Abstract
Purpose
To investigate the relationship between experimental neuroimaging and self-reported measures of urinary incontinence.
Materials and Methods
We evaluated 14 functionally independent, community-dwelling older women (> 60 years) who had moderate to severe urgency urinary incontinence. All underwent detailed clinical assessment (3-day bladder diary, 24-hour pad test, and quality of life assessment), urodynamic testing, and functional brain scanning. Brain activity during reported urgency was assessed using a method that combines fMRI with simultaneous urodynamic monitoring during repeated cycles of bladder filling/emptying. We used SPM2, a statistical parametric mapping program, to correlate brain activity with relevant clinical covariates, including number of urgency incontinent episodes, amount of urine leakage, and psychological burden as assessed by the Urge Impact Scale (URIS-24) questionnaire.
Results
Activity in rostral and subgenual anterior cingulate gyrus, insula, inferior frontal gyrus, orbitofrontal cortex, dorsal and posterior cingulate gyrus, parahippocampus, cuneus and parts of parieto-temporal lobe correlated positively with daytime incontinence frequency and urine loss. Different brain regions correlated with the psychological burden, and the associations were inverse: precuneus/cuneus and posterior cingulate gyrus, superior temporal, supramarginal, and transverse gyrus.
Conclusions
Regional brain activity in the setting of self-reported urgency, as provoked by bladder filling, correlates significantly with the severity of incontinence in daily life as well as the associated psychological burden. Thus, observations made under experimental conditions correlate with patients’ real-life experience and suggest neural correlates of urgency incontinence symptoms that could be used as potential targets for future investigations.
Keywords: urinary incontinence, magnetic resonance imaging, urodynamics
INTRODUCTION
Urgency urinary incontinence (urgency incontinence) is characterized by repeated episodes of urgency, many of them associated with urine leakage.1,2 Its severity is assessed clinically by the number of leakage episodes recorded in the bladder diary and by the amount of urine leaked into absorbent pads.3 Patients with urgency incontinence also experience psychological distress, which is assessed as ‘burden of disease’ using quality of life questionnaires.4 These clinical measures are used to guide therapy and assess its outcome. Unfortunately, over the past 40 years the effectiveness of treatment has improved little, largely because little is known about the etiology of urgency incontinence, as suggested recently in an expert symposium (NIDDK Bethesda, 2009). Indeed, urgency itself has several aspects and is difficult to define.1, 5
Although the brain is essential to control of the lower urinary tract, its role in the pathophysiology of urgency incontinence has been relatively neglected.6 Patients with urgency incontinence usually have no evidence of overt neurological disease. Yet, on urodynamic examination they frequently exhibit detrusor overactivity (DO) during bladder filling suggesting impaired cerebral control, perhaps with peripheral or spinal pathophysiology also. Recent developments in brain imaging, such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have greatly advanced our understanding, revealing many brain regions that are activated during experiments which reproduce components of the micturition cycle or its control.7–10
We have adapted functional brain imaging for urology by adding urodynamics to fMRI.11–14 fMRI produces a BOLD (blood oxygen level dependent) signal which reflects local cerebral blood flow, an indirect measure of regional neuronal activity. Recording of such activity during urodynamic testing facilitates simultaneous monitoring of brain and bladder activity so that continence control can be studied under experimental conditions.
Studies using this methodology have revealed increased overall brain activity (provoked by bladder filling) in older women with urgency incontinence. As shown schematically in Fig. 1, we have seen activation and deactivation of brain regions involved in mapping of body sensations (right insula/somatosensory cortex), emotional processing (anterior cingulate gyrus [ACG]/limbic cortex and parts of frontal cortex) and decision-making (parts of frontal cortex),11–14 implying their potential role in control of continence. The most striking brain responses occur when filling provokes a compelling need to void, suggesting that they might represent a neural correlate of urgency.14 Nevertheless it is not known whether these experimental observations reflect patients’ daily experience, as registered by clinical tests such as bladder diary or pad test. A significant association between observed brain responses and clinical data would suggest that activity recorded under experimental conditions reflects the clinical severity of the disorder in daily life.
Figure 1.
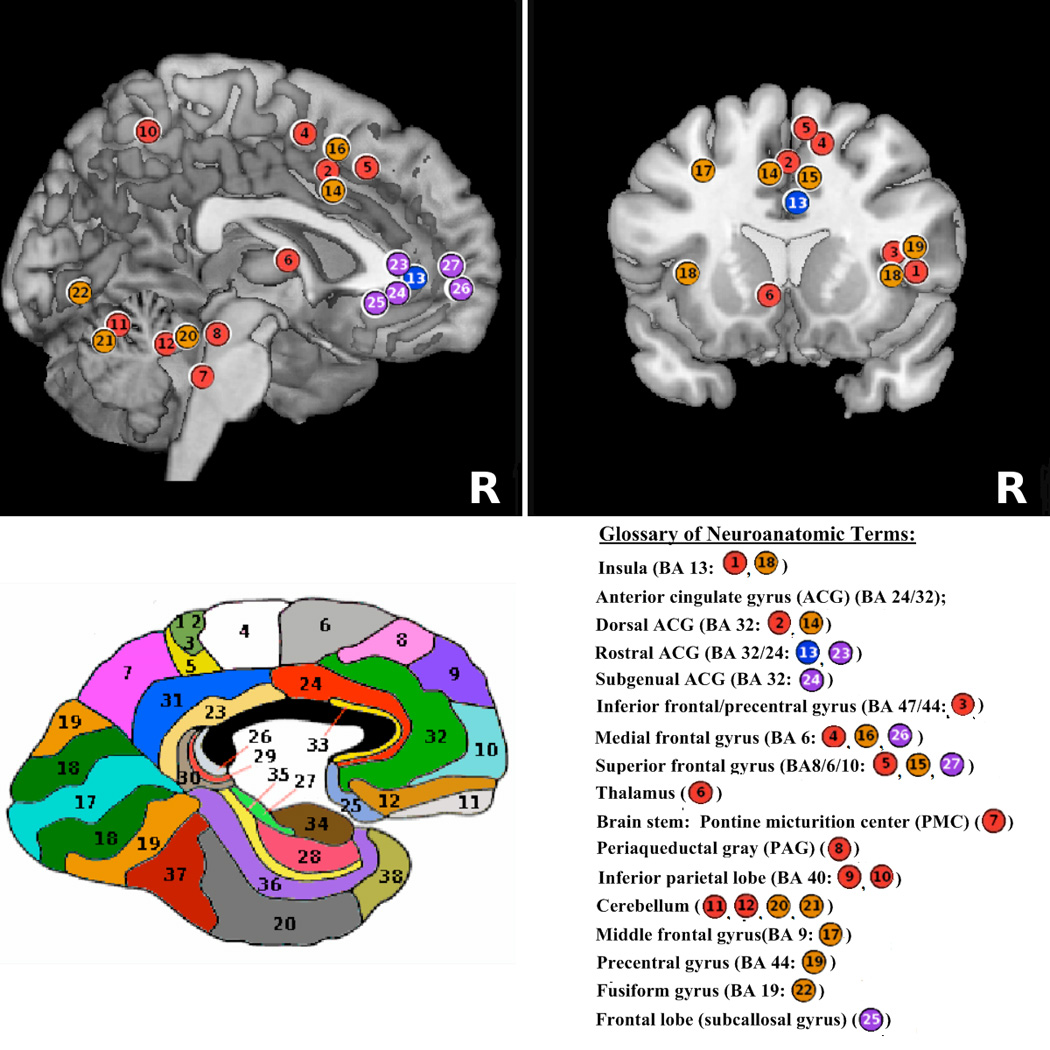
Summative schematic of brain regions activated/deactivated during bladder filling experiments. A. Sagittal section of the brain. B. Coronal section of the brain. C. Functional neuroanatomy map - color coded Brodmann areas (BA) on sagittal section of the brain. Legend: 1–13*– areas previously published by authors (Color coded circles: red - activation, blue – deactivation); 14–27 *– areas reported in this study (also see Table 2) (Color coded circles: orange - activation, purple – deactivation). All figures are shown in neurological convention (right side on right).*Color coded circles are placed in approximate locations for schematic purposes.
To investigate the relationship between experimental neuroimaging and self-reported measures of incontinence, we analyzed older women with urgency incontinence who were first assessed clinically (by 3-day bladder diary, 24-hour pad test, and quality of life questionnaire) and urodynamically, and then underwent fMRI with simultaneous urodynamic monitoring during repeated cycles of partial filling/emptying of the bladder. The primary hypothesis was that the magnitude of the brain responses to bladder filling observed in the scanner during reported sensations of ‘urgency’ would be correlated with the number of episodes of urgency incontinence recorded in the bladder diary and with the severity of leakage measured with pads. Secondary analysis included correlation of brain responses with patients’ reports of the burden of disease.
MATERIALS AND METHODS
Study subjects
Subjects were recruited by advertising. All were functionally independent, community-dwelling women aged 60 years and older with at least two urgency incontinence episodes per week for at least 3 months despite correction of potentially reversible causes. All had urgency incontinence, either pure or with a minor component of stress-related leakage as determined clinically and by voiding diary.
All subjects had to be able to complete a voiding diary and a 24-hour pad test. We excluded women with significant cognitive impairment (Mini-Mental State Examination [MMSE] score ≤24) that might have prevented adherence to the protocol. Women with a history of bladder cancer, spinal cord lesions, multiple sclerosis, pelvic radiation, interstitial cystitis, or sphincter implant and those who were medically unstable were also excluded. We excluded subjects for characteristics that would preclude performing functional MRI, including claustrophobia and the presence of implanted metal or electromagnetic devices. The study was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent.
Clinical assessment 12, 13
All subjects underwent comprehensive clinical evaluation, including detailed history, physical examination that included neurological testing and a pelvic exam, and a test of cognitive status (MMSE). Additional information included laboratory tests, uroflowmetry, measurement of post-void residual urine, and detailed urodynamic assessment. Since presence of DO on urodynamic study in this group may vary, women with urgency incontinence on history whose detrusor overactivity could not be demonstrated were still eligible.
Measures
Bladder diary and pad test
All subjects completed a 3-day bladder diary that included times and amounts voided or leaked and the circumstances of leakage. To assess clinical incontinence severity, we calculated the average number of daytime urgency incontinence episodes recorded in the bladder diary over 3 days. “Daytime” was defined as the period between getting out of bed for the day and going to bed for the night. The amount leaked in one daytime period was determined by re-weighing pre-weighed absorbent pads that the patient changed at each bathroom visit and placed in a zipped bag.
Both measures of incontinence severity were based only on daytime incontinence to avoid the confounding effects of nocturnal polyuria and nocturia which may be related to factors outside the lower urinary tract and its control system.
Psychological burden of incontinence
To assess psychological burden, we used the Urge Impact Scale (URIS-24) questionnaire,15 an urgency incontinence–specific instrument developed using focus groups of older adults (>90% women) with urgency incontinence. 16 It includes 24 questions with replies on a 5-point Likert scale, higher scores reflecting better quality of life.
Scanning session and bladder filling protocol
As described previously,11–13 two 8 F urethral catheters were introduced to allow for bladder filling/emptying and bladder pressure measurement to detect detrusor overactivity (DO). Structural MRI was performed. A small volume of sterile water (10–20 ml) was introduced into the empty bladder, followed by functional MRI while fluid was repeatedly infused into and withdrawn from the bladder (acquisition details: 3 T magnet; one brain scan per 1.5 s; 3 × 3 × 3 mm resolution, field of view 20 cm covering brainstem to near top of anterior cingulate gyrus).
Each infusion/withdrawal cycle comprised: pause (10.5 s); infusion (22 ml in 10.5 s); pause (10.5 s); withdrawal (15 ml in 10.5 s). Four cycles formed one measurement block. After 2 measurement blocks, the bladder was filled quickly, without scanning, until the subject signaled compelling desire to void. With subject’s permission, 1 to 2 further measurement blocks were performed with scanning until subjects stopped the study to void, or developed DO or leakage. The BOLD signal acquired in the final measurement block (or the last block before DO onset) was used for analysis. This measurement block was comparable in different subjects because it was always performed in the same situation: with full bladder and just tolerable sensation. We refer to this situation as ‘urgency’ because it reproduces most aspects (compelling desire to void, difficult to defer1 and associated with fear of leakage,5 although neither not necessarily sudden onset nor DO1).
Data analysis
After image acquisition, pre-processing and further analyses were done using Statistical Parametric Mapping (SPM2; Wellcome Department of Imaging Neuroscience, 2003, http://www.fil.ion.ucl.ac.uk/spm/spm2.html). As in our previous work,11–13 first-level analysis was performed by determining regional brain responses to bladder filling in each subject. This was accomplished by comparing the BOLD signal during fluid infusion with the baseline during fluid withdrawal. This contrast (infusion minus withdrawal) was chosen because (unpublished observations): 1. The BOLD signal is greatest during infusion and usually smallest during withdrawal; 2. the signal during pause conditions is variable from block to block and not suitable for use as a baseline; 3. Reported bladder sensation essentially vanishes during withdrawal; 4. other contrasts usually lead to less significant results. This small contrast is near-normally distributed (a basic assumption of SPM).
Second-level group analyses were then performed. To determine the main effects of bladder filling we combined the single-subject results for the selected measurement block in a standard random-effects analysis. For correlation analyses, the first-level image for this measurement block was selected for each individual, together with the clinical covariate of interest (daytime number of urgency incontinence episodes, daytime amount of urine leakage, or score on URIS-24 questionnaire). A voxel-by-voxel correlation between the images and the covariate was then performed. The statistical maps showing voxels with a significant correlation with each of the clinical measures were thresholded at P = 0.05 or 0.01 (uncorrected for multiple comparisons), corresponding to correlation coefficients r =0.48 and 0.63 respectively. Correction for multiple comparisons was performed at family-wise error (FWE) and cluster levels, both for whole brain and with a small volume correction within previously defined regions of interest (ROI) for dorsal ACG (18 mm radius at 2, 12,42) and right insula (12 mm radius at 38, 16, −6).12
RESULTS
The study population comprised 14 community dwelling older women (Table 1). Seven subjects exhibited detrusor overactivity during urodynamic testing prior to fMRI. Six subjects showed DO during fMRI (four with DO on preceding urodynamic testing and 2 without). Altogether, DO was documented in 9 of 14 subjects.
Table 1.
Characteristics of study subjects.
| Variable | Average | Range |
|---|---|---|
| Age (years) | 76.2 | 64 – 88 |
| MMSE | 29.2 | 27 – 30 |
| Daytime Voiding Frequency | 7.4 | 5.7 – 10 |
| Daytime Incontinent Episodes | 2.3 | 0.7 – 4.7 |
| Daytime Urine Leakage (ml) | 23.6 | 3.3 – 75 |
| Voided Volume/Diary (ml) | 198.1 | 75 – 225 |
| Max Voided Volume/Diary (ml) | 454.6 | 250 – 810 |
| Max Bladder Capacity/Urodynamics (ml) | 522.5 | 164 – 700 |
| Psychological Burden (URIS-24) | 79.2 | 43 – 105* |
scores for URIS-24 range from 24 (minimum) – 120 (maximum)
For the whole group, measurements made during bladder filling when subjects signaled ‘urgency’ (but before DO or urine leakage), revealed that brain activity was provoked in many of the regions found in previous studies and shown in Figure 1 (see Table 2), including the dorsal ACG, insula, frontal cortex, precentral gyrus, fusiform gyrus, and cerebellum. Activation in dorsal ACG was significant at cluster level after small volume correction for multiple comparisons. There also was a trend towards activation of the brainstem or midbrain (data not shown). Bladder filling also led to deactivation in frontal regions, including rostral and subgenual ACG, subcallosal, medial and superior frontal gyrus (Figure 1 and Table 2).
Table 2.
Brain regions with significant activity or deactivation during bladder filling when study subjects reported sensation corresponding to urgency (p<0.01)
| Region | Coordinates | T-Value | Z-Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Activation | |||||
| Dorsal Anterior Cingulate Gyrus (BA32)14 | −8 | 8 | 42 | 9.69 | 5.15 † |
| Superior Frontal Gyrus (BA6) 15 | 8 | 18 | 32 | 7.06 | 4.45 |
| Medial Frontal Gyrus (BA6) 16 | 4 | 12 | 48 | 5.73 | 3.97 |
| Middle Frontal Gyrus (BA9) 17 | −26 | 28 | 28 | 3.25 | 2.73 |
| Insula (BA13) 18 | 38 | 12 | 4 | 4.01 | 3.55 |
| −38 | 12 | 2 | 7.23 | 4.50 | |
| Precentral Gyrus (BA44) 19 | 48 | 2 | 6 | 5.01 | 3.67 |
| Cerebellum (Anterior Lobe) 20 | 20 | −44 | −28 | 3.81 | 3.06 |
| Cerebellum (Posterior Lobe) 21 | 2 | −68 | −22 | 4.16 | 3.25 |
| Fusiform Gyrus (BA19) 22 | 34 | −72 | −12 | 2.89 | 2.49 |
| Deactivation | |||||
| Rostal Anterior Cingulate Gyrus (BA24) 23 | 0 | 34 | 6 | 3.92 | 3.12 |
| Subgenual Anterior Cingulate Gyrus (BA32)24 | −6 | 38 | −4 | 3.34 | 2.78 |
| Frontal Lobe (Subcallosal Gyrus) 25 | −12 | 26 | −10 | 3.06 | 2.60 |
| Medial Frontal Gyrus (BA10) 26 | −4 | 60 | −8 | 3.46 | 2.86 |
| Superior Fronal Gyrus (BA10) 27 | −20 | 54 | 10 | 3.74 | 3.02 |
Note: x, y, z are MNI (Montreal Neurological Institute) coordinates. T- and Z- are statistics (Student’s T and normal variable Z) given by SPM output. Regions are represented by coordinates of most significant voxel.
Significant at cluster level (P < 0.05) after small volume correction for multiple comparisons.
14 – 27 Regions shown in Figure 1.
Some of the regions responding to bladder filling were significantly correlated with daytime incontinence frequency measured by bladder diary. As shown in Figure 2A and Table 3, the correlation was positive in rostral and subgenual ACG (plot shown in Figure 2B), insula, inferior frontal gyrus, orbitofrontal cortex, dorsal ACG, posterior cingulate gyrus, parahippocampus, cuneus, and parts of the parieto-temporal lobe. Correlation of activity in right insula ROI with daytime incontinence frequency was significant at FWE and cluster levels after small volume correction for multiple comparisons. Brain responses were also significantly and positively correlated with the amount of daytime urine loss in similar regions (Table 3). No significant negative correlations with daytime incontinence frequency or amount of leakage were observed.
Figure 2.
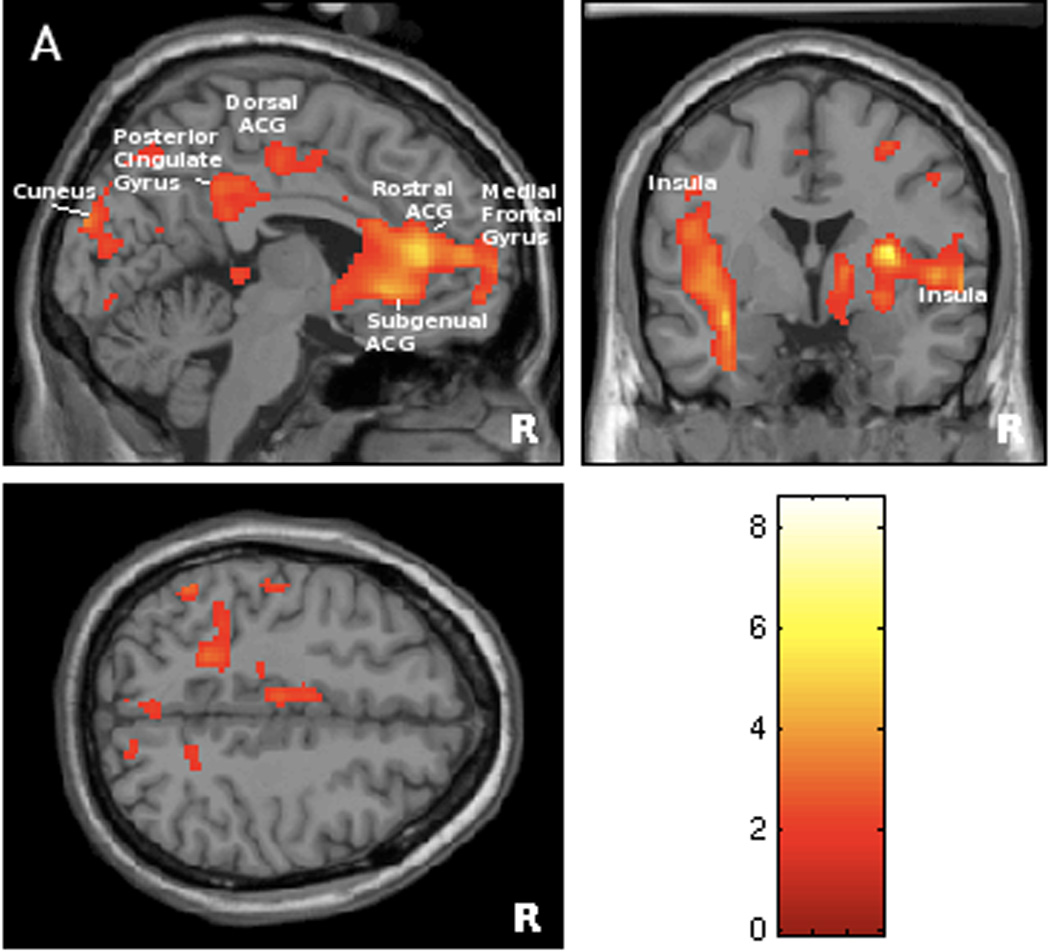
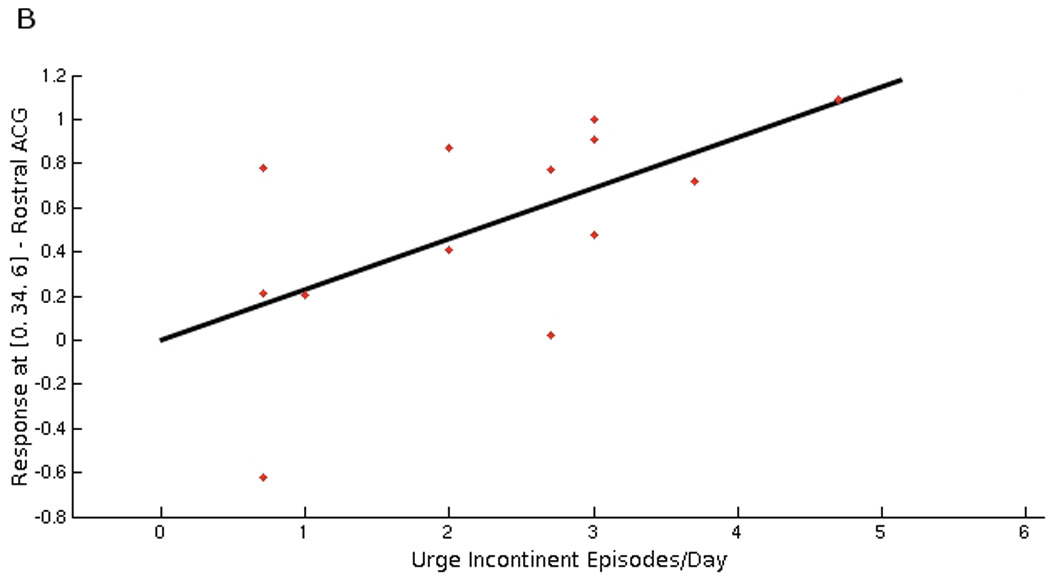
Figure 2A. Statistical map of brain regions where responses to bladder filling during scanning session were significantly associated with the number of daytime urge incontinent episodes measured on the bladder diary (positive correlation at threshold level of p<0.05, r >0.48). Regions’ coordinates and statistics are listed on Table 3.
Figure 2B. Graphical illustration of positive correlation between brain responses in rostral anterior cingulate gyrus (BA 24) and number of daytime urgency incontinent episodes on bladder diary (r=0.60). This region (at MNI coordinates 0, 34, 6) has been selected because it showed significant deactivation during bladder filling (see Figure 1/Table 2).
Table 3.
Brain regions where activity correlated significantly with clinical measures: A. bladder diary reports of daytime urge incontinence episodes (p < 0.05, r > 0.48); B. pad measurements of daytime urine leakage (p < 0.05, r>0.48); and C. psychological burden measured by Urge Impact Scale (URIS-24) (p < 0.01, r > 0.63).
| Region | Coordinates | T-Value | Z-Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| A. (daytime urge incontinence episodes) | |||||
| Inferior Frontal Gyrus (BA47) | 34 | 16 | −12 | 8.59 | 4.65 † |
| Lentiform Nucleus | 30 | −4 | 10 | 8.54 | 4.63 |
| Rostral Anterior Cingulate Gyrus (BA24/32) | −12 | 38 | 12 | 7.55 | 4.39 |
| Subgenual Anterior Cingulate Gyrus (BA24/32) | −8 | 24 | −4 | 4.67 | 3.39 |
| Middle Frontal Gyrus (BA11/10) | −28 | 40 | −14 | 7.13 | 4.27 |
| −26 | 56 | 10 | 4.51 | 3.32 | |
| 26 | 54 | −12 | 4.31 | 3.23 | |
| Medial Frontal Gyrus (BA10) | −8 | 52 | 10 | 3.91 | 3.03 |
| 6 | 40 | −8 | 3.55 | 2.84 | |
| Insula (BA13) | 46 | 0 | 2 | 3.95 | 3.05 |
| Parietal Lobe (BA40) | 34 | −46 | 32 | 5.21 | 3.62 |
| Parahippocampal Gyrus (BA34) | 12 | −12 | −18 | 3.50 | 2.80 |
| Transverse Temporal Gyrus (BA41) | 46 | −30 | 12 | 3.34 | 2.71 |
| Middle Temporal Gyrus (BA21) | −46 | 10 | −30 | 5.71 | 3.60 |
| Posterior Cingulate Gyrus (BA31) | −10 | −40 | 36 | 3.80 | 2.97 |
| Cuneus (BA19) | −6 | −94 | 24 | 3.48 | 2.79 |
| Dorsal Anterior Cingulate Gyrus (BA24) | −8 | −6 | 46 | 2.92 | 2.47 |
| B. (daytime urine leakage) | |||||
| Inferior Frontal Gyrus (BA47) | 22 | 22 | −14 | 3.53 | 2.82 |
| Subgenual Anterior Cingulate Gyrus (BA24/32) | 4 | 24 | −10 | 3.18 | 2.62 |
| Rostral Anterior Cingulate Gyrus (BA24/32) | −6 | 38 | 16 | 2.90 | 2.44 |
| Putamen | 22 | 2 | 12 | 3.03 | 2.53 |
| Medial Frontal Gyrus (BA9, BA10) | −10 | 40 | 18 | 2.99 | 2.50 |
| −8 | 46 | 12 | 2.88 | 2.43 | |
| Insula (BA13) | 40 | 12 | 0 | 3.71 | 2.92 |
| Posterior Cingulate Gyrus (BA31) | −10 | −38 | 42 | 3.38 | 2.73 |
| Cuneus (BA19) | −8 | −92 | 24 | 5.44 | 3.71 |
| Dorsal Anterior Cingulate Gyrus (BA24) | −12 | −4 | 50 | 3.13 | 2.59 |
| C. (psychological burden) | |||||
| Precuneus (BA7) | −18 | −64 | 28 | 6.45 | 4.06 |
| 0 | −50 | 38 | 6.40 | 4.05 | |
| −4 | −72 | 48 | 4.91 | 3.50 | |
| Superior Temporal Gyrus (BA39/13) | 50 | −54 | 28 | 6.16 | 3.97 |
| 40 | −48 | 16 | 5.81 | 3.85 | |
| Supramarginal Gyrus (BA40) | 52 | −48 | 34 | 5.52 | 3.74 |
| Insula (BA13) | −34 | −6 | 16 | 4.94 | 3.51 |
| Transverse Gyrus (BA41) | −36 | −36 | 10 | 4.61 | 3.37 |
| Posterior Cingulate Gyrus (BA23/31) | 2 | −34 | 28 | 4.74 | 3.42 |
| Cuneus (BA19) | −6 | −82 | 30 | 4.40 | 4.06 |
Significant after small volume correction for multiple comparisons at FWE level (P = 0.001) and cluster level (p < 0.01). Note: this location is within the region of interest referred to as insula.
Secondary analysis showed that the baseline score for the psychological burden of urgency incontinence, as represented by the Urge Impact Scale (URIS-24), was significantly correlated with brain responses to bladder filling, albeit in different regions: precuneus/cuneus and posterior cingulate gyrus, superior temporal, supramarginal, and transverse gyrus (see Figure 3A and Table 3). Moreover, the relation was inverse: increased burden of disease correlated with a lesser degree of activation in these brain regions (Figure 3B).
Figure 3.
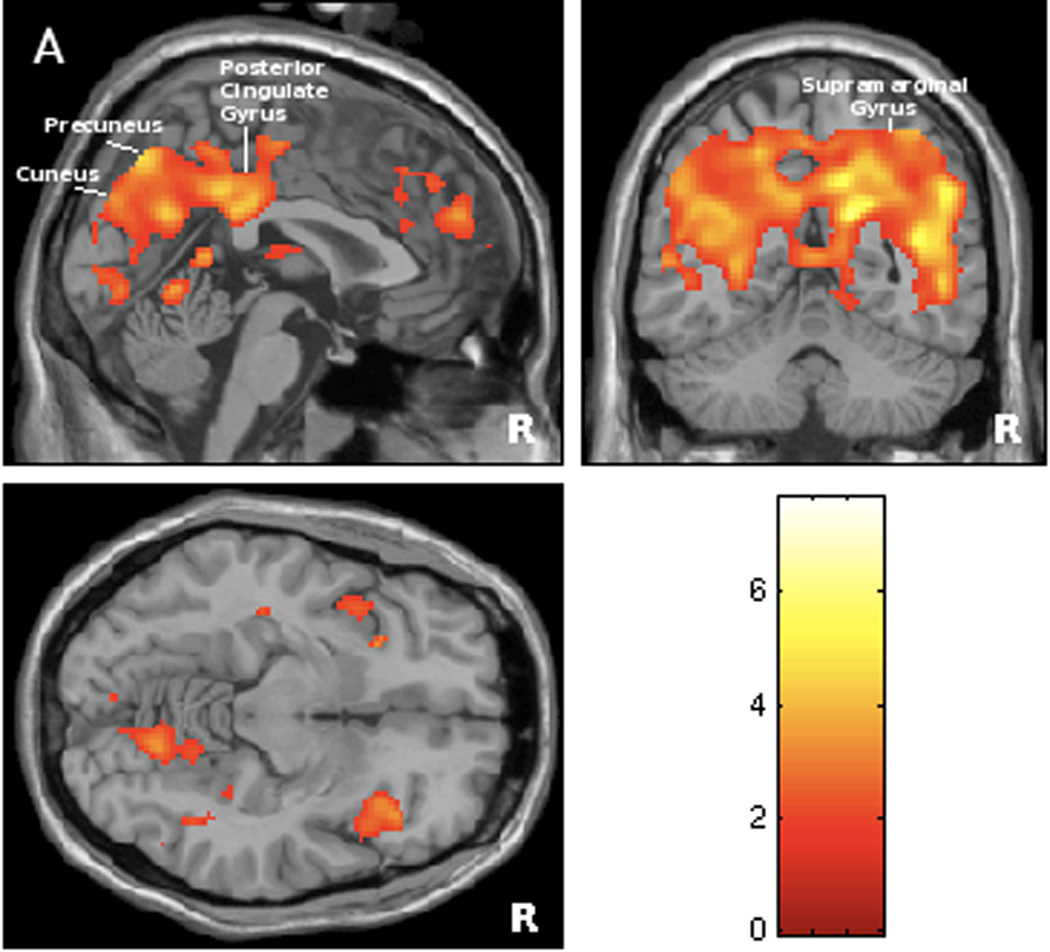
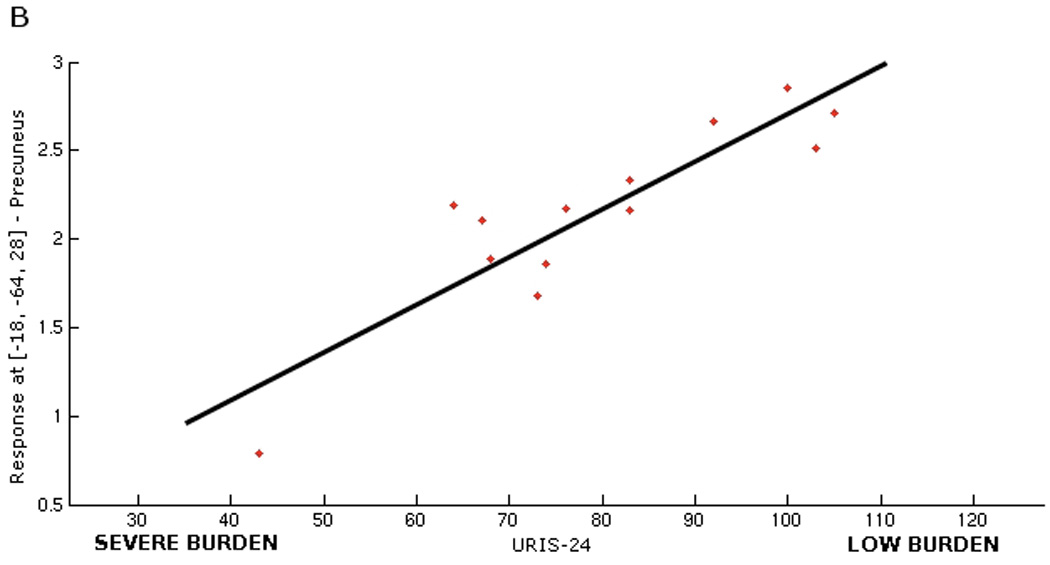
Figure 3A. Statistical map of brain regions where responses to bladder filling during scanning session were significantly associated with psychological burden of urge incontinence measured by Urge Impact Scale (URIS-24) (positive correlation at threshold level of p<0.01, r >0.63). Regions’ coordinates and statistics are listed on Table 3.
Figure 3B. Graphical illustration of correlation between brain responses in precuneus (BA 47) and severity of psychological burden measured by URIS-24 (r= 0.85). Note that URIS-24 has negative scoring system, with lower score indicating more severe burden of the disease, so that brain response is smaller for greater burden.
DISCUSSION
We found that regional brain activity, provoked by bladder filling in the presence of self-reported urgency (but in the absence of DO), correlated significantly with the severity of incontinence in daily life. The brain regions involved are similar, whether the severity of incontinence is represented by the number of daytime incontinence episodes on a 3-day bladder diary or by the weight of urine lost in one daytime period. These findings are consistent with our hypothesis that urgency and changes in brain activity measured during experimental conditions reflect the clinical severity of urgency incontinence.
Bladder control and continence involve complex neural processes, but our findings suggest that neural correlates can be identified for urgency incontinence and its symptoms. For example, increased response to bladder filling in the dorsal ACG, insula and parietal lobe correlates with the severity of incontinence in daily life. We have suggested previously14 that activation of the insula reflects the intensity of bladder filling sensation, while activation of the dorsal ACG – a major component of the limbic system17 – reflects motivation to void and concurrent efferent control and occurs preferentially in urgency-incontinent subjects. (Similarly, in patients with irritable bowel syndrome, abnormal ACG activation is provoked by rectal distension.18) Thus dorsal ACG activation may indicate unpleasantness rather than intensity of sensation19 and our findings suggest that brain activity during urgency may represent both an emotional reaction to imminent loss of bladder control (fear of leakage5) and an attempt to suppress it. Activation of the dorsal ACG in particular may represent a target for future interventions.
The strongest correlations with incontinence severity were observed in brain regions that are normally deactivated during bladder filling (rostral/subgenual ACG and orbitofrontal cortex). Specifically, we observed less prefrontal deactivation in subjects who had more severe incontinence, suggesting that such deactivation is involved with supressing the voiding reflex and that less deactivation may lead to more severe incontinence.14 These regions are also involved in emotional control and decision-making. 20 Thus, monitoring of prefrontal cortex deactivation may provide a surrogate outcome measure for testing interventions aimed at improving bladder control.
Different brain regions were correlated with the subjective burden of urgency incontinence. The direction of the association differs as well, suggesting that subjective and objective measures of incontinence may represent different dimensions of this condition. The regions associated with subjective burden are located predominantly in the posterior cortex, and they are less activated by bladder filling in subjects who report more severe psychological burden. These regions are also reportedly involved in associative cognitive activity, self-orientation and working memory21,22 and are activated by emotional stimuli in the scanner.20 They may represent a neural system that supports emotional processing20 and be part of a mechanism used to cope with the burden of incontinence. If the coping mechanism is less effective, then the associated burden may be greater. Of course, adequate coping may depend on general psychological wellbeing rather than being related specifically to urinary incontinence. Nevertheless, monitoring of posterior cortical activation may provide a target for interventions designed to reduce the burden of the disorder.
This study has limitations. Although sample size is adequate for fMRI, based on experience from studies of our own and others, 23 it is small for reliable interpretation of bladder diary data and spurious correlations are possible. However, the correlation between activity in the insula and incontinence frequency remained significant even after correcting for multiple comparisons. Second, fMRI has inherent limitations.24 For instance, increased fMRI activity may be excitatory or inhibitory, making it difficult to interpret deactivations. Yet, fMRI can facilitate translational studies that bridge the gap between animal observations and human clinical findings.25 In the present study, fMRI has suggested potential neural correlates of important patient-reported symptoms such as ‘urgency’ or ‘burden of disease’. The brain regions involved in these neural correlates represent potential targets for future clinical and pharmacological investigations.
CONCLUSION
Activity observed in a number of brain regions during fMRI evaluation of continence control mechanisms correlates well with patient-derived clinical measures of urgency incontinence severity. Thus, observations made under artificial conditions in an fMRI scanner seem to reflect patients’ real-life experience and may provide useful therapeutic targets and outcome measures.
ACKNOWLEDGEMENTS
We are grateful to Ms. Mary Alice Riley and Mr. Andrew Murrin for assistance during the study. We thank the reviewers for helpful suggestions for improvement.
Supported by: NIH: K23AG031916-01; Kl2RR024154-02; 2R01AG020629-06; John A. Hartford Center of Excellence in Geriatric Medicine.
Glossary of neuroanatomic terms
- BA 13
Insula
- ACG (BA 24/32)
Anterior cingulate gyrus
- BA 32
Dorsal ACG
- BA32/24
Rostral ACG
- BA32
Subgenual ACG
- BA47/44
Inferior frontal/precentral gyrus
- BA6
Medial frontal gyrus
- BA8/6/10
Superior frontal gyrus
- Brain stem
Thalamus
- PMC
Pontine micturition center
- PAG
Periaqueductal gray
- BA40
Inferior parietal lobe
- BA9
Cerebellum; Middle frontal gyrus
- BA44
Precentral gyrus
- BA19
Fusiform gyrus
- subcallosal gyrus
Frontal lobe
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Artibani W, Cardozo L, et al. Reviewing the ICS 2002 terminology report: The ongoing debate. Neurourol Urodyn. 2006;25:293. doi: 10.1002/nau.20737. [DOI] [PubMed] [Google Scholar]
- 3.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 4.Ross S, Soroka D, Karahalios A, et al. Incontinence-specific quality of life measures used in trials of treatments for female urinary incontinence: a systematic review. Int Urogynecol J. 2006;17:272–285. doi: 10.1007/s00192-005-1357-7. [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Blaivas JG, Stanton S, et al. The standardization of terminology of lower urinary tract function. Neurourol Urodyn. 1988;7:403–426. [Google Scholar]
- 6.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- 8.Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- 9.Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- 10.Nour S, Svarer C, Kristensen JK, et al. Cerebral activation during micturition in normal men. Brain. 2000;123:781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths D, Derbyshire S, Stenger A, et al. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths D, Tadic SD, Schaefer W, et al. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadic SD, Griffiths D, Schaefer W, et al. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage. 2008;39:1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths D, Tadic SD. Bladder control, urgency and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 15.Tadic SD, Zdaniuk B, Griffiths D et al. Effect of biofeedback on psychological burden and symptoms in older women with urge urinary incontinence. J Am Geriatr Soc. 2007;55:2010–2015. doi: 10.1111/j.1532-5415.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 16.DuBeau CE, Kiely DK, Resnick NM. Quality of life impact of urge incontinence in older persons: A new measure and conceptual structure. J Am Geriatr Soc. 1999;47:989–994. doi: 10.1111/j.1532-5415.1999.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 17.Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 18.Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 20.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 22.Wagner A, Shannon BJ, Kahn I, et al. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Murphy K, Garavan H. An empirical investigation into the number of subjects required for an event-related fMRI study. NeuroImage. 2004;22:879–885. doi: 10.1016/j.neuroimage.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Jezzard P, Buxton RB. The clinical potential of functional magnetic resonance imaging. J Magn Reson Imaging. 2006;23:787–793. doi: 10.1002/jmri.20581. [DOI] [PubMed] [Google Scholar]
- 25.Detre JA. Clinical applicability of functional MRI. J Magn Reson Imaging. 2006;23:808–815. doi: 10.1002/jmri.20585. [DOI] [PubMed] [Google Scholar]


