Abstract
Inositol 1,4,5-trisphosphate receptors (IP3R) are ubiquitous intracellular Ca2+ channels. IP3binding to the IP3-binding core (IBC) near the N-terminal initiates conformational changes that lead to opening of a pore. The mechanisms are unresolved. We synthesized 2-O-modified IP3 analogues that are partial agonists of IP3R. These are like IP3 in their interactions with the IBC, but they are less effective than IP3 in rearranging the relationship between the IBC and N-terminal suppressor domain (SD), and they open the channel at slower rates. IP3R with a mutation in the SD occupying a position similar to the 2-O-substituent of the partial agonists has a reduced open probability that is similar for full and partial agonists. Bulky or charged substituents from either the ligand or SD therefore block obligatory coupling of the IBC and SD. Analysis of ΔG for ligand binding shows that IP3 is recognised by the IBC and conformational changes then propagate entirely via the SD to the pore.
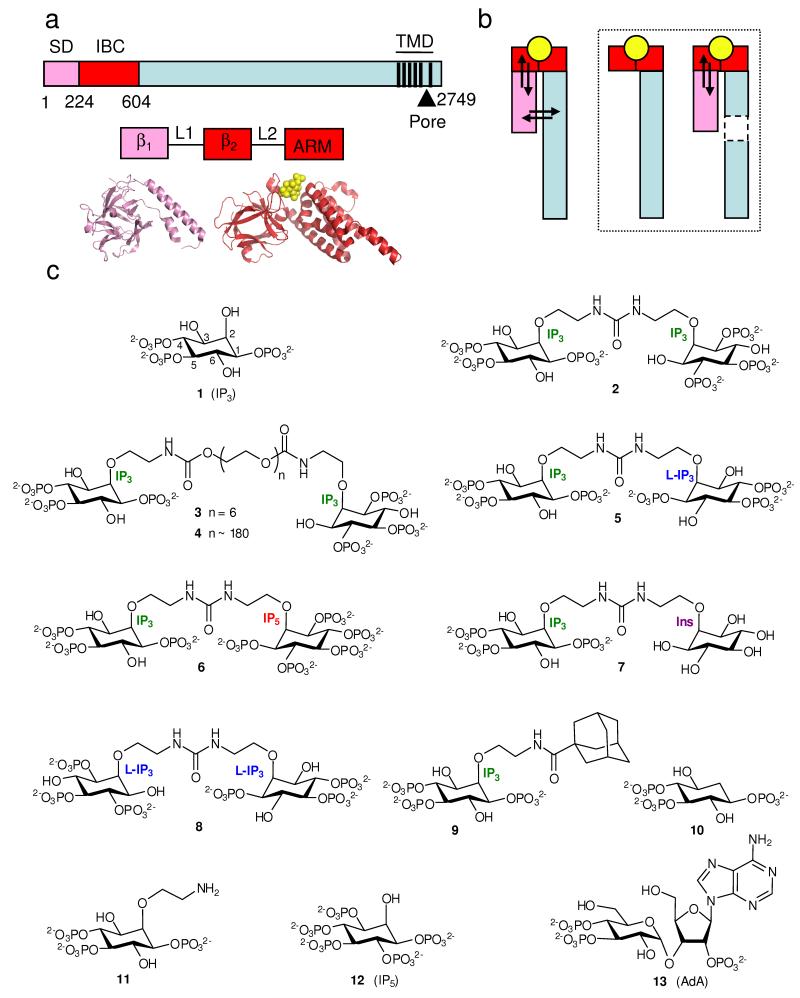
Inositol 1,4,5-trisphosphate receptors (IP3R) are ligand-gated channels. They are expressed in most animal cells and mediate release of Ca2+ from the endoplasmic reticulum in response to the many stimuli that evoke IP3 formation. IP3R are tetrameric, and each subunit of about 2700 residues has an IP3-binding site near the N-terminus and six transmembrane domains (TMD) towards the C-terminus (Fig. 1a)1. The pore is formed by the last pair of TMD and the intervening loop, the pore-loop (“P-loop”), from all four subunits1. The structure of the pore is predicted to be broadly similar to the pores of other tetrameric P-loop channels, like bacterial K+ channels, for which high-resolution structures are available2. IP3 binds to a discrete part of the IP3R, the IP3-binding core (IBC, residues 224-604, Fig. 1a)3. Although the extreme N-terminus (residues 1-223) is not required for IP3 binding, it decreases the affinity for IP3 and has therefore been called the suppressor domain (SD)4. The SD is thought to be required for channel gating because IP3 binds to IP3R without an SD, but it no longer opens the pore4,5. However, the links between IP3 binding and gating are not understood, and nor do we have a structure of the entire IP3R at sufficient resolution to provide insight into these gating mechanisms1,6.
Figure 1.
Structure of the IP3R and its ligands. (a) Key domains of IP3R (numbering from rat IP3R1, accession code NM_001007235). Pink denotes the SD (residues 1-223), red the IBC (224-604), and black vertical lines represent TMD. The SD (β1) and IBC (β2 and armadillo-like repeat, ARM) comprise 3 stably folded domains connected by flexible linkers (L1 and L2)34. Crystal structures are shown below3,33. (b) Agonist binding (yellow) to a discrete site on the receptor (IBC for IP3R, red) evokes conformational changes that propagate through the receptor and which then affect (arrows) the binding site. Removal (boxed diagrams) of a domain through which conformational changes must pass prevents this energetic interplay between conformational changes and binding. (c) Structures of the ligands used.
Activation of ligand-gated ion channels begins with agonist binding to a stable closed state and proceeds via many short-lived intermediates to a state in which the pore is open7. This activation may proceed entirely through a sequence of incremental changes in the receptor8 or be dominated by a single concerted transition between two stable conformations9. Agonists differ in both the strength of their binding (affinity) and in their ability to drive the receptor to its open state (efficacy)10. A ligand with reduced efficacy must occupy more receptors than a full agonist to evoke the same cellular response. Such partial agonists, by occupying receptors, diminish the response to a full agonist11. Partial agonists are particularly useful for exploring the mechanisms of receptor activation because they lie between full agonists and antagonists in their ability to activate receptors7,10. This is true for all receptors, but ligand-gated ion channels are uniquely amenable to such analyses because single-channel recording allows key conformational changes of single receptors to be determined with outstanding temporal resolution7.
For these ligand-gated ion channels, full and partial agonists may differ in either the frequency with which they cause the receptor to visit the fully active open state or they may stabilize different open states that mediate lesser ion fluxes. In both cases, a partial agonist evokes lesser activation. Two subtypes of ionotropic glutamate receptors (iGluR) illustrate the distinction. These receptors, which mediate most excitatory neurotransmission in the brain, are those for which the structural basis of efficacy has been most thoroughly explored12,13. For the AMPA subtype of iGluR, a series of partial agonists, which differ from each other by only a single atom, close the clam-like binding site to varying degrees, but less completely than do full agonists. Partial agonists thereby preferentially open the pore to states with lesser conductance12. For full and partial agonists of the NMDA subtype of iGluR, the conformational changes in the binding site are more subtly different. Both cause similar closure of the clam, and they fully open the pore, but the conformational changes proceed more slowly for partial agonists14,15.
Affinity and efficacy are distinguishable, but the two properties are not independent because energy provided by agonist binding drives the conformational changes that cause channel opening10,16,17. This “binding-gating problem” is a fundamental issue in pharmacology10, but it can also be turned to advantage because the interplay depends upon both the efficacy of the ligand and the presence of the parts of the receptor through which conformational changes must pass. The former because partial agonists divert less binding energy than full agonists into effective conformational changes; and the latter because receptors lacking essential domains are expected to be less able to divert binding energy into conformational changes (Fig. 1b). Analyses of the free energy changes for ligand binding (ΔG = -RTlnKd, where Kd is the equilibrium dissociation constant) can thus provide insight into the conformational changes evoked by agonist binding. Comparisons of ΔG for full and partial agonists, and for agonist binding to normal and truncated IP3R, can therefore contribute to defining the links between IP3 binding and opening of the pore.
Here we synthesize a new series of partial agonists of the IP3R, and, in defining their properties, we identify a novel form of partial agonism that allows us to define key steps in IP3R activation.
RESULTS
Synthesis of 2-O-modified analogues of IP3
All high-affinity agonists of all IP3R have structures equivalent to the vicinal 4,5-bisphosphate and 6-hydroxyl of IP3 (Fig. 1c), but the axial 2-hydroxyl is not required18. The essential phosphate moieties interact predominantly with opposite sides of the clam-like IBC (P-4 with the β2-domain, and P-5 with the ARM domain)3 (Fig. 1a), suggesting that agonists might close the clam in a manner reminiscent of glutamate binding to iGluR6,13.
In seeking to develop novel high-affinity ligands of IP3R that might differ in efficacy, we focused on the 2-OH group of IP3 because earlier structure-activity analyses had suggested that analogues modified at this position retain activity18. The X-ray structure of the IBC with IP3 bound subsequently confirmed that the 2-OH group of IP3 makes no significant contacts with the IBC3.
We began by preparing homo-dimers of IP3 with linkers of various lengths (2, 3 and 4 in Fig. 1c), aiming initially to define the separation of IP3-binding sites within a tetrameric IP3R. However, informed by our initial results19,20 and cognizant that dimeric cGMP is a partial agonist of a cGMP-gated cation channel21, we extended our work to include syntheses of additional 2-O-modified analogues (Fig. 1c) and an assessment of their efficacy.
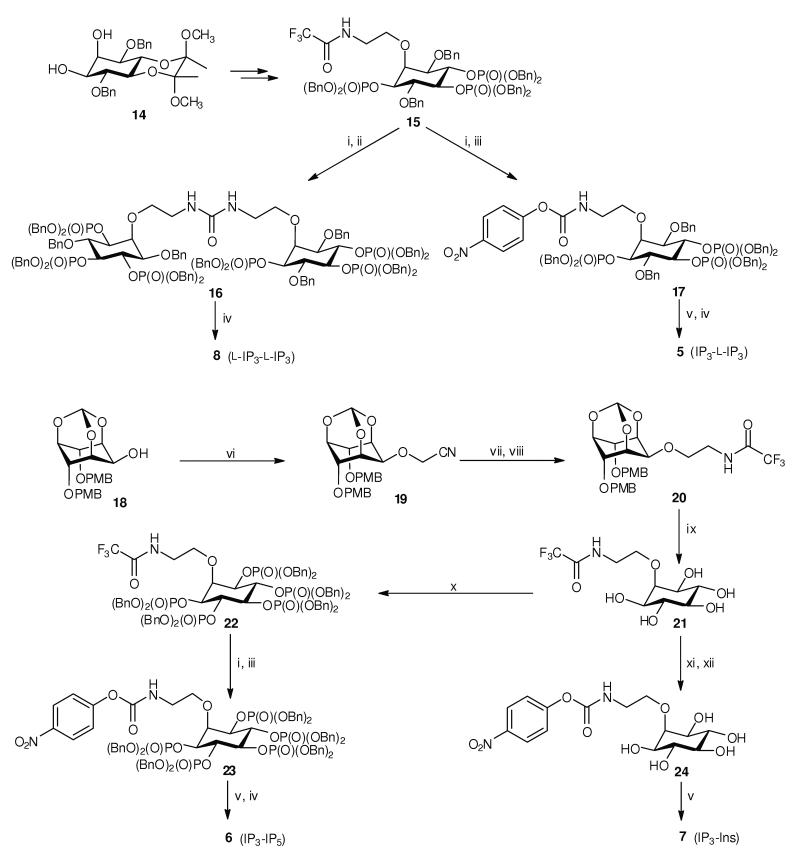
The shortest IP3 dimer (2) is a symmetrically substituted N,N’-diethyl urea, synthesized by cross-linking of a protected D-2-O-(2-aminoethyl)-IP3 building block using bis(4-nitrophenyl) carbonate19. A modification of this synthetic method was used to synthesize hetero-dimers such as 5, 6 and 7, in which the second IP3 moiety is replaced by a different inositol phosphate or by inositol (Scheme 1). We also synthesised an L-IP3 homo-dimer (8, the enantiomer of 2) (Scheme 1) and an IP3-adamantane conjugate (9). The syntheses of 2-deoxy-IP3 (10)22 and 2-O-(2-aminoethyl)-IP3 (11)23 were reported previously. Details of the synthetic procedures and compound characterizations are provided in Supplementary Methods online.
Scheme 1.
Syntheses of hetero-dimers 5, 6 and 7, and L-IP3 dimer 8. Dimers 5 and 8 were synthesized from L-IP3-based building block 15, obtained from diol 1448 (see Supplementary Methods online). The N-trifluoroacetyl protecting group was removed, generating an unstable amine which was reacted with 0.5 equivalents of bis(4-nitrophenyl) carbonate, giving protected L-IP3 dimer 16. Hydrogenolytic deprotection of 16 gave L-IP3 dimer 8. When 1 equivalent of bis(4-nitrophenyl) carbonate was used, the product was 4-nitrophenyl N-alkylcarbamate 17, which could be isolated and conjugated with D-IP3 component 11. The conjugation reaction was carried out in CD3OD and monitored by 31P NMR spectroscopy. Deprotection followed by anion-exchange chromatography then gave IP3–L-IP3 hetero-dimer 5. Dimers 6 and 7 were synthesized from alcohol 1849. Nitrile 19 was reduced, and the amine product was temporarily protected as the N-trifluoroacetamide (20). Acid-labile protecting groups were then removed, giving pentaol 21, which was converted, via 22, into carbamate 23. Carbamate 23 was then conjugated with 11, and deprotection followed by anion-exchange chromatography gave IP3–IP5 hetero-dimer 6. Alternatively, conjugation of carbamate 24 with 11 gave IP3–Ins dimer 7. Reagents and conditions: (i) LiOH, THF, MeOH, H2O; (ii) bis(4-nitrophenyl) carbonate (0.5 equiv), THF; (iii) bis(4-nitrophenyl) carbonate (1 equiv), THF; (iv) H2, Pd(OH)2/C, MeOH, H2O; (v) 11, CD3OD, Et3N; (vi) NaH, BrCH2CN, CH3CN; (vii) LiAlH4, THF; (viii) EtOC(O)CF3, THF; (ix) TFA, H2O; (x) (BnO)2PNiPr2, 1H-tetrazole, CH2Cl2 then 3-chloroperoxybenzoic acid; (xi) Et3N, H2O, reflux; (xii) bis(4-nitrophenyl) carbonate (1 equiv), DMF, Et3N. Bn, benzyl; PMB, 4-methoxybenzyl. All experimental procedures are described in detail in Supplementary Methods online.
2-O-modified IP3 analogues are high-affinity agonists of IP3R
We used a cell line that expresses only recombinant rat IP3R1 (DT40-IP3R1 cells)24 to measure Ca2+ release from intracellular stores, and IP3R1 purified from rat cerebellum to measure IP3 binding. These analyses show that homo-dimers of IP3 linked through the 2-O-positions of the inositol rings are high-affinity agonists of IP3R. The shortest dimer (2) (Fig. 1c) binds to IP3R1 with greater affinity than IP3 (Table 1 and Supplementary Fig. 1a online) and stimulates Ca2+ release from intracellular stores at lower concentrations than does IP3 (Table 1 and Supplementary Fig. 1b online). 2 is the most potent inositol phosphate-based agonist so far identified.
Table 1.
Responses to IP3 analogues. The effects of each analogue on Ca2+ release from the intracellular stores of permeabilized DT40-IP3R1 cells and on 3H-IP3 binding to full-length purified IP3R1 (FL), its N-terminal (NT, residues 1-604) or the IP3-binding core (IBC, residues 224-604) are summarized (n ≥ 4). For dimers, the estimated separation of the two moieties is shown calculated as in50. Results are means ± SEM. *No detectable Ca2+ release with 30μM 8. ND, not determined.
| Ca2+ release |
FL |
NT |
IBC |
||||
|---|---|---|---|---|---|---|---|
| EC50 (nM) | Release % | Kd (nM) | EC50/Kd | Kd (nM) | Kd (nM) | ||
| 1 | IP3 | 20±2 | 77±5 | 12.5±1.06 | 1.6±0.2 | 2.82±0.26 | 0.21±0.03 |
| 13 | AdA | 1.5±0.1 | 75±6 | 1.26±0.11 | 1.2±0.01 | ND | ND |
| 2 | (IP3)2 0.8nm | 2.7±0.4 | 76±6 | 0.45±0.04 | 6.0±1.0 | 0.41±0.03 | 0.18±0.01 |
| 3 | (IP3)2 1.5nm | 4.9±0.3 | 74±6 | 0.86±0.19 | 5.7±1.3 | 0.47±0.09 | 0.18±0.01 |
| 4 | (IP3)2 8nm | 13±1 | 73±7 | 1.39±0.23 | 9.3±1.6 | 1.37±0.20 | 0.48±0.02 |
| 5 | IP3–L-IP3 0.8nm | 5±1 | 70±7 | 0.90±0.12 | 5.6±1.3 | 0.42±0.02 | 0.14±0.02 |
| 6 | IP3–IP5 0.8nm | 5±0 | 72± 5 | 0.89±0.11 | 5.6±0.7 | 0.36±0.03 | 0.20±0.01 |
| 7 | IP3–Ins 0.8nm | 21±1 | 76±4 | 7.82±1.73 | 2.7±0.6 | 2.81±0.22 | 0.33±0.01 |
| 8 | (L-IP3)2 0.8nm | Inactive* | ND | ND | ND | ND | ND |
| 9 | 2-adamantane-IP3 | 30±3 | 71±1 | 7.62±0.51 | 3.9±0.5 | 1.63±.022 | 0.20±0.02 |
| 10 | 2-deoxy-IP3 | 32±7 | 65±3 | 17.1±1.1 | 1.9±0.4 | 4.01±0.38 | 0.22±0.03 |
| 11 | 2-aminoethyl-IP3 | 42±5 | 69±6 | 43.6±4.6 | 0.95±0.02 | 13.5±2.0 | 0.52±0.04 |
A homo-dimer of L-IP3 (8) is, as expected, inactive because L-IP3 does not bind to the IBC18. However, homo-dimers of IP3 with longer linkers (3, 4) also bind to IP3R with greater affinity than IP3, as do hetero-dimers in which IP3 is linked to inositol (inositol-IP3, 7), an unrelated bulky hydrophobic group (adamantane-IP3, 9), or to an inositol phosphate that does not itself bind to the IBC (IP3-IP5, 6; or IP3-L-IP3, 5) (Table 1). The latter (5-7 and 9) demonstrate that high-affinity binding of IP3 dimers does not result from an interaction with a second specific IP3-binding site, nor does it result from alternating association of the two IP3 moieties with the IBC19. Furthermore, the two components of the dimer must be linked, because a high concentration of IP5 (12, 10μM) had no effect on the Ca2+ release evoked by IP3 or 2-deoxy-IP3 (10) (Supplementary Fig. 1c online). We conclude that addition of bulky or charged groups to the 2-O-position of IP3 produces high-affinity agonists of the IP3R.
A new family of partial agonists of IP3R
Because a partial agonist less effectively activates its receptor than a full agonist, it must occupy more receptors to evoke the same cellular response11. In our Ca2+ release assays, where each ligand caused the same maximal Ca2+ release as IP3 (Table 1), we can therefore gain some insight into the efficacy of a ligand by comparing the concentration that causes 50% of the maximal response (EC50) with that which occupies 50% of the binding sites (Kd).
For each ligand, we compared the EC50/Kd ratio using DT40-IP3R1 cells for the functional assays24 (EC50) and purified IP3R1 to measure IP3 binding (Kd). Our results suggest that 2 occupies more IP3R than IP3 to evoke the same Ca2+ release (2 has a higher EC50/Kd ratio, Fig. 2a and Table 1). This indicates that 2 may be a partial agonist. These characteristics, an increase in both affinity and EC50/Kd ratio, are shared by very different 2-O-modified IP3 analogues (Fig. 2a and Table 1). They do not, therefore, depend upon precise structural features: an IP3 moiety that binds to the IBC and a 2-O-substitutent larger than adamantane (9) are sufficient to increase both the affinity and EC50/Kd ratio.
Figure 2.
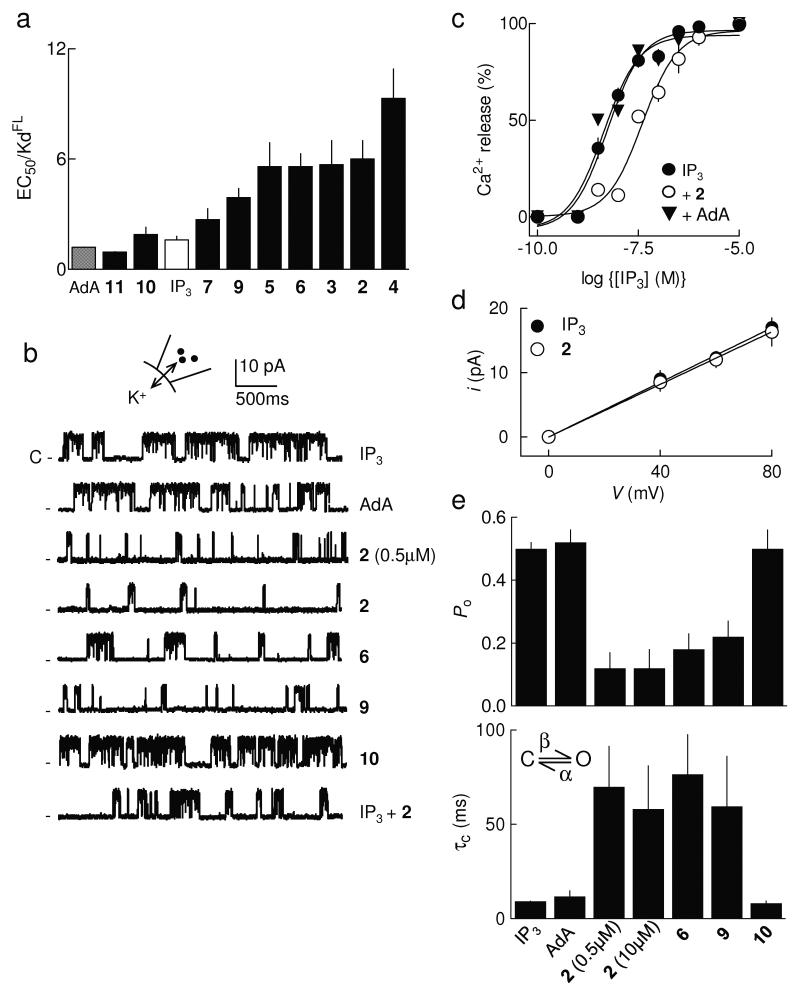
2-O-modified IP3 analogues are partial agonists of IP3R. (a) From experiments similar to those shown in Supplementary Fig. 1a,b online, EC50/Kd ratios (n ≥ 4) were calculated for each ligand. (b) Traces (each typical of at least 3 similar records) show excised nuclear patch-clamp recordings from DT40-IP3R1 cells with the pipette solution containing ATP (0.5mM), a free [Ca2+] of 200nM and the indicated ligands (10μM, except where shown otherwise). The holding potential was +40mV. C denotes the closed state. (c) Cells were treated with IP3 alone, or for 30s with either 0.1nM AdA or 2 and then with the indicated concentrations of IP3. Results (n = 3) show the concentration-dependent release of Ca2+ by IP3. (d) Current-voltage (i-V) relationship for patches stimulated with IP3 or 2 (means ± SEM, n = 3). (e) Summary data showing Po and mean closed time (τc) for IP3R1 stimulated as shown, n = 3-11 (further details in Supplementary Table 1 online). The simplified activation scheme for IP3R shows the transition between closed (C) and open (O) states determined by rate constants, β and α (see Supplementary Methods online). All results (a,c-e) are means ± SEM.
The EC50/Kd ratio for adenophostin A (AdA, 13, Fig. 1c)25, another high-affinity agonist of IP3R, is similar to that for IP3 (Fig. 2a and Table 1). This is consistent with single channel analyses, where IP3 and AdA cause the IP3R to open to the same maximal single channel open probability (Po, the fraction of time that each channel spends in its open state) (Fig. 2b and Supplementary Table 1 online)26. We conclude that IP3 and AdA are full agonists of the IP3R, whereas 2-7 and 9 appear to be partial agonists.
The results shown in Fig. 2c confirm that 2 must occupy more IP3R than the full agonist, AdA, to evoke the same Ca2+ release. DT40-IP3R1 cells were first pre-treated with concentrations of AdA or 2 that caused the same Ca2+ release (5.7 ± 1.0% and 5.4 ± 1.3% of the intracellular stores, respectively) and then stimulated with IP3. More IP3 is required to evoke further Ca2+ release after treatment with 2 than after AdA (EC50 = 44.4 ± 3.19 and 4.37 ± 0.18nM, respectively). This confirms that 2 occupies more IP3R than AdA to evoke the same Ca2+ release.
Because the nuclear envelope is continuous with the endoplasmic reticulum26, we can use patch-clamp recording from the outer nuclear envelope of DT40-IP3R1 cells to resolve the behaviour of single IP3R. To both maximize the amplitude of the currents recorded and to avoid the complexity of feedback regulation of IP3R by Ca2+ passing through them1, we used K+ as the charge-carrier in these experiments26,27. Both the single channel K+ conductance (γK) and the mean channel open time (τo) were the same for all agonists examined (Fig. 2b,d and Supplementary Table 1 online). The open state of the IP3R thus appears to be similar whether it is evoked by binding of a full (AdA, IP3 and 10) or partial agonist (2, 6 and 9).
However, for IP3R activated by maximal concentrations of AdA, IP3 or 10, Po was higher than with 2, 6 or 9 (Fig. 2b,e and Supplementary Table 1 online). Increasing the concentration of 2 (from 0.5 to 10μM, Fig. 2b,e) did not further increase Po, and after stimulation with a mixture of IP3 and 2 (10μM of each) Po was significantly less than with IP3 alone (Fig. 2b and Supplementary Table 1 online). These analyses of single IP3R confirm and extend the results obtained with Ca2+ release and binding assays (Table 1 and Fig. 2a). Analogues of IP3 with bulky additions to the 2-O-position (2-7 and 9) are high-affinity partial agonists of IP3R1. Subsequent analyses of the mechanisms underlying these properties of the 2-O-substituted analogues focus on 2 (Fig. 1c).
Our single channel analysis shows that whereas τo is similar for all agonists (Supplementary Table 1 online), mean channel closed times (τc) were longer for the partial agonists (2, 6 and 9) than for full agonists (IP3, AdA and 10) (Fig. 2e and Supplementary Table 1 online). The latter, assuming a simplified activation scheme (Fig. 2e, Supplementary Methods online), reveals that the rate constant for channel opening (β = 1/τc) with partial agonists is less than with full agonists. The 2-O-modified analogues are the first partial agonists of IP3R for which the basis of their reduced efficacy has been established. They open the channel fully (Fig. 2b,d), the channel closes at the same rate whether it has a partial agonist or IP3 bound (α in Fig. 2e), but the rate constant for channel opening (β) is lower for partial agonists (Fig. 2e and Supplementary Table 1 online). We conclude that these full and partial agonists drive the IP3R into a similar open state, but the partial agonists do so less effectively.
Full and partial agonists differ in how they rearrange the IBC-SD
For IP3R, conformational changes evoked by IP3 binding to the IBC near the N-terminal must be transmitted to the pore formed by residues close to the C-terminal (Fig. 1a). Some of the energy provided by IP3 binding is used to drive the opening of the pore. The Kd (ΔG = -RTlnKd) measured in a binding assay is therefore determined by both the strength of the contacts between IP3 and the IBC (“intrinsic binding affinity”)16 and the ensuing conformational changes10.
The IBC includes all the amino acid residues that contact IP3 (Fig. 1a)3,28 and each 2-O-modified agonist (2-7 and 9-11) (Fig. 1c) retains the groups within IP3 that interact with the IBC. Furthermore, each of these ligands binds with similar affinity to the IBC alone (Fig. 3a and Table 1). Because the full (IP3) and partial agonists (2-7, 9) are both expected to make the same contacts with the IBC and are also observed to bind to it with similar affinity, we suggest that they do not differ in the binding energy they divert into changing the conformation of the IBC. This contrasts with AMPA receptors, where the clam-like binding site closes more fully with more efficacious agonists12,13. The distinction highlights two fundamentally different ways of reducing efficacy, a defining feature of all ligand-receptor interactions10. A partial agonist may fail to make optimal contacts with the binding site and so less effectively activate the receptor (e.g., AMPA receptors12), or it may impair onward transmission of conformational changes. Subsequent experiments demonstrate that our partial agonists (2-7 and 9) belong to the second category. They are thereby useful in defining the steps that follow IP3 binding.
Figure 3.
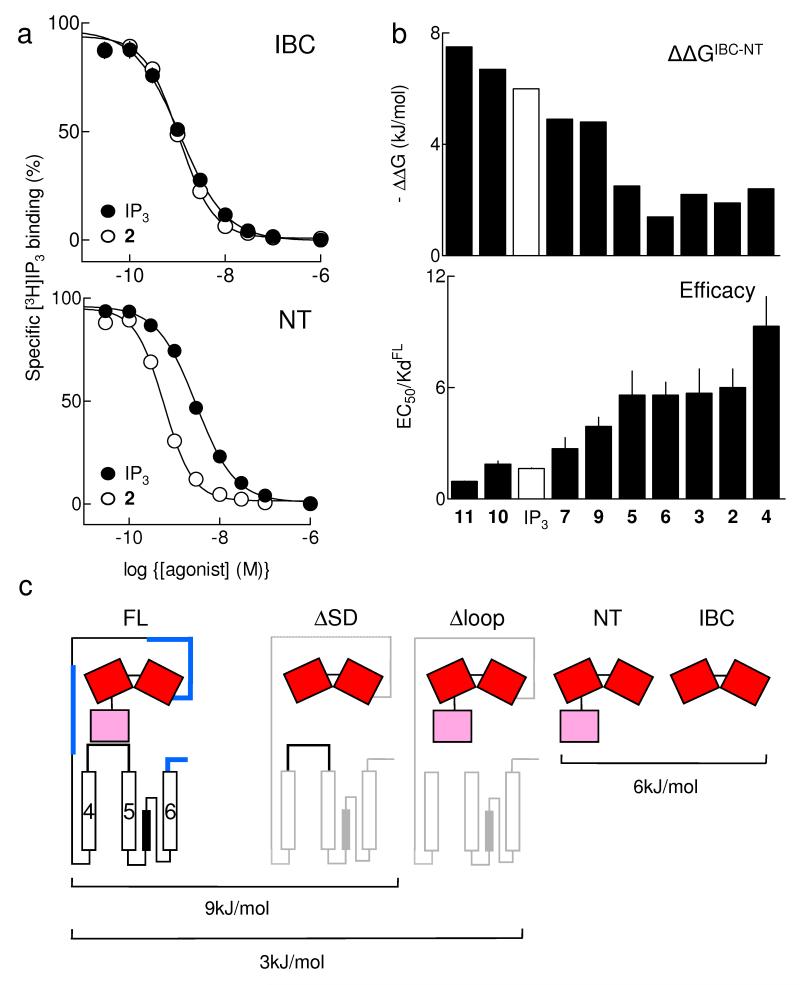
Partial agonists are IP3-like in their interactions with the IBC. (a) Equilibrium-competition binding to IBC (top) and NT (bottom) with 3H-IP3 and either IP3 or 2, n ≥ 17. (b) ΔΔG (ΔGIBC-ΔGNT), reflecting ΔG used to rearrange the IBC-SD relationship, is shown for each ligand, and compared with the efficacy of each (EC50/Kd, with Kd determined for full-length IP3R1). Results (a,b) are means ± SEM. (c) Estimated ΔG for conformational changes associated with IP3R activation. The affinity (Kd) of IP3 for IP3R1 truncated as shown was measured herein (Table 1) or by others: by4 for ΔSD (IP3R1 lacking residues 1-223), and by32 for Δloop (IP3R1 lacking residues 2428-2437); ΔG was then calculated from ΔG = -RTlnKd. The Kd for IP3 was not directly measured in32, but under the conditions used the 4-fold increase in IP3 binding after deletion of residues 2428-2437 (ie Δloop) is likely to reflect a 4-fold decrease in Kd. We assume that deletion of IP3R fragments through which conformational changes must pass increases IP3 affinity because less binding energy is diverted into re-arranging the protein (Fig. 1b). Deletions of many other regions (shown in blue) do not increase IP3 affinity4, suggesting that the IP3-evoked conformational changes do not pass through them. This analysis is consistent with each IP3 binding event diverting ~9kJ/mol into conformational changes of the IP3R, of which ~6kJ/mol rearranges the SD-IBC relationship, and ~3kJ/mol is used by the SD to gate the pore.
For all three IP3R subtypes, IP3 binds to the IBC with greater affinity than to either full-length IP3R or the NT (Fig. 3a, Table 1 and Supplementary Table 2 online)28,29. The SD reduces the IP3 binding affinity through its intramolecular interaction with the IBC28 and appears also to mediate communication between the IBC and pore4,5. We therefore examined the contribution of the SD to the conformational changes initiated by IP3 via analysis of ΔG for ligand binding.
Removal of the SD increases the affinity of the NT for IP3, but it has lesser effects on binding of the partial agonists (Table 1). Efficacy (reported by the EC50/Kd ratio) and the difference in ΔG (ΔG = -RTlnKd) for binding to the IBC and NT (ΔΔG) are inversely correlated (Fig. 3b). Because we suggest that each agonist contributes similar “intrinsic binding energy”16,17 through the similar interactions that each makes with the IBC (Table 1 and Supplementary Table 2 online), the different ΔΔG for binding of full and partial agonists to the NT must reflect the extent to which each uses binding energy to rearrange the relationship between the IBC and SD16,17,30. We conclude that full and partial agonists differ minimally in their interactions with the IBC, but radically in how they rearrange its relationship with the SD.
Conformational changes pass from the IBC entirely via the SD to the pore
IP3 binds only to a small contiguous sequence within the IP3R, the IBC (Fig. 1a). Truncations of the IP3R might therefore disconnect IP3 binding from downstream conformational changes without directly perturbing the IP3-binding site. These truncated IP3R might then reveal, via analysis of ΔG for ligand binding, the parts of the IP3R through which IP3-evoked conformational changes must pass (Fig. 1b).
All full-length IP3R subtypes bind IP3 with only slightly lower affinity than the NT (ΔΔG ca. -3kJ/mol)28, whereas the NT and IBC differ more substantially in their affinities for IP3 (ca. -6kJ/mol) (Table 1 and Supplementary Table 2 online). This suggests that the most costly conformational changes evoked by IP3 occur within the NT (~6kJ/mol) with downstream events requiring less energy (~3kJ/mol) (Fig. 3c). Removing the SD from full-length IP3R increases its affinity for IP3 by an amount (≤ ca. - 9kJ/mol)4 consistent with uncoupling IP3 binding from all the conformational changes downstream of the IBC (Fig. 3c). These analyses suggest that the IBC communicates with the rest of the IP3R entirely via the SD.
A site within the first 340 residues of the IP3R, which includes the SD, appears to interact with a short cytosolic loop linking TMD 4 and 5 (Fig. 3c). This interaction has been proposed to open the pore directly31,32. Disruption of this loop increases the affinity of the IP3R for IP3 by an amount (ca. -3kJ/mol)32 that matches the estimated cost of all conformational changes downstream of the SD (Fig. 3c).
These analyses corroborate our suggestion that conformational changes pass directly and exclusively from the IBC to the SD, and then perhaps directly to the TMD4-5 loop31,32.
Point mutations within the SD mimic partial agonists
Removal of the SD and additions to the 2-O-position of IP3 similarly increase binding affinity (Table 1). The latter, we suggest, because the analogues evoke lesser conformational changes in the IP3R. Both modifications also uncouple ligand binding from gating, although removal of the SD does so more completely4,5 than do the 2-O modifications to IP3. We therefore speculated that 2-O-modified analogues partially mimic removal of the SD by disrupting its interaction with the IBC and that this causes both a decrease in efficacy and an increase in affinity.
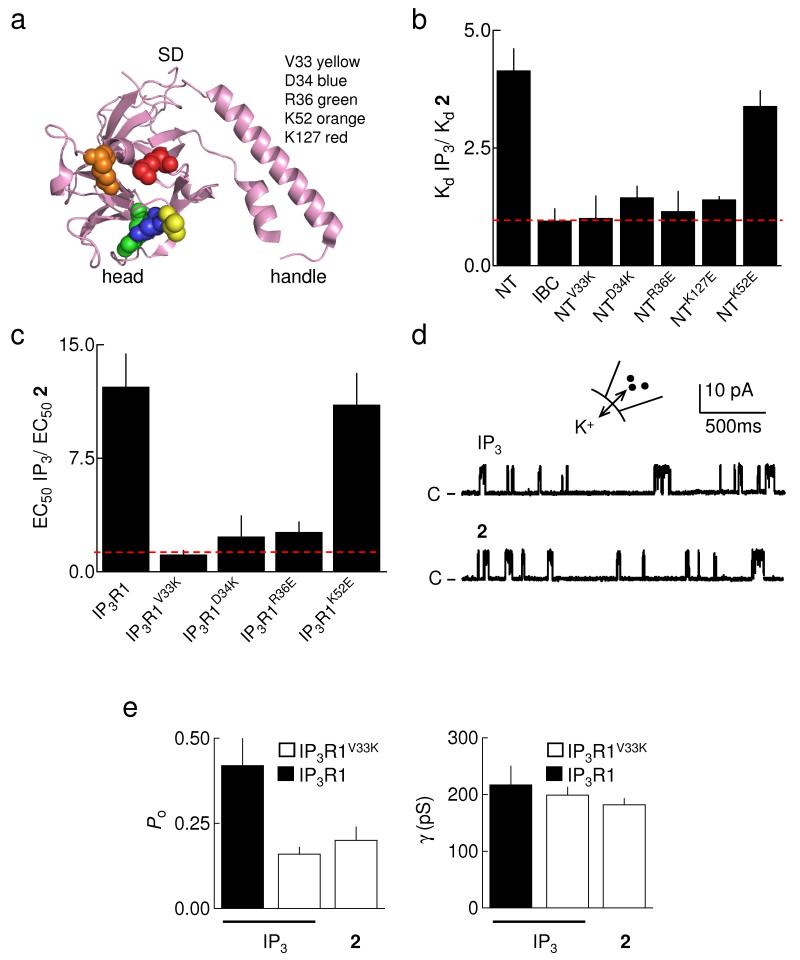
The SD has a structure reminiscent of a hammer with a large head and short handle (described earlier as an “arm”)33 (Fig. 4a). Others33 have shown that removing the handle of the SD (residues 67-108) minimally affects IP3 binding to the NT. But mutation of highly conserved residues on the surface of the head domain, most notably within the β2-β3 loop (loop 2)33, increases the affinity of the NT for IP3. We therefore tested our hypothesis that 2-O-modified analogues of IP3 disrupt the IBC-SD interface by mutagenesis of residues in the β2-β3 loop and of other residues nearby in the 3D structure of the SD (Fig. 4a). As reported33, several mutations increased the affinity of the NT for IP3, with the most effective (V33K) almost mimicking the effect of removing the entire SD. Another mutation (K52E) had no effect (Supplementary Table 3 online)33. Furthermore, and consistent with our suggestion that 2-O-substituents of IP3 disrupt the IBC-SD interaction, the effective mutations had lesser effects on binding of 2 to the NT (Fig. 4b and Supplementary Table 3 online). From these non-additive effects, we conclude that binding of 2 displaces the SD in a manner that mimics its removal or displacement by appropriate mutations.
Figure 4.
Point mutations within the SD mimic partial agonists. (a) The structure of the SD33 is shown highlighting the residues mutated in this study. (b) Relative affinities (Kd) of IP3 and 2 for IBC, NT, and NT with the indicated mutations (Supplementary Table 3 online); n ≥ 5. The dashed line shows KdIP3/Kd2 = 1. (c) Potency (EC50) of IP3 relative to 2 in releasing Ca2+ from permeabilized DT40 cells stably expressing mutant IP3R1 (Supplementary Table 4 online); n ≥ 5. The dashed line shows EC50IP3/EC502 = 1. (d) Typical recordings from excised nuclear patches of DT40-IP3R1V33K cells with 10μM IP3 or 2 in the patch pipette. The holding potential was +40mV. C denotes the closed state. (e) Summary data showing Po and γK for IP3R1 and IP3R1V33K stimulated with 10μM IP3 or 2; n ≥ 3. Results (b,c,e) are means ± SEM.
Our results so far establish that the 2-O-substituents of the IP3 analogues and appropriate point mutations within the SD cause similar increases in binding affinity. These effects mimic removal of the SD, leading us to conclude that they result from disrupted communication between the IBC and SD. Given that the 2-O-substituted analogues are partial agonists, and that the SD is required for IP3 to gate the pore4,5, we speculated that the point mutations might further mimic the analogues and give IP3R that even full agonists are unable to activate fully.
In DT40 cells expressing IP3R1 mutated within the SD (Fig. 4a and Supplementary Fig. 2a,b online), IP3 and 2 evoke Ca2+ release from permeabilized cells and activate IP3R in nuclear patch-clamp recordings (Fig. 4c-e, Supplementary Fig. 2c online, and Supplementary Table 4 online). The properties of these interactions are consistent with our prediction that disrupting the IBC-SD interaction decreases efficacy and increases agonist affinity by blocking propagation of conformational changes from the IBC. In permeabilized DT40 cells expressing IP3R1 with the V33K mutation (IP3R1V33K), IP3 and 2 are equipotent (Supplementary Fig. 2c online), and in single channel recordings each has the same Po (Fig. 4d,e). This Po is similar to that observed for normal IP3R stimulated with 2, but lower than the Po with IP3 (Fig. 2b,e). The less effective mutations have lesser effects (Fig. 4c), consistent with our suggestion that they cause lesser disruption of the IBC-SD interaction.
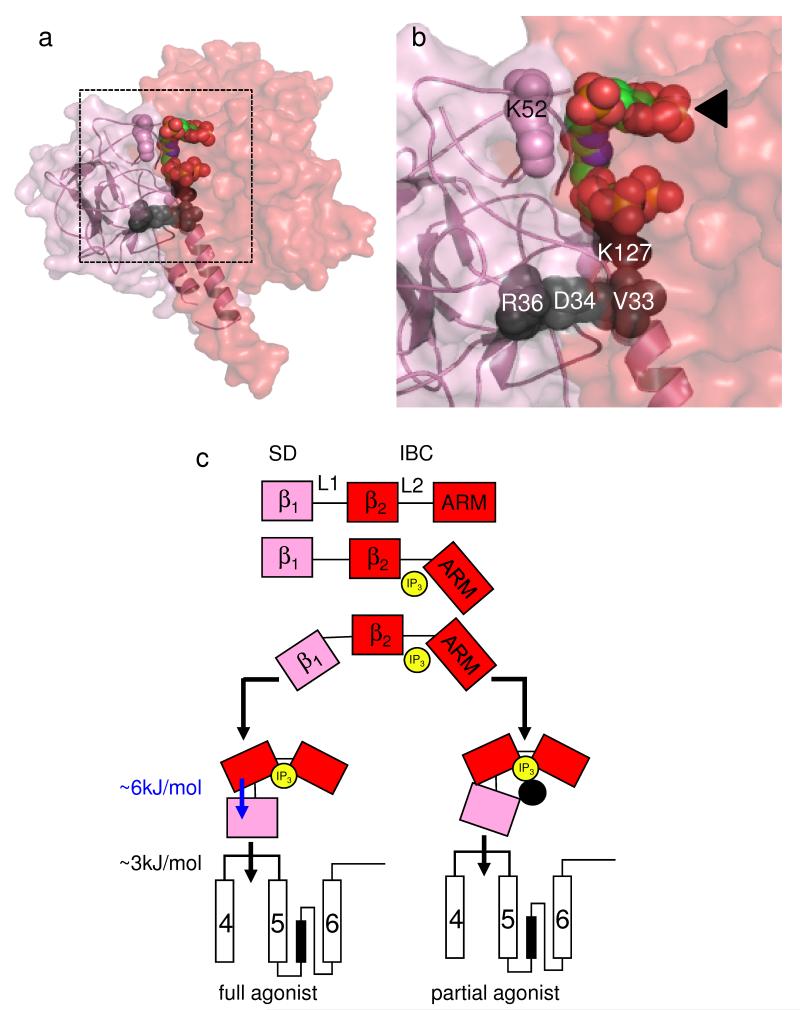
The structures of the IBC-IP3 and SD are known3,33 (Fig. 1a), but not the relationship between them34. We used protein-protein docking to identify a likely relationship between them (Supplementary Methods online). The three IP3R subtypes differ in their affinities for IP3, but their IBC share similar sequences and bind IP3 with the same affinity28. A subtype-specific interaction between the IBC and SD determines the different affinities of the three full-length IP3R28. Because the residues within the SD that confer these subtype-selective interactions28,33 are likely to lie at an IBC-SD interface, this criterion was used to select between possible models of the IBC-SD complex. Our proposed model (Fig. 5a,b and Supplementary Fig. 3 online) is consistent with the radius of the NT-IP3 complex from small-angle X-ray scattering34. In this structure, four of the loops (loops 2 and 5, and part of loops 3 and 7)33 that link the β-strands of the SD interact primarily with loops from the β2-domain of the IBC (Supplementary Fig. 3 online). Within this IBC-SD structure, the second IP3 moiety of 2 lies close to several point mutations in the SD (V33K, D34R, R36E) that reduce efficacy (Supplementary Table 4 online), each lying on the putative IBC-SD interface (within loop 2). The same interface includes the other effective mutation (K127E, within loop 5), but not the ineffective one (K52E) (Fig. 5a,b and Supplementary Fig. 3c,d online).
Figure 5.
IP3 binding to the IBC activates IP3R entirely via the SD. (a,b) Predicted relationship between the SD (pink) and IBC (red) with 2 bound. Residues within the SD that affect efficacy (V33, D34, R36 and K127) are shown in black (see Fig. 4a and Supplementary Fig. 3 online for details). The ineffective residue K52 is shown in pink. Panel b is an enlargement of the boxed area in panel a, with the IBC-bound IP3 moiety indicated by an arrow. (c) IP3 (yellow) rearranges the 2 domains of the IBC (β2 and ARM, red) around its L2 loop causing rearrangement of the SD (= β1, pink) around the L1 loop. The SD is then entirely responsible for transmitting conformational changes towards the pore, probably by directly interacting with the TMD4-5 loop of an adjacent subunit31,32. ΔG associated with rearranging the SD and its subsequent communication with the pore region is shown. Partial agonists effectively rearrange the IBC, but the inositol 2-O-substituent (or point mutations in the SD; black circle) disrupt the IBC-SD interface and so block communication with the SD. The latter is now less likely to contact the TMD4-5 loop, but once it makes contact the channel gates normally.
We conclude that bulky or charged groups introduced into the IBC-SD interface by either the ligand or the SD disrupt essential communication between the IBC and SD and thereby reduce efficacy.
DISCUSSION
We have synthesized and characterized a family of partial agonists of IP3R that differ minimally from full agonists in their interactions with the binding site (IBC), but which have reduced efficacy because they block an obligatory communication between the IBC and SD. These results define two fundamentally different routes to reduced efficacy. A partial agonist may fail to make optimal contacts with the ligand-binding site12,13,35. Alternatively, it may, as we have shown for our partial agonists of IP3R, bind normally and then, through additional interactions, block onward transmission of essential conformational changes. These novel properties of our partial agonists allow us to show that the conformational changes initiated at the IBC pass entirely via the SD to the pore (Fig. 5c).
Our activation scheme is consistent with an earlier proposal that IP3 minimally affects the structures of the three domains of the NT, but rearranges their relationships via flexible linking loops34 (Fig. 5c). We suggest that IP3 first stabilizes interaction of the β2 and ARM domains of the IBC by interacting with residues in each3,36. These interactions require the 4- and 5-phosphate groups of IP3. The IBC then interacts with the SD (=β1 in Fig. 5c) to give a compact structure34 that allows the SD alone to signal onwards to the pore, probably via its interaction with the TMD4-5 loop (Fig. 5c)32.
IP3R are close relatives of ryanodine receptors (RyR), sharing most sequence similarity within their N-termini and pores. The likely structural similarities between the SD of IP3R and the N-terminal of RyR suggests these regions may have similar functions in both families of intracellular Ca2+ channels33. Mutations that cause RyR to become dysfunctional in malignant hyperthermia, central core disease (RyR1) and catecholaminergic polymorphic ventricular tachycardia (RyR2) cluster in four regions that include the N-terminal and a region close to the pore37. Furthermore, 3D reconstructions of RyR have shown that activation is associated with major conformational changes within a region that includes the N-terminus38. For RyR1, the same region includes residues that interact with the dihydropyridine receptor, which is the major physiological regulator of RyR1. From structure-based sequence alignment36, it has been suggested that the SD surface opposite to that which we suggest contacts the IBC (Supplementary Fig. 3e,f online) is most conserved between IP3R and RyR. We speculate that this may be the surface that communicates with the conserved pore region for both IP3R and RyR.
The SD of an IP3R activated by a partial agonist fully engages the structures that open the pore because an open IP3R is the same whether activated by a full or partial agonist (Fig. 2b,d and Supplementary Table 1 online), but it does so less frequently than when activated by a full agonist (Fig. 5c). The many additional proteins that interact with the SD1,33 may exert their effects on IP3R by targeting this essential link between IP3 binding and channel opening.
In conclusion, we have synthesized a family of 2-O-modified analogues of IP3 and shown they are partial agonists of IP3R. IP3 and these partial agonists interact similarly with the IBC, but the 2-O-substituents of the analogues block transmission of essential conformational changes from the IBC to the SD. The partial agonists thereby open the channel less effectively. This unusual form of partial agonism allows us to define two means whereby a ligand may have reduced efficacy: it may either fail to make optimal contacts with the binding site, or it may bind like a full agonist but then interfere with subsequent conformational changes. By combining mutagenesis of IP3R with analyses of the effects of these novel partial agonists, we have shown that the major conformational changes evoked by IP3 occur within the N-terminal and they pass to the pore entirely via the SD (Fig. 5c).
METHODS
Synthesis of ligands
Adenophostin A (AdA, 13)39, inositol 1,3,4,5,6-pentakisphosphate (IP5, 12)40 , IP3 dimers19 2, 3 and 4, D-2-deoxy-IP3 (10)22, and 2-O-(2-aminoethyl)-IP3 (11)23 were synthesized as previously reported. Details of the syntheses of compounds 5–9 are given in Supplementary Methods online. IP3 was from American Radiolabeled Chemicals. [3H]-IP3 (18-23Ci/mmol) was from Amersham Biosciences.
Stable expression of IP3R1 in DT40 cells
Rat IP3R1 were stably expressed in DT40 cells in which the genes for all endogenous IP3R had been disrupted41. The open reading frame42 of rat IP3R1 was amplified by PCR using primers P6 and P7 and cloned as an EcoRI fragment into pcDNA3. The CMV promoter was replaced by the chicken β-actin hybrid promoter, excised from the vector pAneo41, to produce the construct pcDNA3-IP3R1. QuikChange II XL site-directed mutagenesis kit (Stratagene) was used to introduce point mutations in rat IP3R1, which had been previously cloned into the pENTR1A vector. The primers are listed in Supplementary Table 5 online. Mutated IP3R1 was subcloned into pcDNA3.2 by recombination (Gateway, Invitrogen). The sequences of all full-length IP3R constructs were confirmed. DT40 cells stably expressing IP3R1 and its mutants were generated and cultured as described24. Expression of mutant IP3R in DT40 cell lines was quantified by immunoblotting (Supplementary Fig. 2a,b online).
Functional assay of IP3R1 in DT40 cells
A low-affinity Ca2+-indicator (Magfluo-4) trapped within the intracellular Ca2+ stores was used to measure IP3-evoked Ca2+ release24.
Cloning and mutagenesis of N-terminal fragments of IP3R1
Appropriate regions of rat IP3R1 were amplified by PCR from the full-length receptor clone lacking the S1 splice region (S1-). Fragments are numbered by reference to the full-length (S1+) rat IP3R1 (Accession number NM_001007235). PCR used P1 and P2 primers for the fragment including residues 1-604 (NT), and P3 and P2 for residues 224–604 (IBC). Both P1 and P3 insert a thrombin-cleavage site. Fragments were ligated into the pTrcHisA vector at the XhoI/EcoRI sites (Invitrogen) to allow expression of N-terminally tagged His6 proteins. Insertion of the S1 splice region into the IBC fragment used QuikChange mutagenesis kit with P4 and P5 primers. Mutagenesis of residues within the SD used the same kit. The primers are listed in Supplementary Tables 5 and 6 online. The sequences of all constructs were confirmed by DNA sequencing.
Expression of IP3R1 fragments in bacteria
Constructs were transformed into E. coli BL21(DE3)43 and 1ml of the culture was grown overnight at 37°C in Luria-Bertani medium (LBM) with 50μg/ml ampicillin. The inoculum was cultured at 22°C in 100ml of LBM until the OD600 reached 1.0–1.5, isopropyl β-D-thiogalactoside (0.5mM) was added, and after 20h at 15°C, cells were harvested (5000xg, 5min). The pellet was resuspended in Tris/EDTA medium (TEM: 50mM Tris, 1mM EDTA, pH 8.3) supplemented with 10% PopCulture (Novagen), 1mM 2-mercaptoethanol and protease inhibitor cocktail (Sigma). The suspension was incubated with lysozyme (100μg/ml) and RNAase (10μg/ml) for 30min on ice, and the lysate was sonicated for 20s. After centrifugation (30,000xg, 60min), aliquots of supernatant were frozen in liquid nitrogen and stored at -80°C.
For immunoblotting, samples were loaded onto SDS-PAGE gels, transferred to Immobilon membranes (Millipore) and His6-tagged proteins were identified using an anti-His6 antibody. Proteins were cleaved from their His6 tags by incubating bacterial lysates with biotinylated thrombin (Novagen), and thrombin was removed with streptavidin-agarose (Novagen). Cleavage was monitored by immunoblotting using anti-His6, and Ab142 or Ab1.1 antisera for the NT and IBC fragments, respectively (Supplementary Fig. 4 online and Supplementary Methods online).
Purification of IP3R1 from rat cerebellum
IP3R1 was purified at 4°C from cerebella of adult rats using heparin-affinity chromatography44. Frozen cerebella were homogenized in homogenization medium (HM: 1M NaCl, 1mM EDTA, 50mM Tris, 1mM benzamidine, protease inhibitor cocktail tablet (Roche), pH 8.3) and centrifuged (100,000xg, 30min). The pellet was solubilized in HM without NaCl and supplemented with 1.2% CHAPS. After centrifugation (100,000xg, 1h), the NaCl concentration of the supernatant was increased to 250mM before loading onto heparin-agarose beads (Sigma). After 30min, the beads were washed twice in glycerol-containing medium (250mM NaCl, 50mM Tris, 10% glycerol, 1mM 2-mercaptoethanol, 1mM benzamidine, 1mM EGTA, 1% CHAPS, protease inhibitor cocktail, pH 8.0). IP3R were then eluted with elution medium (500mM NaCl, 50mM Tris, 10% glycerol, 1mM 2-mercaptoethanol, 1mM benzamidine, 1mM EGTA, 50mM Tris, 1% CHAPS, pH 8.0), and aliquots frozen in liquid nitrogen before storage at -80°C.
3H-IP3 binding
Equilibrium-competition binding assays were performed at 4°C for 5min in TEM containing 3H-IP3 (18-23Ci/mmol, 0.2-1.5nM), bacterial lysate (5-10μg) or purified IP3R (2.5μg), and competing ligands. Results were analysed by fitting to a Hill equation (GraphPad Prism) from which the IC50, and thereby the Kd, were calculated. The variance of the ratios of mean values (a and b) were calculated from the variances (var) of each45: var(a/b) = (a/b)2[(var(a)/a2)+(var(b)/b2)].
Single channel recording
Patch-clamp recording from excised nuclear patches of DT40 cells used the methods reported previously26,27. IP3R are relatively non-selective cation channels (PBa/PK ~6)1. K+ Ba was therefore used as charge-carrier to increase single channel current amplitudes26 and avoid feedback regulation of IP3R by permeating Ca2+. QuB (http://www.qub.buffalo.edu) was used for analysis of all channel records (Supplementary Methods online).
Molecular modelling
We developed a model of the IBC-SD relationship from the coordinate files for the IBC (1N4K) and SD (1XZZ) using protein-protein docking. Coarse-grained models of the complex were first produced using the program Hex5.1 (http://www.csd.abdn.ac.uk/hex/)46. From these models we selected those in which the linked termini of the SD and IBC were appropriately separated, and then considered only those models in which residues from the SD known to affect binding of IP3 to the IBC28,33 were located at an IBC-SD interface. A representative structure was further refined using a local docking search with RosettaDock47. Detailed methods are given in Supplementary Methods online. Our predicted structure of the IBC-SD complex (Fig. 5a,b and Supplementary Fig. 3 online) has an inertial radius of gyration (26.1Å), which is compatible with the Guinier radius of gyration (30.7Å) obtained by small angle X-ray scattering34.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Dedos, P. da Fonseca, A. Burgen, S. Otto and M. Garcia Alai for helpful comments, and T. Woodman for advice on NMR spectroscopy. Supported by grants from the Wellcome Trust (to CWT, AMR (Bath) and BVLP) and the Biotechnology and Biological Sciences Research Council (to CWT). AMR (Cambridge) holds a Junior Research Fellowship at Queens’ College, Cambridge.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
Note: Supplementary information and chemical compound information is available on the Nature Chemical Biology website.
References
- 1.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacKinnon R. Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture) Angew. Chem. Int. Edn. Engl. 2004;43:4265–4277. doi: 10.1002/anie.200400662. [DOI] [PubMed] [Google Scholar]
- 3.Bosanac I, et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–701. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 4.Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- 5.Szlufcik K, et al. The suppressor domain of inositol 1,4,5-trisphosphate receptor plays an essential role in the protection against apoptosis. Cell Calcium. 2006;39:325–336. doi: 10.1016/j.ceca.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CW, da Fonseca PCA, Morris EP. IP3 receptors: the search for structure. Trends Biochem. Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auerbach A. Gating of acetylcholine receptor channels: Brownian motion across a broad transition state. Proc. Natl. Acad. Sci. USA. 2005;102:1408–1412. doi: 10.1073/pnas.0406787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 10.Colquhoun D. Binding, gating, affinity and efficacy. Br. J. Pharmacol. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson RP. A modification of receptor theory. Br. J. Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouax E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nature Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- 13.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 14.Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nature Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- 15.Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nature Neurosci. 2003;6:476–483. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- 16.Jencks WP. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv. Enzymol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- 17.Burgen ASV. Conformational changes and drug action. Fed. Proc. 1981;40:2723–2728. [PubMed] [Google Scholar]
- 18.Potter BVL, Lampe D. Chemistry of inositol lipid mediated cellular signaling. Angew. Chem. Int. Edn. Engl. 1995;34:1933–1972. [Google Scholar]
- 19.Riley AM, Laude AJ, Taylor CW, Potter BVL. Dimers of D-myo-inositol 1,4,5-trisphosphate: design, synthesis, and interaction with Ins(1,4,5)P3 receptors. Bioconj. Chem. 2004;15:278–289. doi: 10.1021/bc034214s. [DOI] [PubMed] [Google Scholar]
- 20.Riley AM, et al. Interactions of inositol 1,4,5-trisphosphate (IP3) receptors with synthetic poly(ethylene glycol)-linked dimers of IP3 suggest close spacing of IP3-binding sites. J. Biol. Chem. 2002;277:40290–40295. doi: 10.1074/jbc.M206925200. [DOI] [PubMed] [Google Scholar]
- 21.Kramer RH, Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature. 1998;395:710–713. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- 22.Poinas A, et al. Study of the interaction of the catalytic domain of Ins(1,4,5)P3 3-kinase A with inositol phosphate analogues. ChemBioChem. 2005;6:1449–1457. doi: 10.1002/cbic.200400443. [DOI] [PubMed] [Google Scholar]
- 23.Riley AM, Dozol H, Spiess B, Potter BVL. 2-O-(2-aminoethyl)-myo-inositol 1,4,5-trisphosphate as a novel ligand for conjugation: physicochemical properties and synthesis of a new Ins(1,4,5)P3 affinity matrix. Biochem. Biophys. Res. Commun. 2004;318:444–452. doi: 10.1016/j.bbrc.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 24.Tovey SC, Sun Y, Taylor CW. Rapid functional assays of intracellular Ca2+ channels. Nature Protocols. 2006;1:258–262. doi: 10.1038/nprot.2006.40. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M, Tanzawa K, Takahashi S. Adenophostins, newly discovered metabolites of Penicillium brevicompactum, act as potent agonists of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1994;269:369–372. [PubMed] [Google Scholar]
- 26.Dellis O, et al. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 27.Rahman T-U, Skupin A, Falcke M, Taylor CW. Clustering of IP3 receptors by IP3 retunes their regulation by IP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2007;282:12755–12764. doi: 10.1074/jbc.M609833200. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa F, et al. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 30.Williams DH, Zhou M, Stephens E. Ligand binding energy and enzyme efficiency from reductions in protein dynamics. J. Mol. Biol. 2006;355:760–767. doi: 10.1016/j.jmb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Boehning D, Joseph SK. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J. 2000;19:5450–5459. doi: 10.1093/emboj/19.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schug ZT, Joseph SK. The role of the S4-S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J. Biol. Chem. 2006;281:24431–24440. doi: 10.1074/jbc.M604190200. [DOI] [PubMed] [Google Scholar]
- 33.Bosanac I, et al. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol. Cell. 2005;17:193–203. doi: 10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 34.Chan J, et al. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J. Mol. Biol. 2007;373:1269–1280. doi: 10.1016/j.jmb.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 35.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Bosanac I, Michikawa T, Mikoshiba K, Ikura M. Structural insights into the regulatory mechanism of IP3 receptor. Biochim. Biophys. Acta. 2004;1742:89–102. doi: 10.1016/j.bbamcr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 37.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J. Mol. Cell. Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 38.Wagenknecht T, Samsó M. Three-dimensional reconstruction of ryanodine receptors. Front. Biosci. 2002;7:1464–1474. doi: 10.2741/A853. [DOI] [PubMed] [Google Scholar]
- 39.Marwood RD, Correa V, Taylor CW, Potter BVL. Synthesis of adenophostin A. Tetrahedron: Asymmetry. 2000;11:397–403. [Google Scholar]
- 40.Riley AM, et al. Scyllo-inositol pentakisphosphate as an analogue of myo-inositol 1,3,4,5,6-pentakisphosphate: chemical synthesis, physicochemistry and biological applications. ChemBioChem. 2006;7:1114–1122. doi: 10.1002/cbic.200600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardy TJA, Traynor D, Taylor CW. Differential regulation of types 1 and 3 inositol trisphosphate receptors by cytosolic Ca2+ Biochem. J. 1997;328:785–793. doi: 10.1042/bj3280785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa F, et al. High efficient expression of the functional ligand binding site of the inositol 1,4,5-trisphosphate receptor in Escherichia coli. Biochem. Biophys. Res. Commun. 1999;257:792–797. doi: 10.1006/bbrc.1999.0498. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q-X, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. EMBO J. 2002;21:3575–3581. doi: 10.1093/emboj/cdf380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colquhoun D. Lectures in biostatistics. Clarendon Press; Oxford: 1971. [Google Scholar]
- 46.Ritchie DW, Kozakov D, Vajda S. Accelerating and focusing protein-protein docking correlations using multi-dimensional rotational FFT generating functions. Bioinform. 2008;24:1865–1873. doi: 10.1093/bioinformatics/btn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray JJ, et al. Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 2003;331:281–299. doi: 10.1016/s0022-2836(03)00670-3. [DOI] [PubMed] [Google Scholar]
- 48.Riley AM, Correa V, Mahon MF, Taylor CW, Potter BVL. Bicyclic analogues of D-myo-inositol 1,4,5-trisphosphate related to adenophostin A: synthesis and biological activity. J. Med. Chem. 2001;44:2108–2117. doi: 10.1021/jm0005499. [DOI] [PubMed] [Google Scholar]
- 49.Riley AM, Guédat P, Schlewer G, Spiess B, Potter BVL. A conformationally restricted cyclic phosphate analogue of inositol trisphosphate: synthesis and physicochemical properties. J. Org. Chem. 1998;63:295–305. [Google Scholar]
- 50.Riley AM, Potter BVL. Poly(ethylene glycol)-linked dimers of D-myo-inositol 1,4,5-trisphosphate. Chem. Commun. 2000:983–984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.