This study demonstrated a new approach and novel opportunity for treatment of chronic and refractory chorioretinal diseases.
Abstract
Purpose.
A long-lasting, slow-release, crystalline antiviral drug delivery system was initially reported using ganciclovir and cyclic cidofovir as the prototype compounds. The present study was undertaken to investigate the feasibility of applying this system to antiproliferative small molecules.
Methods.
The crystalline lipid prodrugs of hexadecyloxypropyl-arabinofuranosylguanine 5′-monophosphate (HDP-P-AraG), hexadecyloxypropyl 5-fluoro-2′-deoxyuridine cyclic 3′,5′-monophosphate (HDP-cP-5-F-2dUrd), and hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 5′-monophosphate (HDP-P-5-F-2dUrd) were synthesized from their parent compounds arabinofuranosylguanine (AraG) and 5-fluoro-2′-deoxyuridine (5-F-2dUrd). All three compounds were tested at escalating doses in rabbit eyes. Only one eye of each animal was injected with test compound, and the fellow eye was injected with 5% dextrose as the control. The injected eyes were monitored by slit lamp, a handheld tonometer, indirect ophthalmoscopy, electroretinography (ERG), and histology. The selected doses were used for efficacy study with the rat CNV model or the rabbit PVR model.
Results.
The highest nontoxic dose for HDP-P-AraG was 75 μg/eye, and was 70 and 210 μg/eye for HDP-P-5-F-2dUrd and HDP-cP-5-F-2dUrd, respectively. All compounds demonstrated a localized depot of crystalline aggregate in the vitreous with a clear view of vitreous and retina elsewhere. The drug depot of HDP-P-AraG was visible for 4 to 5 weeks; HDP-P-5-F-2dUrd, 8 to 10 weeks; and HDP-cP-5-F-2dUrd longer than 14 weeks. The treatment study showed HDP-P-AraG led to 33% reduction in CNV in the rat (P = 0.015), and HDP-cP-5-F-2dUrd provided 100% prevention of trauma-induced PVR in the rabbit (P = 0.046). The pretreatment study demonstrated a significant protection against intraocular proliferation compared with the 5-FU in a parallel study (P = 0.014).
Conclusions.
The intravitreous injectable lipid prodrugs of AraG and 5-fluoro-2′-deoxyuridine could be long-lasting, slow-release, antiproliferative compounds to treat unwanted intraocular proliferation.
Direct delivery of drug to the posterior segment by intravitreal injection or intravitreal implant has demonstrated advantages in treating acute or chronic vitreoretinal diseases. Contrasted with systemic drug administration, local intravitreal drug administration bypasses the blood–ocular barriers, allowing higher intraocular drug levels and avoiding many of the side effects associated with systemic therapy. We have developed and reported a crystalline lipid prodrug intraocular delivery system.1 We demonstrated that the crystalline lipid prodrugs of ganciclovir (HDP-P-GCV) and cyclic cidofovir (HDP-cCDV) provided longer duration of antiviral effect than the unmodified compounds in a rabbit HSV retinitis model after a single intravitreal injection.1,2 Compared with intravitreal surgical implants, this delivery system involves only a simple intravitreal injection that can be easily performed in an office setting without the complications associated with surgical placement or replacement of intravitreal implants.3 We propose that this intravitreal drug-delivery system may be applicable to certain other compounds for the purpose of developing long-lasting drugs for inhibition of intraocular proliferation and angiogenesis. In this report, we extended this work to an area of unmet medical need; long-acting antiproliferative intraocular drug treatment. We chose to target proliferative vitreoretinopathy (PVR) associated with trauma and/or retinal detachment as well as proliferation associated with age-related macular degeneration (ARMD).
PVR is the most common cause of poor visual outcomes in association with retinal detachment4,5 and ocular trauma. despite extensive advances in vitreoretinal surgical technique.6–8 Pharmacologic interventions have shown limited success with the use of one-time intraoperative treatments with antiproliferative agents such as daunomycin and a combination of 5-fluorouracil and heparin.9,10 In both ocular trauma and retinal detachment, an intravitreal injection of a long-acting nontoxic agent to prevent proliferation and all the attendant disastrous consequences of such proliferation would be of major benefit.
ARMD and choroidal neovascularization (CNV) in particular are the leading causes of irreversible visual loss in the Western world.11,12 The primary pathologic process in CNV associated with ARMD includes a subretinal proliferative neovascular response in the macula. Over time, proliferation results in a fibrous disciform scar causing permanent loss of central vision and legal blindness. Currently available treatments for CNV include thermal photocoagulation for extrafoveal CNV,13,14 and intravitreal injection of antiangiogenesis agents for subfoveal CNV.15,16 These antiangiogenesis treatments require frequent intravitreal injections (8–12 times per year), although the vision can be maintained during the study period with repeated administration.17,18 A long-acting, intravitreal antiproliferation treatment could reduce the necessity for frequent intravitreal injections, and the crystalline lipid prodrugs of antiproliferation may be synergistic with these angiogenesis treatments by reducing scarring and angiogenesis.19
In the present study, we modified the antiproliferative nucleoside analogues arabinofuranosylguanine (AraG) and 2′-deoxy-5-fluorouridine (5-F-2dUrd) by covalent attachment of an alkoxyalkyl phospholipid residue to obtain three lipophilic prodrugs: hexadecyloxypropyl arabinofuranosylguanine 5′-monophosphate (HDP-P-AraG), hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 5′-monophosphate (HDP-P-5F-2dUrd), and hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 3′,5′-cyclic monophosphate (HDP-cP-5-F-2dUrd). These compounds were intravitreally injected into animal eyes, and the intravitreal properties as well as preliminary efficacy were evaluated.
Materials and Methods
Synthetic Chemistry
Synthesis of HDP-P-AraG.
The synthesis of HDP-P-AraG is shown in Figure 1. Efficient coupling of AraG to the alkoxyalkyl phosphate required a blocked derivative of AraG. A suitably blocked AraG derivative (Fig. 1, compound 6) was obtained by using a modified version of the synthesis published by Resmini and Pfleiderer.20 After blocking the reactive groups of guanosine, the 2′ hydroxyl was converted to trifluoromethanesulfonate (Fig. 1, compound 4). Reaction of compound 4 with lithium acetate, followed by partial deblocking provided compound 6. Compound 6 was then coupled to hexadecyloxypropyl phosphate by using dicyclohexylcarbodiimide as the condensing reagent. Complete deblocking afforded the target compound, HDP-P-AraG, as a white crystalline solid. Analytical data are as follows: 1H NMR (DMSO-d6): δ 8.22 (s, 1H), 7.74 (s, 2H), 6.05 (d, 2H), 4.29 (d, 1H), 3.85 (m, 1H), 3.80–3.82 (m, 2H), 3.40 (t, 2H), 3.31 (t, 2H), 1.77 (m, 2H), 1.24 (br s, 28 H), 0.86 (t, 3H); and MS ESI (m/z) 646.15 (M+H)+.
Figure 1.
Synthesis of HDP-P-AraG. Reagents: (a) see Reference 1; (b) 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane, pyridine; (c) trifluoromethanesulfonic acid, pyridine; (d) LiOAc, N,N-DMF; (e) tetrabutylammonium fluoride, HOAc, CH3CN; (f) hexadecyloxypropyl phosphate, dicyclohexylcarbodiimide, pyridine; and (g) three deprotection steps.
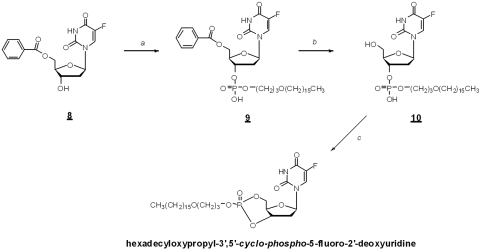
Synthesis of Hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 3′,5′-monophosphate (HDP-cP-5-F-2dUrd).
Hexadecyloxypropyl phosphate was coupled to 5-fluoro-5′-benzoyl-2′-deoxyuridine (Fig. 2, compound 8) to give compound 9. After deblocking, compound 10 (Fig. 2) was treated with 1,3,5-triisopropylbenzenesulfonyl chloride to yield the cyclic phosphate. Analytical data: 1H NMR (CDCl3): 7.50 (d, J = 6 Hz, 1H), 6.30 (d, J = 8 Hz, 1H), 4.88–5.02 (m, 1H), 4.48–4.69 (m, 2H), 4.18–4.33 (m, 2H), 3.82–3.97 (m, 1H), 3.47–3.52 (m, 2H), 3.36–3.42 (m, 2H), 2.51–2.69 (m, 1H), 2.19–2.51 (m, 1H), 1.92–1.97 (m, 2H), 1.50–1.59 (m, 2H), 1.19–1.40 (m, 26H), and 0.87 (t, J = 7 Hz, 3H); 31P NMR (CDCl3): δ −2.68; and MS-ESI (m/z) 591.20 (M+H)+ and 613.27 (M+Na)+.
Figure 2.
Synthesis of hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 3′,5′-cyclic monophosphate. Reagents: (a) hexadecyloxypropyl phosphate, 1,3-dicyclohexylcarbodiimide, pyridine; (b) 2.0 M NH3/MeOH; and (c) 1,3,5-triisopropyl benzenesulfonyl chloride, 1-methylimidazole, pyridine.
Synthesis of Hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 5′-monophosphate (HDP-P-5-F-2dUrd).
HDP-P-5-F-2dUrd was prepared by direct coupling of hexadecyloxypropyl phosphate and 5-fluoro-2-deoxyuridine in the presence of dicyclohexylcarbodiimide (DCC). Analytical data: 1H NMR (dMSO-d6) δ 8.11 (d, 1H), 6.22 (dd, 2H), 3.75–4.00 (m, 4H), 3.42 (t, 2H), 3.33 (t, 2H), 1.77 (pentet, 2H), 1.48 (m, 2H), 1.24 (br s, 28H), 0.87 (t, 3H); and MS-ESI (m/z) 609 (M+H)+.
Water Solubility and Cytotoxicity Assay
To determine aqueous solubility, the lipid prodrugs HDP-P-5-F-2dUrd, HDP-cP-5-F-2dUrd, and HDP-P-AraG (3 mg) and distilled water (1 mL) were added to a vial and shaken at room temperature for 24 hours. The remaining solid was then separated by filtration through a 0.2 μm membrane filter, and the concentration of the compound in the filtrate was determined by UV spectroscopy, using calibration curves prepared in DMSO.
Cytotoxicity (CC50), the concentration of drug that causes 50% reduction in cell viability relative to controls (100% viability) was determined by using the neutral red dye uptake method12 with human foreskin fibroblasts (HFFs), and WST1 or XTT assay using ARPE 19 cells or rMC-1 (rat retina Müller) cells.21 For the neutral red assay, stock solutions of test compounds at 10 mM were prepared in sterile DMSO (Sigma-Aldrich) and then diluted into medium to give final concentrations of 100, 10, 1, 0.1, and 0.01 mM. Primary HFF cells, obtained from American Type Culture Collection (Manassas, VA), were cultured in MEM. The stock cell culture was dissociated with trypsin/EDTA (ATV solution; Invitrogen, Carlsbad, CA), each well of a 96-well microtiter plate was seeded with approximately 5000 cells; and the plate was incubated for 24 hours at 37°C in a 5% CO2 incubator. Culture medium was then removed, 200 μL of fresh medium containing test compounds was added with three replicate wells per concentration, and the plates were incubated for an additional 4 days. The cells were observed microscopically and their condition noted. Medium was removed and the wells were rinsed twice with 100 μL PBS. Medium (100 μL) containing 0.02% neutral red dye (Fisher Scientific, Pittsburgh, PA) was added to each well including six wells for background and incubation continued for 1 hour at 37°C. The dye medium was then aspirated, and the wells were rinsed once with 200 μL PBS and aspirated. To each well was added 100 μL of a solution of 1% acetic acid in 50% aqueous ethanol. The plate was put on shaker for 15 minutes at room temperature and then neutral red uptake in each well was determined by measuring the absorbance at 540 nm with a microplate reader.
For the XTT assay, ARPE-19 cells and rat Müller cells were plated at 1 × 104 per well at 100 μL per well in 10% FBS DMEM/F12 for ARPE-19 and 10% FBS DMEM for rat Müller cells. Six wells were left empty for background readings. The plates were incubated overnight. Drugs were made from a 10-mM stock in DMSO and were diluted with 2% FBS medium to a starting dilution of 100 μM and then diluted 1:10 in three subsequent dilution steps. After overnight incubation, the cells were checked for confluence (∼80%) and the medium was aspirated. Drug dilutions were added at 100 μL, leaving six wells for no drug readings and adding 2% FBS medium instead. Plates were incubated for 5 days.
After 5 days, the plate was read using either the XTT kit for rat Müller cells or WST-1 reagent for ARPE-19 cells. The XTT method consists of adding 100 μL of electron coupling agent to 5 mL XTT reagent and mixing well. This solution (50 μL) is added to all wells including background. The plate is placed on a shaker for 1 minute and incubated for 30 minutes before reading on a microplate reader at OD450. The WST-1 method consists of adding 10 μL of WST-1 reagent to all wells including background. As in the XTT assay, the plate is placed on a shaker for 1 minute, incubated for 30 minutes, and read at OD450 on the plate reader.
Animal Studies
All animal management in this study was in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Intravitreal Toxicity and Prophylactic Treatment of Rat CNV Using HDP-P-AraG.
For the toxicity study, eight New Zealand Red rabbits were used, four for the 2-week observation and the other four for the 8-week observation. Rabbits received 25 μg (0.0387 micromoles) or 125 μg (0.19 micromoles) of HDP-P-AraG in their right eyes and the same volume (50 μL) of 5% dextrose in the left eyes as the control. After the intravitreal injection, the eyes were monitored by slit lamp, indirect ophthalmoscopy, and ERG.1 Intraocular AraG safety was tested in rat eyes. Four Brown Norway rats received 3 μg AraG in 3 μL of 5% dextrose in their right eyes and the fellow eyes received an equal volume of 5% dextrose as the control. The IC50 of AraG on a human T-cell line was reported to be 284 μM,22 which translates into the AraG concentration of 0.08 μg/μL. If we assume 30 μL of rat vitreous volume,23,24 the safe or maximum tolerated dose would be approximately 3 μg/eye. After the intravitreal injection, the eyes were monitored for 2 weeks with indirect ophthalmoscopy and slit-lamp biomicroscopy. ERG was obtained before the animal kill and histology was evaluated using light microscopy. For rat ERGs, the rats were anesthetized with a mixture of ketamine (50 mg/kg) and xylazine (5.5 mg/kg). Responses were obtained from the anesthetized cornea (proparacaine HCl, 0.5%) using a gold loop corneal electrode and reference subdermal electrode at the cheek and ground clip electrode on the ear. Responses were collected and amplified as described for the rabbit ERG procedure.1 At the end of the scheduled observation time points, the animals were killed, and enucleated globes were processed for routine paraffin sections for histology.
For the prophylactic treatment of laser-induced rat CNV, 26 Brown Norway rats were used, 13 for HDP-P-AraG and 13 for AraG. Three days before laser photocoagulation of the retina, 3 μg of 3 μL of HDP-P-AraG suspension or 3 μg of 3 μL of AraG was injected into the right vitreous cavity of each group of 13 rats through the sclera at 1 mm behind the limbus with a sterilized 50-μL syringe on a repeating dispenser (Hamilton, Reno, NV). On a molar basis, the dose of AraG is 2.3-fold higher than the dose of HDP-P-AraG. The drug was injected into the vitreous cavity through a 27-gauge butterfly needle. Three microliters of 5% dextrose was injected into the left vitreous cavity in the same manner as the control. Three days after the injection, the retinas in both eyes were photocoagulated with an 810-nm diode laser. The parameters were a spot size of 75 μm, power of 250 to 300 mW, and exposure time of 100 mS. Five to seven spots were placed around the optic nerve head, 2 to 3 disc diameters away from the optic nerve. Only laser burns that resulted in air bubble formation at the initial application were considered effective burns, and a map of laser burns from each eye was documented by direct viewing through a slit-lamp with a rat cornea contact lens (rat ocular fundus lens, OFA5.4; Ocular Instruments, Inc., Bellevue, WA). In addition, color fundus photographs were obtained from all eyes with a fundus camera (Canon USA, Lake Success, NY).
Two weeks after the laser photocoagulation, fluorescein angiography (FA) was performed on both eyes of each rat. For FA, 0.1 mL of a 24 mg fluorescein sodium/mL solution was injected via sublingual vein. Serial fundus images were acquired in the early, middle, and late phases of FA with a confocal scanning laser ophthalmoscope (HRA; Heidelberg Engineering, Vista, CA). Leakage of the laser burns was determined along with the color fundus photos by reviewing the serial HRA FA frames and identifying the hyperfluorescence increase over time. The leakage was graded by two ophthalmologists in a masked manner as 0 (no leaking), 1 (minimal intensity increase), 2 (moderate intensity increase), and 3 (both intensity and area increase). The average grade of each lesion of the treated eyes from two observers was used to compare the average grade of laser lesions in the fellow eyes using generalized estimating equations (GEEs) for multinomial models (SAS 9.2; SAS Institute, Inc., Cary, NC). All procedures were performed in animals under general anesthesia with intraperitoneal injection of ketamine (56 mg/kg) and xylazine (6 mg/kg). Topical anesthesia with proparacaine-HCl 1% was also used.
Intravitreal Toxicity of HDP-P-5-F-2dUrd and HDP-cP-5-F-2dUrd.
Twenty-four New Zealand Red rabbits were used for the studies. Six were used for the study of HDP-P-5-F-2dUrd and 12 for the study of HDP-cP-5-F-2dUrd. For HDP-P-5F-2dUrd, the doses of 35, 70, and 210 μg/eye were tested, and each dose was tested in two eyes of two rabbits for 8 weeks. For HDP-cP-5-F-2dUrd, the doses of 35, 70, and 210 μg/eye were tested, and each dose was tested in two eyes of two rabbits for 8 weeks; in addition, the doses of 350 and 700 μg/eye were tested in six rabbits, and each dose was tested in 3 eyes of three rabbits for 4 months. For the toxicity study, only one eye of the rabbits was injected with testing drug and the fellow eyes were injected with 5% dextrose as control. After the intravitreal injection, rabbit eyes were examined on day 3 and a 1, 2, 3, 5, and 8 weeks, and then every other 2 weeks, using indirect ophthalmoscope, slit lamp biomicroscope, and tonometer. The findings from the examinations including congestion of anterior segment, clarity of anterior chamber, clarity of lens and vitreous, the size of drug depot relating to the size of the optic nerve head, and fundus changes were documented as previously described.1,25,26 Electroretinography (ERG) was performed on all eyes of all rabbits before death.1 The eye globes were enucleated and fixed in 2% glutaraldehyde/2% paraformaldehyde overnight for histopathology.
Pilot Efficacy Study of HDP-cP-5-F-2dUrd.
Treatment Study.
Nine New Zealand Red rabbits were used for a pilot treatment study with HDP-cP-5-F-2dUrd. Nine eyes of the nine rabbits had the procedures for the controlled wound-induced PVR, which is close to the PVR developed after trauma in a human eye. In brief, under a surgical microscope an 8-mm transscleral incision was made circumferentially 2 mm from limbus. The protruding vitreous was cut and removed before the wound was closed with 8-0 silk interrupted sutures. After confirmation of retinal attachment, 0.4 mL of autologous blood was injected into the vitreous cavity. After the procedure, three eyes received intravitreal injection of 700 μg/eye HDP-cP-5-F-2dUrd, three eyes received 700 μg/eye 5-fluorouracil (5-FU), and the other three eyes received a 5% dextrose intravitreal injection. The intravitreal blood injection precluded performing a reliable fundus examination and PVR grading. Careful evaluation of the vitreoretinal condition was performed on the enucleated eye globes under a dissecting microscope. Three weeks after the procedure, ERGs and IOPs were obtained from all eyes of all rabbits before death, and the eye globes were enucleated and fixed in 1.25% glutaraldehyde and 1% paraformaldehyde overnight to better preserve the anatomic relationships of the ocular tissue to avoid producing tissue artifacts.27 The globes were sagittally cut into two halves through the optic nerve, and intraocular tissue relationships were inspected and documented under a dissecting microscope. Retinal detachment was graded into a percentage of the whole retina area. The inferior retina accounted for approximately 60% of the whole retina and the superior retina accounted for approximately 40% of the retina in rabbit eyes due to the optic nerve location. The dose of 700 μg of 5-FU was chosen because the maximum tolerated dose in rabbit eyes was reported to be either 500 or 1000 μg.28,29 On a molar basis, this dose is 4.6-fold higher than the dose of HDP-cP-5-F-2dUrd.
Pretreatment Study.
Eight rabbits were used. Four rabbits had their right eyes injected with 700 μg 5-FU and the other four rabbits had their right eyes injected with 700 μg HDP-cP-5-F-2dUrd 3 weeks before the induction of experimental PVR trauma, as described in the treatment study. Each rabbit had only one eye used for PVR induction; the fellow eye was untouched. Three weeks after the surgery, the rabbits' eyes were examined exactly the same as the description in the treatment study.
Results
In Vitro Solubility and Cytotoxicity Assays
The solubility study showed that HDP-P-5F2Urd was completely soluble with the solubility greater than 3 mg/mL (4.9 mM). For HDP-P-AraG and HDP-cP-5F2Urd, the solubility was 0.5 mM and less than 0.1 mM, respectively.
Cytotoxicity of the three lipid prodrugs and their parent compounds was determined with human HFFs, ARPE19 cells, and rMC-1 cells by measuring cell viability with the neutral red dye uptake assay, the WST1 assay, and the XTT assay. The results are summarized in Table 1. All three lipid analogues were found to be slightly more potent than the corresponding parent compound.
Table 1.
Cytotoxicity of Nucleoside Analogues in Various Cell Lines
| Compound | CC50, μM (HFF)* | CC50, μM (ARPE19)† | CC50, μM (rMC-1)‡ |
|---|---|---|---|
| 5-Fluorouracil | 59 ± 21 (3) | >100, 39 | 0.22, 0.1 |
| HDP-P-5F-2dUrd | 5.1 ± 1.3 (3) | >100 (2) | <0.00001 (2) |
| HDP-cP-5F-2dUrd | 17 ± 3.5 (3) | 90 ± 11 (3) | 0.04 (2) |
| Ara-G | 86 ± 18 (3) | >100 (3) | >100, >100 |
| HDP-P-Ara-G | 49 ± 1.4 (3) | 33 ± 19 (3) | 30, 65 |
Mean ± SD; numbers in parenthesis are the number of replications.
Neutral red assay.
WST1 assay.
XTT assay.
Intravitreal Toxicity of HDP-P-AraG in Rabbit Eyes
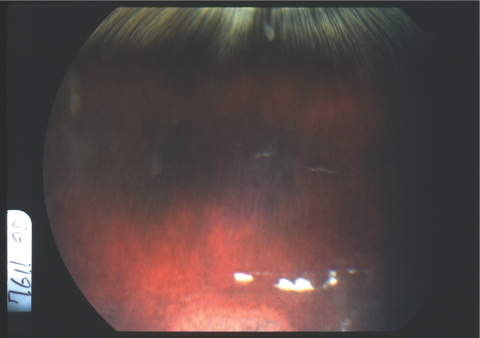
After intravitreal injection, the drug formed a depot in the vitreous cavity with clear vitreous elsewhere. The drug depot was observable for 2 weeks with the low dose (25 μg/eye) and for 4 to 5 weeks with the high dose (125 μg/eye). No toxicity was observed except for the local retinal toxicity that presented as a local retinal disturbance of fundus pigmentation where the drug depot touched the retina in the eyes at high doses (Fig. 3). ERGs were normal in all eyes including the ones with local retinal toxicity. Pathology showed no toxicity in the eyes with the 25-μg intravitreal injections. In the eyes with the 125-μg intravitreal injections, localized retinal structural disturbance, and proliferation or hypertrophy of retinal pigment epithelium were noted. The retina was normal elsewhere.
Figure 3.
Fundus photograph of a rabbit eye at the second week after an intravitreal injection of crystalline HDP-P-AraG (125 μg). Note the white drug depot in the vitreous and a depigmented area of the retina beneath the white crystal drug.
Prophylactic Treatment of Rat CNV Using HDP-P-AraG
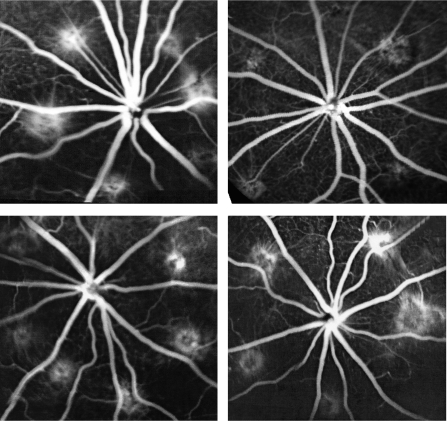
For the prophylactic treatment for laser-induced rat CNV study, 3 μg per rat eye was used. This dose was based on 75 μg per rabbit eye which was between 25 and 125 μg doses tested in rabbit eyes, assuming rat vitreous volume of 56 μL24 and rabbit vitreous volume of 1400 μL. The dose of 3 μg per rat eye did not show any toxicity in any tested rat eye. The prophylactic study revealed that treated eyes had a roughly three times higher chance of having a lower leakage grade than that in the control eyes (OR, 2.89; 95% CI, 1.35–6.19; P = 0.015, GEE). Grade 3 leakage was also examined across the treated and control eyes. Of all the burns, 40% were actively leaking (grade 3) in the treated eyes versus 60% in the control eyes (OR, 2.28; 95% CI, 1.02–5.06; P = 0.043, GEE). In addition, the leaking of the laser burns in the rat eyes injected with AraG was compared with the leaking in the fellow eye, and no significant difference was found (P = 0.57, GEE). Data from both groups were pooled and further analyzed across the three groups (HDP-AraG, AraG, and a pooled control group). HDP-AraG-treated eyes had roughly a 3.5 times higher chance of having a lower leaking grade than that of eyes that had an intravitreal injection of AraG (OR, 3.48; 95% CI, 1.58–8.04; P = 0.004, GEE). The drug led to a 33% reduction of CNV formation in this rat CNV model, judged by fluorescein angiograph (Fig. 4).
Figure 4.
Fluorescein angiographs (FA) taken in a late stage (5 minutes) at 2 weeks after laser coagulation. Top left: data from the left eye, which received 3 μL of 5% dextrose; top right: data from the fellow eye (right eye), which received 3 μg HDP-P-AraG 3 days before laser. Compared with the left eye (six laser burns), the right eye (five laser burns) showed less hyperfluorescein staining of the laser burns with minimal leakage. Bottom left: left eye, which that received 3 μL of 5% dextrose; bottom right: the fellow eye (right eye), which received 3 μg of AraG 3 days before laser photocoagulation. FA leakage from the laser burns on both eyes was comparable (six burns in the left eye and five burns in the right eye).
Intravitreal Toxicity of HDP-P-5-F-2dUrd and HDP-cP-5-F-2dUrd in Rabbit Eyes
The eyes injected with HDP-P-5-F-2dUrd at doses of 35 and 70 μg/eye showed no toxicity, and the drug depot manifested as a localized haze in the vitreous, with a clear view of the retina. The dose of 210 μg/eye HDP-P-5-F-2dUrd was toxic, causing localized retinal damage and late retinal detachment. The highest nontoxic dose was 70 μg/eye.
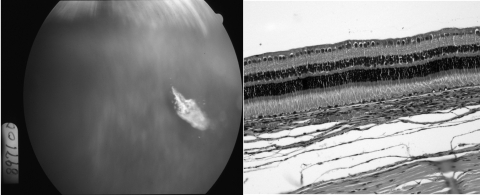
The eyes injected with HDP-cP-5-F-2dUrd, including the highest dose of 210 μg/eye, showed no toxicity, and a drug depot of crystalline aggregate in the vitreous (Fig. 5) or on the retina was observed with normal histology (Fig. 5). In the additional six eyes with even higher doses (350 and 700 μg/eye), no toxicity was found until 3.5 months after injection, after which two eyes, one with a 350-μg and the other with a 700-μg injection, showed subtle variable pigment disturbance on the inferior retina near the drug depot. The pathology of the corresponding location showed low-grade vitritis and occasional subretinal macrophage infiltration (Fig. 6). However, the IOP and ERGs of those eyes with 350 and 700 μg/eye doses was normal.
Figure 5.
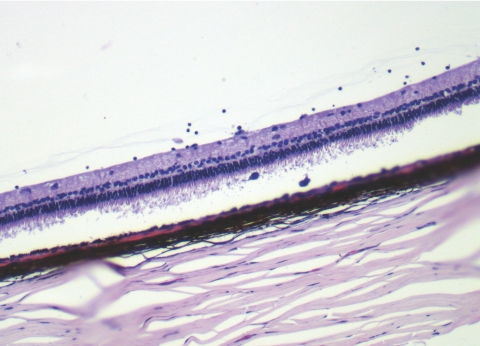
Left: a fundus photograph taken 2 weeks after intravitreal injection of 210 μg HDP-cP-5-F-2dUrd, showing crystalline drug depot in the vitreous with a clear view of normal retina. Right: light micrograph from the part of visual streak of the same eye at 8 weeks after drug injection, showing normal retinal morphology and structure. Magnification, ×62.5.
Figure 6.
A light micrograph of an eye with a 350-μg intravitreal injection of HDP-cP-5-F-2dUrd at 5 months after the injection, showing mild inflammatory cells in the inferior vitreous at the site of the drug depot and two pigment-laden macrophages in the corresponding subretinal space. The retinal detachment in the image was an artifact from the histology processing. Magnification, ×25.
Pilot Efficacy Study of HDP-cP-5-F-2dUrd
Treatment Study.
Three eyes injected with 700 μg/eye of HDP-cP-5-F-2dUrd showed normal ERGs, IOPs, and no occurrence of any degree of retinal detachment. In contrast, three eyes injected with 5% dextrose and three eyes injected with 700 μg 5-FU developed partial or full retinal detachment (Fig. 7) with low IOPs and abnormal ERGs (Table 2).
Figure 7.
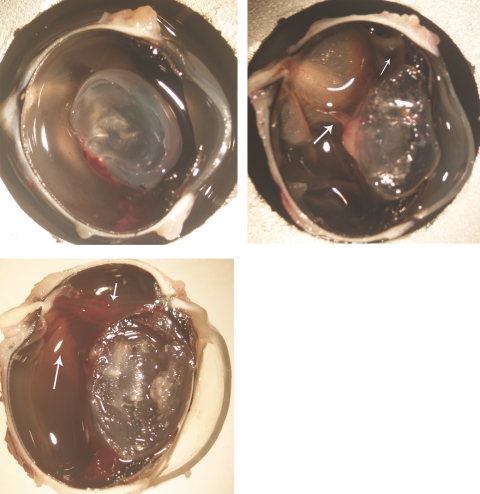
Top left: an eye that had PVR-induced trauma along with intravitreal injection of 700 μg HDP-cP-5-F-2dUrd, showing a normal medullary ray and attached retina at 3 weeks. Top right: an eye that had the trauma procedure but with an intravitreal injection of 5% dextrose, showing a proliferating tissue band between the lens and medullary ray (large arrow) and detached medullary ray and retina. The retina was also pulled to the incision site (small arrow). Bottom: an eye that had PVR-induced trauma and intravitreal injection of 700 μg 5-FU, showing a proliferating stiff strand (small arrow) between the incision and the optic nerve head along with the detached medullary ray and the surrounding retina (large arrow). Magnification, ×12.
Table 2.
Results of Treatment with HDP-cP-5-F-2dUrd and 5-Fluorouracil
| Animal ID | Group | IOP Difference from the Fellow Eye (mm Hg) | ERG Amp Difference from the Fellow Eye (μV) | Tractional Retinal Detachment |
|---|---|---|---|---|
| 59 | HDP-cP-5-F-2dUrd | 1 | 20 | No |
| 60 | HDP-cP-5-F-2dUrd | 0 | 5 | No |
| 61 | HDP-cP-5-F-2dUrd | 1 | 20 | No |
| 62 | Control | 10 | 90 | Yes (100%) |
| 63 | Control | 8 | 35 | Yes (15%) |
| 64 | Control | 24 | 50 | Yes (10%) |
| 55 | 5-Fluorouracil | 5 | 70 | Yes (11%) |
| 6 | 5-Fluorouracil | 8 | 20 | Yes (50%) |
| 57 | 5-Fluorouracil | 8 | 50 | Yes (30%) |
| P | 0.017* | 0.049* | 0.018† |
Amp, amplitude (peak of b-wave to trough of a-wave).
Wilcoxon/Kruskal Wallis rank sum test, one-tailed.
Fisher's exact test, one-tailed.
Pretreatment Study.
The four eyes pretreated with 700 μg HDP-cP-5-F-2dUrd showed no or minimal intravitreal or epiretinal proliferation, and only one eye had retinal detachment, whereas all four eyes pretreated with 700 μg 5-FU demonstrated retinal detachment with obvious intravitreal and epiretinal proliferation (Table 3, Fig. 8).
Table 3.
Results from the Pretreatment Using HDP-cP-5F2dUrd Fluorouracil
| Animal ID | Group | IOP Difference from the Fellow Eye (mm Hg) | ERG Amp Difference from the Fellow Eye (μV) | Retinal Detachment | Tractional Retinal Detachment (%) |
|---|---|---|---|---|---|
| R27 | HDP-cP-5-F-2dUrd | 5 | 50 | N | 0 |
| R61 | HDP-cP-5-F-2dUrd | 6 | 35 | Y | 17 |
| R86 | HDP-cP-5-F-2dUrd | 1 | 5 | N | 0 |
| R37 | HDP-cP-5-F-2dUrd | 3 | 40 | N | 0 |
| R77 | 5-Fluorouracil | 3 | 60 | Y | 30 |
| R90 | 5-Fluorouracil | 8 | 110 | Y | 90 |
| R58 | 5-Fluorouracil | 7.3 | 170 | Y | 50 |
| R01 | 5-Fluorouracil | 10.3 | 80 | Y | 15 |
| P | 0.05* | 0.02* | 0.07† | 0.014* |
Amp, amplitude (peak of b-wave to trough of a-wave).
Wilcoxon/Kruskal Wallis rank sum test, one tail.
Fisher's exact test, one-tail.
Figure 8.
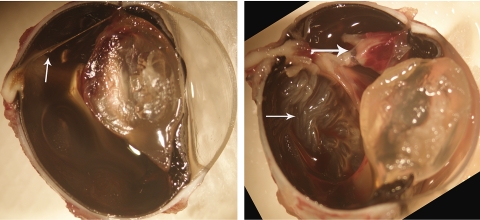
Left: an eye that received 700 μg HDP-cP-5-F-2dUrd intravitreal injection 3 weeks before the trauma procedure, showing a thick strand (arrow) between the incision and the optic nerve head but with no medullary ray lifting or retinal detachment noted. Right: in contrast, an eye with a 700-μg intravitreal injection of 5-FU 3 weeks before the trauma procedure, showing massive proliferation (large arrow) and retinal detachment (small arrow). Magnification, ×12.
Discussion
The underlying factor in many vision-threatening vitreoretinal diseases is unwanted intraocular cell proliferation. At the current time, there is little success in pharmacologic treatment or prevention of these proliferative vitreoretinal diseases. A major disadvantage of currently available antiproliferation drugs is the short vitreous half-life. For example, perioperative infusion with 5-fluorouracil (5-FU) has failed to demonstrate significant benefit,30 but a 5-FU implant has demonstrated significant preventative effects in experimental PVR models.31 Therefore, sustained intraocular release of antiproliferative agents is the key to achieving the goal of PVR treatment or prevention.
Our goal is to develop intravitreally injectable long-lasting slow release compounds to treat intraocular diseases. We previously described a crystalline intraocular drug delivery system.1,2 The advantage of this drug delivery system is that drug can be delivered by a simple intravitreal injection and achieve sustained intravitreal drug release while eliminating the invasiveness and the complications associated with ocular surgeries. In fact, triamcinolone acetonide is such a crystalline drug and has been extensively used as an intravitreal long-lasting therapeutic to treat many of retinal diseases.32 We hypothesized that this approach1 could be applied to the delivery of other small nucleoside analogues such as AraG and 5-fluoro-2′-deoxyuridine. As demonstrated in the current studies, HDP-P-AraG, HDP-P-5-F-2dUrd, and HDP-cP-5-F-2dUrd all formed vitreous drug depot after intravitreal injection and appeared to be long-lasting. The key to achieving a long-lasting depot appears to be the insolubility of the compound. HDP-P-5-F-2dUrd was the most soluble. To further increase the vitreous residence time of HDP-P-5-F-2dUrd, we synthesized HDP-cP-5-F-2dUrd by bonding the phosphate to 3′ oxygen of the ribose to close the ring that makes the compound less soluble by masking the negative charge of the phosphate moiety. Our results supported the above theory, showing that the highest nontoxic dose of HDP-P-5-F-2dUrd (70 μg/eye) lasted approximately 8 weeks in the vitreous, whereas HDP-cP-5-F-2dUrd demonstrated a much higher nontoxic dose (210 μg/eye or higher) and a much longer vitreous residence time (over 4 months with 350- and 700-μg/eye doses). In contrast, it has been reported that 5-FU had 7.7 hours of half-life in rabbit vitreous.33 We acknowledge that a free-floating vitreous drug depot might cause direct touch of the retina and the attendant local toxicity, which probably would not be shown by ERG. Therefore, to identify the highest nontoxic dose is important for this type of drug delivery. The highest nontoxic dose leads to an ideal drug depot that is not large enough to cause visual disturbance and is dense and heavy enough to cause direct touching of the retina and the local toxicity.
In addition being long-lasting in the vitreous of the lipid derivatized nucleoside, the potency of these compounds against cell proliferation was changed, too. For AraG, HDP-P-AraG had slightly increased potency (roughly doubled, CC50 = 86 μm for AraG versus CC50 = 49 μM for HDP-P-AraG on HFF cells proliferation). However, HDP-P-5-F-2dUrd or HDP-cP-5-F-2′dUrd was 11.6 or 3.5 times as potent as 5-FU on the proliferation of HFF cells. The similar potency increase was also observed with lipid derivatized antiviral compounds such as cidofovir and ganciclovir (GCV).2,34 The underlying mechanism could be related to the conjugated lipid chain which enhances the cellular uptake of the compound.35 Our previous study showed that HDP-cidofovir and HDP-cyclic cidofovir were taken up rapidly by MRC-5 human lung fibroblasts in vitro, but uptake of cidofovir and cyclic cidofovir was much slower; analysis of cellular metabolites showed that levels of cidofovir diphosphate (CDV-DP), the active antiviral compound, were >100 times greater with HDP-cidofovir than levels observed with cidofovir.35 These compounds exert their antiproliferative activity by cellular conversion of the liponucleotides to the corresponding nucleoside triphosphates, which inhibit cellular DNA and RNA synthesis. We previously demonstrated that incubating a lipid prodrug of GCV, HDP-P-GCV, in rabbit vitreous or heat inactivated rabbit vitreous led to a much higher level of GCV in normal vitreous than that in the heat-inactivated vitreous.36 The interpretation is that vitreous cells in normal vitreous are functional and take up the lipid prodrug and convert the prodrug into the parent drug in the cells.
It is possible that there are other structure-associated functionality changes in lipid derivatizing of small molecules that may increase or decrease the parental drug potency. These changes should be investigated further. In addition, different cell types may have different sensitivities to these lipid prodrugs, although rapidly proliferating cells are usually more susceptible to antimetabolites. In the current in vitro experiments, HDP-P-AraG showed similar CC50 on the three cell lines; however, HDP-cP-5-F-2-dUrd was less toxic to the human RPE19 cell line, which was shown to retain marked features of the retinal pigment epithelium. In fact, many types of the retinal cells are quiescent in the normal retina and should be less sensitive to those lipid prodrugs of antimetabolites than the immortalized cell lines are.
The present study was designed as a pilot study to confirm our hypothesis that crystalline intraocular drug delivery is a system1 that can be applied to other small molecule compounds, such as AraG and 5-F-2dUrd and very possibly to some biologically active peptides. Manipulation of the particle sizes of these compounds to customize the intravitreal residence time to eliminate toxicity and detailed solubility studies as well as pharmacokinetic studies should be performed to further characterize their intraocular property. However, a small pilot efficacy study would be informative before committing resources and effort on a large scale, even though the in vitro efficacy studies were very encouraging. Our in vivo efficacy studies were indicative that both HDP-P-AraG and HDP-cP-5-F-2dUrd could be clinically useful in inhibiting the development of CNV and PVR. Notably, HDP-cP-5-F-2dUrd demonstrated a significant antiproliferation effect in both the treatment and pretreatment experiments. Further studies to determine the optimal formulation and intraocular pharmacokinetics, as well as full-scale efficacy studies are warranted.
Footnotes
Supported by National Institutes of Health Grant EY 018589 (WRF, KH, LC) and unrestricted research funds to the UCSD Jacobs Retina Center (WRF, LC).
Disclosure: L. Cheng, None; K. Hostetler, None; N. Valiaeva, None; A. Tammewar, None; W.R. Freeman, None; J. Beadle, None; D.-U. Bartsch, None; K. Aldern, None; I. Falkenstein, None
References
- 1.Cheng L, Hostetler KY, Lee J, et al. Characterization of a novel intraocular drug-delivery system using crystalline lipid antiviral prodrugs of ganciclovir and cyclic cidofovir. Invest Ophthalmol Vis Sci 2004; 45: 4138–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng L, Hostetler KY, Chaidhawangul S, et al. Treatment or prevention of herpes simplex virus retinitis with intravitreally injectable crystalline 1-O-hexadecylpropanediol-3-phospho-ganciclovir. Invest Ophthalmol Vis Sci 2002; 43: 515–521 [PubMed] [Google Scholar]
- 3.Guembel HO, Krieglsteiner S, Rosenkranz C, Hattenbach LO, Koch FH, Ohrloff C. Complications after implantation of intraocular devices in patients with cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol 1999; 237: 824–829 [DOI] [PubMed] [Google Scholar]
- 4.Esmaeli B, Elner SG, Schork MA, Elner VM. Visual outcome and ocular survival after penetrating trauma: a clinicopathologic study. Ophthalmology 1995; 102: 393–400 [DOI] [PubMed] [Google Scholar]
- 5.Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol 2004; 137: 1105–1115 [DOI] [PubMed] [Google Scholar]
- 6.Pieramici DJ, Sternberg P, Jr, Aaberg TM, Sr, et al. A system for classifying mechanical injuries of the eye (globe). The Ocular Trauma Classification Group. Am J Ophthalmol 1997; 123: 820–831 [DOI] [PubMed] [Google Scholar]
- 7.Szijarto Z, Gaal V, Kovacs B, Kuhn F. Prognosis of penetrating eye injuries with posterior segment intraocular foreign body. Graefes Arch Clin Exp Ophthalmol 2008; 246: 161–165 [DOI] [PubMed] [Google Scholar]
- 8.Waters T, Vollmer L, Sowka J. Proliferative vitreoretinopathy as a late complication of blunt ocular trauma. Optometry 2008; 79: 197–202 [DOI] [PubMed] [Google Scholar]
- 9.Iqbal M, Charteris DG, Cooling RJ, Mackintosh GI. Conservative management of double penetrating ocular injuries. Eye 2000; 14: 249–251 [DOI] [PubMed] [Google Scholar]
- 10.Charteris DG. Ocular injuries caused by a suction toy. J R Coll Surg Edinb 1989; 34: 338–339 [PubMed] [Google Scholar]
- 11.Maberley DA, Hollands H, Chuo J, et al. The prevalence of low vision and blindness in Canada. Eye 2006; 20: 341–346 [DOI] [PubMed] [Google Scholar]
- 12.Gunnlaugsdottir E, Arnarsson A, Jonasson F. Five-year incidence of visual impairment and blindness in older Icelanders: the Reykjavik Eye Study. Acta Ophthalmol Published online, February 11, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Freund KB, Yannuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol 1993; 115: 786–791 [DOI] [PubMed] [Google Scholar]
- 14.Gelfand YA, Linn S, Miller B. The application of the macular photocoagulation study eligibility criteria for laser treatment in age-related macular degeneration. Ophthalmic Surg Lasers 1997; 28: 823–827 [PubMed] [Google Scholar]
- 15.Schouten JS, La Heij EC, Webers CA, Lundqvist IJ, Hendrikse F. A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2009; 247: 1–11 [DOI] [PubMed] [Google Scholar]
- 16.Kourlas H, Abrams P. Ranibizumab for the treatment of neovascular age-related macular degeneration: a review. Clin Ther 2007; 29: 1850–1861 [DOI] [PubMed] [Google Scholar]
- 17.Takeda AL, Colquitt J, Clegg AJ, Jones J. Pegaptanib and ranibizumab for neovascular age-related macular degeneration: a systematic review. Br J Ophthalmol 2007; 91: 1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008; 145: 239–248 [DOI] [PubMed] [Google Scholar]
- 19.Yanase K, Yoshiji H, Ikenaka Y, et al. Synergistic inhibition of hepatocellular carcinoma growth and hepatocarcinogenesis by combination of 5-fluorouracil and angiotensin-converting enzyme inhibitor via anti-angiogenic activities. Oncol Rep 2007; 17: 441–446 [PubMed] [Google Scholar]
- 20.Resmini M, Pfleiderer W. Synthesis of arabinonucleoside phosphoramidite building blocks. Helvetica Chim Acta 1993; 76: 158–167 [Google Scholar]
- 21.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci 1998; 39: 212–216 [PubMed] [Google Scholar]
- 22.Bjerke M, Balzarini J, Karlsson A. Reduced levels of mitochondrial DNA increases the toxicity of 9-beta-D-arabinofuranosylguanine (araG). Nucleosides Nucleotides Nucleic Acids 2008; 27: 746–749 [DOI] [PubMed] [Google Scholar]
- 23.Dureau P, Bonnel S, Menasche M, Dufier JL, Abitbol M. Quantitative analysis of intravitreal injections in the rat. Curr Eye Res 2001; 22: 74–77 [DOI] [PubMed] [Google Scholar]
- 24.Berkowitz BA, Lukaszew RA, Mullins CM, Penn JS. Impaired hyaloidal circulation function and uncoordinated ocular growth patterns in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 1998; 39: 391–396 [PubMed] [Google Scholar]
- 25.Cheng L, Hostetler KY, Gardner MF, et al. Intravitreal toxicology in rabbits of two preparations of 1-O-octadecyl- sn-glycerol-3-phosphonoformate, a sustained-delivery anti-CMV drug. Invest Ophthalmol Vis Sci 1999; 40: 1487–1495 [PubMed] [Google Scholar]
- 26.Cheng L, Hostetler KY, Chaidhawangul S, et al. Intravitreal toxicology and duration of efficacy of a novel antiviral lipid prodrug of ganciclovir in liposome formulation. Invest Ophthalmol Vis Sci 2000; 41: 1523–1532 [PubMed] [Google Scholar]
- 27.Margo CE, Lee A. Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Graefes Arch Clin Exp Ophthalmol 1995; 233: 366–370 [DOI] [PubMed] [Google Scholar]
- 28.Stern WH, Guerin CJ, Erickson PA, Lewis GP, Anderson DH, Fisher SK. Ocular toxicity of fluorouracil after vitrectomy. Am J Ophthalmol 1983; 96: 43–51 [DOI] [PubMed] [Google Scholar]
- 29.Leon JA, Britt JM, Hopp RH, Mills RP, Milam AH. Effects of fluorouracil and fluorouridine on protein synthesis in rabbit retina. Invest Ophthalmol Vis Sci 1990; 31: 1709–1716 [PubMed] [Google Scholar]
- 30.Charteris DG, Aylward GW, Wong D, Groenewald C, Asaria RH, Bunce C. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of established proliferative vitreoretinopathy. Ophthalmology 2004; 111: 2240–2245 [DOI] [PubMed] [Google Scholar]
- 31.Cardillo JA, Farah ME, Mitre J, et al. An intravitreal biodegradable sustained release naproxen and 5-fluorouracil system for the treatment of experimental post-traumatic proliferative vitreoretinopathy. Br J Ophthalmol 2004; 88: 1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maia M, Farah ME, Belfort RN, et al. Effects of intravitreal triamcinolone acetonide injection with and without preservative. Br J Ophthalmol 2007; 91: 1122–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarus G, Blumenkranz M, Hernandez E, Sossi N. Clearance of intravitreal fluorouracil: normal and aphakic vitrectomized eyes. Ophthalmology 1985; 92: 91–96 [DOI] [PubMed] [Google Scholar]
- 34.Beadle JR, Hartline C, Aldern KA, et al. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob Agents Chemother 2002; 46: 2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldern KA, Ciesla SL, Winegarden KL, Hostetler KY. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol 2003; 63: 678–681 [DOI] [PubMed] [Google Scholar]
- 36.Cheng L, Hostetler KY, Toyoguchi M, et al. Ganciclovir release rates in vitreous from different formulations of 1-O-hexadecylpropanediol-3-phospho-ganciclovir. J Ocul Pharmacol Ther 2003; 19: 161–169 [DOI] [PubMed] [Google Scholar]