Abstract
L-buthionine sulfoximine (BSO) is a potent inhibitor of glutathione biosynthesis and studies have shown that it is capable of enhancing the apoptotic effects of several chemotherapeutic agents. Previous studies have shown that long term antihormonal therapy leads to acquired drug resistance and that estrogen, which is normally a survival signal, is a potent apoptotic agent in these resistant cells. Interestingly, we have developed an antihormone resistant breast cancer cell line, MCF-7:2A, which is resistant to estrogen-induced apoptosis but has elevated levels of glutathione. In the present study, we examined whether BSO is capable of sensitizing antihormone resistant MCF-7:2A cells to estrogen-induced apoptosis. Our results showed that treatment of MCF-7:2A cells with 1 nM E2 plus 100 μM BSO combination for 1 week reduced the growth of these cells by almost 80-90% whereas the individual treatments had no significant effect on growth. TUNEL and DAPI staining showed that the inhibitory effect of the combination treatment was due to apoptosis. Our data indicates that glutathione participates in retarding apoptosis in antihormone-resistant human breast cancer cells and that depletion of this molecule by BSO may be critical in predisposing resistant cells to estrogen-induced apoptosis.
Keywords: Antihormone resistance, low dose estrogen therapy, apoptosis, breast cancer, L-buthionine sulfoximine, glutathione
1. Introduction
Breast cancer continues to be the most common malignancy affecting women. Although great strides have been made in the treatment and cure of early stage breast cancer, metastatic breast cancer remains incurable resulting in 40,000 deaths per year in the United States alone [1]. Approximately two-thirds of all breast cancers contain the estrogen receptor (ER) and/or progesterone receptor (PgR) and are termed hormonally sensitive disease. A significant proportion of these hormonally sensitive breast cancers are dependent upon estrogenic stimulation for survival and growth [2].
Historically, various techniques employing estrogen deprivation have been utilized to exploit this feature in the treatment of hormonally sensitive breast cancers. Until recently, tamoxifen has been considered to be the hormonal therapy of choice for the treatment of estrogen receptor positive breast cancers [3]. Now, survival benefits have been demonstrated for the third generation aromatase inhibitors [4] and the pure antiestrogen, Fulvestrant, that causes degradation of the estrogen receptor [5].
The use of exhaustive anti-estrogen therapies has consequences for the tumor [6]. With continued long term estrogen deprivation, these initially hormonally sensitive breast cancer cells become sequentially resistant to further anti-estrogen therapy [7-9], indicating that they develop sophisticated survival mechanisms to sustain growth in estrogen-deprived environments (Fig. 1). Jordan and colleagues have demonstrated that when estrogen receptor positive breast cancer cells are grown and maintained in long term estrogen deprived (LTED) environments, they can ultimately develop enhanced responsiveness to greatly diminished levels of estrogen [6, 7, 10]. These pre-clinical animal models show that initially, estrogen receptor expressing tumors are stimulated by estrogen and respond appropriately to tamoxifen with tumor regression. However, with continued exposure to tamoxifen, the tumors become resistant and re-grow [9]. Additionally, treatment of these LTED tumors with post-menopausal levels of estrogen inhibits tumor growth as well as causes regression of established tamoxifen resistant tumors [7, 8, 11, 12] (Fig. 1).
Figure 1.
Evolution of drug resistance to selective estrogen receptor modulations (SERMs). Acquired resistance occurs during long-term treatment with a SERM and is evidenced by SERM-stimulated breast tumor growth. Tumors also continue to exploit estrogen for growth when the SERM is stopped, so a dual signal transduction process develops. The aromatase inhibitors prevent tumor growth in SERM-resistant disease and fulvestrant that destroys the estrogen receptor (ER) is also effective. This phase of drug resistance is referred to as phase I resistance. Continued exposure to a SERM results in continued SERM stimulated growth, but eventually autonomous growth occurs that is unresponsive to fulvestrant or aromatase inhibitors. The event that distinguishes phase I from phase II acquired resistance is a remarkable switching mechanism that now causes apoptosis, rather than growth, with physiologic levels of estrogen.
Clinical data supports the use of estrogen to treat hormonally sensitive breast cancers. In the past, pharmacologic doses of estrogen were a commonly employed therapy that resulted in durable responses with regression of disease [13] with as high as 40% response rate as first-line treatment in patients with hormonally sensitive breast cancer with metastatic disease [3] and approximately 31% (44% clinical benefit rate) in patients heavily pre-treated with previous endocrine therapies [14]. Long-term survival data for pharmacologic estrogen treatment in the patients treated as first-line therapy for hormonally sensitive metastatic breast cancer has yielded a statistically significant 5-year survival benefit in favor of estrogen when compared to tamoxifen, 35% and 16% respectively. This clinical data is consistent with the pre-clinical models of Jordan and colleagues that show that after exhaustive anti-hormonal treatment, estrogen treatment produces tumor apoptosis and rapid tumor regression [8, 9].
Therefore, we have hypothesized that treatment with a defined course of estrogen in post-menopausal women with estrogen receptor positive metastatic breast cancer whose disease has progressed after initial response to sequential anti-estrogen therapies, will result in clinical responses and may potentially reverse hormonally refractory disease, resulting in additional clinical benefit with further endocrine treatment such as an aromatase inhibitor, in this heavily endocrine pre-treated population. We are currently evaluating the optimal dose of daily estradiol therapy to reverse antihormonal resistance [6] but the goal is to enhance the estradiol-induced apoptotic response.
Increased intracellular glutathione has long been associated with tumor cell resistance to various cytotoxic agents. Studies have shown that L-buthionine sulfoximine (BSO) (Fig. 2), a potent inhibitor of glutathione biosynthesis [15], sensitizes tumor cells to apoptosis induced by standard chemotherapeutic drugs in vitro and in vivo [16, 17]. We previously reported the development of a long-term estrogen deprived breast cancer cell line, MCF-7:2A [18], which appeared to be resistant to estradiol-induced apoptosis but expressed elevated levels of glutathione. We believe that the combination of BSO and estradiol could possibly be used to improve the efficacy of estradiol as an apoptotic agent if glutathione depletion is fundamental to tumor cell survival. Our goal is to address the hypothesis that by altering glutathione levels, we may be able to enhance estrogen-induced apoptosis and have employed BSO as our agent of choice.
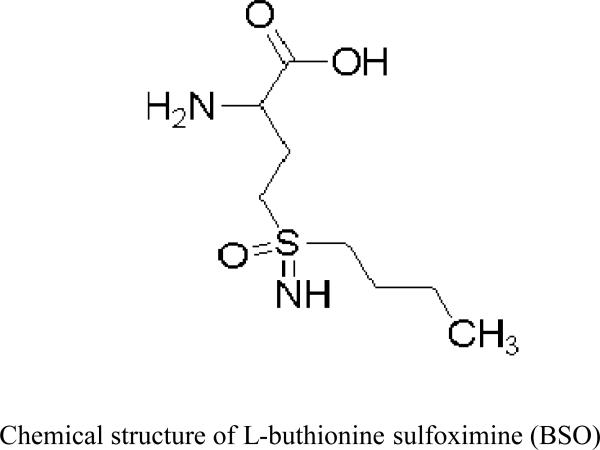
Figure 2.
Chemical structure of L-buthionine sulfoximine
In the current study, we investigated the in vitro effect of the combination of BSO and estradiol (E2) on MCF-7:2A cell viability in relation to apoptosis. We found that BSO or E2, as individual treatments, did not significantly alter the viability of MCF-7:2A cells nor induced apoptosis. However, the combined treatment of BSO and E2 depleted glutathione content and induced significant apoptosis in MCF-7:2A cells. In contrast, similar experiments performed in wild-type hormone responsive MCF-7 cells showed no apoptosis or growth inhibition following the combination treatment of BSO and E2. Our data indicates that glutathione participates in retarding apoptosis in antihormone-resistant human breast cancer cells and that depletion of this molecule by BSO may be critical in predisposing resistant cells to E2-induced apoptotic cell death. We suggest that these data may form the basis of improving therapeutic strategies for the treatment of antihormone resistant ER-positive breast cancer.
2. Materials and methods
2.1. Cell culture and reagents
The MCF-7 human breast cancer cell line was obtained from Dr. Dean Edwards (University of Texas, San Antonio, TX) and was maintained in phenol red RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1X non-essential amino acids and bovine insulin at 6 ng/mL. The clonal cell line, MCF-7:2A [18], was derived by growing MCF-7 cells in estrogen-free media for more than 1 year, followed by two rounds of limiting dilution cloning. These cells were grown in phenol red-free RPMI 1640 medium supplemented with 10% 4X dextran-coated, charcoal-treated FBS (SFS). All reagents for cell culture were obtained from Invitrogen. L-Buthionine sulfoximine (BSO) and 17 beta-estradiol (E2) were from Sigma.
2.2. Cell proliferation
Prior to the start of the cell growth assay, parental MCF-7 cells were grown in estrogen-free RPMI media containing 10% SFS for 3 days. This procedure was performed in order to remove any endogenous estrogen from the serum. On the day of the experiment, MCF-7 and MCF-7:2A cells were seeded in estrogen-free RPMI media containing 10% SFS at a density of 5×105 cells per 15-cm dish. After 24 hours, cells were treated with nothing (control), 10-9 M E2, increasing concentrations of BSO (10 μM to 2.5 mM) either alone or combined with 10-9 M E2 for 1 week with retreatment on alternate days. At the indicated time point, the DNA content of the cells was determined as previously described [8] using a Fluorescent DNA Quantitation kit (Bio-Rad). For each analysis, six replicate wells were used, and at least three independent experiments were performed.
2.3. TUNEL staining for apoptosis
Apoptosis was determined by the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay using an in situ cell death detection kit, POD (Roche Molecular Biochemicals), according to the manufacturer's instructions. Briefly, fixed cells were washed, permeabilized, and then incubated with 50 μL of terminal deoxynucleotidyl transferase end-labeling cocktail for 60 min at 37 °C in a humidified atmosphere in the dark. For signal conversion, slides were incubated with 50 μl of converter-POD (anti-fluorescein antibody conjugated with horse-radish peroxidase) for 30 min at 37 °C, rinsed with PBS, and then incubated with 50 μl of DAB substrate solution for 10 min at 25 °C. The slides were then rinsed with PBS, mounted under glass coverslips, and analyzed under a light microscope (Inverted Nikon TE300).
2.4. DAPI staining for apoptosis
MCF-7:2A cells were grown (overnight) in RPMI medium containing 10% dextran coated charcoal stripped fetal bovine serum (SFS) and then treated with ethanol vehicle (i.e., control), 1 nM estradiol, 100 μM BSO, or BSO + E2 for 72 hours. The cells were then washed in PBS, fixed with 4% paraformaldehyde for 20 minutes at room temperature, and washed again in PBS. Cells were then treated with 1 μg/mL of 4',6-diamidino-2-phenylindole (DAPI) (Sigma Chemical Co.) for 30 minutes, washed again with PBS for 5 minutes, and treated with 50 μL of VectaShield (Vector Laboratories, Burlingame, CA). Stained nuclei were visualized and photographed using a Zeiss fluorescence microscope (Provis AX70; Olympus Optical Co., Japan). Apoptotic cells were morphologically defined by cytoplasmic and nuclear shrinkage and by chromatin condensation or fragmentation.
2.5. Glutathione assay
Total cellular glutathione was measured using the Total Glutathione Colorimetric microplate assay Kit (Oxford Biomedical Research), according to the manufacture's protocol. Cells were plated at 0.5 × 106/well of a six-well plate and allowed to recover overnight. After appropriate treatments, cells were washed in PBS and then lysed in 100–150 μl of buffer (100 mM NaPO4, 1 mM EDTA, pH 7.5) containing 0.1% Triton X-100 and frozen at –80°C until analysis. To measure total glutathione, proteins were precipitated with sulfosalicylic acid at a final concentration of 1%. Samples were then spun for 10 min in a microcentrifuge to pellet proteins, and supernatant was diluted 1:20 in buffer before being measured. For all measurements, 50-μl triplicates of each sample were used for glutathione determination. The GSH level was obtained by comparing with the GSH standards and represented as nmol/mg of protein.
2.6. Statistical analysis
Statistical analysis was performed using Student's t test, and a P value of <0.05 was considered significant. Data are expressed as the mean ± SE. The mean value was obtained from at least three independent experiments.
3. Results
3.1. Glutathione levels are elevated in estrogen deprived MCF-7:2A breast cancer cells
Previous studies have shown that GSH levels in primary breast tumors are more than twice the levels found in normal breast tissue, and levels in lymph node metastases are more than four times the levels in normal breast tissue [19]. Recently, we reported the development of an estrogen deprived breast cancer cell line MCF-7:2A that is resistant to estrogen-induced apoptosis and expresses high levels of the glutathione synthetase gene GSS. To determine whether GSH levels were elevated in our apoptosis-resistant MCF-7:2A breast cancer cell line glutathione assays were performed on these cells. Fig.3A shows that MCF-7:2A cells had significantly higher levels of GSH at 24, 48, and 72 hours (11.9 nmol/mg protein to 15.8 nmol/mg protein) compared to wild-type MCF-7 cells (7.8 nmol/mg protein to 7.6 nmol/mg protein) and this trend continued up to day 7 (data not show). We next examined whether the GSH synthesis inhibitor BSO was capable of suppressing GSH levels in these cells. Fig. 3B shows that treatment with 100 μM of BSO for 48 hours suppressed GSH levels by ~55% in MCF-7 cells and by ~75% in MCF-7:2A cells. Longer treatment with BSO (>48 hours) yielded similar levels of inhibition (data not shown). These results indicate a possible link between elevated GSH levels and resistance to estrogen-induced apoptosis and they suggest that suppression of GSH by BSO has the ability to reverse the resistant phenotype of the MCF-7:2A cells.
Figure 3.
Intracellular glutathione levels in wild-type MCF-7 cells and antihormone-resistant MCF-7:2A breast cancer cells. (A) Cells were seeded at 2 × 106 cells per 100 mm culture plates in estrogen-free media and total cellular glutathione was measured over a 72 hour time period using a glutathione colorimetric assay kit, as described in “Materials and methods.” *, P < .0001, with respect to MCF-7 cells. (B) BSO reduces glutathione levels in MCF-7 and MCF-7:2A cells. For experiment, cells were treated with 100 μM BSO for 48 hours and levels of glutathione were measured as described in “Materials and methods.” Bars, ± SE
3.2. Glutathione suppression by BSO sensitizes antihormone resistant MCF-7:2A cells to estrogen-induced apoptosis
We next examined whether depletion of glutathione by BSO has the ability to sensitize MCF-7:2A cells to estrogen-induced apoptosis. Wild-type MCF-7 cells and estrogen deprived MCF-7:2A cells were seeded in estrogen-free media, and after 24 hours, were treated with nothing (control), 1 nM estradiol, or 10 μM to 10 mM BSO in the presence or absence of 1 nM estradiol for 7 days. Fig. 4A shows that the growth of MCF-7 cells was stimulated 5-fold over the control cells by 1 nM estradiol during the course of the 7-day assay and that treatment with BSO, either alone or in combination with estradiol, did not significantly alter the growth of these cells except at very high concentrations (> 1 mM). In contrast, MCF-7:2A cells treated with the combination of BSO and estradiol showed a significant concentration dependent decrease in cell growth relative to cells treated with estradiol or BSO alone (Fig. 4B). It is noteworthy that 100 μM BSO, as a single agent, did not cause growth inhibition of MCF-7:2A cells. However, when combined with 1 nM estradiol the combination caused an 80-90% decrease in growth (Fig. 4B). The cell killing effect of BSO and estradiol was observed as early as 48 hours after treatment and persisted over the time course of the experiment with maximum cell death at the 7-day time point. The concentration of BSO used in this study is already known to be clinically achievable without significant side effects [20, 21].
Figure 4.
BSO enhances the growth inhibitory effect of estradiol in antihormone-resistant MCF-7:2A cells. (A) MCF-7 cells were grown in estrogen-free media for 3 days prior to the start of the growth assay. On the day of the experiment, cells were seeded in 24-well plates and after 24 hours were treated with various concentrations (10 μM- 10 mM) of BSO in the presence or absence of 1 nM (10-9 M) E2 for 7 days. At the indicated time points, cells were harvested and total DNA (ng/well) was quantitated as described in Materials and methods. (B) MCF-7:2A cells were treated similarly as described above. The data represents the mean of three independent experiments.
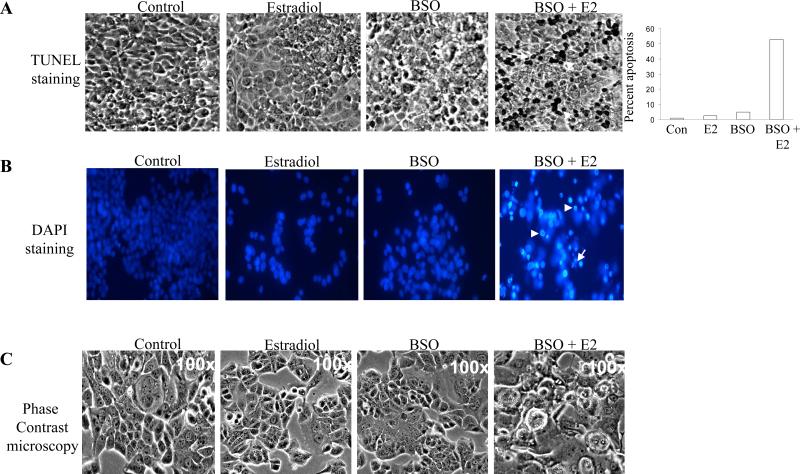
Based on the above finding, we next determined whether MCF-7:2A cells underwent apoptotic cell death following BSO plus estradiol treatment. TUNEL assay was performed on cells treated with 100 μM BSO, 1 nM estradiol, or 100 μM BSO plus 1 nM estradiol for 72 hours to detect fragmentation of DNA, a characteristic of apoptotic cell death. Fig. 5A shows that the percentage of TUNEL-positive cells significantly increased with the combination of BSO and estradiol but not with estradiol or BSO alone. After treatment with BSO and estradiol (72 hours), as many as 53% of cells displayed TUNEL-positive staining, whereas, only 1% of the control cells and 5% of the estradiol treated cells were TUNEL-positive (Fig. 5A). BSO-treated cells looked similar to control cells. As expected, wild-type MCF-7 cells showed very little TUNEL-positive staining in the presence of estradiol alone or BSO plus estradiol combined (data not shown), thus indicating a lack of apoptosis in these cells. DAPI staining of MCF-7:2A cells treated with BSO and estradiol further confirmed that the cells were undergoing apoptosis (Fig. 5B). In addition, phase contrast microscopy of MCF-7:2A cells showed morphological changes associated with apoptosis following BSO and estradiol treatment (Fig. 5C). Overall, these results indicate that BSO, as a single agent, causes neither growth inhibition nor cell death, but is capable of sensitizing antihormone resistant MCF-7:2A cells to estradiol induced apoptosis at clinically achievable concentrations.
Figure 5.
BSO enhances the apoptotic effect of estradiol in MCF-7:2A breast cancer cells. (A) Cells were treated with 1 nM E2, 100 μM BSO, or 1 nM E2 + 100 μM BSO for 72 hours and TUNEL staining for apoptosis was performed as described in “Materials and methods.” Slides were photographed through brightfield microscope under 100X magnification. TUNEL-positive cells were stained black (white arrows). Columns (right), mean percentage of apoptotic cells (annexin V-positive cells) from three independent experiments done in triplicate; bars, SEs. (B). Fluorescent microscopic analysis of apoptotic cells stained with 4',6-diamidino-2-phenylindole (DAPI). MCF-7:2A cells were treated with 1 nM E2, 100 μM BSO, or 1 nM E2 + 100 μM BSO as described above for 72 hours. To assess the number of cells undergoing apoptosis, round and/or shrunken nuclei of DAPI-stained cells were counted (white arrows). At least 200 cells per slide were counted by two individuals to control for subjective variability. Experiments were repeated three times with similar results. Representative slides are shown. Scale bars = 50 μm. (C) Phase contrast microscopy of MCF-7:2A cells treated with 1 nM E2, 100 μM BSO, or 1 nM E2 + 100 μM BSO for 72 hours.
4. Discussion
In the current study, we investigated whether suppression of the antioxidant glutathione by BSO has the ability to sensitize antihormone resistant MCF-7:2A breast cancer cells to estradiol-induced apoptosis. Our results showed that glutathione levels were significantly elevated in antihormone-resistant MCF-7:2A breast cancer cells compared to wild-type MCF-7 cells and that the combination treatment of BSO and estradiol caused a dramatic increase in apoptosis whereas the individual treatments had no effect on growth. Noteworthy, the killing effect of BSO and estradiol occurred at clinically achievable concentrations and was observed as early as 48 hours. These findings are consistent with previous studies which have shown that the cytotoxicity of a number of chemotherapeutic drugs, including melphalan [22], doxorubicin [23], and bleomycin [24], are significantly enhanced when glutathione is depleted by BSO.
Our laboratory has previously demonstrated that when estrogen receptor positive breast cancer cells are grown and maintained in long term estrogen deprived (LTED) environments, they can ultimately develop enhanced responsiveness to greatly diminished levels of estrogen [7, 9]. These pre-clinical animal models show that initially, estrogen receptor expressing tumors are stimulated by estrogen and respond appropriately to tamoxifen with tumor regression. However, with continued exposure to tamoxifen, the tumors become resistant and re-grow [9]. Additionally, treatment of these LTED tumors with post-menopausal levels of estrogen inhibits tumor growth as well as causes regression of established tamoxifen resistant tumors [7, 9, 11, 12] (Fig. 1). Mechanistic studies indicate that the apoptotic action of estrogen is due to its ability to either activate the fasR/FasL death receptor pathway [11, 25] or to disrupt mitochondrial function through activation of the bcl-2 family proteins [7]. The paradoxical action of estrogen in these resistant cells is hypothesized to be due to increased sensitivity to estrogen due to adaptation to estrogen deprivation caused either by tamoxifen or an aromatase inhibitor [26]. It is believed that this “estrogen hypersensitivity” helps to explain the effectiveness of high-dose estrogen in patients with extensive prior endocrine therapy [14].
Interestingly, our present findings indicate that the ability of estradiol to induce apoptosis in antihormone resistant cells is influenced by the level of glutathione present in the cells. Glutathione levels were elevated ~ 1.4- to 1.6-fold in antihormone-resistant MCF-7:2A cells compared to wild-type MCF-7 cells and these cells failed to undergo apoptosis following 1 week of treatment with physiological concentrations of estradiol alone. In the presence of BSO, however, which depleted intracellular glutathione by ~60-70%, the combination treatment of BSO and estradiol caused a dramatic increase in apoptosis which was observed as early as 48 hours with maximum induction observed at day 7. Previous studies have shown that glutathione is an important component of tumor drug resistance [21] and that depletion of intracellular glutathione by BSO significantly enhances the cytotoxicity of many cytotoxic agents, principally alkylating agents [15, 20, 27] and platinating compounds [16] but also irradiation [28] and anthracyclines [29]. The concentration of BSO used in our study was within the range of 10 μM to 1 mM, which is similar to what has previously been reported in the literature. However, we did observe some toxicity at higher concentrations of BSO (> 1 mM) in wild-type MCF-7 and antihormone-resistant MCF-7:2A cells (Fig. 4). It should be noted that BSO, at a clinically achievable concentration of 100 μM, was used for all of our combination experiments with estradiol since this concentration, as an individual treatment, did not significantly alter the growth of MCF-7:2A cells.
Glutathione, a sulfhydryl containing tripeptide, is involved in detoxifying cells from various toxins including chemotherapeutic agents [30, 31]. Several lines of evidence have shown that in tissue culture studies of cancer cell line made resistant to selected chemotherapy agents, glutathione levels correlated with increasing chemotherapy resistance [32]. This resistance was not limited to the particular chemotherapy agent used to induce resistance, but was also evident when other chemotherapeutic agents were tested for cross-resistance [32]. Additionally, translational studies of in vitro cell lines derived from patients with chemorefractory disease were found to have elevated glutathione levels [33]. BSO inhibits γ-glutamylcysteine synthetase (γ-GCS), the rate limiting enzyme in the production of glutathione, thus depleting glutathione levels within the cell [34]. Both, GSH as well as resultant increase in γ-GCS levels as a result of BSO treatment can be monitored peripherally in patients by analysis of peripheral mononuclear cells (PMNs) [35]. BSO also exhibits selectivity in that in vitro studies have demonstrated greater depletion of glutathione levels in tumor tissues than sampled normal tissues [30]. Based on its ability to target intracellular glutathione and reverse therapeutic resistance in refractory cancers, BSO is thought to be a potential antineoplastic agent and/or “therapeutic sensitizer” worthy of clinical evaluation.
Early phase clinical trials of BSO at doses resulting in both peripheral and tumor GSH depletion show that BSO can be safely administered to patients with refractory disease. BSO was administered intravenously twice daily either alone or together with chemotherapy to cancer patients whose disease who disease had progressed despite multiple lines of previous chemotherapy [35, 36]. In these patients treated with escalating doses of BSO, nausea and vomiting amenable to anti-emetic therapy were the main toxicities. Bone marrow suppression correlating with extent of previous chemotherapy exposure was found to be the rate limiting toxicity in the combination studies. No other significant toxicities were noted. Intracellular glutathione levels measured in PMNs decreased in a linear manner with repeated doses of BSO to a maximum of approximately 10-40% of baseline values [35, 36]. When tested in sequential tumor biopsies, glutathione was also found to be depleted to a variable extent in a similarly predictable pattern [36]. Additionally, BSO administration resulted in an initial rapid inhibition of γ–GCS activity followed by γ–GCS recovery during the intervening time between dosings. In fact, γ–GCS levels mirrored peripheral BSO concentrations in patients thus demonstrating targeted delivery of BSO. Clinically, responses to treatment, including complete responses, have been achieved [27, 35, 36].
In this present study, we demonstrated that glutathione depletion by BSO sensitized antihormone-resistant MCF-7:2A human breast cancer cells to estradiol-induced apoptosis in vitro. Taken together, it would be reasonable to incorporate this data into our working translational model for clinical evaluation (Fig. 6). We therefore propose utilizing BSO together with estrogen in patients for a defined therapeutic course in patients with hormonally sensitive metastatic breast cancer whose disease has progressed on prior antihormonal therapies to significantly reduce their disease burden, while potentially reversing resistance to antihormonal therapies. This would then be followed by continuing treatment with an aromatase inhibitor for maintenance of additional clinical benefit for these patients (Fig. 6). Our future goal will be to address this hypothesis in the context of a clinical trial based on these new pre-clinical findings.
Figure 6.
Clinical protocol to investigate the efficacy of estradiol plus BSO combination treatment to induce apoptosis in long-term endocrine refractory breast cancer. An anticipated treatment plan for third-line endocrine therapy. Patients must have responded and experience treatment failure with two successive antihormone therapies to be eligible for a course of low-dose estradiol combined with BSO therapy for 3 months. The anticipated response rate is 30% and responding patients will be treated with anastrozole until relapse. The overall goal is to increase response rates and maintain patients for longer on antihormone strategies before chemotherapy is required.
Acknowledgments
This work was supported by the Department of Defense Breast Program under award number BC050277 Center of Excellence (VCJ); Fox Chase Cancer Center Core Grant NIH P30 CA006927 (VCJ); Weg Fund of Fox Chase Cancer Center (VCJ); the American Cancer Society Grant IRG-92-027-14 (JSLW); the Hollenbach Family Fund (JSLW), and the NIH Career Development Grant 1K01CA120051-01A2 (JSLW). The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Cancer Facts and Figures. 2005 www.cancer.org.
- 2.Lippman M, Bolan G, Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer research. 1976;36(12):4595–4601. [PubMed] [Google Scholar]
- 3.Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, Nichols WC, Creagan ET, Hahn RG, Rubin J, Frytak S. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. The New England journal of medicine. 1981;304(1):16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19(3):881–894. doi: 10.1200/JCO.2001.19.3.881. [DOI] [PubMed] [Google Scholar]
- 5.Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, Vergote I, Erikstein B, Webster A, Morris C. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 6.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26(18):3073–3082. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, Bell E, Chandel NS, Jordan VC. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. Journal of the National Cancer Institute. 2005;97(23):1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 8.Lewis JS, Osipo C, Meeke K, Jordan VC. Estrogen-induced apoptosis in a breast cancer model resistant to long-term estrogen withdrawal. The Journal of steroid biochemistry and molecular biology. 2005;94(1-3):131–141. doi: 10.1016/j.jsbmb.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O'Regan RM, Jordan VC. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6(5):2028–2036. [PubMed] [Google Scholar]
- 10.Lewis JS, Cheng D, Jordan VC. Targeting oestrogen to kill the cancer but not the patient. British journal of cancer. 2004;90(5):944–949. doi: 10.1038/sj.bjc.6601627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. Journal of the National Cancer Institute. 2003;95(21):1597–1608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 12.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, Yue W, Wang J, Santen RJ. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. Journal of the National Cancer Institute. 2001;93(22):1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 13.Haddow WJA, Paterson E. Influence of synthetic oestrogens upon advanced malignant disease. BMJ. 1944;2:393–398. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, Mella O, Howell A. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast cancer research and treatment. 2001;67(2):111–116. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 15.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chemico-biological interactions. 1998;111-112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 16.Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer research. 2003;63(2):312–318. [PubMed] [Google Scholar]
- 17.Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer. 2000;89(7):1440–1447. [PubMed] [Google Scholar]
- 18.Pink JJ, Jiang SY, Fritsch M, Jordan VC. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer research. 1995;55(12):2583–2590. [PubMed] [Google Scholar]
- 19.Perry RR, Mazetta JA, Levin M, Barranco SC. Glutathione levels and variability in breast tumors and normal tissue. Cancer. 1993;72(3):783–787. doi: 10.1002/1097-0142(19930801)72:3<783::aid-cncr2820720325>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Anderson CP, Seeger RC, Satake N, Monforte-Munoz HL, Keshelava N, Bailey HH, Reynolds CP. Buthionine sulfoximine and myeloablative concentrations of melphalan overcome resistance in a melphalan-resistant neuroblastoma cell line. J Pediatr Hematol Oncol. 2001;23(8):500–505. doi: 10.1097/00043426-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Bailey HH. L-S,R-buthionine sulfoximine: historical development and clinical issues. Chemico-biological interactions. 1998;111-112:239–254. doi: 10.1016/s0009-2797(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 22.Kramer RA, Greene K, Ahmad S, Vistica DT. Chemosensitization of L-phenylalanine mustard by the thiol-modulating agent buthionine sulfoximine. Cancer research. 1987;47(6):1593–1597. [PubMed] [Google Scholar]
- 23.Dusre L, Mimnaugh EG, Myers CE, Sinha BK. Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells. Cancer research. 1989;49(3):511–515. [PubMed] [Google Scholar]
- 24.Russo A, DeGraff W, Friedman N, Mitchell JB. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer research. 1986;46(6):2845–2848. [PubMed] [Google Scholar]
- 25.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, Loweth J, McKian K, De Los Reyes A, Wing L, Jordan VC. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. Journal of the National Cancer Institute. 2003;95(21):1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 26.Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. The Journal of clinical endocrinology and metabolism. 1995;80(10):2918–2925. doi: 10.1210/jcem.80.10.7559875. [DOI] [PubMed] [Google Scholar]
- 27.Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy RT, Wilding G. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. Journal of the National Cancer Institute. 1997;89(23):1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- 28.Rosi A, Grande S, Luciani AM, Palma A, Giovannini C, Guidoni L, Sapora O, Viti V. Role of glutathione in apoptosis induced by radiation as determined by 1H MR spectra of cultured tumor cells. Radiation research. 2007;167(3):268–282. doi: 10.1667/RR0578.1. [DOI] [PubMed] [Google Scholar]
- 29.Benderra Z, Trussardi A, Morjani H, Villa AM, Doglia SM, Manfait M. Regulation of cellular glutathione modulates nuclear accumulation of daunorubicin in human MCF7 cells overexpressing multidrug resistance associated protein. Eur J Cancer. 2000;36(3):428–434. doi: 10.1016/s0959-8049(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 30.Calvert P, Yao KS, Hamilton TC, O'Dwyer PJ. Clinical studies of reversal of drug resistance based on glutathione. Chemico-biological interactions. 1998;111-112:213–224. doi: 10.1016/s0009-2797(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 31.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40(13):1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton TC, Winker MA, Louie KG, Batist G, Behrens BC, Tsuruo T, Grotzinger KR, McKoy WM, Young RC, Ozols RF. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochemical pharmacology. 1985;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- 33.Ozols RF, Louie KG, Plowman J, Behrens BC, Fine RL, Dykes D, Hamilton TC. Enhanced melphalan cytotoxicity in human ovarian cancer in vitro and in tumor-bearing nude mice by buthionine sulfoximine depletion of glutathione. Biochemical pharmacology. 1987;36(1):147–153. doi: 10.1016/0006-2952(87)90392-3. [DOI] [PubMed] [Google Scholar]
- 34.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) The Journal of biological chemistry. 1979;254(16):7558–7560. [PubMed] [Google Scholar]
- 35.Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol. 1994;12(1):194–205. doi: 10.1200/JCO.1994.12.1.194. [DOI] [PubMed] [Google Scholar]
- 36.O'Dwyer PJ, Hamilton TC, LaCreta FP, Gallo JM, Kilpatrick D, Halbherr T, Brennan J, Bookman MA, Hoffman J, Young RC, Comis RL, Ozols RF. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol. 1996;14(1):249–256. doi: 10.1200/JCO.1996.14.1.249. [DOI] [PubMed] [Google Scholar]