentration of 0.5mg/mL collagen, 10% v/v FCS in 1.8-times MEM containing 6 × 105 cells/mL. 1mL of this mixture was placed on top of the basal collagen gels within individual wells of a 24-well culture dish and maintained at 37°C until the gel had solidified, after which the gels were overlaid with DMEM medium supplemented with 10% v/v FCS. On day 1 of culture (gel preparation, above, was day 0), the gels were released from the edge of the wells, as described above. On day 6 of culture, the culture medium was removed and replaced with culture medium appropriate to the epithelial cell line to be used (below), supplemented with 0.1% v/v chelated newborn calf serum (NCS) and incubated for one hour. Meanwhile epithelial cells (IEC 6, IPI-21, CRL-2102) were prepared and resupended in culture medium appropriate to the epithelial cell line supplemented with 0.1% v/v chelated NCS, and a small volume containing 5 × 105 cells added to each well, incubated for two hours after which more of the same culture media was added. On day 8 of culture, the culture medium was removed and replaced with the culture medium appropriate to the epithelial cell line, supplemented with 0.1% v/v NCS. On day 10 of culture, the culture medium was removed and replaced with the culture medium appropriate to the epithelial cell line, supplemented with 2% v/v NCS. At the same time the gels were moved to the air-liquid interface. This was done by placing a disk of perforated polypropylene (as above) approximately 30mm in diameter within individual wells (diameter c. 34mm) of 6-well culture dishes. The collagen-Matrigel gels were removed from their wells with a spatula and then transferred to the top of the 30mm diameter plastic disks. Medium was then added to the well to a level such that the top of the gels was at the air-liquid interface. At the end of the experiment (usually day 15) the medium was removed and the gels rinsed in PBS, the PBS removed and the gels fixed in an excess of 10% v/v neutral buffered formalin. These fixed samples were processed for standard histological analysis and the sections stained with haematoxylin and eosin. To generate chelated NCS, 100mL of NCS was mixed with 20g Chelex 100 (Sigma, UK) for one hour at room temperature, after which the NCS was decanted and stored at −20°C.
Results
Characterization of the cell lines
The morphology of Rat-2 cells grown in submerged monoculture was that typically seen for cultured fibroblasts (Figure 1a), and they grew rapidly with a cell doubling time of c.20 hours. Cells of line IEC 6 and IPI-21 each had homogenous cell morphology (Figure 1b, c). Line IEC 6 grew as tessellated-like sheets of cells, whereas IPI-21 cells grew with a looser arrangement. The estimated cell doubling time was c. 23 and 31 hours for IEC 6 and IPI-21, respectively. Cells of line CRL-2102 appeared to have a diversity of sizes and shapes with a heterogeneous morphology (Figure 1d). Cells commonly appeared to contain vacuoles and refractile inclusions. The cells appeared to strongly adhere to each other, which was apparent when they were being released from their growth substrate. Cell doubling time was c.51 hours.
Figure 1.
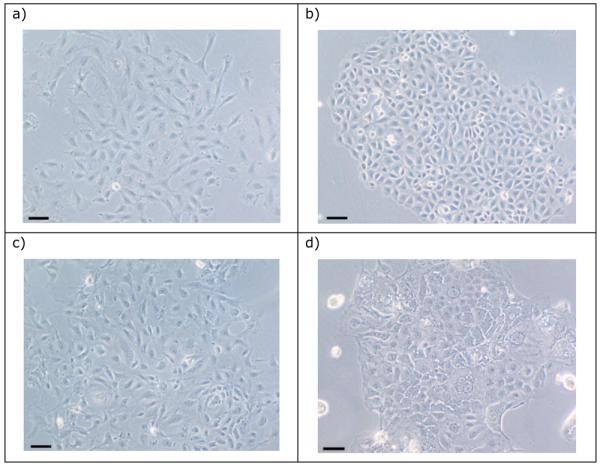
The morphology of (a) Rat-2, (b) IEC 6, (c) IPI-21 and (d) CRL-2012 when grown in submerged monoculture. Bar = 10μm.
The results of the immunohistochemical labelling of monolayers of Rat-2, IEC 6, IPI-21 and CRL-2102 for cytokeratin and vimentin are summarized in Table I. This shows that Rat-2 expressed vimentin but not cytokeratin, as would be expected for stromal cells. Analogously, CRL-2102 expressed cytokeratin but not vimentin, as would be expected for epithelial cells. In contrast the lines of putative epithelial cells IEC 6 and IPI-21 appeared to express both vimentin and cytokeratin.
Table I.
Immunohistochemical expression of vimentin and cytokeratin by cell lines Rat-2, IEC 6, IPI-21 and CRL-2102.
| Cell line | Vimentin | Cytokeratin |
|---|---|---|
| Rat-2 | + | − |
| IEC 6 | + | + |
| IPI-21 | + | + |
| CRL-2102 | − | + |
2D co-culture of cell lines
Co-culture of IEC 6, IPI-21 and CRL-2102 separately and with Rat-2 at different ratios, and subsequent immunohistochemical detection of cytokeratin showed that cells of each of the three epithelial cell lines grew at all ratios. At ratios in which the fibroblast line dominated, the epithelial cells grew as focal islands within the majority fibroblast population (Figure 2). Note, the density at which these co-cultures were initiated, required that all cells had to undergo substantial growth until the cells were confluent. Therefore this observed morphology is a result of cell growth rather than the initial starting ratios per se.
Figure 2.
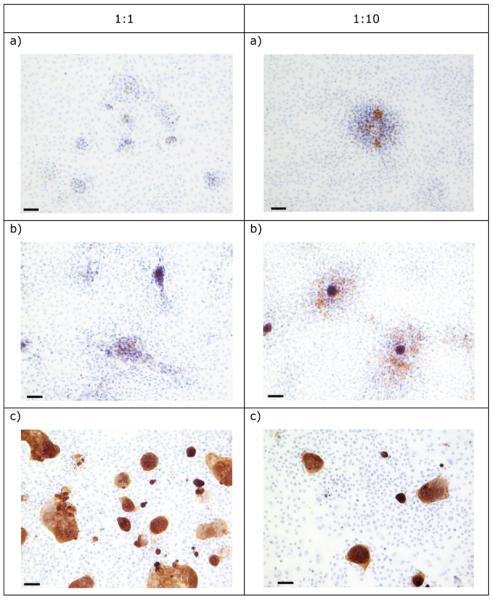
The morphology of 2D co-cultures of Rat-2 with (a) IEC 6, (b) IPI-21 and (c) CRL-2102 at different epithelial cell : Rat-2 ratios, immunolabelled for cytokeratin expression. Bar = 10μm.
3D co-culture on Promogran collagen supports
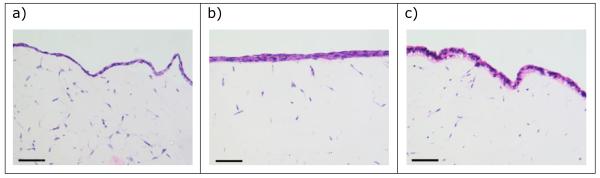
Cell lines IEC 6, IPI-21 and CRL-2102 grew as monocultures and as co-cultures with Rat-2 on Promogran collagen supports. The growth of cells on the surface of the Promogran was better when the Promogran co-cultures were held at the air-liquid interface. In the IEC 6 and Rat-2, and IPI-21 and Rat-2 co-cultures held at the air-liquid interface, cells (presumably Rat-2 fibroblasts) were scattered throughout the matrix and there was a monolayer of presumed epithelial cells on the Promogran surface (Figure 3a, b). This surface growth was better in co-culture compared with IEC 6 and IPI-21 monoculture. For cells both within the matrix and at the surface, there was histological evidence of nuclear condensation and fragmentation within individual cells consistent with the characteristic morphological appearance of apoptosis. Comparison of 7- and 14-day-old cultures showed that the epithelial layer was often thicker in 7-, compared with 14-day-old, cultures. When the cultures were initiated with 1 : 5 or 1 : 10 IEC 6 or IPI-21 : Rat-2 there was no obvious difference in cell and tissue growth compared with 1 : 1 ratios (data not shown).
Figure 3.
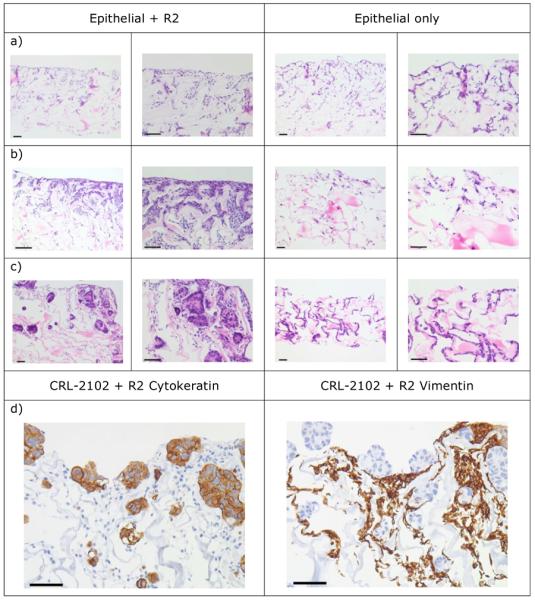
The morphology of seven-day-old 3D mono- and Rat-2-co-cultures of (a) IEC 6, (b) IPI-21 and (c) CRL-2102 on Promogran held at the air-liquid interface and (d) immunohistochemical labeling for vimentin and cytokeratin of (c). Bar = 10μm.
The CRL-2102 and Rat-2 co-cultures on Promogran held at the air-liquid interface also had cells (presumably Rat-2 fibroblasts) scattered throughout the matrix. This was overlaid by a CRL-2102 epithelial surface layer often more than one cell thick. In places an acinar arrangement of the cells was be observed (Figure 3c). Immunohistochemical labelling for cytokeratin and vimentin expression showed that the surface epithelial layer expressed cytokeratin (consistent with these consisting of CRL-2102 cells, Table I) while the non-epithelial cells in the Promogran expressed vimentin (consistent with Rat-2 cells) (Figure 3d). There was no observed difference in cell and tissue growth between cultures in which the Rat-2 cells were at ratios of 1, 5 or 10 of the CRL-2102 cells (data not shown). In summary, good epithelial cell growth over a fibroblast layer was achieved by co-culture of Rat-2 and CRL-2102 on a Promogran collagen matrix held at the air-liquid interface for six days.
3D co-culture on collagen gels
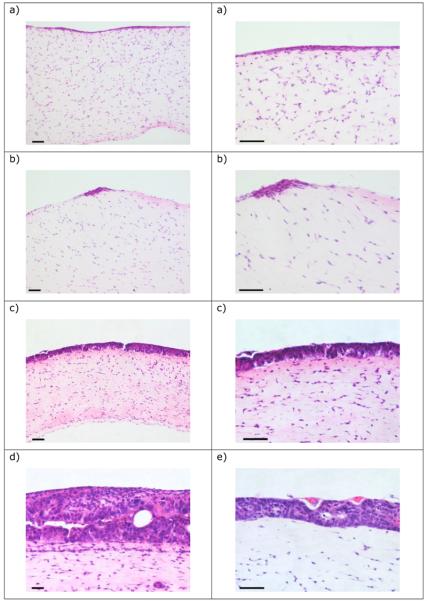
The IEC 6 cells grew as a monolayer on the surface of the collagen (containing Rat-2 fibroblasts) (Figure 4a). IPI-21 grew as a multi-cell deep epithelial layer on the collagen; there was substantial cell death morphologically consistent with apoptosis (Figure 4b). CRL-2102 also grew as a multi-layered epithelium (Figure 4c). The depth of this epithelial growth of CRL-2102 was often thickest at the edges of the collagen disk. For all epithelial cell lines, there was no clear difference in the cell growth in the presence or absence of Rat-2 cells within the collagen gels.
Figure 4.
The morphology of 3D co-cultures on collagen gels of Rat-2 with (a) IEC 6, (b) IPI-21 and (c) CRL-2102. Bar = 10μm.
3D co-culture on collagen-Matrigel gels
The IEC 6 and IPI-21 cell lines grew as a mono- or oligo-layer on top of the gel (Figure 5a, b). The IPI-21 cells also grew as multi-cell deep foci on the gel. In 15 day old cultures the CRL-2102 cells grew as a multi-cellular layer across the entire gel, with a substantial epithelial layer commonly up to 3-4 cells deep, and deeper still in places (Figure 5c), with evidence of acinar growth reminiscent of aspects of the morphology of intestinal epithelium. Substantially greater epithelial growth was often observed in isolated areas (Figure 5d) and there was occasional putative invasion by the CRL-2102 cells into the collagen-Matrigel gel (possibly due to imperfections etc. within the gel) appearing as space lined by CRL-2102 cells. The cultures could successfully be maintained until days 20 (Figure 5e) and 25 (data not shown).
Figure 5.
The morphology of 3D co-cultures on collagen-Matrigel gels of Rat-2 with (a) IEC 6, (b) IPI-21, and CRL-2102 at (c) and (d) 15 and (e) 20 days. Bar = 10μm.
Discussion
We have investigated the co-culture of three different intestinal epithelial cell lines with a stromal fibroblast cell line using a range of collagen-based supports for the 3D cultures. The most extensive epithelial growth was achieved by the cell line CRL-2102 on both Promogran held at the air-liquid interface and on collagen-Matrigel gels. This epithelial growth of CRL-2102, compared with that of IEC 6 and IPI-21, in these co-cultures was consistent with the typical epithelial cell-like immunohistochemical characterization of CRL-2102 as an epithelial cell line (Table I). This is in contrast to the lines IEC 6 and IPI-21 which showed an immunohistochemical profile not always typical of epithelial cells, and which resulted in no, or poor, epithelial growth in co-culture. Vimentin expression by putative epithelial cell lines has been observed before [4,7]. Specifically, rat intestinal epithelial cell primary cultures could be stained for cytokeratin, but with increasing time of culture these cells also began to express vimentin [4]. Freshly isolated human retinal pigment epithelial cells could not be labelled for vimentin, but this was detected when cells were seeded in culture [9]. Analogous observations were made during the isolation of bovine epithelial cell cultures [10], but it was also observed that the epithelial cell expression of vimentin was constitutive. In further support of this, cells of rabbit intestinal Peyer's patches, as well as other some other intestinal cells also constitutively express vimentin [11]. Epithelial cells can also revert to a more fibroblast-like state (so called epithelial-mesenchymal transition) during normal morphogenesis and epithelial repair, such that vimentin is expressed in epithelial cells [12, 13, 14]. Therefore, the observation that two of the epithelial cell lines used here expressed vimentin is in fact consistent with these cells being of epithelial origin, but perhaps subsequently losing this identity, at least in part.
The growth of CRL-2102 on Promogran at the air-liquid interface and in collagen-Matrigel gels was typically characterized by the formation of a continuous epithelial layer, which was often more than one cell thick and which showed heterogeneous morphology. The CRL-2102 cells often grew in an acinar-like arrangement, reminiscent of true intestinal crypt epithelium. This degree and nature of the growth of CRL-2102 required the presence of the Rat-2 stromal cells seeded together onto the Promogran matrix. Immunohistochemical analyses of the cultures of CRL-2102 and Rat-2 on Promogran show that these two cell types self-organize within the matrix. This results in the vimentin-expressing Rat-2 cells growing predominantly within the matrix while the cytokeratin-expressing CRL-2102 cells grow predominantly on the matrix surface. Together these observations imply that there may be some chemical and, or physical interaction between the stromal and epithelial cells, which promotes the epithelial-like growth of the CRL-2102 cells. The 2D co-culture experiments showed that each of the epithelial cells lines grew successfully with the stromal Rat-2 cells, even when the former were in the numerical minority. A priori, this is somewhat surprising given the far greater growth rate of the Rat 2 cells in monoculture, compared with all of the epithelial cell lines. Based on a simple comparison of the growth rates of the lines, the Rat 2 cells may be expected to outgrow and overgrow the epithelial cells when in co-culture. The fact that this does not occur, is consistent with the idea that there is cross-talk between the two cell populations and that the epithelial cell lines may slow the growth of the stromal cells.
Proliferation and differentiation of cultured cells in artificial 3D environments are affected by cellular and physical stimuli. For example, co-cultures of keratinocytes and fibroblasts are able to proliferate and segregate into epithelial and stromal components when cultured on polystyrene fibres at an air liquid interface [15]. Such a physical promoter of epithelial formation has been exploited for many years in the production of human skin equivalents [16, 17] and other epithelia [18, 19]. Overall, this has shown the need for mesenchymalepithelial interaction coupled to some physical promotion of epithelial formation [20]. In the co-culture work presented here there is no native basement membrane. However, collagen is present, which will aid attachment; further, these co-cultures contain cells which may, given time, produce basement membrane proteins [21], which could significantly promote the development of a functional epithelium as observed elsewhere [22, 23]. In the small intestine, stem cells are in the villus crypts. Their division and differentiation to transit amplifying cells and onwards to become differentiated cells of the villi have been shown to be under the control of multiple signalling pathways involving multiple villus cell types [24, 25].
Overall, our results are therefore in agreement with other work based on co-culturing epithelial and stromal cells using collagen gels for the in vitro growth of other epithelia, such as lung [26] and mammary tissue [27]. It is of note that in our work established cell lines were used.
This study has shown that it is possible to produce a 3D construct with some intestinal-like epithelial-type features using a combination of two established epithelial and stromal cell lines and commercially available collagen. These are promising steps towards producing an in vitro model which, with further development, may prove useful for a range of biomedical studies.
Executive Summary
Introduction
There are few existing in vitro engineered intestinal tissue models.
Such models would aid many in vitro investigations of intestinal epithelium.
Methods
Established lines of intestinal fibroblasts and epithelial cells were co-cultured.
These cells were co-cultured on Promogran and on collagen-based gels under a wide range of conditions.
Tissue constructs were immunohistochemically labelled for vimentin and cytokeratin.
Results
Some intestinal epithelial cell lines examined showed both vimentin and cytokeratin expression.
Optimal tissue growth was achieved when fibroblasts were embedded in a collagen gel supplemented with Matrigel, over which cytokeratin-only expressing epithelial cells were layered, and the whole construct held at an air liquid interface.
In these constructs there was a continuous epithelial layer, including acinar-like arrangement of cells reminiscent of normal intestinal crypt epithelium.
Discussion
This work has shown that tissue engineered multi-cellular intestinal epithelial constructs can be produced by co-culture of established intestinal epithelial and stromal cell lines.
These engineered constructs may have a range of in vitro applications spanning, for example, in vitro drug toxicity testing through to the in vitro maintenance of intestinal pathogens which cannot survive outside of an animal host.
Acknowledgments
We would like to thank Nicola Green (University of Sheffield) for her help with the collagen gel constructs, Sheila Jones (School of Clinical Veterinary Science, University of Bristol) for histological processing, Jodi Miller (Animal Health Trust, Newmarket) for performing the immunohistochemical studies and Louise Hughes, William Spinner and Fiona Thompson (University of Bristol) for laboratory help. Preparation of this paper by MEV was undertaken during the tenure of a fellowship at the Institute for Advanced Study, La Trobe University, Melbourne. This work was supported by a grant from the Wellcome Trust.
References
- 1.Gabe SM, Day RM, Boccaccini A. Tissue engineering of the small intestine. In: Bowlin GL, Wnek G, editors. Encyclopaedia of Biomaterials and Biomedical engineering. Marcel Dekker Inc.; New York, USA: 2004. pp. 1661–1671. [Google Scholar]
- 2.Gardner-Thorpe J, Grikscheit TC, Ito H, et al. Angiogenesis in tissue-engineered small intestine. Tissue Eng. 2003;9:1255–1261. doi: 10.1089/10763270360728161. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestine – current status. Biomacromolecules. 2006;7:2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- 4.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell. Sci. 1992;101:219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 5.Fukamachi H. Proliferation and differentiation of fetal rat intestinal epithelial cells in primary serum-free culture. J. Cell. Sci. 1992;103:511–519. doi: 10.1242/jcs.103.2.511. [DOI] [PubMed] [Google Scholar]
- 6.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J. Cell. Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaeffer B, Bottreau E, Velge P, Pardon P. Epithelioid and fibroblastic cell lines derived from the ileum of an adult histocompatible miniature boar (d/d haplotype) and immortalized by SV40 plasmid. Eur. J. Cell. Biol. 1993;62:52–162. [PubMed] [Google Scholar]
- 8.Andl CD, Mizushima T, Nakagawa H, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J. Biol. Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 9.Hunt RC, Davis AA. Altered expression of keratin and vimentin in human retinal pigment epithelial cells in vivo and in vitro. J. Cell. Phys. 1990;145:187–199. doi: 10.1002/jcp.1041450202. [DOI] [PubMed] [Google Scholar]
- 10.Rusu D, Loret S, Peulen O, et al. Immunochemical, biomolecular and biochemical characterization of bovine epithelial intestinal primocultures. BMC Cell Biol. 2005;6:42. doi: 10.1186/1471-2121-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez C, Gebert A. Vimentin-positive cells in the epithelium of rabbit ileal villi represent cup cells but not M-cells. J. Histochem. Cytochem. 2003;51:1533–1544. doi: 10.1177/002215540305101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasiliev JM. Reorganization of molecular morphology of epitheliocytes and connective-tissue cells in morphogenesis and carcinogenesis. Biochemistry (Mosc.) 2008;73:528–531. doi: 10.1134/s0006297908050052. [DOI] [PubMed] [Google Scholar]
- 13.Borthwick LA, Parker SM, Brougham KA, et al. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax. 2009 doi: 10.1136/thx.2008.104133. in press. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Halberg RB, Burch RP, et al. Intestinal adenomagenesis involves core molecular signatures of the epithelial-mesenchymal transition. J. Mol. Hist. 2008;39:283–294. doi: 10.1007/s10735-008-9164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun T, Mai S, Norton D, et al. Self-organization of skin cells in three-dimensional electrospun polystyrene scaffolds. Tissue Eng. 2005;11:1023–1033. doi: 10.1089/ten.2005.11.1023. [DOI] [PubMed] [Google Scholar]
- 16.Gangatirkar P, Paquet-Fifield S, Li A, et al. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Prot. 2007;2:178–186. doi: 10.1038/nprot.2006.448. [DOI] [PubMed] [Google Scholar]
- 17.Ralston DR, Layton C, Dalley AJ, et al. Keratinocytes contract human dermal extracellular matrix and reduce soluble fibronectin production by fibroblasts in a skin composite model. Br. J. Plast. Surg. 1997;50:408–415. doi: 10.1016/s0007-1226(97)90327-1. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan MB, Ramirez RD, Wright WE, et al. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee MK, Yoo JW, Lin H, et al. Air-liquid interface culture of serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Drug Deliv. 2005;12:305–311. doi: 10.1080/10717540500177009. [DOI] [PubMed] [Google Scholar]
- 20.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Ghalbzouri A, Ponec M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004;12:359–367. doi: 10.1111/j.1067-1927.2004.012306.x. [DOI] [PubMed] [Google Scholar]
- 22.Hosokawa T, Betsuyaku T, Nishimura M, et al. Differentiation of tracheal basal cells to ciliated cells and tissue reconstruction on the synthesized basement membrane substratum in vitro. Connect. Tissue Res. 2007;48:9–18. doi: 10.1080/03008200601017488. [DOI] [PubMed] [Google Scholar]
- 23.Segal N, Andriani F, Pfeiffer L, et al. The basement membrane microenvironment directs the normalization and survival of bioengineered human skin equivalents. Matrix Biol. 2008;27:163–170. doi: 10.1016/j.matbio.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 25.Ishizuya-Oka A. Epithelial-connective tissue cross-talk is essential for regeneration of intestinal epithelium. J. Nip. Med. Sch. 2005;72:13–18. doi: 10.1272/jnms.72.13. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan MB, Ramirez RD, Wright WE, et al. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 27.Krause S, Maffini MV, Soto AM, Sonnenschein C. A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng. 2008;14:261–271. doi: 10.1089/ten.tec.2008.0030. [DOI] [PubMed] [Google Scholar]