Abstract
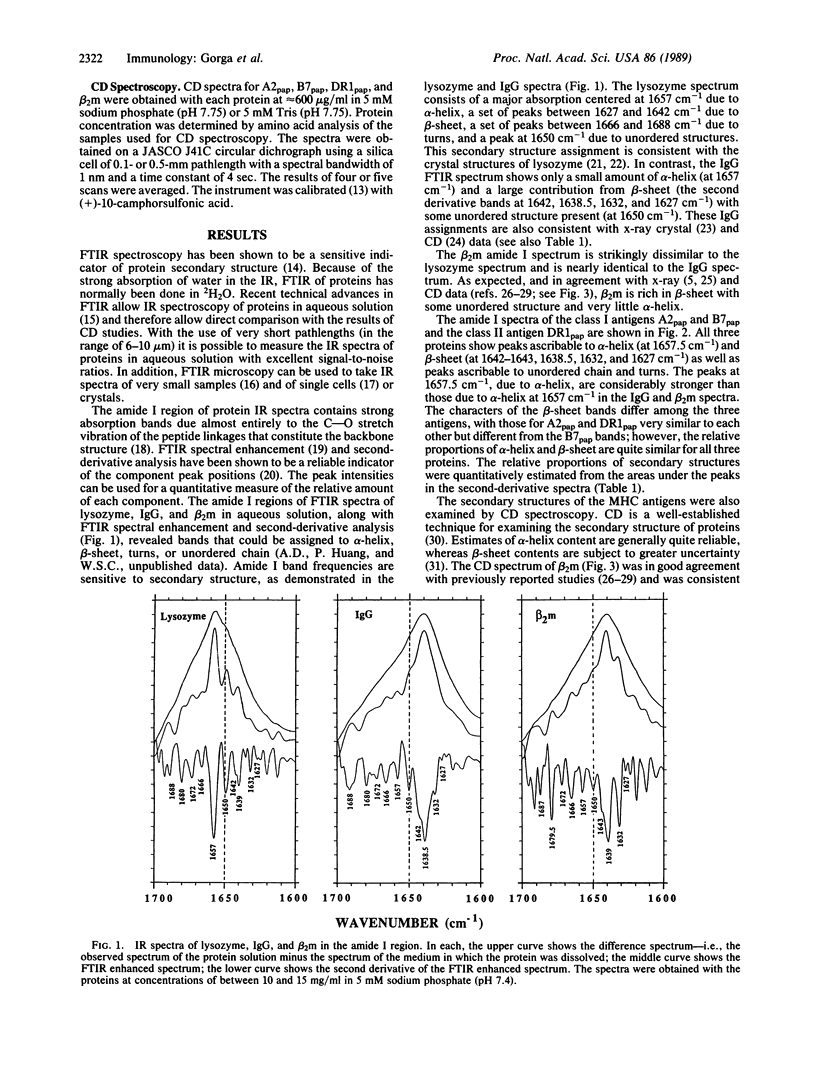
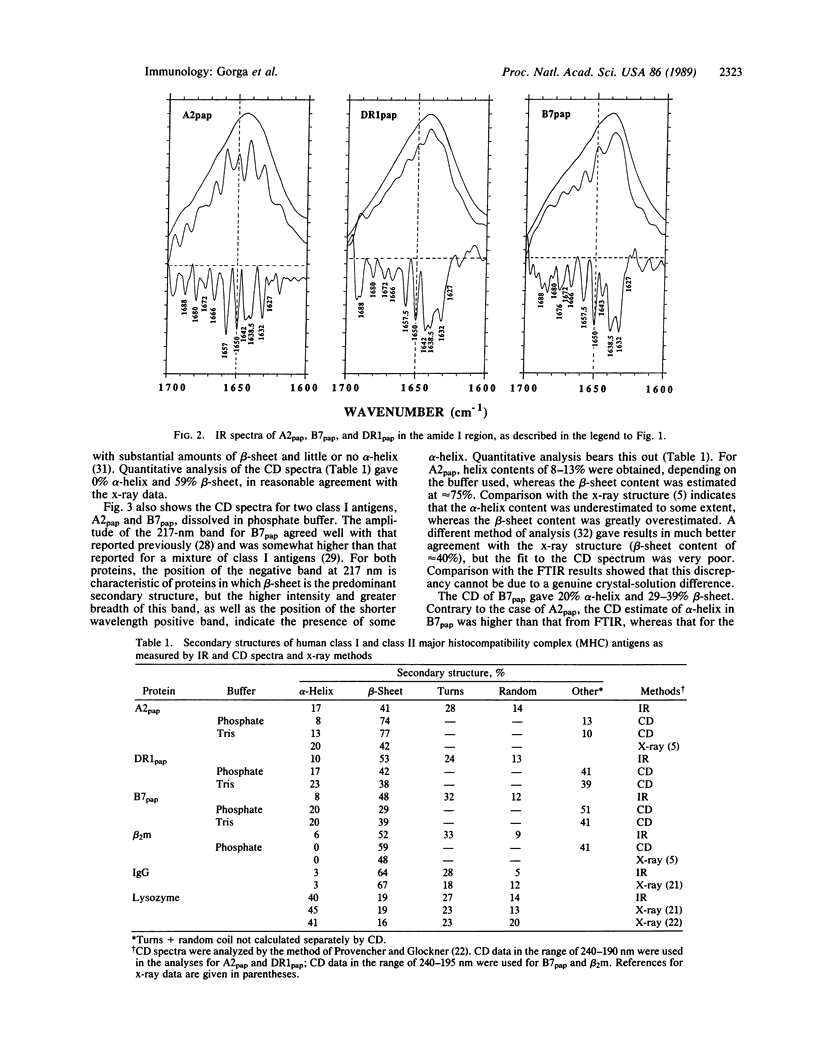
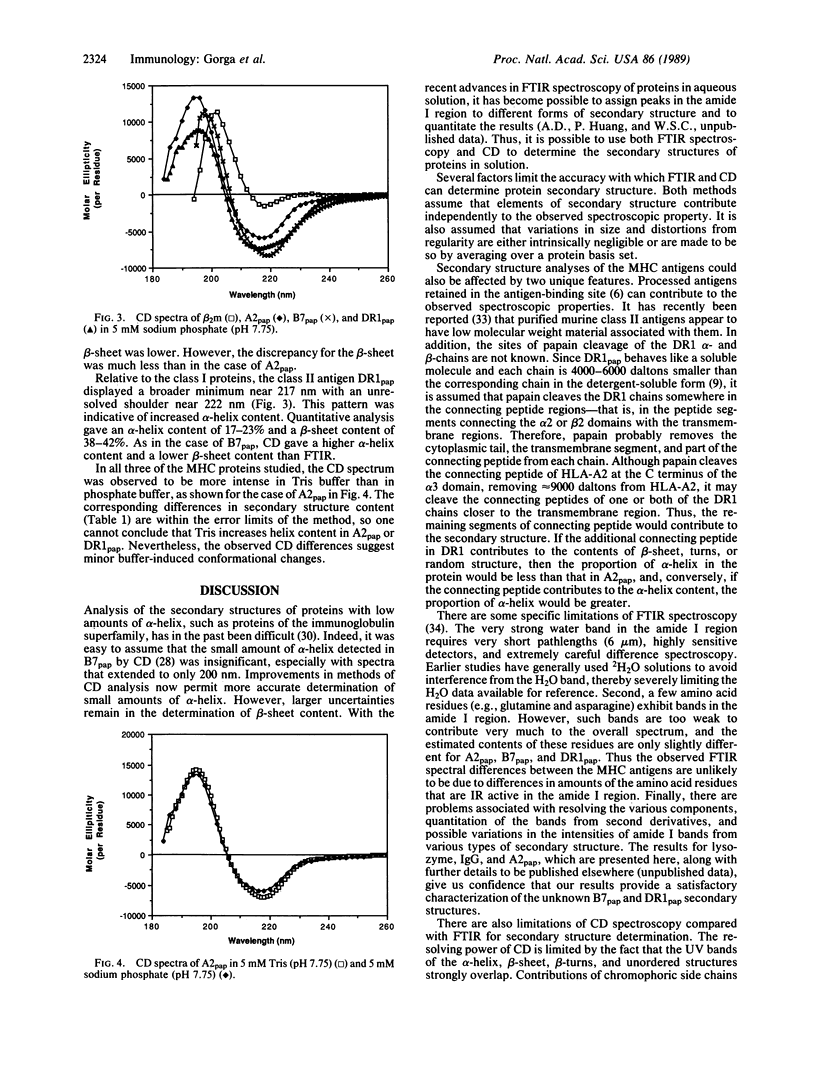
We have examined the secondary structures of human class I and class II histocompatibility antigens in solution by Fourier transform infrared spectroscopy and circular dichroism in order to compare the relative amounts of alpha-helix, beta-sheet, and other structures, which are crucial elements in the comparison of the protein structures. Quantitation of infrared spectra of papain-solubilized HLA-A2, HLA-B7, and DR1 in phosphate buffer gave alpha-helix contents of 17%, 8%, and 10% and beta-sheet contents of 41%, 48%, and 53%, respectively. By circular dichroism, papain-solubilized HLA-A2, HLA-B7, and DR1 were also found to have comparable alpha-helix contents (e.g., 8%, 20%, and 17%, respectively). Circular dichroism analysis for beta-sheet gave 29% for papain-solubilized HLA-B7 and 42% for papain-solubilized DR1. The value for papain-solubilized HLA-A2 (74%) was anomalous. It is proposed that Trp-107 of HLA-A2, missing in both HLA-B7 and DR1, may be responsible for much of the anomaly. Due to the uncertainties inherent in quantitation of the amounts of secondary structures by both spectral methods, the differences in the contents of alpha-helix and beta-sheet in the three proteins are not considered significant. However, differences in the nature of the beta-sheet structures are suggested by infrared spectroscopy. These results provide physical evidence for an overall structure of class II antigens modeled on that of class I antigens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Lee D. C., Baldwin S. A., Chapman D. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J Biol Chem. 1987 Mar 15;262(8):3502–3509. [PubMed] [Google Scholar]
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4225–4229. doi: 10.1073/pnas.82.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Strominger J. L., Wiley D. C. Crystallization and X-ray diffraction studies on the histocompatibility antigens HLA-A2 and HLA-A28 from human cell membranes. J Mol Biol. 1985 Nov 5;186(1):205–210. doi: 10.1016/0022-2836(85)90271-2. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Rothschild K. J. Fourier transform infrared techniques for probing membrane protein structure. Annu Rev Biophys Biophys Chem. 1988;17:541–570. doi: 10.1146/annurev.bb.17.060188.002545. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Day L. A. Circular dichroism and ultraviolet absorption of a deoxyribonucleic acid binding protein of filamentous bacteriophage. Biochemistry. 1973 Dec 18;12(26):5329–5339. doi: 10.1021/bi00750a017. [DOI] [PubMed] [Google Scholar]
- Dong A., Messerschmidt R. G., Reffner J. A., Caughey W. S. Infrared spectroscopy of a single cell--the human erythrocyte. Biochem Biophys Res Commun. 1988 Oct 31;156(2):752–756. doi: 10.1016/s0006-291x(88)80907-0. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Hazzard J. H., Caughey W. S. Determination of anesthetic molecule environments by infrared spectroscopy. I. Effects of solvating molecule structure on nitrous oxide spectra. Arch Biochem Biophys. 1985 Aug 1;240(2):734–746. doi: 10.1016/0003-9861(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Horejsí V., Johnson D. R., Raghupathy R., Strominger J. L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987 Nov 25;262(33):16087–16094. [PubMed] [Google Scholar]
- Green N. M., Melamed M. D. Optical rotatory dispersion, circular dichroism and far-ultraviolet spectra of avidin and streptavidin. Biochem J. 1966 Sep;100(3):614–621. doi: 10.1042/bj1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey J. P., Jr, Johnson W. C., Jr Information content in the circular dichroism of proteins. Biochemistry. 1981 Mar 3;20(5):1085–1094. doi: 10.1021/bi00508a007. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Drake A. F., Tamiya N. An analysis of the 225-230-nm CD band of elapid toxins. Biopolymers. 1988 Jan;27(1):113–122. doi: 10.1002/bip.360270109. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Painter R. H., Dorrington K. J. The structure and function of immunoglobulin domains: studies with beta-2-microglobulin on the role of the intrachain disulfide bond. Proc Natl Acad Sci U S A. 1975 Feb;72(2):548–552. doi: 10.1073/pnas.72.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Jr Secondary structure of proteins through circular dichroism spectroscopy. Annu Rev Biophys Biophys Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- Kappes D., Strominger J. L. Human class II major histocompatibility complex genes and proteins. Annu Rev Biochem. 1988;57:991–1028. doi: 10.1146/annurev.bi.57.070188.005015. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A. Physical-chemical properties of beta 2-microglobulin. Immunochemistry. 1974 Mar;11(3):111–114. doi: 10.1016/0019-2791(74)90207-9. [DOI] [PubMed] [Google Scholar]
- Krimm S., Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. doi: 10.1016/s0065-3233(08)60528-8. [DOI] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M., Greer J. Automatic identification of secondary structure in globular proteins. J Mol Biol. 1977 Aug 5;114(2):181–239. doi: 10.1016/0022-2836(77)90207-8. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. Adv Immunol. 1986;38:1–30. doi: 10.1016/s0065-2776(08)60005-x. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Rupp F., Acha-Orbea H., Hengartner H., Zinkernagel R., Joho R. Identical V beta T-cell receptor genes used in alloreactive cytotoxic and antigen plus I-A specific helper T cells. 1985 May 30-Jun 5Nature. 315(6018):425–427. doi: 10.1038/315425a0. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988 Jan 29;952(2):115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986;130:290–311. doi: 10.1016/0076-6879(86)30015-6. [DOI] [PubMed] [Google Scholar]
- Susi H. Infrared spectroscopy--conformation. Methods Enzymol. 1972;26:455–472. doi: 10.1016/s0076-6879(72)26024-4. [DOI] [PubMed] [Google Scholar]
- Trägårdh L., Curman B., Wiman K., Rask L., Peterson P. A. Chemical, physical-chemical, and immunological properties of papain-solubilized human transplatation antigens. Biochemistry. 1979 May 29;18(11):2218–2226. doi: 10.1021/bi00578a013. [DOI] [PubMed] [Google Scholar]
- Turner M. J., Cresswell P., Parham P., Strominger J. L., Mann D. L., Sanderson A. R. Purification of papain-solubilized histocompatibility antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1975 Jun 25;250(12):4512–4519. [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]



