Specific aspects of accommodative dynamics are shown to change with age from preschool to adulthood, whereas other aspects remain stable over these ages in response to a range of near stimulus demands.
Abstract
Purpose.
To study variations in dynamic measures of accommodation and disaccommodation with age in subjects ranging from preschool to adulthood.
Methods.
Accommodative responses to a step stimulus cartoon movie alternating from distance to near were recorded with a dynamic infrared photorefractor. Subjects viewed at least three stimulus cycles of far and near for four near stimulus demands (2, 3, 4, and 5 D). Latencies, peak velocities, and the magnitude of accommodative microfluctuations were calculated from the responses and compared in 41 subjects from 3 to 38 years of age.
Results.
Mean accommodative and disaccommodative latencies decreased linearly with age. The magnitude of accommodative microfluctuations during sustained near accommodation had a significant quadratic relationship to age, with subjects in the first decade of life having the largest fluctuations and subjects in the third decade of life having the smallest for all stimulus demands. Accommodative peak velocities were fastest in subjects in the first two decades of life, compared with subjects in the third and fourth decades; however, disaccommodative peak velocities showed no significant age differences.
Conclusions.
Age-related changes in dynamics occur in accommodative and disaccommodative latencies, accommodative peak velocities, and accommodative microfluctuations, all of which decrease with increasing age from preschool to adulthood. Disaccommodative peak velocities showed no change with age.
Accommodation is a dynamic process that includes many descriptive components, such as the time to initiate a response (latency), the maximum speed of a response (peak velocity), and the stability of accommodation during a sustained response (microfluctuations). Accommodation can be investigated by dynamically recording accommodative responses, such as with infrared photorefraction.1–3 Understanding age-related changes in the accommodative dynamics is of interest to develop a better understanding of the accommodative mechanism and its changes with age, with respect to visual system development and presbyopia.
The majority of studies investigating accommodative dynamics have been performed on adult subjects exploring relationships such as dynamic measurements and age, response amplitude, and pupil diameter.3–15 In recent years studies have recorded dynamic accommodative responses in young infants and found longer latencies and greater microfluctuations of accommodation in infants than in adult subjects, suggesting that some aspects of accommodation develop beyond infancy into adulthood.16–18 However, only a few studies of accommodative dynamics have included individuals in the first two decades of life,3,9,11,15,19 the majority of which had the youngest participants in their teenage years.9,11,15,19 Thus, there is still much that remains unknown about age-related changes in accommodative dynamics between the years of infancy and early adulthood.
One confounding factor when comparing accommodative performance across age groups is the decrease in maximum accommodative amplitude with increasing age. Direct comparisons of accommodative performance at a given stimulus demand may not represent similar accommodative efforts for subjects of widely differing age. This factor is of particular importance when comparing dynamic aspects that are shown to vary with response amplitude, such as microfluctuations and peak velocity.6,10,13,14 A recent study of age-related changes in dynamics avoided this problem by recording accommodative responses to a large range of stimulus demands, rather than comparing responses to only one stimulus demand.15 For example, one particular analysis compared peak velocity of accommodation as a function of response amplitude to define the main sequence function of accommodation and disaccommodation in subjects of two different age groups and compared the differences between the two functions. This approach allows a more general analysis of changes related to age that might otherwise be confounded by differences in maximum amplitude.
The goal of the present study was to investigate the effect of age (from preschool to adulthood) on dynamic accommodative measures (latency, peak velocity, and microfluctuations) performed with a dynamic infrared photorefractor in response to step-stimuli of multiple stimulus demands. Evaluating accommodative dynamics from childhood to adulthood will provide a better understanding of the age at which specific aspects of accommodative function become adultlike, as well as the age at which certain aspects of accommodative function begin to decline, such as the decline in maximum accommodative amplitude with increasing age that ultimately ends in presbyopia.
Methods
This study adhered to the tenets of the Declaration of Helsinki and was approved by the University of Houston Committee for the Protection of Human Subjects. Informed consent was obtained from all adult participants and the parents' consent and the child's assent was obtained for all participants less than 18 years of age.
Subjects
Fifty subjects aged 3 to 38 years were recruited for the study from the University of Houston College of Optometry students, staff, clinic patients, and family members. This age range was selected to compare accommodative responses across four decades of life before the onset of presbyopia. The lower age limit of 3 years was selected because of the inability of younger children to sit for the study tasks. An upper recruitment age of 40 was selected to exclude presbyopic subjects; the oldest subject who enrolled in the study was 38. Subjects were excluded from participation if they had a history of significant eye or head injuries, intraocular surgery, strabismus or amblyopia or were using any medications suspected of interfering with accommodation. Of the 50 subjects recruited, some of various ages were excluded from data analysis due to poor-quality photorefraction images due to pupil diameter less than 3 mm, downward-pointing eyelashes, or upper eyelids obstructing the view of the pupil (n = 8), and one 3-year-old subject was excluded due to unwillingness to place his chin in the chinrest. Forty-one subjects between the ages of 3 and 38 years were included in the final analysis with the following age distribution: 16 subjects aged 3 to 9 years, 8 subjects aged 11 to 19 years, 7 subjects aged 20 to 29 years, and 9 subjects aged 32 to 38 years.
All subjects were first screened with a nondilated vision assessment that included distance visual acuities of an age-appropriate acuity task, either the Bailey-Lovie high-contrast acuity chart20 for older subjects, or the Lea symbols acuity matching test21 for younger subjects. All 41 subjects included in the analysis had 20/20 acuity or better. Ocular alignment was assessed with both distance and near cover testing, and distance refraction was assessed with either retinoscopy or autorefraction.
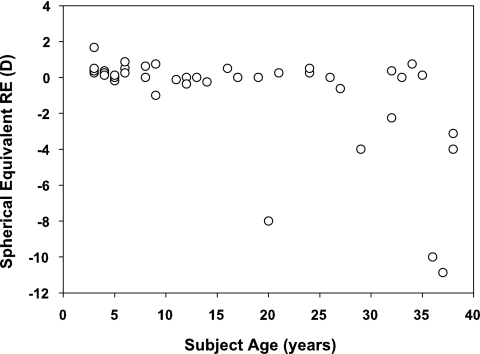
Subjects with spectacles or contact lenses wore their refractive correction for all study measurements. One subject was also included who had undergone LASIK refractive surgery to correct myopia. The distribution of refractive errors is shown in Figure 1. The subjects were not recruited for this study based on refractive error, and thus the distribution of myopia (refractive error < −0.50 D) in the subjects from this study may not match that of the general population in the United States. Typically, myopia (≤ −0.50 D) begins to occur at ∼7 to 8 years of age and it increases in prevalence throughout the teenage years.22 By the ages of 20 to 39 years, the prevalence of myopia in the United States is approximately 36%.23 In this study, the prevalence of myopia among the subjects aged 20 to 38 years was 43% and most of those subjects were in the upper age bin of 32 to 38 years.
Figure 1.
Distribution of subject ages and spherical equivalent refractive errors.
The study included 32 emmetropic subjects (spherical equivalent RE −0.50 to +0.75 D with no more than −0.75 D cylinder in all but a 3-year-old subject who had −1.00 DC), 1 subject with uncorrected hyperopia who had just turned 3 years of age (+1.75, −0.25 × 180), 4 subjects with myopia corrected with contact lenses (RE, −1.00 to −11.00 D), 1 subject with myopia corrected with LASIK (presurgical RE, −10.00 D), and 3 subjects with myopia corrected with spectacles (RE −1.00, −2.25, and −3.12 D). For the subjects with spectacle-corrected vision (all myopic), effectivity at the cornea due to lenses at the spectacle plane would impact the accommodative demand of the near stimulus; however, the largest effect would be a 0.37 D reduction at the highest accommodative demand tested (5 D) for the subject with the greatest amount of myopic spectacle correction (−3.12 D for a 13-mm vertex distance). Because of this negligible difference, subjects with spectacles were not eliminated from the analysis. Measurements of refraction and accommodative response were corrected for the corneal plane due to the effectivity of the spectacle lenses from the individual calibrations performed for each subject.
Stimulus Presentation
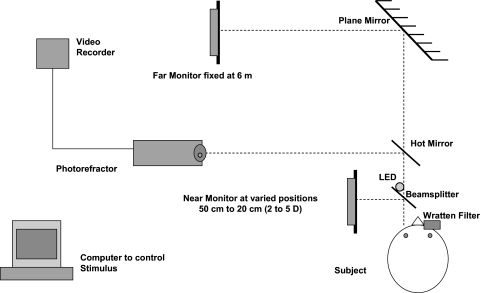
Subjects monocularly viewed with the left eye a continuously playing cartoon movie with the room lights off. The movie stimulus was controlled by a computer that alternately presented the movie on one far and one near 19-in. LCD flat-screen computer monitor in a step-wise fashion. The movie stimulus had a mean luminance ∼ 85 cd/m2 on each monitor. The switch between the monitors occurred instantaneously and was indicated to the examiner by a white light LED illuminated during near stimulus presentation by a signal sent from the computer's printer port. The far-stimulus monitor was placed a linear distance of 6 m from the subject and viewed by the subject as reflecting off a front-surfaced mirror angled at 45° and placed 4.9 m in front of the subject and aligned with the subject's line of sight. The near-stimulus monitor was positioned at various distances, 90° to the left of the subject to create near demands of 2, 3, 4, and 5 D. The subject viewed the near stimulus in primary gaze by viewing the reflection of the near-monitor reflected off a 45° angled, 5-cm2 beamsplitter (50% reflectance/50% transmittance) placed 8 cm in front of the subject's left eye. The reflected image of the near stimulus was aligned with the reflected image of the far stimulus so that the subject could view both the far or near target with the left eye in primary gaze. The subject's right eye was occluded with a gelatin filter (89B Wratten filter; Eastman Kodak Company, Rochester, NY) that blocks visible light below 720 nm and transmits infrared light. The use of this filter allowed both eyes to be visible to the infrared photorefractor, but occluded the subject's view of the movie with the right eye so that all accommodative responses were monocularly driven by the left eye. The experimental setup is shown in Figure 2.
Figure 2.
The experimental setup.
The movie stimulus filled the entire far monitor (visual angle 2.864° vertical and 3.628° horizontal when viewed from 6 m). The near stimulus was reduced in size to enable the movie to fit within the 5-cm2 beamsplitter. The size of the near stimulus was not altered, as the near stimulus was presented at closer viewing conditions and thus subtended a relatively greater visual angle with increasing proximity. The primary reason for not matching the visual angle of the stimulus at all positions was the desire to maintain as many cues to accommodation as possible to improve the subject's performance of the task, since peripheral visual cues, proximity cues, and vergence cues in this viewing environment are limited or absent. A secondary reason for not matching the visual angle at all stimulus positions was that the small size necessary to match the visual angle at the closest demands would have greatly diminished the resolution of the movie stimulus, as it would have covered only 1.3 × 1 cm of the computer monitor display.
One cycle of stimulus presentation was defined as the presentation of the movie on the far monitor, the near monitor, and back to the far monitor. A stimulus cycle lasted 10 seconds, and the switch from far to near occurred instantaneously at a random time during the 10-second cycle with a minimum of 2 seconds' presentation at each far and near. The time to switch between far and near was randomized to avoid artificially reducing latencies due to subject anticipation.24 Multiple cycles were presented for each near stimulus demand beginning with 2 D and repeating the process with increasing demand up to 5 D.
Infrared Photorefraction Measurements
Accommodative responses were recorded from the viewing eye (left eye) using a custom-built infrared (IR) photorefractor which followed the design of Schaeffel et al.3 and has been used extensively in previous studies.13,15,25 The IR photorefractor consisted of five rows of IR (890 nm) LEDs (20 LEDs in total) mounted in a triangular arrangement on a knife-edged, semicircular aperture placed on the front of a lens (MicroNikkor, 55 mm 1:2.8; Nikon Corp., Tokyo, Japan) with a UV filter (L37c, 52 mm; Nikon), mounted on an IR-sensitive CCD camera (4910 Series RS-170 Monochrome; Cohu, Inc. San Diego, CA). The photorefractor was placed a total linear distance of 100 cm from the subject. The 55 mm lens mounted on the CCD camera was focused for the 1-m viewing distance. A 4 × 5-in. hot mirror (90% visible light transmitting, 90% infrared reflecting) was positioned at a 45° angle in front of the subject and in line with the IR camera. This setup allowed IR light from the photorefractor LEDs to reflect into the eyes of the subject and the image of the subject's eyes to reflect back to the photorefractor. The photorefractor was positioned off to the left so as not to block the subject's view of the movie stimulus (Fig. 2). All measurements were performed with the room lights off, so that the edges of both the beamsplitter and hot mirror were not visible to the subject in the dark room, giving the movie stimulus the appearance that is was floating in space either at the far end of the room or immediately in front of the subject at a close distance. Performing measurements in a dark room also increased pupil dilation and enhanced the quality of the photorefraction images by providing more contrast to the images of the pupil luminance profile.
Photorefraction images from the CCD camera were recorded onto tapes (DVCam; Sony Corp., Tokyo, Japan) with a digital videocassette recorder (DVCam DSR-20 MD; Sony Corp.) at 30 Hz (30 frames/s). The digital videocassette recorder was connected to a black and white video monitor (SSM-910; Sony) to allow the experimenter to view live images as they were captured during the experiment. Auditory prompts such as the subject's identification code and the stimulus demand condition were recorded on the tapes using a wireless FM microphone. The recorded images were analyzed off-line with a photorefraction analysis program written in image analysis software (Optimas; Media Cybernetics, Bethesda, MD). The slope of the brightness profile in the pupil was determined from a linear fit to the average of two vertical sampling lines in the pupil for each individual video frame and output into a spreadsheet (Excel; Microsoft, Redmond, WA) along with the image frame number, stimulus status (far or near as indicated by the white light LED), pupil diameter, and mean pupil luminance. The slope of the brightness profile was then later converted to refraction based on an individual trial lens calibration performed on each subject3 and plotted as a function of time as an accommodative response to each step stimulus cycle. For the calibration, each subject viewed the far stimulus with the right eye (so that accommodation remained relaxed) while the left eye was occluded with the Wratten filter. Trial lenses of known power were introduced in front of the filter in front of the left eye in 1-D steps ranging from −2 to +6 D, which would alter the brightness profile in the pupil of the left eye without eliciting an accommodative response from the subject. Some of the less patient younger subjects had lenses from 0 to +6 DS introduced, but all subjects had at least six data points to determine their calibration function and all accommodative responses recorded in the study were within the measurement range of the trial lens calibrations. The slope of the brightness profile was determined for three video frames from each of the lenses and averaged to provide a mean slope for each known lens power. Data were plotted with slope on the x-axis and lens power on the y-axis and regression analysis was performed (Excel; Microsoft). All subjects had significant linear fits to the data, with r2 > 0.93. For subjects with linear regression r2 ≥ 0.99 (n = 19), the linear equation was used to convert slope to refraction. The other subjects (n = 21) had either second- or third-order polynomials fit to the data to obtain calibration functions with r2 ≥ 0.99, and these equations were used to convert slope to refraction. One subject's regression fit did not improve by attempting second- or third-order polynomials, and so her calibration function was based on the linear regression (r2 = 0.97).
If a subject was uncooperative during a portion of the taped experimental session (i.e., backing out of the chinrest or moving excessively), those segments of video were not analyzed, and thus responses analyzed were from video in which the subject was cooperative and looking straight ahead. During a blink, the image-analysis software assumed the pupil to be in the same position and instead took a measurement of the brightness of the eyelid; thus, video frames with blinks during times of good subject cooperation had to be removed from the data set. We identified the video frames in which blinks occurred by plotting both pupil size and mean luminance versus time for each subject. Blinks were easily identified in this manner, as both the mean luminance and pupil size decreased dramatically during the segment of time when the eyelid was closed. The slope values for these frames were eliminated from the data. Some accommodative or disaccommodative responses in which blinks occurred during the response were unable to be used for subsequent analysis. For example, for latency calculations, responses in which a blink occurred during the onset or offset of the near stimulus were excluded. Likewise, for velocity calculations, responses with blinks occurring during the initial rapid increase or decrease of the accommodative response were eliminated from analysis.
Data Analysis
Latency Calculations.
Latencies of accommodation were calculated for each response by determining the time between the onset of the near stimulus and the initiation of the accommodative response. Disaccommodative latencies were calculated as the time between the offset of the near stimulus and the initiation of the disaccommodative response. For these latency calculations, the data were smoothed by calculating the average of three consecutive data points and re-plotting the averaged data versus time. For example, the data at time tn was obtained from an average of the data from tn−1, tn, and tn+1. This three-point smoothing of the data reduced the noise without temporally shifting the data and allowed the initiation of the accommodative and disaccommodative responses to be more clearly identified. To identify the initiation of the accommodative response, we visually inspected the smoothed data for an increase after the onset of the near stimulus, using a technique similar to the one described by Schor et al.26 The start of the first series of five data points that increased consecutively was defined as the initiation of the response. Accommodative latency was quantified as the time between the onset of the stimulus and the first point in the series of five increasing data points. We used the same technique to calculate disaccommodative latencies, except that the series of five points were all of decreasing magnitude.
Mean Accommodative Response Calculations.
Mean accommodative response amplitudes were calculated for each individual accommodative response from the 2-second time interval occurring 1 second after the onset of the near stimulus. In addition, to ensure that the interval did not include data recorded after the termination of the near stimulus, only responses in which at least 2 seconds of data were available before the termination of the near stimulus were included in the analysis. The duration of the responses was visually verified to ensure that the responses included were typical responses in which the accommodative response persisted for the entire duration of the near stimulus presentation.
Accommodative Microfluctuation Calculations.
Root mean square (RMS) deviation was used to quantify the magnitude of the accommodative microfluctuations during the sustained accommodative responses to the near stimulus, by the formula:
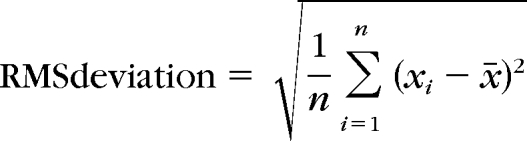 |
where n is the number of accommodation values, xi, is each individual accommodative value, and x̄ is the mean accommodative value. This technique has been reported in previous studies in which the magnitude of accommodative microfluctuations was investigated and provides a single number to indicate the average amount of fluctuation in diopters about the mean accommodative response for a specified period.27 RMS deviations were calculated for the same 2-second interval of steady state accommodative responses used for the mean accommodative response analysis.
Peak Velocity Calculations.
The speed of the accommodative and disaccommodative responses was assessed by calculating the peak velocity (Vmax) for each response. Peak velocity was calculated from the response amplitude (a) and the time constant (τ) derived from first-order exponential functions fit to the data (Vmax = a/τ).13,28,29 The equations used to fit the responses were: Accommodation:
Disaccommodation:
where y is the response in diopters, yo is the starting point of the response (after latency is removed) in diopters, a is the amplitude of the response in diopters, t is time in seconds, and τ is the time constant (the time to reach 63% of the overall response amplitude). Exponential functions were fit to each response with the user-defined statistical regression analysis tool in commercial software (SigmaPlot ver. 8.0; SPSS, Inc.). Data preceding the start of the response were removed before fitting the functions to the accommodative and disaccommodative responses. In addition, accommodation data were adjusted to a mean 0 starting point by the mean of 10 consecutive data points before the onset of the near stimulus. This adjustment corrected the data for any small variations in starting position and provided a mean 0 starting point for each of the accommodative responses for velocity calculations. For each exponential function, the residuals of the data points (difference between each response point and the exponential fit) were plotted versus time to assess the fit of the function. A poor fit was defined as any response with residuals greater than 1.25 D, and these responses were eliminated from the analysis (average residuals of all responses were approximately ±0.50 D). The pattern of the residuals was also visually inspected and any responses with distinguishable nonrandom patterns were eliminated from the analysis. This technique was similar to that reported by Kasthurirangan et al.13 and only resulted in the elimination of 1.8% of the responses.
Results
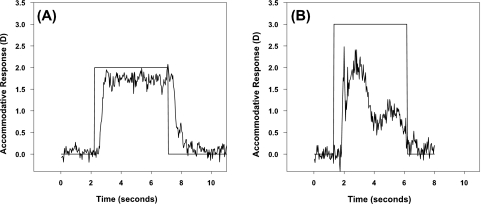
Figures 3A and 3B show two examples of accommodative stimuli and responses plotted as a function of time. Figure 3A represents a typical response to the step stimulus in which accommodation increases after the onset of the near stimulus and is sustained until the offset of the near stimulus. This sustained response pattern is representative of 90.8% of the total number of accommodative responses collected from the subjects (757/834 total responses). Figure 3B is from a 3-year-old subject and represents an atypical response. Atypical responses varied greatly in shape and included responses in which (1) no identifiable increase in accommodation occurred to the onset of the near stimulus (21/834 responses; 2.5%); (2) an initial increase in accommodation to the near stimulus which peaked and decreased before the offset of the near stimulus (8/834 responses = 1%) as shown in Figure 3B; and (3) responses in which an increase in accommodation was present, but the overall response was variable (48/834 responses; 5.7%). Of all the atypical responses recorded, 55% (42/77 responses) were recorded from subjects between the ages of 3 and 5 years and 5% (4/77 responses) were recorded from subjects 20 years of age and older. Typical responses were recorded from all subjects, indicating that of the subjects tested, both children and adults were capable of making typical sustained accommodative responses to a step stimulus. Only the typical responses were used in the analyses.
Figure 3.
A typical accommodative response to the step stimulus is shown for a 32-year-old subject (A) and an atypical accommodative response for a 3-year-old subject (B). All subjects demonstrated at least some portion of typical responses, although a larger portion of atypical responses were present in the younger subjects (55% of atypical responses were from subjects 3–5 years of age). No atypical responses were used in the analysis.
Latencies
Accommodative latencies from all four stimulus demands were pooled by decade of life and plotted as a function of accommodative response amplitude (data not shown). There were no significant linear relationships between accommodative latency and response amplitude (P > 0.6) or disaccommodative latency and response amplitude (P > 0.06) in any of the four age groups. Accommodative and disaccommodative latencies for all response amplitudes were then plotted separately as a function of age, and both were found to decrease linearly with increasing age (r2 = 0.025, P = 0.0007 and r2 = 0.068, P < 0.0001, respectively). One-way ANCOVA testing revealed no significant differences between accommodative and disaccommodative latencies (F = 0.06, df = 1, 882, P = 0.799). Table 1 shows mean accommodative and disaccommodative latencies for subjects grouped by decade of life.
Table 1.
Mean Accommodative and Disaccommodative Latencies for Subjects Binned by Decade of Life
| Subject Age (y) | Accommodative Latencies | Disaccommodative Latencies |
|---|---|---|
| 3–9 | 330 ± 107 | 337 ± 154 |
| 11–19 | 311 ± 130 | 362 ± 137 |
| 20–29 | 311 ± 98 | 302 ± 118 |
| 32–38 | 288 ± 101 | 251 ± 123 |
Data are expressed in mean milliseconds ± SD.
Mean Accommodative Responses
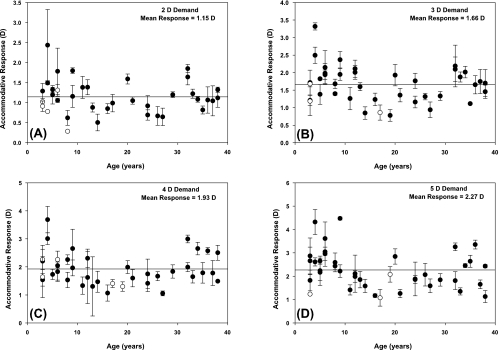
Mean accommodative responses for individual subjects are shown in Figure 4 for each of the four stimulus demands. Accommodative responses were low, indicating poor accommodative accuracy across subject ages in response to the near movie stimulus. The average accommodative response of the subjects did increase with increasing demand, although only by 0.36 D per diopter increase in demand. There was no significant linear trend of accommodative response with age for any of the demands tested, although visual inspection of Figure 4 suggests a possible quadratic trend with lower accommodative responses observed for subjects in the middle age range (late teens to 20s). A significant main effect of age2 on accommodative response was found in a mixed-model analysis, with age as the between-subjects factor and demand as the within-subjects factor (F = 4.72, df = 1, 31, P = 0.0375). The interaction of demand and age2 was nonsignificant indicating the shape of the function was similar for all demands, and interpretation of the main effect of age2 was appropriate.
Figure 4.
Accommodative response amplitudes for the four stimulus demands tested: (A) 2, (B) 3, (C) 4, and (D) 5 D. (●) Mean responses for subjects with three responses; (○) subjects with only one or two responses. Error bars, SD for subjects with more than one response. Solid line: the mean response for all subjects combined.
To investigate the possibility of a relationship between refractive error and accommodative response, Bonferroni-adjusted paired t-test analysis was performed to compare the mean accommodative responses of the five myopic subjects (RE range, −2.25 to −11.00 D) versus the four emmetropic subjects (RE range, plano to +0.75 D) in the oldest age group. Mean differences (emmetropic subjects − myopic subjects) were −0.08 D for the 2-D demand, 0.02 D for the 3-D demand, 0.20 D for the 4-D demand, and 0.35 D for the 5-D demand. None of these differences reached statistical significance (P > 0.5), indicating no differences in accommodative response related to refractive error. The other age bins included few or no myopic subjects and would not be expected to have differences in accommodative response related to refractive error (Fig. 1). It is possible that the lack of a significant difference between accommodative responses of myopic versus emmetropic subjects in the older age group was due to a lack of power from the small number of subjects compared; however, the mean differences were all small, particularly for the first three demands, and thus were unlikely to be clinically meaningful.
Microfluctuations
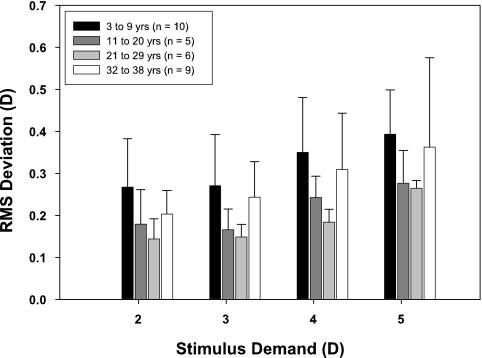
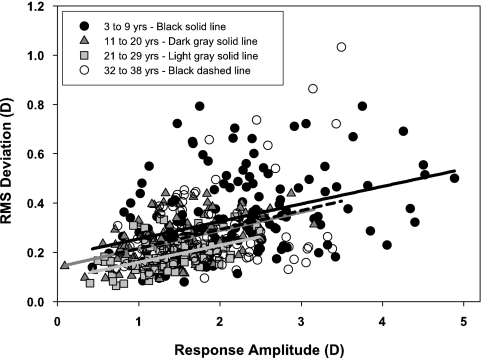
Because stimulus durations were varied randomly, only the 2 seconds of accommodative response occurring 1 second after stimulus onset were analyzed. For the analysis, RMS deviation was calculated only for subjects who had at least three usable, nonatypical responses for each of the four stimulus demands (n = 30). Mean RMS deviations for each subject were age binned by decade. Figure 5 shows the mean and standard deviations for each age bin at each stimulus demand. Visual inspection of the age trend of mean RMS deviation reveals that the youngest subjects have the largest magnitude fluctuations at all stimulus demands, and that RMS deviations appear to decrease with increasing age into the third decade of life and then increase again by the fourth decade of life. A post hoc quadratic regression analysis was performed to confirm the observation that for all four demands there was a quadratic relationship between age and RMS deviation (contrast test, 2-D demand: F = 5.34, df = 1, 26, R2 = 0.15, P = 0.0291; 3-D demand: F = 8.66, df = 1, 26, R2 = 0.24, P = 0.0068; 4-D demand: F = 7.82, df = 1, 26, R2 = 0.21, P = 0.0096; 5-D demand: F = 4.24, df = 1, 26, R2 = 0.14, P = 0.0496). RMS deviation also shows a significant linear increase with increasing stimulus demand for all age groups (3–9 years: R2 = 0.167, P = 0.0087; 11–20 years: R2 = 0.299, P = 0.0126; 21–29 years: R2 = 0.599, P < 0.0001; 32–38 years: R2 = 0.183, P = 0.0091). The relationship between RMS deviation and response amplitude rather than stimulus demand is shown in Figure 6 and confirms the trend of greatest RMS deviation in the youngest age bin.
Figure 5.
RMS deviations of accommodative microfluctuations for 2-second responses age-binned by decade for each stimulus demand. Error bars, SD.
Figure 6.
RMS deviations of accommodative microfluctuations as a function of accommodative response amplitude. RMS deviation increased linearly with response amplitude for all age groups (P ≤ 0.0126), although the range of measured response amplitudes varied with age (Fig. 4).
To ensure that the microfluctuations measured in this study were not a result of instrument noise, recordings were made on an emmetropic model eye. The RMS deviation of the model eye was 0.05 ± 0.002 D, which is much smaller than the magnitude of the RMS deviations reported in this study, indicating that the findings were not due to the measurement method.
Peak Velocities
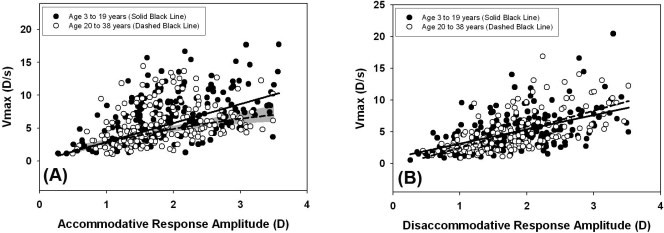
To investigate the speed of accommodative and disaccommodative responses as a factor of age, we considered peak velocities for response amplitudes from 0.5 to 3.5 D of subjects divided into two age groups: younger (age, 3–19 years) and older (age, 20–38 years). This range of response amplitudes was selected because it included most of the responses measured from all subjects and did not exceed the maximum response amplitude of either group. The results are plotted in Figures 7A and 7B, as a main sequence of accommodation and disaccommodation with Vmax plotted as a function of the amplitude for each response. For accommodative peak velocities, data were fitted with nonlinear models of Vmax versus response amplitude. To derive the models, we plotted time constant (τ) as a function of response amplitude for each age group (data not shown). For the older age group, time constant increased linearly with response amplitude (r2 = 0.031, P < 0.02) and the statistically significant linear relationship was used to calculate the modeled Vmax from the formula Vmax = a/τ. This modeling is the same as that described in previous studies of age-related changes in accommodative velocities.13,15 For the younger age group, there was no linear increase in time constant with response amplitude, and thus mean τ was used to calculate Vmax for all stimulus response amplitudes. In Figure 7A, the 95% confidence interval for the modeled Vmax was calculated for the older group from the 95% confidence interval of the linear fit to time constant versus response amplitude. Peak velocities in the older group were significantly slower than those in the younger group for response amplitudes greater than approximately 1.75 D, as evidenced by the lack of overlap between the model of the younger group and the model 95% confidence interval of the older group.
Figure 7.
Pooled peak velocities for response amplitudes ranging from 0.5 to 3.5 D. Accommodative peak velocities (A) were slower in subjects aged 20 to 38 at response amplitudes of approximately 1.75 D and higher as indicated by the 95% confidence interval (gray shading) for this group. Disaccommodative peak velocities (B) did not differ significantly between subject age groups.
For disaccommodative responses there was a significant linear increase in peak velocity with response amplitude for both age groups (younger: r2 = 0.28, P < 0.0001; older: r2 = 0.40, P < 0.0001). Figure 7B demonstrates that there were no age-related differences in disaccommodative peak velocity for the two groups which was confirmed statistically by using one-way ANCOVA analysis (F = 0.27, df = 2, 376, P = 0.6).
Discussion
Multiple stimulus demands were used to measure accommodative dynamics across a range of response amplitudes to characterize the potential changes that occur with age. Overall, the measured accommodative responses in this study were low across all ages tested. Some possible explanations for the low responses could be the instructions given to the subjects (“watch the movie”), or that a movie stimulus might not have been a strong stimulus for accommodation. The movie stimulus was selected due to its ability to maintain the attention of the youngest subjects; however, differences in subject attention and interest in the stimulus may also provide a potential explanation for the variability in accommodative responses observed with age in this study. Of interest, in general, it was the younger subjects who showed the stronger accommodative responses to the movie stimulus. The low mean accommodative responses observed in this study could also be related to the unnatural conditions of viewing a reflection of the near stimulus monocularly in a dark room without any apparent peripheral visual cues or vergence cues to indicate the location of the stimulus. The low response amplitudes were not due to the inaccuracy of the photorefractor, as this instrument has been demonstrated to have a low level of noise (SD of 0.05 D) and trial lens calibrations over a +6 D range were performed in all subjects with r2 ≥ 0.97. Although the low response amplitudes limit the range over which comparisons of dynamic measures between younger and older subjects can be made (0–3.5 D), this range of responses is within the maximum amplitude of subjects for the ages presented (0–38 years) and represents a typical functional range of intermediate and near working distances.
The subjects in the present study were all volunteers rather than a random sample of the general population. This selection bias could limit the generalizability of the findings. Subjects in this study were recruited from the University of Houston College of Optometry students, staff, clinic patients, and family members. Most of the young subjects (<20 years of age) were family members of faculty, students, and staff and would not be expected to have knowledge of accommodation beyond that of the general population. A majority (63%) of the adult subjects; however, were faculty and students at the optometry school and would be expected to have knowledge about topics related to the purpose of this study which represents a possible limitation to the study. However, if it were possible for additional knowledge about the study's purpose to bias adult subjects in their voluntary efforts to perform study tasks, then the age-related decreases in accommodative response and peak velocity observed in this study would be predicted to be even greater when comparing young children to adults with no knowledge about the study purpose.
This study included subjects with different refractive errors, primarily myopic, and emmetropic. The distribution of myopia varied throughout the four age bins and was greatest in the oldest age bin (32–38 years). Accommodative responses were not found to differ between the subjects with myopia and emmetropia in this group, however, and thus measurements of accommodative dynamics would not be predicted to differ by refractive error in this study as they are strongly related to response amplitude. Other potential differences between subjects include levels of higher order aberrations that have been documented to change with age.30,31 These potential differences in ocular aberrations between subjects may have affected the accommodative responses measured in this study, as it has been suggested that an individual's accommodative response is linked to an effort to maximize retinal image quality by balancing the defocus from higher order aberrations that increase with increasing accommodation.31 The authors are not aware of any prior studies that have demonstrated a relationship between higher order ocular aberrations and accommodative dynamics, aside from the effect on accommodative response. As higher order ocular aberrations were not measured in this study, the authors are unable to investigate these potential relationships.
Latencies
The mean accommodative and disaccommodative latencies reported in this study are in agreement with previously reported studies.4,11,15,19,28 The relationship between subject age and latency, however, differs among studies. Sun et al.11 and Heron et al.12 reported no differences in accommodative or disaccommodative latencies with age, while Kasthurirangan et al.15 reported no differences in accommodative latency, but a significant linear increase in disaccommodative latency with increasing age from 14 to 45 years.15 The present study did not find an increase in disaccommodative latencies with increasing age, although the oldest subject tested was 38 years of age. Tondel and Candy16 found longer accommodative and disaccommodative latencies in subjects 8 to 20 weeks old when compared with adults. These findings are in agreement with the findings from the present study and suggest that a developmental change in reaction time occurs between birth and adulthood.
Microfluctuations
Previous studies of microfluctuations have reported a significant relationship between small pupil size (<3 mm) and an increase in the magnitude of microfluctuations, as well as a relationship between stimulus demand and the magnitude of microfluctuations.6,10,27 Thus, it is important to determine whether these factors varied as a function of age in the present study to determine whether the reported age-related differences in microfluctuations can be explained by differences in pupil size or response amplitude. For the present study, pupil sizes were all greater than 3 mm, which is greater than the size at which the magnitude of microfluctuations should be impacted by pupil size,27 and thus should not have influenced the findings. RMS deviation increased with increasing response amplitude, as previously reported6 and age-related differences were observed both when RMS deviation was plotted as a function of response amplitude and as a function of stimulus demand (Figs. 5, 6).
In the present study, the greatest RMS deviations were observed in children in the first decade of life; they decreased over the middle two decades and began to increase again in the fourth decade of life. This trend of greater magnitude fluctuations in the youngest subjects is in agreement with a study comparing infants and adults.18 Other studies of microfluctuations as a function of age have also reported decreases in fluctuations with increasing age; however, they did not report the increase in fluctuations in the fourth decade of life found in the present study.8,32 The age range of subjects in these previous studies varied between 16 and 48 and 21 and 50 years. The reported trend of continually decreasing magnitude of microfluctuations into the fourth and fifth decades of life may not have been observed in the present study due to the younger age of the oldest subject (38 years), and also due to the differences in stimulus demands tested. The greatest demand tested by Heron and Schor8 was 2 D, as was the greatest demand for which formal analysis was reported by Mordi and Ciuffreda.32
Candy and Bharadwaj18 explored several different explanations for the differences in microfluctuations between infants and adults, the most likely of which they reported was related to the mechanical properties of the lens. They suggest that the more compliant infant lens may be more susceptible to small changes in the ciliary muscle and thus may fluctuate with greater magnitude than in adult subjects whose crystalline lenses become stiffer with age.33,34 This explanation is also in agreement with the decreasing trend in microfluctuations observed in the first three decades of life in the present study.
Peak Velocities
We found an increase in accommodative and disaccommodative peak velocities with increasing response amplitude for all subject groups when responses were pooled by age. These findings are in agreement with those in two previous studies of peak velocities in adults.14,35 However, it has also been demonstrated that both accommodative and disaccommodative peak velocities are more strongly influenced by the starting point of the response rather than the overall magnitude of the response, and thus a different shape of the main sequence plot may occur if different starting points are used.25 In the present study, the starting point was always infinity for accommodative responses, but varied for disaccommodative responses depending on the stimulus demand presented and the subject's response to that demand. This difference in starting point between accommodation and disaccommodation may provide an explanation for the observation of a nonlinear increase in peak velocity with response amplitude for accommodative responses versus the linear increase in peak velocity observed for disaccommodative responses.
In the present study, accommodative peak velocities were found to decrease with age for response amplitudes greater than approximately 1.75 D, whereas no change with age was observed in disaccommodative peak velocities. The results of this study agree well with those in a previous study that demonstrated steeper main sequences in younger adult subjects than in subjects approaching the onset of presbyopia.15 In addition, the findings of the present study are in accordance with those in a recent study that reported that infants match velocity changes in a sinusoidally moving near target,16 suggesting that the velocity component of accommodation develops at an early age. Other studies of the speed of accommodation and disaccommodation have reported varied trends with age. Schaeffel et al.3 reported a linear decrease in accommodative and disaccommodative peak velocities with increasing age; however, the study did not indicate whether an individual subject's response amplitudes were considered and it is possible that the observed decreases were related to differences in the magnitude of the responses between subjects. Other studies have reported no change in accommodative velocity with age; however, these studies either varied the stimulus demand by subject age rather than plotting a main sequence from a range of stimulus demands, or they used very small stimulus demands (<2 D) that match the range in which this study found similar peak velocities with age.12,19,32
The present study's observation of age-related changes in peak velocity for accommodative but not disaccommodative responses supports the previously reported idea that separate mechanisms dominate accommodative versus disaccommodative responses and are affected differently with increasing age.15 In vivo ultrasonographic studies have been used to develop a biomechanical model for accommodation and disaccommodation that indicates that the response time of accommodation is driven largely by the viscoelastic properties of the crystalline lens, whereas the response time of disaccommodation is driven not only by the lens, but also by the viscoelastic properties of the choroid and lens zonules.9,29 Although the mechanical properties of the lens and choroid have been shown to change with age,33,34,36 the properties of the zonules do not appear to change with age over the first four decades of life36 and this may provide an explanation for the decrease in speed of accommodation with age, but the lack of change with age for disaccommodative responses.15
Conclusions
The findings of this study demonstrate that some dynamic aspects of accommodation and disaccommodation change with age, whereas others remain the same during the first four decades of life. Accommodative and disaccommodative reaction times were both found to decrease linearly with increasing age, whereas the magnitude of accommodative microfluctuations varied significantly with increasing age in a quadratic manner with subjects in the first decade of life demonstrating the greatest fluctuations. Accommodative peak velocities were found to decrease with increasing age, whereas disaccommodative peak velocities showed no change with age in these subjects aged 3 to 38 years.
Acknowledgments
The authors thank Hope Queener, Chris Kuether, and Sanjeev Kasthurirangan, for assistance with computer programming and experimental setup.
Footnotes
Supported by Grants T32 EY07024 and P30 EY07551 from the National Eye Institute and by the A. O. F. Ezell Fellowship.
Disclosure: H.A. Anderson, None; A. Glasser, None; R.E. Manny, None; K.K. Stuebing, None
References
- 1.Howland HC. Optics of photoretinoscopy: results from ray tracing. Am J Optom Physiol Optics 1985; 62(9): 621–625 [PubMed] [Google Scholar]
- 2.Schaeffel F, Farkas L, Howland HC. Infrared photoretinoscope. Appl Opt 1987; 26(8): 1505–1509 [DOI] [PubMed] [Google Scholar]
- 3.Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol 1993; 461: 301–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell FW, Robson JG. High-speed infrared optometer. J Opt Soc Am 1959; 49(3): 268–272 [DOI] [PubMed] [Google Scholar]
- 5.Campbell FW, Westheimer G. Dynamics of accommodation responses of the human eye. J Physiol 1960; 151: 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotulak JC, Schor CM. Temporal variations in accommodation during steady-state conditions. Opt Soc Am A 1986; 3(2): 223–227 [DOI] [PubMed] [Google Scholar]
- 7.Gray LS, Winn B, Gilmartin B. Accommodative microfluctuations and pupil diameter. Vision Res 1993; 33(15): 2083–2090 [DOI] [PubMed] [Google Scholar]
- 8.Heron G, Schor C. The fluctuations of accommodation and ageing. Ophthalmol Physiol Opt 1995; 15(5): 445–449 [PubMed] [Google Scholar]
- 9.Beers APA, Van der Heijde GL. Age-related changes in the accommodation mechanism. Optom Vis Sci 1996; 73(4): 235–242 [DOI] [PubMed] [Google Scholar]
- 10.Stark LR, Atchison DA. Pupil size, mean accommodation response and the fluctuations of accommodation. Ophthalmic Physiol Opt 1997; 17(4): 316–323 [PubMed] [Google Scholar]
- 11.Sun F, Stark L, Nguyen A, Wong J, Lakshminarayanan V, Mueller E. Changes in accommodation with age: static and dynamic. Am J Optom Physiol Opt 1988; 65(6): 492–498 [DOI] [PubMed] [Google Scholar]
- 12.Heron G, Charman WN, Gray LS. Accommodation responses and ageing. Invest Ophthalmol Vis Sci 1999; 40: 2872–2883 [PubMed] [Google Scholar]
- 13.Kasthurirangan S, Vilupuru AS, Glasser A. Amplitude dependent accommodative dynamics in humans. Vis Res 2003; 43: 2945–2956 [DOI] [PubMed] [Google Scholar]
- 14.Bharadwaj SR, Schor CM. Acceleration characteristics of human ocular accommodation. Vis Res 2005; 45: 17–28 [DOI] [PubMed] [Google Scholar]
- 15.Kasthurirangan S, Glasser A. Age related changes in accommodative dynamics in humans. Vis Res 2006; 46: 1507–1519 [DOI] [PubMed] [Google Scholar]
- 16.Tondel GM, Candy TR. Human infants' accommodation responses to dynamic stimuli. Invest Ophthalmol Vis Sci 2007; 48(2): 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tondel GM, Candy TR. Accommodation and vergence latencies in human infants. Vis Res 2008; 48: 564–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candy TR, Bharadwaj SR. The stability of steady state accommodation in human infants. J Vision 2007; 7(11): 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heron G, Charman WN, Schor C. Dynamics of the accommodation response to abrupt changes in target vergence as a function of age. Vis Res 2001; 41(4): 507–519 [DOI] [PubMed] [Google Scholar]
- 20.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt 1976; 53(11): 740–745 [DOI] [PubMed] [Google Scholar]
- 21.Hyvarinen L, Nasanen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol (Copenh) 1980; 58(4): 507–511 [DOI] [PubMed] [Google Scholar]
- 22.Mutti DO, Zadnik K. Refractive Error. In: Zadnik K. ed. The Ocular Examination Measurements and Findings Philadelphia: WB Saunders; 1997: 51–86 [Google Scholar]
- 23.Vitale S, Ellwein L, Cotch MF, Ferris FL, Sperduto R. Prevalence of refractive error in the United States 1999–2004. Arch Ophthalmol 2008; 126(8): 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips S, Shirachi D, Stark L. Analysis of accommodative response times using histogram information. Am J Optom Arch Am Acad Optom 1972; 49(5): 389–401 [DOI] [PubMed] [Google Scholar]
- 25.Kasthurirangan S, Glasser A. Influence of amplitude and starting point on accommodative dynamics in humans. Invest Ophthalmol Vis Sci 2005; 46(9): 3463–3472 [DOI] [PubMed] [Google Scholar]
- 26.Schor CM, Lott LA, Pope D, Graham AD. Saccades reduce latency and increase velocity of ocular accommodation. Vision Res 1999; 39: 3769–3795 [DOI] [PubMed] [Google Scholar]
- 27.Gray LS, Winn B, Gilmartin B. Effect of target luminance on microfluctuations of accommodation. Ophthalmic Physiol Optics 1993; 13: 258–265 [DOI] [PubMed] [Google Scholar]
- 28.Shirachi D, Liu J, Lee M, Jang J, Wong J, Stark L. Accommodation dynamics I. Range nonlinearity. Am J Optom Physiol Opt 1978; 55(9): 631–641 [DOI] [PubMed] [Google Scholar]
- 29.Beers APA, Van der Heijde GL. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vision Res 1994; 34(21): 2897–2905 [DOI] [PubMed] [Google Scholar]
- 30.Buehren T, Collins MJ. Accommodation stimulus-response function and retinal image quality. Vision Res 2006; 46: 1633–1645 [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Gil N, Fernandez-Sanchez V, Legras R, Montes-Mico R, Lara F, Nguyen-Khoa JL. Accommodation-related changes in monochromatic aberrations of the human eye as a function of age. Invest Ophthalmol Vis Sci 2008; 49(4): 1736–1743 [DOI] [PubMed] [Google Scholar]
- 32.Mordi JA, Ciuffreda KJ. Dynamic aspects of accommodation: age and presbyopia. Vision Res 2004; 44(6): 591–601 [DOI] [PubMed] [Google Scholar]
- 33.Glasser A, Campbell MCW. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res 1999; 39: 1991–2015 [DOI] [PubMed] [Google Scholar]
- 34.Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis 2004; 10: 956–963 [PubMed] [Google Scholar]
- 35.Ciuffreda KJ, Kruger PB. Dynamics of human voluntary accommodation. Am J Optom Physiol Opt 1988; 65(5): 365–370 [DOI] [PubMed] [Google Scholar]
- 36.Van Alphen GWHM, Graebel WP. Elasticity of tissues involved in accommodation. Vision Res 1991; 31(7/8): 1417–1438 [DOI] [PubMed] [Google Scholar]