SUMMARY
BACKGROUND
The human immunodeficiency virus (HIV) is a key factor responsible for the high rates of tuberculosis (TB) in sub-Saharan Africa. Treatment of TB with rifampicin (R, RMP) containing short-course regimens is highly effective in HIV-infected adults. We conducted a study to compare the efficacy and safety of intermittent ethambutol (E, EMB) with two RMP-containing regimens to treat pulmonary TB in HIV-infected patients.
SETTING
National Tuberculosis Treatment Centre, Mulago Hospital, Kampala, Uganda.
DESIGN
This was a prospective cohort compared to two non-randomised control groups. The study group and the two control arms were treated with 2 months of isoniazid (H), RMP, pyrazinamide (Z) and EMB followed by 6 E3H3 for the study group and 4HR or 6HR for controls.
RESULTS
Between April 1993 and March 2000, 136 patients were enrolled in the 2EHRZ/E3H3 arm, 147 in the 2EHRZ/4HR arm and 266 in the 2EHRZ/6HR arm. The relapse rate was 18.2 per 100 person-years observation (PYO) for the study regimen compared to 9.7/100 PYO (P = 0.0063) and 4.8/100 PYO (P = 0.0001) in patients treated with 2 EHRZ/4HR or 2EHRZ/6HR, respectively.
CONCLUSION
The 2EHRZ/6E3H3 regimen is safe and effective but has a significant risk of relapse.
Keywords: tuberculosis, treatment, HIV/AIDS, ethambutol, rifampicin
Tuberculosis (TB) is the leading cause of death due to an identifiable infectious pathogen among human immunodeficiency virus (HIV) infected individuals globally.1,2 HIV infection is a key factor responsible for increasing rates of TB in developing countries in sub-Saharan Africa.3 The current prevalence of HIV infection among new TB patients at Mulago Hospital, Kampala, Uganda, is approximately 50%.4,5 The World Health Organization (WHO) estimates that 38% of all patients with newly-diagnosed TB in the African region are HIV-co-infected.6
Treatment of pulmonary TB (PTB) with 6-month regimens using rifampicin (R, RMP) throughout treatment is highly effective in HIV-infected adults.6–9 The DOTS-based programs in Africa use RMP during the first 2 months of TB treatment, where all doses are supervised. Due to limited resources to provide supervision throughout the entire course of treatment and concerns about the potential for developing acquired RMP resistance, many National TB Programs (NTPs) use 6EH during the continuation phase. The thiacetazone-containing regimen previously used in East Africa is less expensive, but it has been associated with lower cure rates and a high frequency of cutaneous drug reactions in areas with a high prevalence of HIV infection.6,8,10–12 This led in part to the recommendations by the WHO to include ethambutol (E, EMB) in the continuation phase of treatment, to be used in an 8-month regimen.13
Although regimens using 6EH (H, INH = isoniazid) in the continuation phase are widely used in developing countries, their efficacy has not been well studied. We studied the efficacy and safety of an 8-month regimen using thrice-weekly INH and EMB in the continuation phase following an initial intensive phase consisting of daily INH, RMP, pyrazinamide (Z, PZA) and EMB in HIV-infected adults with initial episodes of sputum smear-positive, culture-confirmed PTB. Response to therapy and relapse with this regimen were compared with results from two other cohorts of HIV-infected adults treated at Mulago Hospital with regimens containing RMP throughout treatment.
Objectives
The objectives of the study were: 1) to evaluate the relapse rate of a regimen utilizing intermittent EH in the continuation phase compared to regimens utilizing daily RH; 2) to describe the adverse drug reactions associated with the intermittent EMB regimen; and 3) to assess patient adherence to the study regimen.
METHODS
Study design
Three cohort studies of HIV-associated TB were conducted between 1993 and 2000 at the National Tuberculosis Treatment Centre, Kampala, by the Uganda-Case Western Reserve Research Collaboration. Each cohort treated patients with 2 months of daily (2HRZE), but in the continuation phase the study cohort received EMB and INH thrice weekly for 6 months (6E3H3)* (Table 1), one cohort was given RMP and INH for 6 months: (6RH14) and the other was given RMP and INH for 4 months (4 RH15). Recruitment and follow-up evaluation were similar in all studies.
Table 1.
Dose, frequency and administration of continuation phase of treatment for the three cohorts included in the analysis
| Cohort | 6E3H3 cohort | 6RH cohort | 4RH cohort |
|---|---|---|---|
| Sample size | 136 | 266 | 147 |
| Study period | 1995–1996 | 1993–1998 | 1995–2000 |
| Dose | 900 mg INH 1800 mg EMB |
300 mg INH 600 mg RMP |
300 mg INH 600 mg RMP |
| Frequency | 3 times per week | Daily | Daily |
| DOT | DOT | Self-administered | Self-administered |
E, EMB = ethambutol; H, INH = isoniazid; R, RMP = rifampicin; DOT = directly observed therapy.
Patient population
Adults with initial episodes of smear-positive PTB were evaluated for enrollment in all of the study cohorts. Patients were eligible for this analysis if they were HIV-positive, acid-fast bacilli (AFB) smear-positive, had at least one sputum culture-positive for Mycobacterium tuberculosis, and had chest X-ray (CXR) findings consistent with TB. Persons with suspected miliary and meningeal TB, previous treatment of TB and pregnant women were excluded. No patients were receiving antiretroviral therapy during the course of the study. The studies were approved by the institutional review boards of Case Western Reserve University and University Hospitals of Cleve-land and the Ugandan National AIDS Research Subcommittee of the National Council for Science and Technology. All study participants gave informed consent and received pre- and post-test HIV counseling.
Study treatment regimen
In the intensive phase of treatment, all patients received 2HRZE, while treatment in the continuation phase varied according to cohort (Table 1). In the 6E3H3 cohort, INH (900 mg/dose) and EMB (1800 mg/dose) were administered thrice-weekly for 6 months. Two of three doses of anti-tuberculosis drugs were administered each week by directly observed therapy (DOT) throughout the 8 months of treatment. In both the 4HR and the 6HR cohorts, INH (300 mg) and RMP (600 mg) were given daily as a self-administered dose in the continuation phase. Adherence to treatment in all cohorts was monitored through self-report, pill counts, missed clinic appointments and urine INH metabolite testing.
Study measurements
A standard medical history and physical examination was completed for study subjects. HIV testing was performed using enzyme immunoassay (Recombigen HIV-1 env + gag enzyme immunoassay, Cambridge Bioscience, Cambridge, MA).
Eligible subjects underwent Mantoux skin testing. Induration was measured and recorded between 48 and 72 h. Postero-anterior CXRs were performed at study entry, end of anti-tuberculosis treatment, and end of study. Radiographic extent of disease was graded.16 Sputum specimens were collected from each patient for AFB smear and culture at baseline, monthly during treatment, at 3 months after completion of treatment and every 6 months thereafter in patients able to spontaneously produce sputum. All specimens were stained for AFB with both Ziehl-Neelsen and fluorescent auramine stains. Smears were graded based on the number of AFB.17 Specimens were cultured on Löwenstein-Jensen (LJ) slants and examined weekly until positive or for a maximum of 8 weeks. Susceptibility testing for INH, RMP and EMB was performed using standard methods18 on LJ slopes containing INH 0.2 μg/ml. For the 6E3H3 cohort, 20% of the susceptibility results were rechecked using BACTEC radiometric methods.19 Isolates were stored at −70°C for subsequent molecular analysis if necessary. Paired initial sputum isolates and isolates obtained at the time of suspected relapsed TB were compared using standard insertion sequence (IS) 6110 DNA restriction fragment length polymorphism (RFLP) analysis.20 Urine INH metabolite testing was done at least monthly (Myco-Dyn Uritest, Dynagen, Cambridge, MA).
Study outcomes
The primary study outcome was the rate of relapse of TB occurring during the 24 months following cure. Relapse was defined as the development of active TB after successful completion of an initial course of treatment.
Secondary outcomes included 2-month sputum culture conversion rate, treatment failure rate, mortality, development of acquired drug resistance, and toxicity.
Statistical analysis
Baseline characteristics for the three cohorts were compared using χ2 tests for categorical data and analysis of variance (ANOVA) for the continuous variables. Incidence rates for TB relapses were calculated using the person-years of observation (PYO) method. Overall survival after end of therapy for the three cohorts was estimated using Kaplan-Meier methods and compared using the log-rank test. For the analysis of TB relapse, patients were censored at the time of death, withdrawal, loss to follow-up, or the end of study. Cox proportional hazard models were fit using TB-free survival as the dependent variable. The hazard ratio (with 95% confidence intervals [CI]) for relapse among cohorts was estimated using 4RH as the reference treatment.
RESULTS
Study populations
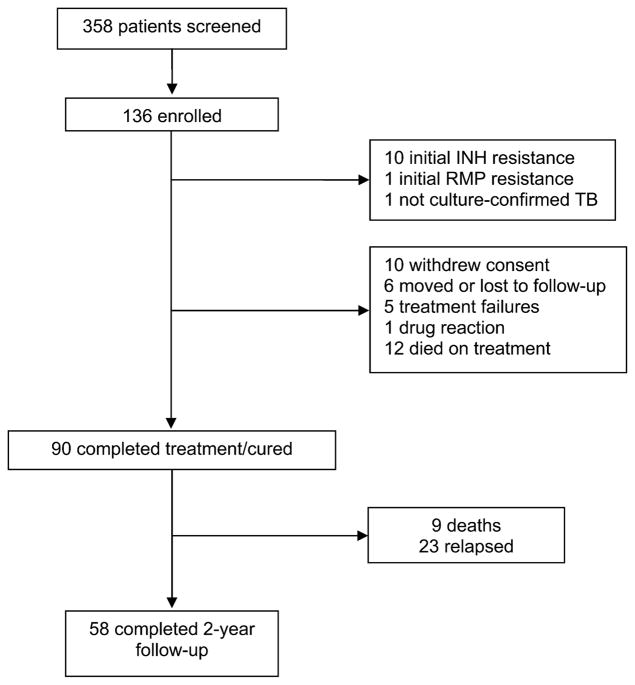
Between April 1993 and March 2000, 549 HIV-infected adults with smear-positive PTB were enrolled in the studies. Details of the patient populations for the 4RH and 6RH cohorts have been published previously.13,14 In the 6E3H3 cohort, 136 patients were eligible for the analysis. Details of enrollment and follow-up are shown in Figure 1.
Figure 1.
Status of patients screened and enrolled in the 6E3H3 cohort. H, INH = isoniazid; RMP = rifampicin; E = ethambutol.
The patients in the three cohorts were similar in terms of sex, baseline body mass index, Karnofsky performance status, CXR presentation, Mantoux reaction size, and presence of cavitary disease (Table 2). The 4RH cohort was older than the other cohorts (P = 0.0001). The mean hemoglobin levels in the 6RH cohort were greater than in the other cohorts (P = 0.001). The total white blood cell (WBC) count was greater in the 6E3H3 cohort than the other two cohorts (P = 0.001).
Table 2.
Baseline characteristics of the three study cohorts
| Characteristics | 6E3H3 (n = 136) | 6RH (n = 266) | 4RH (n = 147) | P value |
|---|---|---|---|---|
| Male sex (%) | 70 (51) | 128 (48) | 77 (52) | 0.666 |
| Age (years; mean ± SD) | 28.2 (6.4) | 29.2 (6.2) | 33.3 (7.7) | 0.0001 |
| BMI (kg/m2; mean ± SD) | 18.9 (2.5) | 19.1 (2.7) | 19.2 (2.3) | 0.69 |
| Karnofsky scale score (%: mean ± SD) | 80 (7.1) | 82 (6.7) | 81 (8.2) | 0.018 |
| Weight (kg: mean ± SD) | 50.3 (7.3) | 50.9 (7.6) | 52.1 (7.0) | 0.297 |
| Hemoglobin (gm/dl; mean ± SD) | 10.8 (1.9) | 13.4 (2.3) | 11.1 (2.9) | 0.001 |
| Total WBC count (mm3; mean ± SD) | 7.2 (3.5) | 5.4 (1.7) | 6.3 (2.4) | 0.001 |
| PPD (mm; mean ± SD) | 15.3 (6.9) | 14.5 (6.8) | 13.1 (6.0) | 0.0241 |
| Chest X-ray findings | ||||
| Extent of disease, n (%) | ||||
| Normal | 1 (0.7) | 1 (0.4) | 4 (3) | 0.229 |
| Minimal disease | 10 (7.4) | 33 (12.5) | 16 (11) | |
| Moderately advanced disease | 48 (36) | 89 (33.6) | 55 (37) | |
| Far advanced disease | 76 (56) | 142 (53.5) | 72 (49) | |
| Cavitary disease, n (%) | 82 (61) | 148 (56) | 69 (47) | 0.057 |
E = ethambutol; H = isoniazid; R = rifampicin; SD = standard deviation; BMI = body mass index; WBC = white blood count; PPD = purified protein derivative.
In the 6E3H3 cohort, 76% of all urine INH metabolite tests were positive; 82% of the subjects received 80% or more of their drugs and 80% attended at least 80% of their scheduled clinic appointments. Similar levels of adherence were present in the other cohorts.
Study outcomes
In all three cohorts, over 94% of patients had negative sputum mycobacterial cultures by the end of 2 months (Table 3). Treatment failure and mortality rates during treatment did not significantly differ among cohorts. By 1 year, there was a trend toward higher mortality in the 4RH cohort (24.3%; P = 0.10) that persisted at 2 years (36.8%; P = 0.06) (Table 3).
Table 3.
Events of treatment outcomes
| Outcome | 6E3H3* (n = 136) n (%) | 6RH (n = 266) n (%) | 4RH (n = 147) n (%) |
|---|---|---|---|
| Culture-negative at 2 months | 101 (94) | 238 (98) | 126 (95) |
| Treatment failure | 8 (5.8) | 7 (2.6) | 5 (3.4) |
| Completed therapy | 89 (65) | 195 (73) | 122 (83) |
| Mortality | |||
| During treatment | 15 (12.4) | 20 (8.4) | 16 (10.9) |
| 1 year | 21 (17.7) | 38 (16.3) | 35 (24.3) |
| 2 years | 27 (23.5) | 62 (27.9) | 51 (36.8) |
| Median follow-up (days) | 661 | 533 | 512 |
| Person-years observation | 126 | 289 | 165 |
| Relapse (n) | 23 | 14 | 16 |
| Relapse rate (95% CI) | 18.2 (8.5–22.5) | 4.8 (2.6–8.1) | 9.7 (5.5–15.8) |
E = ethambutol; H = isoniazid; R = rifampicin; CI = confidence interval.
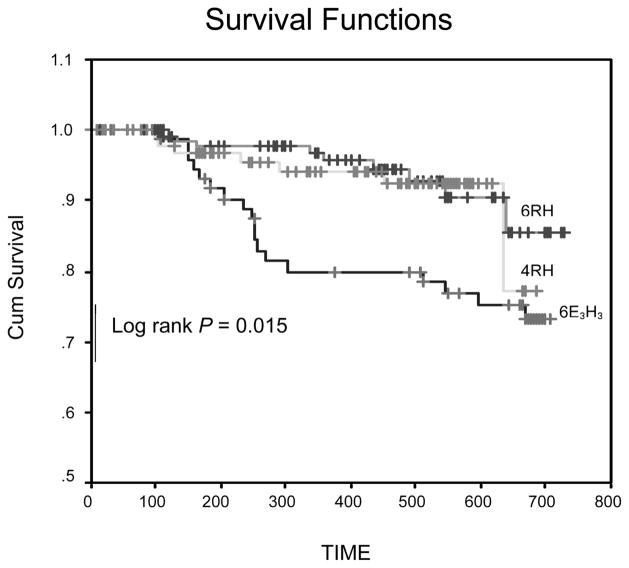
At the end of successful treatment, 90 patients were available for follow-up in the 6E3H3 cohort, 195 in the 6RH cohort, and 122 in the 4RH cohort. In the 6E3H3 cohort, 23 patients developed active TB during follow-up, yielding a relapse rate of 18.2 cases per 100 PYO (Table 3) compared to 14 patients in 6RH yielding a relapse rate of 4.8/100 PYO and 16 patients in the 4RH cohort for a relapse rate of 9.7/100 PYO. The time from cure to relapse was significantly shorter for patients in the 6E3H3 cohort compared to those in the 4HR cohort (log rank P = 0.015). However, there was no difference in time to relapse between the 4HR and 6HR cohorts (Figure 2). In a Cox proportional hazards regression analysis controlling for presence of residual lower lung infiltrates at the end of therapy, and using 4RH as the reference group, the relative risk of relapse for 6E3H3 was 2.2 (95%CI 0.98–5.06) and for 6RH was 0.62 (95%CI 0.28–1.39).
Figure 2.
Kaplan Meier curve showing time to relapse of patients in the three cohorts. Cohort 1 = 4RH; cohort 2 = 6RH; cohort 3 = 6E3H3. R = rifampicin; H = isoniazid; E = ethambutol; Cum = cumulative.
One patient relapsed with an acquired INH-resistant strain of M. tuberculosis 16 months after initial cure in the 6E3H3 arm and responded well clinically and microbiologically to retreatment with the WHO standard retreatment regimen. In five of the relapse cases, pre-treatment and relapse isolates were identical.
Safety of ethambutol regimen
The 6E3H3 regimen was well tolerated by most study patients. There were no serious drug reactions attributed to EMB. One patient developed a moderately severe pruritic skin rash attributed to RMP and five patients reported arthralgias attributed to PZA.
DISCUSSION
Although this is not a controlled clinical trial, we believe that the three cohorts are comparable in terms of the likelihood of relapse at baseline. Although there were differences in baseline characteristics, such as age, Karnofsky performance status, hemoglobin and WBC count, these characteristics were not associated with the development of relapse, suggesting that baseline imbalances do not introduce a bias in the analysis. We used a contemporary parallel cohort design to guard against cohort effects in the population. As all studies were performed in one center, referral patterns were likely similar for each cohort. Moreover, clinical evaluations and data collection were uniform across studies because a single clinic member of staff evaluated patients and collected data using standard data collection procedures. The major design limitation to this analysis is that treatment was not randomized to patients; we therefore cannot exclude a hidden bias that accounts for the observed findings.
In this study, all three regimens performed well, as clearance of M. tuberculosis from sputum at 2 months and treatment failure rates were similar for each regimen. Compared with the regimen containing RMP for 4 months in the continuation phase, the intermittent HE regimen had relapse rates that were two-fold higher. Also, when the RH continuation phase was extended to 6 months, the relapse rate tended to be lower than for standard short course therapy by 38%; however, because of overlapping 95%CIs for these regimens, we cannot definitely conclude that the longer continuation phase reduced relapse rates.
The rationale for using EMB in the continuation phase is that it offers an inexpensive, widely available alternative to RMP when combined with INH. Furthermore, when EMB is used in the continuation phase, it limits exposure to RMP and hence reduces the risk of developing RMP-resistant stains of M. tuberculosis. EMB has moderate early bactericidal activity21 but contributes little to sterilizing active TB lesions.22 It is, however, advantageous for longer treatment because resistance to EMB develops slowly and may prevent the emergence of resistance to other, more active anti-tuberculosis drugs.
The responses at 2 months suggest that the three cohorts of patients were similar clinically and that there was no excess of initial drug resistance in any cohort. There was also minimal variation in treatment failure rates, suggesting that EMB was an effective substitute for RMP.
After completion of treatment, however, differences emerged among the three cohorts. In the EMB cohort, the relapse rates for TB were two times greater than those observed in the cohorts receiving RMP-containing regimens. With a relapse rate of 18.2 cases/100 PYO, one would expect to retreat one relapse case for every six patients treated with EMB in the continuation phase. These data are consistent with a recently reported International Union Against Tuberculosis and Lung Disease comparative controlled trial, in which unfavorable outcomes in HIV-infected patients were greater in 8-month EMB-containing regimens (27%) than in a 6-month RMP-containing regimen (5%).23 In non-HIV-infected patients, unfavorable outcomes in the EMB regimens were 9%, and in the RMP regimen, 2%.
The limited information about DNA fingerprinting suggests that patients receiving 6E3H3 developed recurrent disease with the original isolate due to failure to sterilize tissues rather than exogenous re-infection.12,24
This finding should be interpreted with caution because recurrence rates are only one factor in deciding which regimen should be used by NTPs. While the EMB regimen may impose greater financial costs on an NTP through the retreatment of one in six HIV-infected TB cases, a program must balance these costs against the costs of delivering an RMP-based regimen with DOT and the risk of emergence of multidrug-resistant TB. NTPs facing logistical and fiscal constraints may opt for the higher costs of the EMB regimen until it can guarantee proper delivery of RMP-containing regimens using DOT.
In conclusion, this analysis shows that treatment of smear-positive PTB in HIV-infected adults with an intermittent EMB-containing regimen is safe but has a higher relapse rate than RMP-containing regimens. The choice of a standard regimen for NTPs must balance effectiveness, safety and costs; however, using RMP throughout treatment will result in better treatment outcomes.25
Acknowledgments
We would like to thank the staff of the Uganda National Tuberculosis and Leprosy Programme, the Uganda-Case Western Reserve University Research Collaboration TB Project Clinic and the Uganda Tuberculosis Investigations Bacteriological Unit-Wandegeya for their invaluable assistance in the conduct of the study. We greatly acknowledge the kind assistance of Drs M D Cave and K Eisenach of the University of Arkansas for Medical Sciences who performed the IS6110 DNA fingerprinting of the M. tuberculosis isolates. We are enormously indebted to the patients who participated in the study and their families.
Footnotes
Numbers before the letters indicate the duration in months of the phase of treatment; numbers in subscript indicate the number of times the drug is taken each week.
References
- 1.Narain JP, Raviglione MC, Kochi A. HIV associated tuberculosis in developing countries. Epidemiology and Strategies for prevention. Tubercle Lung Dis. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 3.World Health Organization. Tuberculosis advocacy. A practical guide 1998. WHO/TB 98.239. Geneva, Switzerland: WHO; 1998. [Google Scholar]
- 4.Okwera A, Luzze H, Nsubuga P, et al. Trends of HIV-1 infection among pulmonary tuberculosis in adults 1993–1997, Kampala, Uganda (Abstract 610/13273). Geneva, Switzerland. 12th International AIDs Conference; 1998. [Google Scholar]
- 5.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 6.Nunn P, Kibuga D, Gathua S, et al. Cutaneous hypersensitivity reactions due to thiacetazone in HIV-1 seropositive patients treated for tuberculosis. Lancet. 1991;337:627–630. doi: 10.1016/0140-6736(91)92447-a. [DOI] [PubMed] [Google Scholar]
- 7.Small PM, Schecter GF, Goodman PC, Sande MA, Chaisson RE, Hopewell PC. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Eng J Med. 1991;324:289–294. doi: 10.1056/NEJM199101313240503. [DOI] [PubMed] [Google Scholar]
- 8.Okwera A, Whalen C, Byekwaso F, et al. Randomised trial of thiacetazone and rifampicin- containing regimens for pulmonary tuberculosis in HIV-infected Ugandans. Lancet. 1994;344:1323–1328. doi: 10.1016/s0140-6736(94)90693-9. [DOI] [PubMed] [Google Scholar]
- 9.Ackah AN, Coulibaly D, Digbeu H, et al. Response to treatment, mortality and CD4 lymphocyte counts in HIV-infected persons with tuberculosis in Abidjan, Côte d’Ivoire. Lancet. 1995;345:607–610. doi: 10.1016/s0140-6736(95)90519-7. [DOI] [PubMed] [Google Scholar]
- 10.Perriens JH, Colebunders RL, Karahunga C, et al. Increased mortality and tuberculosis treatment failure rate among human immunodeficiency virus (HIV) seropositive compared with HIV seronegative patients with pulmonary tuberculosis treated with ‘standard’ chemotherapy in Kinshasha Zaire. Am Rev Respir Dis. 1991;144:750–755. doi: 10.1164/ajrccm/144.4.750. [DOI] [PubMed] [Google Scholar]
- 11.Chintu C, Luo C, Bhat G, Raviglione M, DuPont H, Zumla A. Cutaneous hypersensitivity reactions due to thiacetazone in the treatment of tuberculosis in Zambian children infected with HIV-1. Arch Dis Child. 1993;68:665–668. doi: 10.1136/adc.68.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawken M, Nunn P, Gathua S, et al. Increased recurrence of tuberculosis in HIV-1 infected patients in Kenya. Lancet. 1993;342:332–337. doi: 10.1016/0140-6736(93)91474-z. [DOI] [PubMed] [Google Scholar]
- 13.Maher D, Chaulet P, Spinaci S, et al. Treatment of tuberculosis: guidelines for national programmes. Geneva Switzerland: World Health Organization; 1997. [Google Scholar]
- 14.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. Aids. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guwatudde D, Nakaketo M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diagnostic standards of classification of tuberculosis. Chapter 6. New York, NY: National Tuberculosis and Respiration Disease Association; 1969. Classification of pulmonary tuberculosis. [Google Scholar]
- 17.International Union Against Tuberculosis and Lung Disease. Technical guide for sputum examination for tuberculosis by direct microscopy. Bull Int Union Tuberc. 1986;61 (Suppl 2):1–16. [Google Scholar]
- 18.Canetti G, Fox W, Khomenko A, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi S. Radiometric (BACTEC) tests for slowly growing mycobacteria. In: Isenberg HD, editor. Clinical microbiology procedures handbook. Washington DC: American Society for Microbiology; 1992. [Google Scholar]
- 20.van Embden JD, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikusova K, Slayden RA, Besra GS, Brennan PJ. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother. 1995;39:2484–2489. doi: 10.1128/aac.39.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botha FJ, Sirgel FA, Parkin DP, van de Wal BW, Donald PR, Mitchison DA. Early bactericidal activity of ethambutol. Pyrazinamide and the fixed combination of isoniazid, rifampicin and pyrazinamide (Rifater) in patients with pulmonary tuberculosis. S Afr Med J. 1996;86:155–158. [PubMed] [Google Scholar]
- 23.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364:1244–1251. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 25.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37:101–112. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]