This study shows that application of BDNF to the eye and brain after optic nerve injury provides a greater level of retinal neuroprotection than treatment of the eye alone.
Abstract
Purpose.
To determine whether application of BDNF to the eye and brain provides a greater level of neuroprotection after optic nerve injury than treatment of the eye alone.
Methods.
Retinal ganglion cell survival and pattern electroretinographic responses were compared in normal cat eyes and in eyes that received (1) a mild nerve crush and no treatment, (2) a single intravitreal injection of BDNF at the time of the nerve injury, or (3) intravitreal treatment combined with 1 to 2 weeks of continuous delivery of BDNF to the visual cortex, bilaterally.
Results.
Relative to no treatment, administration of BDNF to the eye alone resulted in a significant increase in ganglion cell survival at both 1 and 2 weeks after nerve crush (1 week, 79% vs. 55%; 2 weeks, 60% vs. 31%). Combined treatment of the eye and visual cortex resulted in a modest additional increase (17%) in ganglion cell survival in the 1-week eyes, a further significant increase (55%) in the 2-week eyes, and ganglion cell survival levels for both that were comparable to normal (92%–93% survival). Pattern ERG responses for all the treated eyes were comparable to normal at 1 week after injury; however, at 2 weeks, only the responses of eyes receiving the combined BDNF treatment remained so.
Conclusions.
Although treatment of the eye alone with BDNF has a significant impact on ganglion cell survival after optic nerve injury, combined treatment of the eye and brain may represent an even more effective approach and should be considered in the development of future optic neuropathy–related neuroprotection strategies.
Damage to the optic nerve results in the retrograde degeneration of ganglion cells within the retina. One of the mechanisms thought to underlie this cell loss is a reduction in the level of trophic materials that these neurons receive from their target sources. The importance of the relation between ganglion cells and their target neurons has been demonstrated in the cat by studies showing that reducing the number of target neurons in the dorsal lateral geniculate nucleus (LGN), either by neonatal damage to visual cortex1–3 or by direct application of kainic acid to the LGN,4,5 leads to a significant loss of RGCs, in the absence of any direct insult to the retinogeniculate axons themselves. That this loss may represent a decrease in trophic factor availability derives from the many studies that have reported enhanced ganglion cell survival after direct application of various factors to the injured eye.6–8
Over the past few years several studies, including our own, have shown that brain-derived neurotrophic factor (BDNF) is a potent ganglion cell neuroprotectant in the mammalian retina after optic nerve injury.9–16 More recently, we demonstrated that BDNF not only promotes ganglion cell survival after optic nerve injury, but that it also plays an important role in preserving the structural integrity and visual responsiveness of these neurons.17,18 Nevertheless, its role as a potential retinal therapeutic remains equivocal, primarily because, even with multiple applications to the eye, significant ganglion cell loss occurs within 2 weeks of nerve injury.10,11,13–16
To some extent, the inability of BDNF to provide a sustained level of neuroprotection reflects a self-induced downregulation of the TrkB receptor used by the drug to activate intracellular survival pathways.19–23 However, a second contributing factor may be the means by which the neuroprotective potential of BDNF has been assessed. In most studies, either axotomy or severe nerve crush has been used as the nerve injury model, and in all cases, BDNF treatment has been restricted to the eye. Thus, unlike many optic neuropathies, such as glaucoma, in which a large number of ganglion cells retain connections with their primary target neurons during the early stages of the disease process, these models cause an abrupt and complete separation of ganglion cells from their postsynaptic targets and place the full burden of ganglion cell survival on the actions of BDNF at the level of the eye alone.
Based on these factors, we sought to re-examine the neuroprotective potential of BDNF, by using a different experimental approach. Instead of an axotomy or a severe crush, we applied a mild, unilateral, crush to the cat's optic nerve, the result being that at 1 week after injury, approximately half of the retinal ganglion cells survived and retained working connections with the visual thalamus, as demonstrated by their ability to retrogradely transport horseradish peroxidase. Using this model, we then examined the possibility that providing treatment to both the retinal ganglion cells and their target neurons in the LGN may afford a more significant level of neuroprotection than attained by treating the eye alone. Our results indicate that combined applications of BDNF to the eye and central visual pathway enhanced ganglion cell survival and function relative to treatment of the eye alone. Furthermore, they suggest that this combined treatment approach may provide a more sustained level of BDNF neuroprotection than has been achieved previously.
Methods
Animals and Surgical Procedures
Thirty-seven adult cats of both sexes were used. The cats were maintained in a free-run environment, and food and water were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Michigan State University, and all adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
For surgery, initial anesthesia was provided as a mixture of 4% isoflurane (IsoFlo; Abbott Laboratories, Abbott, IL) in pure oxygen (3 L/min). The cat then was intubated and anesthesia maintained with a 2.5% isoflurane and oxygen mixture (1 L/min). Analgesia and sedation consisted of an intramuscular injection of glycopyrrolate (0.005 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), and subcutaneous injections of butorphanol tartrate (0.2 mg/kg; Butler, Columbus, OH) and acepromazine (0.04 mg/kg; Butler). Heart, respiratory rate, percent specific oxygen, and body temperature were monitored continuously with a digital monitor (Vet/Ox G2; Heska, Loveland, CO) and recorded every 15 minutes. Body temperature was maintained at 37°C with an electric heating pad. The head of each animal was stabilized with either a stereotaxic apparatus or vacuum-activated restraining device (Olympic Vac; Olympic Medical, Seattle, WA). The dorsal surface of the skull was shaved and scrubbed with betadine solution (povidone-iodine, 7.5%). The eyes were treated with 0.5% proparacaine HCl (Alcaine; Alcon Laboratories, Ft. Worth, TX) and then 2.5% methylcellulose (Gonak; Akorn, Inc., Buffalo Grove, IL), to prevent corneal drying.
Using sterile procedures, the bone over the left frontal sinus was removed, exposing the roof of the bony orbit. All sinus openings were sealed with bone wax to avoid disturbing the cat's olfactory senses. A fine-tipped scalpel blade then was used to open the dorsal surface of the orbit, and blunt dissection of the overlying tissues exposed the optic nerve (ON) without disturbing the nerve sheath or retinal artery. The left ON received a mild crush with a vascular clamp (∼15 g of force), 2 to 3 mm distal to the globe for 15 minutes. Observation of the fundus indicated a normal retinal vasculature. After removal of the clamp, the bone wax plugs were checked, and the frontal sinus was packed with foam (Gelfoam; Upjohn, Kalamazoo, MI) soaked in sterile saline. The overlying skin then was sutured and treated with betadine solution, the eyes were treated with sterile ophthalmic ointment, and the cat was placed on a heating pad until it recovered from the anesthesia. Postoperative pain medication (buprenorphine, 0.02 mg/kg) was provided, and the cat was monitored every 2 hours for the first 8 hours and then daily for the remainder of the survival period.
BDNF Application
Twelve cats received the optic nerve injury and no treatment, and the remaining 25 cats received one of two neuroprotective treatment strategies: In 13 animals, immediately after the nerve crush, 90 μL of sterile recombinant BDNF (1 μg/μL; Cell Sciences, Canton, MA) was injected directly into the vitreal chamber with a syringe (Hamilton, Reno NV) and a 30-gauge needle.9 Care was taken to avoid hitting the lens.24 The injection was made over a 1-minute period, and the needle left in place for an additional 20 seconds to allow the drug to diffuse away from the injection site. The remaining 12 animals were placed in a stereotaxic apparatus. After the eye surgery and intravitreal injection of BDNF, a pair of small openings (<1 mm diameter) were made through the skull overlying each visual cortex at approximately the representation of central vision (P4.0; L2.0).25 Brain infusion cannulae (21 gauge) connected to osmotic minipumps (Alzet model 1002; Durect Corp., Cupertino, CA) containing 100 μL of BDNF (0.3 μg/μL) were inserted into the visual cortex with micromanipulators and secured to the skull with dental cement. The BDNF was delivered at a rate of 0.25 μL/hour for either 1 week (42 μL; 12.6 μg) or 2 weeks (84 μL; 25.2 μg).
PERG Recordings
After the prescribed survival period, the cats were anesthetized with a mixture of ketamine HCl (20 mg/kg IM) and xylazine (3 mg/kg IM) and prepared for noninvasive electroretinographic testing of visual function. Anesthesia was maintained with ketamine (5 mg/kg IM) as needed. Each animal was then placed on a thermostatically controlled heating pad with its head held upright. Body temperature was monitored via a rectal thermometer and maintained at 37 ± 1°C. Accommodation of the lens was blocked with tropicamide HCl (1%), and pupillary dilation and retraction of the nictitating membranes were achieved with phenylephrine HCl (2.5%). The eyes were treated with proparacaine HCl (0.5%), and a fiber electrode (DTL Plus; Diagnosys, LLC, Lowell, MA) moistened with 1% carboxymethylcellulose sodium was centered across the cornea of each eye and covered with a gas-permeable contact lens. The eyes were refracted with a retinoscope, and final focus of the eye on a video display monitor located 50 cm away was achieved with spectacle lenses. A hand-held transilluminator was used to back-project the fundus, check refraction, center the area centralis (AC) on the video display monitor, and check eye position during recording.26 Each eye was tested separately, with the fellow eye covered with a light-proof cloth. The covered eye served as a reference, and a needle electrode inserted under the skin of the neck, served as the ground electrode.
Pattern visual stimuli (4700° K color temperature) were displayed on an RGB monitor (model HL7955SKF; Sony, Tokyo, Japan) running at a frame rate of 100 Hz. Stimuli were luminance modulations of either a uniform field or contrast reversal of grating patterns. Square-wave luminance modulations were used in both the spatial and temporal domains.27 Stimuli of spatial frequencies ranging from 0.063 to 2 cyc/deg were presented at 1 Hz (two contrast reversals per second) temporal frequency, where maximum amplitudes are generated.27 At the viewing distance of 50 cm, the test field subtended a visual angle of 42° horizontally and 37° vertically. Photopic luminance (in candelas per square meter) was calibrated with a spot photometer (LS-100; Konica Minolta, Ramsey, NJ). The minimum and maximum luminances were 2 and 96 cd/m2, resulting in a stimulus contrast of 95%.
Signals were amplified, DC filtered (300 Hz), and digitized (1 kHz) with a resolution of 0.1 μV. Responses were averaged over 50 stimulus presentations. The largest Fourier component close to 60 Hz was removed digitally to simulate a notch filter, with no phase distortion having a bandwidth of <0.02 Hz. Repeated three-point weighted smoothing (0.25, 0.5, and 0.25) was sometimes used to eliminate noise at frequencies >250 Hz.
Tissue Processing
After the electrophysiology sessions and without recovery from the ketamine/xylazine anesthesia, the animals were euthanatized with an intravenous injection of pentobarbital sodium (>50 mg/kg) and perfused transcardially with 0.5 L of physiological saline (0.9%), followed by 2.0 L of a solution containing 1.5% paraformaldehyde and 2.0% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were placed into the same fixative for future processing. The eyes were removed and bisected at the ora serrata and the resulting posterior eye cups postfixed overnight. The retinas were dissected and wholemounted onto gelatin-coated glass slides. Care was taken during mounting to make the relief cuts in the superior temporal retina as shallow as possible, to avoid any distortion in the region from which the ganglion cell samples would be obtained. The retinas then were dehydrated in graded alcohols, defatted, stained with cresyl violet, and coverslipped. The optic nerves were postfixed with 1% osmium tetroxide in 0.1 M phosphate buffer, and embedded in resin (LX-112-Araldite; Ladd Research Industries, Inc, Burlington, VT). Semithin (∼1.0 μm) cross sections of the optic nerves were cut from a region approximately 8 to 10 mm distal to the optic nerve head to avoid the crush region, mounted on glass slides, stained with 1% p-phenylenediamine (PPD: P-6001; Sigma, St. Louis, MO), and coverslipped.
Ganglion Cell Measurements
Retinal ganglion cell counts from all eyes were obtained with a computer-based imaging system. The region selected for quantitative analysis occupied approximately 1.66 mm2 and was located 3 mm above and 1.5 mm temporal to the AC.9 This region was chosen primarily because of the relatively constant size and density of ganglion cells in this location of the cat retina compared with regions more proximal to the AC. In addition, it is devoid of the high density of ganglion cell fibers that occupy the regions superior and inferior to the AC, it avoids the highly variable pigmented region of the inferior retina, and the ganglion cells within this region contribute to the visual responses obtained when the AC is centered on the pattern stimulation monitor during the PERG tests.1,28,29 Finally, examination of the optic nerves showed a diffuse pattern of nerve degeneration, with no indication of selective sparing of ganglion cells in this midtemporal region. A rotating stage and stage digitizer (Applied Scientific Instrumentation, Eugene, OR) were used to properly orient each retina on the microscope30 and to standardize the starting point and stage movements used for sampling. From the starting point, 10 digital images (16,600 μm2/image) were obtained systematically with a high-resolution color video camera (Microfire; Optronics, Inc., Goleta, CA) and 20× objective. The retinal images were collected as two dorsal–ventral passes composed of five images each. Double counting was avoided by separating each sample column horizontally by 500 μm and vertically by using the previous image as a reference. The number and size of the cells were measured directly from the digital images with image-analysis software (Image Pro Plus; Media Cybernetics, Bethesda, MD). Neurons were classified as ganglion cells based on our previous work9 and the criteria of Stone.29,31 In particular, they had to display a distinct nucleus and nucleolus and have a continuous ring of cytoplasm surrounding the nucleus. Although this most likely resulted in the exclusion of some small ganglion cells,32–34 it also reduced the inclusion of nonganglion cells.35,36
Optic Nerve Measurements
Comparisons of the optic nerves were made by qualitative observation and axon counting. For quantitative comparison, each nerve cross section was divided into quadrants, and eight digital images (two images/quadrant), representing approximately 2.12 × 105 μm2, or 10.3% of the total nerve area for both the normal (2.04 × 106 μm2) and affected (2.07 × 106 μm2) nerves, were obtained. Since retinotopy is not well maintained in the cat optic nerve with increasing distance from the optic nerve head,37 the locations of the sample regions within each quadrant were identified randomly. Myelinated axons in each digital image were counted manually (2800×) with the image-analysis software (Image Pro Plus; Media Cybernetics). Estimates of the total number of fibers were determined from the sample area axon counts and the percentage of total nerve area that they represented. Axons displaying signs of degeneration (e.g., swollen, unwrapped, and filled with debris) were excluded from the counts.
Statistical Analyses
All data are presented as the mean ± SE. The retinal cell counts and mean soma sizes were normalized by taking the square root of each measurement, determining the difference between the experimental and normal fellow eye of the same animal, and comparing these differences across the various experimental conditions and survival times with a one-way ANOVA followed by the Bonferroni test for multiple comparisons (Prism 5.0; GraphPad, Inc., San Diego, CA). Cell size distributions, normalized for differences in the number of eyes examined under each condition, were compared by the Kruskal-Wallis nonparametric test, with the Dunn test used for multiple comparisons. These analyses also were used to compare differences with respect to the axon counts (Prism 5.0; GraphPad, Inc.). Differences in the mean PERG responses were compared by using a two-tailed Student's t-test (SigmaPlot; SPSS, Inc., Chicago, IL). In all cases, P < 0.05 was used as the level of significance.
Results
Qualitative Observations
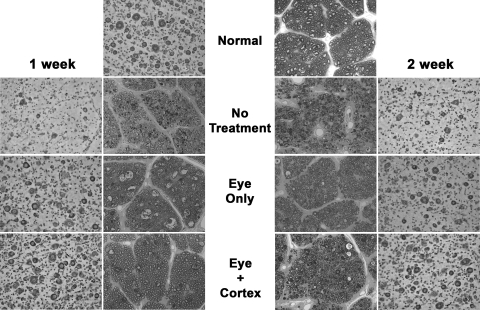
The photomicrographs in Figure 1 compare the retina and optic nerve morphologies for each of the conditions studied. The normal eyes were characterized by darkly stained ganglion cells with well-defined membranes, oval-shaped somata, and centrally located nuclei. The normal optic nerves contained discrete bundles of well-myelinated axons that varied widely in size.
Figure 1.
Morphologic comparison of the retinas and optic nerves from normal cats, cats that received an optic nerve crush and no treatment (NT), or those that received either a single intravitreal injection of BDNF (eye), or the injection combined with 1 or 2 weeks of continuous infusion of BDNF into the visual cortex (eye+cortex).
At 1 to 2 weeks after nerve injury without treatment, there was a significant decrease in the number of ganglion cells within the sample region. Most of those present contained significant amounts of Nissl substance; however, some displayed crenulated somata and asymmetric located nuclei. The optic nerves from the 1-week eyes contained discrete bundles of axons that varied in size; however, many atrophic profiles were present. By 2 weeks after crush, there was a significant increase in the number of atrophic axonal profiles, and the remaining axons were mostly small and were no longer arranged in discrete bundles. After the nerve crush and treatment of the eye alone with BDNF, there was a significant increase in the number of surviving ganglion cells in the sample regions of both the 1- and 2-week animals relative to their untreated counterparts. Present in both, but more so in the 1-week eyes, were several pale staining neurons with irregular outlines, considered to be incompletely degenerated ganglion cells.29 Although the nerves from these animals contained atrophic profiles, the axons varied in size, were well myelinated, and were organized into discrete bundles. Combining treatment of the eye with continuous infusion of BDNF to visual cortex resulted in retinas that were comparable to normal in appearance. Although some pale-staining cells remained, most contained large amounts of well-stained Nissl substance, had round somata with centrally located nuclei, and were comparable in density to those in the normal eyes. This level of normality within the retina was paralleled by an enhanced appearance of the optic nerve.
Quantitative Observations
Ganglion Cell Survival.
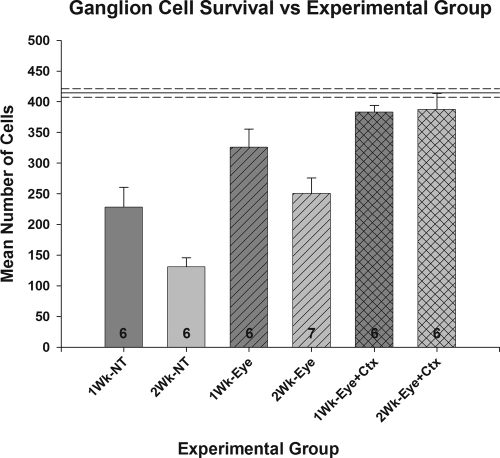
In the normal retinas, the sample region was found to contain, on average, 418 ± 7 ganglion cells (Fig. 2). After the optic nerve injury and no treatment, ganglion cell survival was reduced to 55% of normal (228 ± 32) in the 1-week animals and 31% of normal (131 ± 15) in those that had a 2-week survival period (P < 0.001 for both). Treatment of the eye with 90 μg BDNF at the time of the nerve injury resulted in a significant increase in ganglion cell survival at both time periods relative to that measured in the nontreatment condition; survival in the 1-week animals increased to 79% of normal (328 ± 27), a change of approximately 44%, whereas that in the 2-week eyes increased to 60% of normal (250 ± 25), a change of 92% (P < 0.05 for both). Combining treatment of the eye at the time of injury with continuous infusion of BDNF to visual cortex for the duration of the survival period resulted in an additional, although modest (17%), increase in ganglion cell survival in the 1-week animals relative to treatment of the eye alone, resulting in a final survival rate of 92% (383 ± 11 vs. 418 ± 7; NS). For those animals receiving the combined treatment and a 2-week survival, there was about a 55% increase in ganglion cell survival compared with those receiving the eye treatment alone (387 ± 26 vs. 250 ± 25; P < 0.05). This resulted in a final level of survival (93%) comparable to that of the 1-week animals, and for both it was statistically similar to normal (P > 0.05).
Figure 2.
Comparison of mean ganglion cell survival under each experimental condition tested. Solid line: the mean number of cells counted in the normal fellow eyes. Mean ± SE (NT versus N: P < 0.001; Eye versus NT: P < 0.05; Eye+Ctx versus Eye: 1 week, NS; 2 week, P < 0.05; Eye+Ctx versus N: P > 0.05).
Ganglion Cell Size.
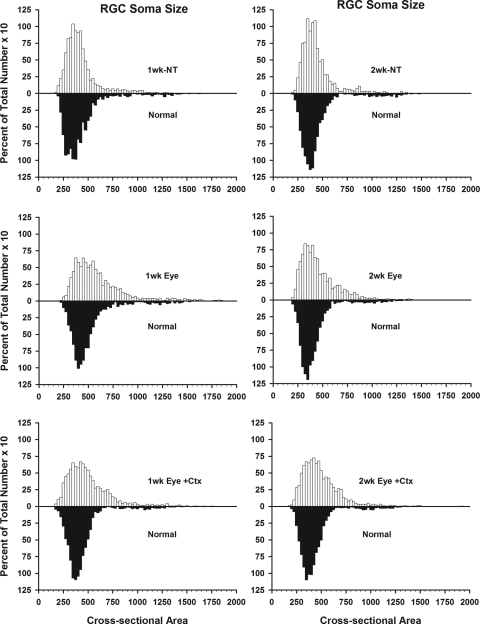
The mean soma size of the population of ganglion cells within the sample region of the normal eyes was approximately 429 ± 8.3 μm2. Similar values were measured in the affected eyes of the untreated animals (1 week, 415 ± 4.7 μm2; 2 weeks, 442 ± 8.5 μm2), as well as those receiving an intravitreal injection and having a 2-week survival period (445 ± 4.7 μm2). Although the mean cell size within the sample region of those cats receiving an intravitreal injection with a 1-week survival period was significantly larger than that of the normal eyes in general (556 μm2 vs. 429 μm2). It was not significantly different when compared with that of the fellow eyes from these same animals (475 ± 5.8μm2). We also did not see any significant shifts in neuronal populations when the cells in each sample region were divided into small, medium, or large, based on previous studies in the cat.17,32–34,38 In the normal retinas, 5% of the ganglion cells in the sample region had small (145–250 μm2) somata, 85% had medium-sized cell bodies (250–600 μm2), and 10% contained large (>600 μm2) somata. After the optic nerve lesion and no treatment, the proportion of neurons in each size group was 8%, 80%, and 12%, respectively. Treatment of the eye alone had little effect on the proportion of small neurons (4% of total), but did result in a decrease in the proportion of medium-sized ganglion cells (72%) and an increase in the proportion of large (25% of total) cells. These proportions were little changed after combined treatment of the eye and visual cortex (6%, 74%, and 21%, respectively). The changes in the representation of medium and large neurons in the sample populations of the treated animals was due primarily to an increase in the proportion of ganglion cells with somata diameters in the 500- to 800-μm2 range, as indicated in Figure 3.
Figure 3.
Size distributions of ganglion cells in the experimental (top) vs. normal fellow (bottom) eyes as a percentage of the total number of ganglion cells in the sample region.
Optic Nerve Measurements.
Overall, the optic nerves from the normal and nerve-crush eyes were not significantly different in size. The mean cross-sectional area of the normal nerves was 2.04 ± 0.05 mm2, whereas that of the nerve-crush eyes was 2.07 ± 0.04 mm2. The estimated total numbers of axons in the optic nerves under each condition are presented in Table 1.
Table 1.
Estimated Total Number of Axons
| Experimental Condition | Axons (n) |
|---|---|
| Normal | 78,222 ± 2,294 |
| 1-wk NT | 42,126 ± 5,477 |
| 2-wk NT | 39,946 ± 3,286 |
| 1-wk eye only | 38,320 ± 2,676 |
| 2-wk eye only | 51,691 ± 3,345 |
| 1-wk eye+cortex | 51,621 ± 5,065 |
| 2-wk eye+cortex | 59,110 ± 4,578 |
Data are presented as mean ± SE
There was no significant difference between the untreated (NT) and 1-week eye-only–treated animals, or between the 2-week eye-only– and eye-plus-cortex–treated animals (P > 0.05). The estimated number of surviving axons in the latter three groups of animals, however, was significantly greater than those measured in the former three (P < 0.05). All the experimental groups were significantly different from normal (P < 0.05).
Visual Responses
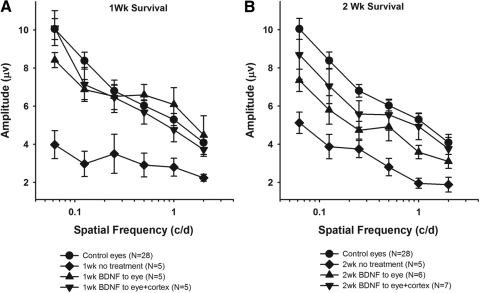
Visual responses were obtained with either luminance modulations of a uniform field or contrast reversal of square-wave gratings presented at 1 Hz.39 In all cases, examination of the uniform field responses showed a normal b-wave (not shown), suggesting that the optic nerve injury did not have a significant effect on outer retinal function. The pattern-evoked visual responses measured under each experimental condition for animals with 1- and 2-week survival periods are shown in Figures 4A and 4B, respectively. For the 1 week animals, the PERG responses after treatment of the eye alone and treatment of the eye combined with visual cortex were significantly greater than those from eyes receiving no treatment (P < 0.01), and were not different from each other or the responses of the control fellow eyes over all spatial frequencies tested (P > 0.15). After the 2-week survival period, the PERG responses of the normal and treated animals again were significantly greater than those in the untreated animals (P < 0.01).
Figure 4.
PERG responses to visual stimuli of different spatial frequencies under each experimental condition. At 1 week, treatment of both the eye and eye+cortex is comparable to normal (A), whereas after a 2-week survival period, only the responses from animals receiving treatment of both the eye and cortex are normal (B). Mean ± SE.
Although the responses under the different BDNF treatment conditions were not different from each other (P > 0.13), the responses measured in the dual-treatment animals were consistently greater than those from animals receiving only treatment of the eye for the 2-week animals. Statistically, the responses measured in the dual-treatment animals were not different from normal (P > 0.1), whereas those from animals receiving treatment of the eye alone were significantly different at four of the six spatial frequencies tested (0.5 cyc/deg: P = 0.12; 2.0 cyc/deg: P = 0.17).
Discussion
The goal of this study was to examine the neuroprotective potential of BDNF after optic nerve injury with an experimental paradigm different from that used in most previous studies—namely, the application of a mild versus severe crush or complete nerve section injury40 and treatment of not only the eye directly, but also indirectly via continuous application of BDNF to the visual cortex, the primary target of the visual thalamic neurons that serve as the main postsynaptic targets of retinal ganglion cell axons. The rationale for this approach was that most optic neuropathies do not involve a sudden and complete disconnect between the retina and its postsynaptic targets, and that the benefits of a sustained relation between pre- and postsynaptic neurons, in both the developing and adult nervous system, are well documented.
The data presented herein suggest that combined treatment of both the retinal ganglion cells and their target neurons results in a significant enhancement in ganglion cell survival relative to no treatment, or treatment of the eye alone. More important, however, they demonstrate ganglion cell survival levels that not only are significantly higher than either of these two conditions, but also are comparable to normal, and furthermore they show preservation of this level of ganglion cell survival 2 weeks after injury, a time at which previous retina-only treatment studies have indicated a significant reduction in ganglion cell survival relative to that measured at 1 week after injury and treatment.10,11,13,14,16
It is well-known that retinal ganglion cells contain the TrkB tyrosine kinase-based receptors used by BDNF. Thus, in agreement with previous severe nerve injury studies, it is not surprising that direct application of BDNF to the eye produced a significant increase in ganglion cell survival relative to that without treatment (78% vs. 55%). This result was most likely due to activation of the different intracellular signaling pathways involved in the upregulation of survival and the suppression of apoptotic mechanisms in the retina.7 In addition, it is likely that BDNF had a direct excitatory effect on the surviving ganglion cells,41 thus increasing their levels of cAMP,42 and resulting in the recruitment of additional TrkB receptors to the membrane. Because of the semi-intact optic nerve in these animals versus those examined in previous studies, this increase in retinal excitation also could have a postsynaptic effect at the level of the LGN, which could involve either the anterograde transport and release of BDNF from the retinogeniculate axon terminals and the subsequent increase in intracellular signaling, or an increase in baseline activity. Either of these could serve to stabilize retinogeniculate synapses that may otherwise be lost due to a reduced level of interactions with postsynaptic neurons.42–47
As demonstrated previously, however, the neuroprotective effect of BDNF treatment to the eye alone was temporary10,11,13–16; at 2 weeks after injury, the level of ganglion cell survival had fallen to 60% of normal and no longer was significantly different from the 1-week untreated eyes (55%). This decrease in responsiveness to BDNF most likely resulted from a downregulation in TrkB receptor availability, due to an injury-induced decrease in TrkB gene expression,10,48 to internalization of the bound receptors with a reduction in replacement in the cell membrane due to reduced visual responsiveness and decreased intracellular levels of cAMP23,42,49 (Chen and Weber, unpublished data, 2002), or to competitive interactions with adjacent truncated TrkB receptors.50
Previous studies in which ganglion cell survival after axotomy and treatment of the eye alone with BDNF have been examined have reported survival levels of 22% to 55% at 2 weeks after injury.11–16 The exception is the work of Cheng et al.,10 who achieved a 2-week survival level of 76%, but only by combining the BDNF injection with transfection of ganglion cells with the TrkB gene using a viral vector. In the present study, allowing the retinal ganglion cells to retain connections with their primary target and providing neuroprotection to both, we achieved a final survival level of 93% at 2 weeks after injury. Although the results of these different studies are not directly comparable because of the differences in methodologies used, our data suggest that such a combined treatment strategy could have a significant impact if applied during the early stages of an optic neuropathy when many ganglion cell-target connections remain. That the increased survival was not simply due to the less severe nerve injury is evident from the progressive level of degeneration if no treatment is provided (Fig. 2). In addition, more recent work indicates that treatment of the visual cortex alone also is insufficient. We hypothesize that the enhanced survival we achieved is the result of contributions from both the eye and LGN; direct treatment of the eye is necessary to counter the initial insult and short-term degenerative effects, whereas treatment of the target provides more long-term stability. As noted, the survival response at the level of the eye most likely involves rapid activation of intracellular signaling pathways and perhaps a limited postsynaptic effect via the injured nerve, especially if the ganglion cell gives priority to sustaining the cell body and dendrites over distal axonal connections. Unlike the retinal treatment, the application of BDNF to the visual cortex could have strong anterograde and retrograde effects at the level of the LGN. The retrograde effect would involve uptake of the BDNF and transport to the LGN. The anterograde effect, conveyed by the large reciprocal corticogeniculate projection in the cat,51–54 could involve upregulation of survival signals in the LGN through the transport and release of BDNF, as well as increased excitation of the LGN relay cells, either by the increase in exogenous BNDF levels, or its excitatory effects within the cortex itself.41–47 By compensating for a decrease in activation from the retina, cortical activation of the LGN could serve to stabilize retinogeniculate connections by affecting the dendritic trafficking, translocation, and translation of TrkB mRNA in the postsynaptic neurons. Part of this stabilization may involve cAMP facilitated movement of TrkB into postsynaptic densities, thereby preserving and strengthening existing active retinogeniculate synaptic contacts.47 It is important to note again, however, that preliminary studies suggest that treatment at the level of the visual cortex alone is not effective, most likely because of the differences in the time course of retinal degeneration after the nerve injury versus upregulation of the LGN-based neuroprotective influence. Thus, it appears that intravitreal treatment is necessary to sustain ganglion cells during the immediate postinsult period.
In agreement with previous studies,9,55,56 we found that ganglion cells of all sizes were lost, with the greatest loss related to medium-sized cells, which comprise the largest proportion of ganglion cells in the cat retina. Although fewer in number, the losses of large and small ganglion cells, as well as cell shrinkage, were such that there was no significant shift in the proportions of small, medium, and large cells under any of the conditions studied. The consistent increase in the proportion of cells with somata in the 500- to 800-μm2 range in the BDNF-treated animals is difficult to understand. Since there are not enough large cells to account for this increase, it seems likely that it includes the preservation and/or enlargement of ganglion cells in the upper range of the medium-size class.
The optic nerve data indicate that the neuroprotective effects of BDNF were not restricted to retinal ganglion cells, but to their axons as well. In addition, they suggest that the effect may be delayed; treatment of the eye alone showed little effect at 1 week after treatment, but there was a significant increase in axons at 2 weeks. This increase also appeared to occur in those animals receiving the nerve crush and treatment of both the eye and visual cortex, where the axon counts were higher in the 2-week vs. 1-week animals. That the axon counts did not directly reflect our measurements of ganglion cell survival was not surprising, given the selected region of retina analyzed, the difficulty of matching ganglion cell location and axon position in the distal optic nerve, and the lack of an a priori reason to assume that events at the cell soma and axon follow the same time course.
Although our estimates of the number of total axons are in agreement with Donovan,57 they are considerably lower than those of Hughes and Wässle (193,000).58 As noted by the latter authors, this difference most likely reflects an underestimation of very small fibers in our light microscopic versus their electron microscopic examination and not the amount or area of the nerve sampled. Doubling our sample area to 20.6% of the total nerve yielded only a 4.8% change in the number of axons.
In addition to demonstrating that application of BDNF to the eye and brain provides a greater level of ganglion cell survival after optic nerve injury than treatment of the eye alone, the data also show that this novel neuroprotection strategy resulted in a sparing of ganglion cell function (Fig. 4) at 2 weeks after injury. This effect was important to show, since previous retinal studies have focused primarily on the morphologic effects of BDNF treatment and because one cannot assume a direct relation between neuronal survival and maintenance of function.44 Future studies will be conducted to determine whether this treatment paradigm is sufficient to sustain long-term ganglion cell survival and function, or whether a more extended period of treatment is needed; recent anatomic work indicates that treatment of the eye and visual cortex for 4 weeks after injury yields ganglion cell levels equal to those measured here at 2 weeks. Because most optic neuropathies are diagnosed only after some period of retinal degeneration has occurred, it also is important to determine the effectiveness of this approach when treatment is delayed relative to the time of the nerve injury. Finally, studies are currently under way to obtain a better understanding of the cellular mechanisms involved.
Footnotes
Supported by National Institutes of Health/National Eye Institute Grant EY11159 (AJW).
Disclosure: A.J. Weber, None; S. Viswanáthan, None; C. Ramanathan, None; C.D. Harman, None
References
- 1.Kalil RE. Removal of visual cortex in the cat: effects on the morphological development of the retino-geniculo-cortical pathway. In: Stone J, Dreher B, Rapaport D. eds. Development of Visual Pathways in Mammals: Proceedings of a satellite symposium of the XXIX International Congress of the Union of Physiological Sciences, Sydney, Australia, August 24–27, 1983 New York: Alan R. Liss; 1984: 257–274 [Google Scholar]
- 2.Pearson HE, Labar DR, Payne BR, Cornwell P, Aggarwal N. Transneuronal retrograde degeneration in the cat retina following neonatal ablation of visual cortex. Brain Res 1981; 212: 470–475 [DOI] [PubMed] [Google Scholar]
- 3.Weber AJ, Kalil RE, Stanford LR. Morphology of single, physiologically identified Y retinogeniculate axons in the cat following damage to visual cortex at birth. J Comp Neurol 1989; 289: 446–455 [DOI] [PubMed] [Google Scholar]
- 4.Pearson HE, Sonstein WJ, Stoffler DJ. Selectivity of kainic acid as a neurotoxin within the dorsal lateral geniculate nucleus of the cat: a model for transneuronal retrograde degeneration. J Neurocytol 1991; 20: 376–386 [DOI] [PubMed] [Google Scholar]
- 5.Pearson HE, Stoffler DJ. Retinal ganglion cell degeneration following loss of postsynaptic target neurons in the dorsal lateral geniculate nucleus of the adult cat. Exp Neurol 1992; 116: 163–171 [DOI] [PubMed] [Google Scholar]
- 6.Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem 2003; 88: 57–75 [DOI] [PubMed] [Google Scholar]
- 7.Lebrun-Julien F, DiPolo A. Molecular and cell-based approaches for neuroprotection in glaucoma. Optometry Vis Sci 2008; 85: E417–E424 [DOI] [PubMed] [Google Scholar]
- 8.Harvey AR, Hu Y, Leaver SG, et al. Gene therapy and transplantation in CNS repair: the visual system. Prog Retin Eye Res 2006; 25: 449–489 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci 2001; 42: 966–974 [PubMed] [Google Scholar]
- 10.Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci 2002; 22: 3977–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A 1998; 95: 3978–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klöcker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signaling. J Neurosci 2000; 20: 6962–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A 1994; 91: 1632–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res 1993; 602: 304–317 [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci 2002; 43: 3319–3326 [PubMed] [Google Scholar]
- 16.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells: a quantitative in vivo study. Invest Ophthalmol Vis Sci 1996; 37: 489–500 [PubMed] [Google Scholar]
- 17.Weber AJ, Harman CD. BDNF Preserves the dendritic morphology of α and β ganglion cells in the cat retina after optic nerve injury. Invest Ophthalmol Vis Sci 2008; 49: 2456–2463 [DOI] [PubMed] [Google Scholar]
- 18.Weber AJ, Harman CD, Viswanathan S. Effects of optic nerve injury, glaucoma, and neuroprotection on the survival, structure, and function of ganglion cells in the mammalian retina. J Physiol 2008; 586: 4393–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Weber AJ. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Research 2004; 1011: 99–106 [DOI] [PubMed] [Google Scholar]
- 20.Frank L, Ventimiglia R, Anderson K, Lindsay R, Rudge J. BDN F down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur J Neurosc 1996; 8: 1220–1230 [DOI] [PubMed] [Google Scholar]
- 21.Frank L, Wiegand SJ, Siuciak JA, Lindsay RM, Rudge JS. Effects of BDNF infusion on the regulation of TrkB protein and message in adult rat brain. Exp Neurol 1997; 145: 62–70 [DOI] [PubMed] [Google Scholar]
- 22.Knusel B, Gao H, Okazaki T, et al. Ligand-induced down-regulation of trk messenger RNA, protein and tyrosine phosphorylation in rat cortical neurons. Neuroscience 1997; 78: 851–862 [DOI] [PubMed] [Google Scholar]
- 23.Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding: evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem 2000; 275: 8982–8990 [DOI] [PubMed] [Google Scholar]
- 24.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 2000; 20: 4615–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tusa RJ, Palmer LA, Rosenquist AC. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol 1978; 177: 213–236 [DOI] [PubMed] [Google Scholar]
- 26.Fernald R, Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res 1971; 11: 95–96 [DOI] [PubMed] [Google Scholar]
- 27.Hess RF, Baker CL, Zrenner E, Schwarzer J. Differences between electroretinograms of cat and primate. Neurophysiology 1986; 56: 747–768 [DOI] [PubMed] [Google Scholar]
- 28.Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol 1974; 240: 397–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone J. The number and distribution of ganglion cells in the cat's retina. J Comp Neurol 1978; 180: 753–771 [DOI] [PubMed] [Google Scholar]
- 30.Vakkur GJ, Bishop PO, Kozak W. Visual optics in the cat, including posterior nodal distance and retinal landmarks. Vision Res 1963; 61: 289–314 [DOI] [PubMed] [Google Scholar]
- 31.Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol 1965; 124: 337–352 [DOI] [PubMed] [Google Scholar]
- 32.Berson DM, Isayama T, Pu M. The eta ganglion cell type of cat retina. J Comp Neurol 1999; 408: 204–219 [PubMed] [Google Scholar]
- 33.Berson DM, Pu M, Famiglietti EV. The zeta cell: a new ganglion cell type in cat retina. J Comp Neurol 1998; 399: 269–288 [DOI] [PubMed] [Google Scholar]
- 34.Isayama T, Berson DM, Pu M. Theta ganglion cell type of cat retina. J Comp Neurol 2000; 417: 32–48 [DOI] [PubMed] [Google Scholar]
- 35.Hughes A. Population magnitudes and distribution of the major modal classes of cat retinal ganglion cell as estimated from HRP filling and a systematic survey of the soma diameter spectra for classical neurones. J Comp Neurol 1981; 197: 303–339 [DOI] [PubMed] [Google Scholar]
- 36.Wong RO, Hughes A. The morphology, number, and distribution of a large population of confirmed displaced amacrine cells in the adult cat retina. J Comp Neurol 1987; 255: 159–177 [DOI] [PubMed] [Google Scholar]
- 37.Naito J. Course of retinogeniculate projection fibers in the cat optic nerve. J Comp Neurol 1986; 251: 376–387 [DOI] [PubMed] [Google Scholar]
- 38.Stein JJ, Johnson SA, Berson DA. Distribution and coverage of beta cells in the cat retina. J Comp Neurol 1996; 372: 597–617 [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci 2000; 41: 2797–2810 [PubMed] [Google Scholar]
- 40.Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol 1998; 116: 906–910 [DOI] [PubMed] [Google Scholar]
- 41.Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 1999; 401: 918–921 [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Franke A, Wilkinson GA, Kruttgen A, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 1998; 21: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altar C, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997; 389: 856–860 [DOI] [PubMed] [Google Scholar]
- 44.Caleo M, Medini P, von Bartheld C, Maffei L. Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J Neurosci 2003; 23: 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caleo M, Menna E, Chierzi S, Cenni M, Maffei L. Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr Biol 2000; 10: 1155–1161 [DOI] [PubMed] [Google Scholar]
- 46.Fawcett JP, Bamji SX, Causing CG, et al. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci 1998; 18: 2808–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci 2005; 28: 464–471 [DOI] [PubMed] [Google Scholar]
- 48.Cui Q, Tang LS, Hu B, So KF, Yip HK. Expression of trkA, trkB, and trkC in injured and regenerating retinal ganglion cells of adult rats. Invest Ophthalmol Vis Sci 2002; 43: 1954–1964 [PubMed] [Google Scholar]
- 49.Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci 2005; 46: 3197–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci 1996; 16: 3123–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert CD, Kelly JP. The projection of cells in different layers of the cat's visual cortex. J Comp Neurol 1975; 163: 81–106 [DOI] [PubMed] [Google Scholar]
- 52.Kawamura S, Sprague JM, Niimi K. Corticofugal projections from the visual cortices to the thalamus, pretectum, and superior colliculus in the cat. J Comp Neurol 1974; 158: 339–362 [DOI] [PubMed] [Google Scholar]
- 53.Updyke BV. Topographic organization of the projection from cortical area 17, 18, and 19 onto the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol 1977; 173: 81–122 [DOI] [PubMed] [Google Scholar]
- 54.Weber AJ, Kalil RE. Development of corticogeniculate synapses in the cat. J Comp Neurol 1987; 264. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe M, Inukai N, Fukuda Y. Survival of retinal ganglion cells after transection of the optic nerve in adult cats: a quantitative study within two weeks. Vis Neurosci 2001; 18: 137–145 [DOI] [PubMed] [Google Scholar]
- 56.Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience 2003; 116: 733–742 [DOI] [PubMed] [Google Scholar]
- 57.Donovan A. The nerve fiber composition of the cat optic nerve. J Anat 1967; 101: 1–11 [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes A, Wässle H. The cat optic nerve: fibre total count and diameter spectrum. J Comp Neurol 1976; 169: 171–184 [DOI] [PubMed] [Google Scholar]