Abstract
BACKGROUND
Used in combination with antiretroviral therapy, subcutaneous recombinant interleukin-2 raises CD4+ cell counts more than does antiretroviral therapy alone. The clinical implication of these increases is not known.
METHODS
We conducted two trials: the Subcutaneous Recombinant, Human Interleukin-2 in HIV-Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) study and the Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT). In each, patients infected with the human immunodeficiency virus (HIV) who had CD4+ cell counts of either 50 to 299 per cubic millimeter (SILCAAT) or 300 or more per cubic millimeter (ESPRIT) were randomly assigned to receive interleukin-2 plus antiretroviral therapy or antiretroviral therapy alone. The interleukin-2 regimen consisted of cycles of 5 consecutive days each, administered at 8-week intervals. The SILCAAT study involved six cycles and a dose of 4.5 million IU of interleukin-2 twice daily; ESPRIT involved three cycles and a dose of 7.5 million IU twice daily. Additional cycles were recommended to maintain the CD4+ cell count above predefined target levels. The primary end point of both studies was opportunistic disease or death from any cause.
RESULTS
In the SILCAAT study, 1695 patients (849 receiving interleukin-2 plus antiretroviral therapy and 846 receiving antiretroviral therapy alone) who had a median CD4+ cell count of 202 cells per cubic millimeter were enrolled; in ESPRIT, 4111 patients (2071 receiving interleukin-2 plus antiretroviral therapy and 2040 receiving antiretroviral therapy alone) who had a median CD4+ cell count of 457 cells per cubic millimeter were enrolled. Over a median follow-up period of 7 to 8 years, the CD4+ cell count was higher in the interleukin-2 group than in the group receiving antiretroviral therapy alone — by 53 and 159 cells per cubic millimeter, on average, in the SILCAAT study and ESPRIT, respectively. Hazard ratios for opportunistic disease or death from any cause with interleukin-2 plus antiretroviral therapy (vs. antiretroviral therapy alone) were 0.91 (95% confidence interval [CI], 0.70 to 1.18; P = 0.47) in the SILCAAT study and 0.94 (95% CI, 0.75 to 1.16; P = 0.55) in ESPRIT. The hazard ratios for death from any cause and for grade 4 clinical events were 1.06 (P = 0.73) and 1.10 (P = 0.35), respectively, in the SILCAAT study and 0.90 (P = 0.42) and 1.23 (P = 0.003), respectively, in ESPRIT.
CONCLUSIONS
Despite a substantial and sustained increase in the CD4+ cell count, as compared with antiretroviral therapy alone, interleukin-2 plus antiretroviral therapy yielded no clinical benefit in either study. (ClinicalTrials.gov numbers, NCT00004978 [ESPRIT] and NCT00013611 [SILCAAT study].)
The CD4+ cell count remains the best single indicator of immunodeficiency related to infection with the human immunodeficiency virus (HIV) and is an important determinant of clinical events defining the acquired immunodeficiency syndrome (AIDS) and other serious diseases.1,2 Interleukin-2 is a cytokine secreted by activated T cells that regulates the proliferation, differentiation, and survival of T cells. Early studies showed that Escherichia coli–expressed recombinant interleukin-2, given intravenously or subcutaneously in combination with antiretroviral therapy, increased the CD4+ cell count significantly as compared with antiretroviral therapy alone.3–11 The cell expansions occur because of an increase in CD4+ T-cell survival (with half-lives that can exceed 3 years) and are characterized by an increase in numbers of both naive and central memory cells.12–14 Absolute increases were greater in patients with higher baseline CD4+ cell counts. The level of HIV-associated immune activation, as reflected in T-cell turnover, was decreased in interleukin-2 recipients.15
The clinical impact of CD4+ T-cell increases associated with the use of interleukin-2 is unknown. Any possible beneficial effects from interleukin-2 would need to be sufficiently large to mitigate the effect of its known toxicity. Since the net effects might differ between patients with higher CD4+ cell counts and those with lower counts, two trials were conducted involving patients receiving combination antiretroviral therapy.
METHODS
STUDY DESIGN
The Subcutaneous Recombinant, Human Interleukin-2 in HIV-Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) study and the Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT) were multicenter, international trials. The studies were open-label because the almost universal and typical side effects of interleukin-2 made blinding impossible. Patients were randomly assigned, in equal numbers, to receive interleukin-2 plus antiretroviral therapy or antiretroviral therapy alone.
Recombinant interleukin-2 was administered subcutaneously in cycles. In the SILCAAT study, one cycle consisted of a dose of 4.5 million IU twice daily for 5 consecutive days. Six cycles of interleukin-2 were planned to be given, approximately 8 weeks apart, within the first 12 months of the study (the induction phase). The induction phase of ESPRIT consisted of three cycles of interleukin-2 given at a dose of 7.5 million IU twice daily. After the induction phase, additional cycles of interleukin-2 therapy were recommended to maintain CD4+ cell counts above the predefined target levels. Guidelines for the management of interleukin-2 toxicity included reductions of the dose of interleukin-2 in decrements of 1.5 million or 3.0 million IU per dose. The minimum dose of interleukin-2 administered was 1.5 million IU twice daily. In the SILCAAT study, after the third cycle, the dose of interleukin-2 could be increased to 6.0 million or 7.5 million IU.
ESPRIT was funded and sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). The SILCAAT study was originally sponsored by Chiron. In February 2003, after completing enrollment, Chiron announced that it would no longer support the trial for business reasons owing to its inability to gain accelerated approval from the Food and Drug Administration on the basis of changes in CD4+ cell count. To complete the study, trial management was transferred to the SILCAAT Scientific Committee and the investigators conducting ESPRIT. NIAID provided regulatory sponsorship, and Chiron — and subsequently Novartis, after acquiring Chiron — provided funds for the SILCAAT study from February 2003 forward. Chiron–Novartis provided the interleukin-2 used in both trials.
The paper was written by a writing group representing the leaders of each study. Neither Chiron nor Novartis was involved in the data analysis or interpretation or in the preparation of the manuscript. Chiron and members of the SILCAAT Scientific Committee designed the SILCAAT study; members of the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) Executive Committee designed ESPRIT. For the entire duration of ESPRIT and since 2003 for the SILCAAT study, oversight of data collection at clinical sites was performed by international coordinating centers working with a central coordinating center at the University of Minnesota, which managed and analyzed the data for both studies. The authors vouch for the accuracy and completeness of the data and analyses.
The ESPRIT design and methods have been reported previously.16 Additional information on methods is given in the Supplementary Appendix, available with the full text of this article at NEJM.org.
STUDY POPULATIONS
Both trials included adult patients with confirmed HIV-1 infection. Patients with a CD4+ cell count of 50 to 299 per cubic millimeter (in the SILCAAT study) or 300 or more per cubic millimeter (in ESPRIT) were enrolled. Patients in the SILCAAT study were also required to have an HIV RNA level of less than 10,000 copies per milliliter. Protocols were approved by the institutional review board at each site. Written informed consent was obtained from all patients.
ASSESSMENTS
Patients were seen every 4 months for a targeted history taking and clinical evaluation and measurement of the CD4+ cell count and plasma HIV RNA level. Follow-up continued until a common closing date (November 15, 2008).
DEFINITIONS OF END POINTS
The primary end point of each study was opportunistic disease or death from any cause. Secondary end points included death from any cause and grade 4 clinical events, defined as potentially life-threatening events (excluding opportunistic diseases) requiring medical intervention (see toxicity table at http://rcc.tech-res.com). Grade 4 events were reported irrespective of their perceived relationship to the use of interleukin-2 or antiretroviral therapy and were coded according to the Medical Dictionary for Regulatory Activities (version 12.0).
INTERIM MONITORING OF SAFETY AND EFFICACY
An independent data and safety monitoring board reviewed interim analyses from the SILCAAT study and ESPRIT. On November 27, 2007, at their final meeting, the board recommended that ESPRIT continue until its planned completion time (when 320 primary events had occurred) and that the SILCAAT study continue until ESPRIT was closed.
STATISTICAL ANALYSIS
In both trials, the primary analysis was based on the intention-to-treat principle. Time-to-event methods were used to compare the groups receiving interleukin-2 plus combination antiretroviral therapy and combination antiretroviral therapy alone, with regard to major end points.17 Follow-up data were censored when patients were lost to follow-up before or on November 15, 2008.
The hazard ratios for the comparisons of interleukin-2 plus antiretroviral therapy and antiretroviral therapy alone were estimated from Cox models with a single indicator for treatment group. We tested the proportional-hazards assumption by including an interaction term between treatment group and natural-log–transformed follow-up time.
Data on the primary end point were summarized for prespecified subgroups defined according to baseline characteristics. A total of 12 subgroup analyses were prespecified. The heterogeneity of hazard-ratio estimates between subgroups was assessed by including an interaction term between treatment and subgroup in expanded Cox models. The results of subgroup analyses should be interpreted with caution; a significant interaction could be due to chance, because there was no adjustment made to the type 1 error for the number of subgroups examined.
Cox models were also used to obtain an estimate of the association between the time-updated follow-up CD4+ cell count (the levels last measured before the event, hereafter called the latest levels) after log10 transformation and the primary end point among recipients of antiretroviral therapy alone. Estimates of parameters in Cox models and average differences in the CD4+ cell count between treatment groups during the follow-up period were used to obtain predicted hazard ratios for comparison with observed hazard ratios.
Statistical analyses were performed using SAS software (version 9.1). P values are two-sided.
RESULTS
BASELINE CHARACTERISTICS
A total of 1695 patients (849 receiving interleukin-2 plus antiretroviral therapy and 846 receiving antiretroviral therapy alone) in the SILCAAT study and 4111 patients (2071 receiving interleukin-2 plus antiretroviral therapy and 2040 receiving antiretroviral therapy alone) in ESPRIT were enrolled and had data included in the analysis (Table 1, and Fig. Ia and Ib in the Supplementary Appendix). The two treatment groups were well balanced with respect to baseline characteristics (Tables Ia and Ib in the Supplementary Appendix).
Table 1.
Baseline Characteristics of Participants in SILCAAT and ESPRIT.*
| Characteristic | SILCAAT Study (N = 1695) |
ESPRIT (N = 4111) |
|---|---|---|
| Age (yr) | ||
| Median | 40 | 40 |
| Interquartile range | 36–47 | 34–46 |
| Female sex (%) | 16.5 | 18.6 |
| Race or ethnic group (%)† | ||
| Black | 8.4 | 9.1 |
| White | 79.8 | 75.3 |
| Other or unknown | 11.8 | 15.5 |
| CD4+ cell count (per mm3) | ||
| Median | 202 | 457 |
| Interquartile range | 151–254 | 372–584 |
| CD4+ cell-count nadir (per mm3) | ||
| Median | 60 | 197 |
| Interquartile range | 26–107 | 91–306 |
| HIV RNA ≤500 copies/ml (%) | 81.4 | 79.7 |
| AIDS event (%) | 32.5 | 25.9 |
| Body-mass index‡ | ||
| Median | 23.9 | 23.7 |
| Interquartile range | 21.8–26.1 | 21.9–25.9 |
| Previous antiretroviral therapy (%) | ||
| PI | 85.5 | 72.4 |
| NNRTI | 57.7 | 57.9 |
| NRTI, PI, and NNRTI | 44.3 | 38.5 |
| Time since first prescribed antiretroviral drugs (yr) |
||
| Median | 3.9 | 4.2 |
| Interquartile range | 1.8–7.2 | 2.2–6.4 |
| Current antiretroviral regimen (%) | ||
| Includes PI | 65.6 | 49.0 |
| Includes NNRTI | 45.8 | 46.3 |
| Includes NRTI, PI, and NNRTI | 13.9 | 7.9 |
AIDS denotes the acquired immunodeficiency syndrome, HIV human immunodeficiency virus, NNRTI nonnucleoside reverse-transcriptase inhibitor, NRTI nucleoside reverse-transcriptase inhibitor, and PI protease inhibitor.
Race or ethnic group was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
COMPLETENESS OF FOLLOW-UP
Approximately 5700 patient-years and 14,000 patient-years of follow-up were accrued in each group in the SILCAAT study and in ESPRIT, respectively. (The median duration of follow-up was 7.6 years for the SILCAAT study and 7.0 years for ESPRIT.) In the SILCAAT study, the status of the primary end point was unknown for 91 of the 849 patients (10.7%) receiving interleukin-2 and antiretroviral therapy and for 100 of the 846 patients (11.8%) receiving antiretroviral therapy alone. In ESPRIT, the status of the primary end point was unknown for 118 of the 2071 patients (5.7%) receiving interleukin-2 and antiretroviral therapy and for 134 of the 2040 patients (6.6%) receiving antiretroviral therapy alone.
USE OF INTERLEUKIN-2
In the SILCAAT study, 72.3% of patients receiving interleukin-2 plus antiretroviral therapy completed six cycles of interleukin-2 therapy; 2.1% never received interleukin-2. In ESPRIT, 83.4% of the patients receiving interleukin-2 plus antiretroviral therapy completed at least three cycles of interleukin-2 therapy; 3.7% of patients never received interleukin-2. The median number of cycles was 7 (interquartile range, 5 to 9) in the SILCAAT study and 4 (interquartile range, 3 to 6) in ESPRIT.
CD4+ CELL COUNT
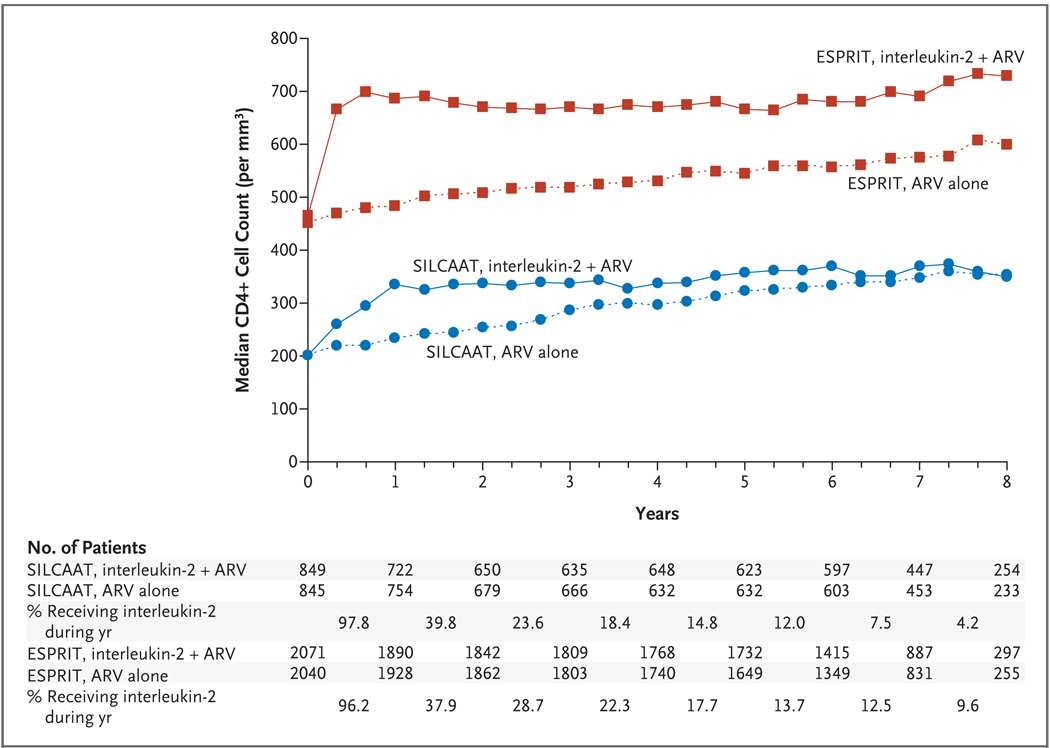
Median CD4+ cell counts are given in Figure 1. In the SILCAAT study, at 1 year, the median CD4+ cell count in the group receiving interleukin-2 plus antiretroviral therapy had increased from the baseline level by 131 per cubic millimeter (interquartile range, 52 to 215). For SILCAAT patients receiving antiretroviral therapy alone, the increase in the CD4+ cell count over the baseline value at 1 year was 32 per cubic millimeter (interquartile range, −11 to 78). The median difference in CD4+ cell count between the two SILCAAT groups declined from 99 per cubic millimeter at 1 year to 38 per cubic millimeter at 6 years. This decline paralleled the percentage of patients receiving interleukin-2 during each year (97.8% in year 1 and 12.0% during year 6). On average, over the follow-up period, the CD4+ cell count was higher with interleukin-2 plus antiretroviral therapy than with antiretroviral therapy alone, by 53 per cubic millimeter (95% confidence interval [CI], 40 to 66).
Figure 1. Median CD4+ Cell Counts during the Study Period, According to Study and Treatment Group.
The median CD4+ cell counts are shown for the groups receiving interleukin-2 plus antiretroviral therapy (ARV) and the groups receiving ARV alone in the SILCAAT study and ESPRIT. The counts during the first 30 days after a cycle of interleukin-2 are not stable and therefore were excluded. Also shown are the percentages of patients assigned to receive interleukin-2 who were taking the drug during each year of the study.
In ESPRIT, the median CD4+ cell count was increased over the baseline value at 1 year, by 206 cells per cubic millimeter (interquartile range, 55 to 376) in the group receiving interleukin-2 plus antiretroviral therapy as compared with 21 cells per cubic millimeter (interquartile range, −64 to 114) in the group receiving antiretroviral therapy alone. This difference between the two ESPRIT groups of 185 cells per cubic millimeter at 1 year declined to 113 cells per cubic millimeter at 6 years. This decrease in the difference between the two groups paralleled the decline in receipt of interleukin-2 — from 96.2% of patients during the first year to 13.7% during the sixth year. On average, during the follow-up period, the CD4+ cell count was higher with interleukin-2 plus antiretroviral therapy, by 159 per cubic millimeter (95% CI, 145 to 174), as compared with antiretroviral therapy alone.
ANTIRETROVIRAL THERAPY AND HIV RNA LEVELS
During the follow-up period, the use of antiretroviral therapy and HIV RNA levels were similar for the groups receiving interleukin-2 plus antiretroviral therapy and the groups receiving antiretroviral therapy alone (Fig. IIa and IIb in the Supplementary Appendix). More than 80% of patients had HIV RNA levels at or below 500 copies per milliliter at each visit.
PRIMARY END POINT AND OTHER MAJOR CLINICAL OUTCOMES
Opportunistic Disease or Death from Any Cause (Primary End Point)
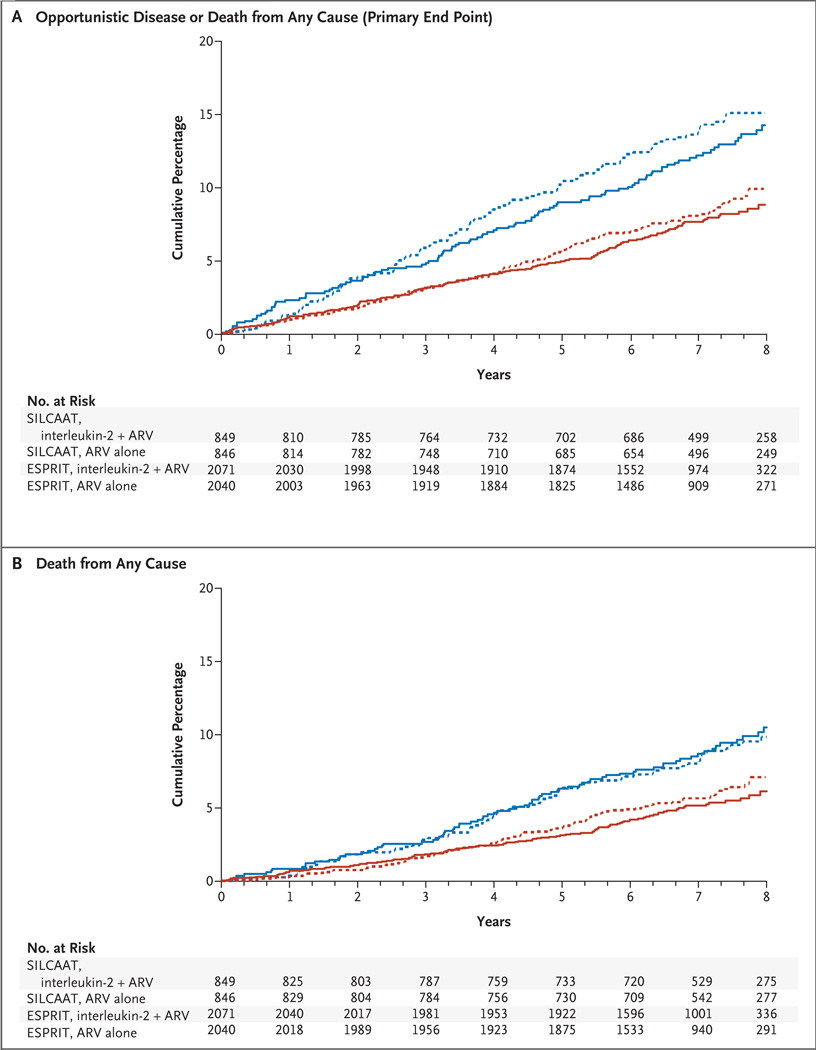
In the SILCAAT study, 110 patients receiving interleukin-2 plus antiretroviral therapy and 119 receiving antiretroviral therapy alone had an opportunistic disease or died (Table 2 and Fig. 2A, and Tables IIa and IIIa in the Supplementary Appendix) (hazard ratio for this primary end point with interleukin-2, 0.91; 95% CI, 0.70 to 1.18; P = 0.47). This hazard ratio did not vary significantly over the follow-up period (P = 0.34).
Table 2.
Hazard Ratios for the Primary End Point and Major Secondary End Points in SILCAAT and ESPRIT, According to Treatment Group.
| End Point* | Interleukin-2 + Antiretroviral Therapy |
Antiretroviral Therapy Alone |
Hazard Ratio for Interleukin-2 (95% CI) |
P Value |
|---|---|---|---|---|
| no. of patients (rate/100 person-yr) | ||||
| SILCAAT | ||||
| Primary end point: opportunistic disease or death from any cause |
110 (1.94) | 119 (2.13) | 0.91 (0.70–1.18) | 0.47 |
| Death from any cause | 81 (1.38) | 77 (1.31) | 1.06 (0.77–1.44) | 0.73 |
| Opportunistic disease | 49 (0.86) | 66 (1.18) | 0.73 (0.51–1.06) | 0.10 |
| Grade 4 event | 203 (3.93) | 186 (3.58) | 1.10 (0.90–1.34) | 0.35 |
| ESPRIT | ||||
| Primary end point: opportunistic disease or death from any cause |
159 (1.14) | 165 (1.21) | 0.94 (0.75–1.16) | 0.55 |
| Death from any cause | 107 (0.75) | 116 (0.83) | 0.90 (0.69–1.17) | 0.42 |
| Opportunistic disease | 68 (0.49) | 63 (0.46) | 1.05 (0.75–1.48) | 0.78 |
| Grade 4 event | 466 (3.80) | 383 (3.09) | 1.23 (1.07–1.41) | 0.003 |
Grade 4 clinical events were defined as potentially life-threatening events (excluding opportunistic diseases) requiring medical intervention (see toxicity table at http://rcc.tech-res.com).
Figure 2. Cumulative Percentages of Patients with Opportunistic Disease or Death from Any Cause, According to Study and Treatment Group.
Panel A shows data for opportunistic disease or death from any cause (primary end point); and Panel B, for death from any cause. ARV denotes antiretroviral therapy.
In ESPRIT, 159 patients receiving interleukin-2 plus antiretroviral therapy and 165 receiving antiretroviral therapy alone had an opportunistic disease or died (Table 2, and Tables IIb and IIIb in the Supplementary Appendix). The hazard ratio for this primary end point with interleukin-2 was 0.94 (95% CI, 0.75 to 1.16; P = 0.55) (P = 0.40 for test of the proportional-hazards assumption).
We predicted the hazard ratios for the primary end point with interleukin-2 therapy on the basis of the overall differences in the CD4+ cell count between the two treatment groups in each study (on the log10 scale, 0.065 cells per cubic millimeter for the SILCAAT study and 0.099 cells per cubic millimeter for ESPRIT) and the relationship between the latest log10-transformed CD4+ cell count and the risk of opportunistic disease or death in the group receiving antiretroviral therapy alone in each study (Cox coefficient [±SE], −3.339±0.233 for the SILCAAT study and −3.049±0.187 for ESPRIT). The predicted hazard ratios for the SILCAAT study and ESPRIT were 0.80 (95% CI, 0.78 to 0.83) and 0.74 (95% CI, 0.71 to 0.77, respectively). Each of the predicted hazard ratios is smaller than the corresponding observed hazard ratio (which was 0.91 for the SILCAAT study and 0.94 for ESPRIT).
Death from Any Cause
In the SILCAAT study, 81 patients receiving interleukin-2 and antiretroviral therapy and 77 receiving antiretroviral therapy alone died (hazard ratio with interleukin-2, 1.06; 95% CI, 0.77 to 1.44; P = 0.73) (Table 2, Fig. 2B, and Table IIIa in the Supplementary Appendix). The hazard ratio for deaths not attributable to opportunistic diseases (which occurred in 70 patients receiving interleukin-2 plus antiretroviral therapy and 60 receiving antiretroviral therapy alone) was 1.17 with interleukin-2 (95% CI, 0.83 to 1.66; P = 0.36).
In ESPRIT, 107 patients receiving interleukin-2 and antiretroviral therapy and 116 receiving antiretroviral therapy alone died (hazard ratio with interleukin-2, 0.90; 95% CI, 0.69 to 1.17; P = 0.42) (Table 2, and Table IIIb in the Supplementary Appendix). The hazard ratio for deaths not attributable to opportunistic diseases (which occurred in 97 patients receiving interleukin-2 and antiretroviral therapy and 106 receiving antiretroviral therapy alone) was 0.89 (95% CI, 0.68 to 1.17; P = 0.41) with interleukin-2.
Opportunistic Diseases
In the SILCAAT study, an opportunistic disease developed in 49 patients receiving interleukin-2 and antiretroviral therapy and 66 receiving antiretroviral therapy alone (hazard ratio with interleukin-2, 0.73; 95% CI, 0.51 to 1.06; P = 0.10). In ESPRIT, an opportunistic disease developed in 68 patients receiving interleukin-2 and antiretroviral therapy and 63 receiving antiretroviral therapy alone (hazard ratio with interleukin-2, 1.05; 95% CI, 0.75 to 1.48; P = 0.78) (Table 2, and Fig. III in the Supplementary Appendix).
Grade 4 Events
In the SILCAAT study, 203 patients receiving interleukin-2 and antiretroviral therapy and 186 receiving antiretroviral therapy alone had a grade 4 event (hazard ratio with interleukin-2, 1.10; 95% CI, 0.90 to 1.34; P = 0.35) (Table 2, and Fig. IV in the Supplementary Appendix). In the interleukin-2 and antiretroviral therapy group, the 203 patients had a total of 342 grade 4 events, 78.4% of which occurred more than 60 days after the last dose of interleukin-2 was administered. Gastrointestinal disorders and psychiatric disorders were more common in the interleukin-2 group (P = 0.02 and P = 0.03, respectively) (Table IVa in the Supplementary Appendix).
In ESPRIT, grade 4 adverse events occurred in 466 patients receiving interleukin-2 and antiretroviral therapy and 383 receiving antiretroviral therapy alone (hazard ratio with interleukin-2, 1.23; 95% CI, 1.07 to 1.41; P = 0.003) (Table 2). In the interleukin-2 and antiretroviral therapy group, the 466 patients had a total of 711 grade 4 events, 82.4% of which occurred more than 60 days after the last dose of interleukin-2 was given. Differences between the two treatment groups were seen for the category of vascular disorders as well as the category of general disorders and administration site conditions (Table IVb in the Supplementary Appendix). Vascular events were seen in 40 patients receiving interleukin-2 and antiretroviral therapy and in 14 receiving antiretroviral therapy alone (hazard ratio with interleukin-2, 2.80; 95% CI, 1.53 to 5.15; P<0.001). The most frequent type of vascular event was deep-vein thrombosis (affecting 10 patients receiving interleukin-2 and antiretroviral therapy and 2 receiving antiretroviral therapy alone).
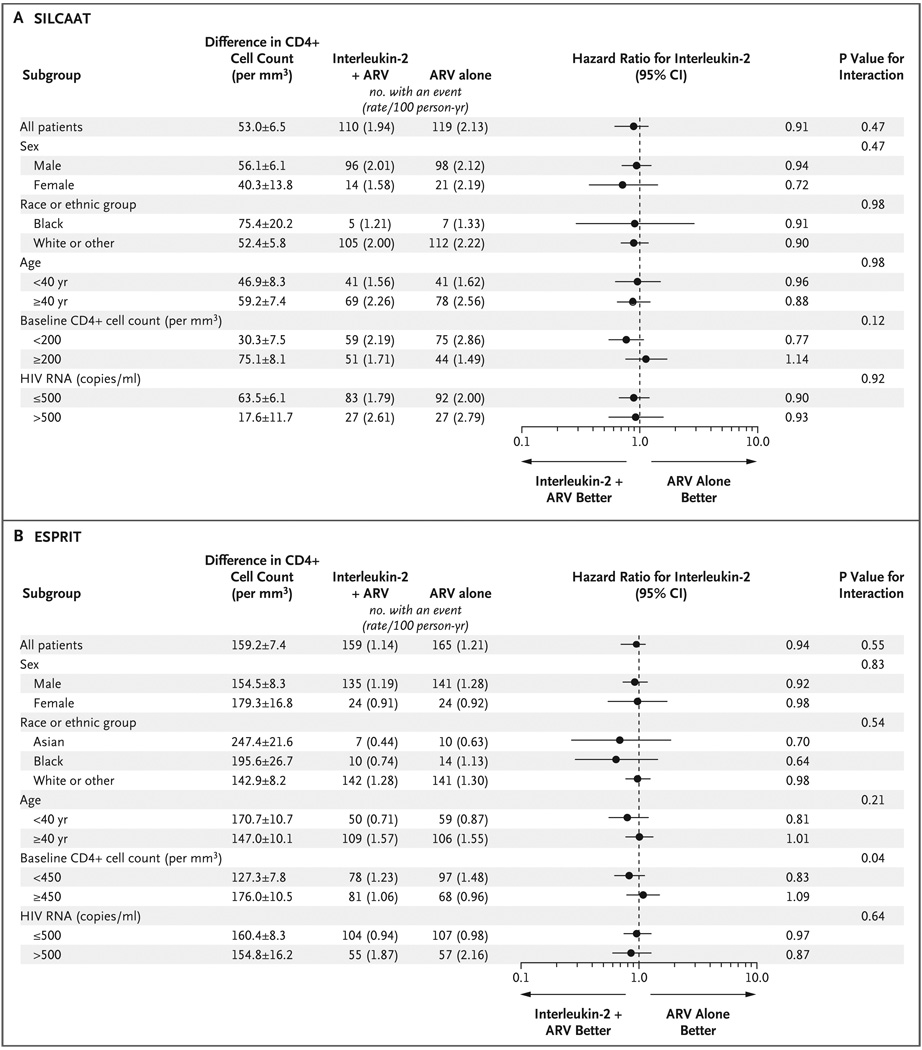
SUBGROUP FINDINGS
In both studies, hazard ratios for the primary end point with interleukin-2 were similar across demographic subgroups (Fig. 3). In ESPRIT, among patients with a baseline CD4+ cell count below 450, the hazard ratio was 0.83 (95% CI, 0.62 to 1.12), whereas among those with counts of 450 or more, the hazard ratio was 1.09 (95% CI, 0.79 to 1.50) (P = 0.04 for the interaction between the CD4+ cell count and treatment group) (Fig. 3B). For these two baseline CD4+ cell-count subgroups in ESPRIT, the hazard ratios for death with interleukin-2 also differed significantly (P = 0.003): 0.68 (95% CI, 0.47 to 0.98) for a count below 450 and 1.25 (95% CI, 0.85 to 1.84) for a count of 450 or more.
Figure 3. Between-Group Differences in the CD4+ Cell Count and Hazard Ratios for Opportunistic Disease or Death from Any Cause (Primary End Point), According to Subgroup.
Panel A shows data for the SILCAAT study; and Panel B, for ESPRIT. The differences in the CD4+ cell count were calculated by subtracting the count for the group receiving antiretroviral therapy (ARV) alone from the count for the group receiving interleukin-2 plus ARV and are expressed as means ±SE. Race or ethnic group was self-reported; the “other” category in Panel A consists of 1.2% Asians, 9.7% Hispanics, 0.8% other, and 0.1% unknown and in Panel B of 4.4% other and 0.3% unknown. The baseline CD4+ cell count is the approximate median value. In ESPRIT, one patient receiving ARV alone who had an event had missing data for baseline HIV RNA level.
DISCUSSION
These studies confirm that intermittent use of interleukin-2 is associated with substantial, sustained increases in CD4+ cell count. However, despite the increases in the CD4+ cell count, there was no clinical benefit, as measured by the reduction in the risk of opportunistic diseases or death, with interleukin-2 plus antiretroviral therapy as compared with antiretroviral therapy alone.
On the basis of the associations between the latest CD4+ cell count and the occurrence of opportunistic disease or death in the groups receiving antiretroviral therapy alone, the difference in the CD4+ cell count between the groups receiving interleukin-2 and antiretroviral therapy and those receiving antiretroviral therapy alone resulted in predicted hazard ratios for the primary end point with interleukin-2 of 0.80 for the SILCAAT study and 0.74 for ESPRIT. The predicted hazard ratios would be even smaller with adjustment for regression dilution bias resulting from variability in the measurement of the CD4+ cell count.18 It is unlikely that treatment differences of these predicted magnitudes were missed.
There are at least two hypotheses that could explain our results. The first and simplest is that the CD4+ T cells induced by interleukin-2 have no role in host defense. The second is that the cells are at least partially functional or that interleukin-2 has some modest beneficial effect not mediated through CD4+ cells but negative effects of interleukin-2 neutralize any improvements in host defense conferred by the therapy.
The value of a given CD4+ T cell to its host is the net sum of the predetermined antigenic specificity of that cell and the effector functions it expresses once activated by its antigen. T cells with receptors for irrelevant antigens or T cells that fail to exert protective effector functions on activation are of little value to the host. Interleukin-2 is known to induce a polyclonal expansion of preexisting CD4+ T cells that have predominantly naive or central-memory phenotypes. Antiretroviral therapy leads to expansions of preexisting effector memory, central memory, and naive cells. In this regard, it is possible that, despite the capacity to respond in vitro to certain antigens and mitogens10 the antigenic specificities of cells expanded with the use of interleukin-2 contribute little to the immediate needs of the host, whereas cells expanded as a result of antiretroviral therapy include those of greatest current value — namely, those in the effector memory pool. In addition, the CD4+ cells expanded by means of interleukin-2 express intermediate levels of CD25+, the alpha chain of the interleukin-2 receptor, as well as moderate levels of the transcriptional regulator forkhead box P3 (FOXP3). In this regard, the CD4+ cells are similar, but not identical, to regulatory T cells — a subset of T cells associated with suppressor-cell activity. Thus, it is possible that even if correct antigenic specificities are present, effector functions exhibited by these cells could be different from those provided by CD4+ cells that are expanded in patients receiving antiretroviral therapy.
With regard to the second hypothesis, that benefits of interleukin-2 are counteracted by negative effects of interleukin-2, in both the SILCAAT study and ESPRIT, patients who were receiving interleukin-2 plus antiretroviral therapy had more grade 4 events than those receiving antiretroviral therapy alone. Although many grade 4 events occurring in the interleukin-2 group occurred more than 60 days after the completion of an interleukin-2 cycle, they nonetheless appear to be related to receipt of interleukin-2. The association between occurrence of thromboembolic events and use of interleukin-2 found in ESPRIT, coupled with the association between elevated d-dimer levels and death from any cause in patients with HIV infection19 suggests a possible mechanism for a negative effect of interleukin-2 on clinical outcome. In ESPRIT, patients with higher baseline CD4+ cell counts had the greatest expansions of CD4+ T cells but also had a greater relative risk of having the primary end point or death from any cause. If this finding is not due to chance, it suggests that there may be clinically deleterious effects of interleukin-2 that are more pronounced in patients with higher baseline CD4+ cell counts or greater increases in CD4+ T cells after the use of interleukin-2. The mechanisms behind these deleterious effects remain unclear but could be related to the effects of T regulatory cells, greater proinflammatory effects of interleukin-2 in patients with higher numbers of CD4+ cells, or both.
Earlier randomized trials of interleukin-2 were conducted in patients receiving mono- or dual-nucleoside therapy, a different setting from that in the SILCAAT study and ESPRIT. In these earlier studies, most patients had HIV RNA levels above 10,000 copies per milliliter, and the groups receiving antiretroviral therapy alone had declining CD4+ cell counts.4,20,21 A pooled analysis of the results from these earlier studies suggested that patients treated with interleukin-2 plus antiretroviral therapy, as compared with antiretroviral therapy alone, had higher CD4+ cell counts, lower viral loads, and a trend toward fewer opportunistic infections and death.22 A more recent study in patients with advanced HIV infection also showed a trend toward fewer AIDS-defining illnesses with the use of interleukin-2.23 One possible explanation for the differences between findings in the previous studies and our results is that interleukin-2 has some net beneficial effect in a small subgroup of patients who have ongoing viral replication and a lower CD4+ cell count. A more likely explanation is that the treatment differences in the earlier studies were chance findings. This emphasizes the importance of conducting adequately powered, randomized trials to evaluate novel therapeutic strategies.
Surrogate markers often do not accurately predict the clinical effects of a treatment. The peripheral-blood total CD4+ cell count only partially explains the beneficial effects of antiretroviral therapy.24,25 These studies reaffirm that effects of a novel intervention that positively perturb levels of prognostic markers need to be assessed and validated in trials with clinical end points before those markers can be deemed reliable surrogates regarding that intervention. This requirement is consistent with experiences in other diseases.26
In summary, the results of the SILCAAT study and ESPRIT indicate that interleukin-2 offers no clinical benefit as compared with antiretroviral therapy alone. Whether these findings are relevant to other immunotherapies, such as interleukin-7,27 is uncertain. The precise role of the immune system in the pathogenesis of HIV infection may benefit from a reevaluation as a consequence of our results. Our data indicate that all CD4+ cells may not be equal with respect to host defense and that improvement in the prognostic or surrogate value of CD4+ counts requires refinement in measurement.
Supplementary Material
Acknowledgments
Supported by grants from the NIAID for ESPRIT (U01 AI46957 and U01 AI068641) and from Chiron and Novartis for ESPRIT and the SILCAAT study (to Dr. Neaton). For both studies, interleukin-2 was provided by Chiron–Novartis.
Dr. Losso reports receiving consulting and lecture fees from Bristol-Myers Squibb and Abbott and grant support from Tibotec, Schering-Plough, and Pharmaceutical Product Development; and Dr. Emery, consulting fees from Tibotec and Gilead, lecture fees from Gilead, and grant support from Gilead, Merck Research Laboratories, Abbott Laboratories, and Bristol-Myers Squibb. Dr. Lane reports being named as a coinventor on a patent issued to the U.S. government for the use of interleukin-2 in HIV infection and receiving research support from Novartis as part of a Cooperative Research and Development Agreement with the National Institutes of Health (NIH). Dr. Lundgren reports receiving consulting fees, lecture fees, and grant support from Boehringer Ingelheim, Bristol-Myers Squibb, Abbott, Gilead, GlaxoSmithKline, Merck Research Laboratories, Roche, Pfizer, and Tibotec; Dr. Mitsuyasu, grant support from Bionor Immuno, Pfizer, and Johnson & Johnson; Dr. Phillips, consulting fees from Bristol-Myers Squibb and lecture fees from Gilead; and Dr. Routy, consulting fees from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, and Cytheris and lecture fees from Abbott, Bristol-Myers Squibb, and GlaxoSmithKline. No other potential conflict of interest relevant to this article was reported.
We thank the many ESPRIT and SILCAAT investigators (listed in the Supplementary Appendix) who collected the data, the INSIGHT Executive Committee (J.D. Neaton, D. Abrams, A. Babiker, J. Baxter, D.A. Cooper, C.J. Cohen, D. Cohn, J.H. Darbyshire, W. El-Sadr, S. Emery, F. Gordin, H.C. Lane, G. Larson, M.H. Losso, J.D. Lundgren, J. Nadler, A.N. Phillips) for their oversight of ESPRIT and valuable editorial assistance on a draft of the manuscript, and the SILCAAT Scientific Committee (Y. Lévy, D. Abrams, A. Babiker, P. Cahn, B. Clotet, N. Clumeck, D.A. Cooper, J.H. Darbyshire, S. Emery, U. Hengge, H.C. Lane, J. Lange, G. Levi, J.D. Lundgren, R. Mitsuyasu, J.D. Neaton, J.P. Routy, G. Tambussi) for their oversight of the SILCAAT study and valuable editorial assistance on a draft of the manuscript.
APPENDIX
The affiliations of the writing group for the INSIGHT–ESPRIT Study Group and the SILCAAT Scientific Committee are as follows: D. Abrams (cochair), University of California, San Francisco, San Francisco; Y. Lévy (cochair), INSERM Unité 955, Université Paris 12, and Assistance Publique–Hôpitaux de Paris, Groupe Henri Mondor–Albert Chenevier, Paris; M.H. Losso (cochair), Hospital General de Agudos J.M. Ramos Mejia, Buenos Aires; A. Babiker and J. Darbyshire, Medical Research Council, and A. Phillips, University College London Medical School — both in London; G. Collins, J.D. Neaton, and D. Wentworth, University of Minnesota, Minneapolis; D.A. Cooper and S. Emery, National Centre in HIV Epidemiology and Clinical Research, Sydney; L. Fox and H.C. Lane, NIAID, Bethesda, MD; F. Gordin, Washington Veterans Medical Center, Washington, DC; J.D. Lundgren, Rigshospitalet and University of Copenhagen, Copenhagen; R. Mitsuyasu, University of California, Los Angeles, Los Angeles; J.P. Routy, Royal Victoria Hospital, McGill University Health Centre, Montreal; and G. Tambussi, Fondazione San Raffaele del Monte Tabor, Milan.
REFERENCES
- 1.Guiguet M, Porter K, Phillips A, Costagliola D, Babiker A. Clinical progression rates by CD4 cell category before and after the initiation of combination antiretroviral therapy (cART) Open AIDS J. 2008;2:3–9. doi: 10.2174/1874613600802010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection: a preliminary study. N Engl J Med. 1995;332:567–575. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs JA, Vogel S, Albert JM, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 5.Davey RT, Jr, Murphy RL, Graziano FM, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA. 2000;284:183–189. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 6.Levy Y, Capitant C, Houhou S, et al. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: a randomized controlled trial. Lancet. 1999;353:1923–1929. doi: 10.1016/s0140-6736(98)07345-0. [DOI] [PubMed] [Google Scholar]
- 7.Losso MH, Belloso WH, Emery S, et al. A randomized, controlled, phase II trial comparing escalating doses of subcutaneous interleukin-2 plus antiretrovirals versus antiretrovirals alone in human immunodeficiency virus-infected patients with CD4+ cell counts ≥350/mm3. J Infect Dis. 2000;181:1614–1621. doi: 10.1086/315430. [DOI] [PubMed] [Google Scholar]
- 8.Ruxrungtham K, Suwanagool S, Tavel JA, et al. A randomized, controlled 24-week study of intermittent subcutaneous interleukin-2 in HIV-1 infected patients in Thailand. AIDS. 2000;14:2509–2513. doi: 10.1097/00002030-200011100-00013. [DOI] [PubMed] [Google Scholar]
- 9.Abrams DI, Bebchuk JD, Denning ET, et al. Randomized, open-label study of the impact of two doses of subcutaneous recombinant interleukin-2 on viral burden in patients with HIV-1 infection and CD4+ cell counts of ≥300 mm3: CPCRA 059. J Acquir Immune Defic Syndr. 2002;29:221–231. doi: 10.1097/00126334-200203010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Levy Y, Durier C, Krzysiek R, et al. Effects of interleukin-2 therapy combined with highly active antiretroviral therapy on immune restoration in HIV-1 infection: a randomized controlled trial. AIDS. 2003;17:343–351. doi: 10.1097/00002030-200302140-00008. [DOI] [PubMed] [Google Scholar]
- 11.Arduino RC, Nannini EC, Rodriguez-Barradas M, et al. CD4 cell response to 3 doses of subcutaneous interleukin 2: meta-analysis of 3 Vanguard studies. Clin Infect Dis. 2004;39:115–122. doi: 10.1086/421775. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs JA, Lempicki RA, Sidorov IA, et al. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. J Clin Invest. 2005;115:2139–2148. doi: 10.1172/JCI23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read SW, Lempicki RA, Di Mascio M, et al. CD4 T cell survival after intermittent interleukin-2 therapy is predictive of an increase in the CD4 T cell count of HIV-infected patients. J Infect Dis. 2008;198:843–850. doi: 10.1086/591250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sereti I, Sklar P, Ramchandani MS, et al. CD4+ T cell responses to interleukin-2 administration in HIV-infected patients are directly related to the baseline level of immune activation. J Infect Dis. 2007;196:677–683. doi: 10.1086/520087. [DOI] [PubMed] [Google Scholar]
- 15.Sereti I, Anthony KB, Martinez-Wilson H, et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004;104:775–780. doi: 10.1182/blood-2003-12-4355. [DOI] [PubMed] [Google Scholar]
- 16.Emery S, Abrams DI, Cooper DA, et al. The evaluation of subcutaneous Proleukin (interleukin-2) in a randomized international trial: rationale, design, and methods of ESPRIT. Control Clin Trials. 2002;23:198–220. doi: 10.1016/s0197-2456(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. New York: John Wiley; 2002. [Google Scholar]
- 18.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 19.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr A, Emery S, Lloyd A, et al. Australian IL-2 Study Group. Outpatient continuous intravenous interleukin-2 or subcutaneous, polyethylene glycol-modified interleukin-2 in human immunodeficiency virus-infected patients: a randomized, controlled, multicenter study. J Infect Dis. 1998;178:992–999. doi: 10.1086/515653. [DOI] [PubMed] [Google Scholar]
- 21.de Boer AW, Markowitz N, Lane HC, et al. A randomized controlled trial evaluating the efficacy and safety of intermittent 3-, 4-, and 5-day cycles of intravenous recombinant human interleukin-2 combined with antiretroviral therapy (ART) versus ART alone in HIV-seropositive patients with 100–300 CD4+ T cells. Clin Immunol. 2003;106:188–196. doi: 10.1016/s1521-6616(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 22.Emery S, Capra WB, Cooper DA, et al. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J Infect Dis. 2000;182:428–434. doi: 10.1086/315736. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuyasu R, Gelman R, Cherng DW, et al. The virologic, immunologic, and clinical effects of interleukin 2 with potent antiretroviral therapy in patients with moderately advanced human immunodeficiency virus infection: a randomized controlled clinical trial — AIDS Clinical Trials Group 328. Arch Intern Med. 2007;167:597–605. doi: 10.1001/archinte.167.6.597. [DOI] [PubMed] [Google Scholar]
- 24.Hughes MD, Daniels MJ, Fischl MA, Kim S, Schooley RT. CD4 cell count as a surrogate endpoint in HIV clinical trials: a meta-analysis of studies of the AIDS Clinical Trials Group. AIDS. 1998;12:1823–1832. doi: 10.1097/00002030-199814000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Delta Coordinating Committee and Virology Group. An evaluation of HIV RNA and CD4 cell count as surrogates for clinical outcome. AIDS. 1999;13:565–573. [PubMed] [Google Scholar]
- 26.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Levy Y, Lacabaratz C, Weiss L, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.