Abstract
Amyloid-β peptide (Aβ) plays an essential pathophysiological role in Alzheimer disease (AD) and elevation of luteinizing hormone (LH) levels during aging has been implicated in its pathogenesis. To assess the effect of LH receptor deficiency on Aβ accumulation, we generated a bigenic mouse model APPsw+/Lhr−/− that expresses human amyloid precursor protein (APPsw) in the background of LH receptor (Lhr) knockout. Genetic ablation of Lhr resulted in a significant decrease in the number of Aβ plaques and protein content in the hippocampus and cerebral cortex in both male and female mice. Accordingly, several Aβ deposition-related neuropathologic features and functionally relevant molecules were markedly improved, including decreased astrogliosis, reductions of elevated phosphorylated tau, c-fos, α7-nicotinic acetylcholine receptor, and restoration of the altered neuropeptide Y receptors Y1 and Y2. Diminution of Aβ accumulation in the absence of LHR supports the contention that dysregulation of LH may impact the pathogenesis of AD. The APPsw+/Lhr−/− mouse may be a useful tool for advancing understanding of the role of LH-mediated events in AD and a model in which to test therapeutic interventions.
Keywords: Aging, Alzheimer disease, Amyloid plaque, APP transgenic mice, Luteinizing hormone, LH receptor knockout
INTRODUCTION
Alzheimer disease (AD) is an age-associated disorder marked by cognitive decline coupled with distinct neuropathological hallmarks that include extracellular senile plaques and intracellular neurofibrillary tangles (NFTs) (1, 2). Although the majority of cases of AD are sporadic, familial and sporadic AD cases have similar clinical manifestations and neuropathological features. Amyloid-β peptide (Aβ) is derived from enzymatically processed fragments of amyloid precursor protein (APP) by β- and γ-secretase complexes (3–5) and is the principal constituent of senile plaques. The overproduction, accumulation and aggregation of Aβ in plaque cores are characteristic of AD (6, 7).
Overwhelming evidence obtained from in vitro and in vivo studies using neuronal cell lines and APP transgenic animal models indicates that Aβ is a primary cytotoxic molecule in the brain and plays a central role in AD pathogenesis (8–10). A cascade initiated by Aβ is thought to result in aberrant phosphorylation of tau protein and its subsequent aggregation to form NFTs (11). Decline in learning and memory functions correlate with progressive Aβ deposition and NFT formation in the limbic and associated cortices during the course of AD (1, 2). Cognitive impairment also correlates with astrogliosis, neuritic dystrophy, synaptic deficits, and imbalance of neuronal network activity. Additionally, there are alterations of numerous functionally relevant molecules, notably the immediate early gene product c-fos, the neurotransmitter neuropeptide Y (NPY), and neurotransmitter receptors neuropeptide Y receptor Y1 (NPY1R), neuropeptide Y receptor Y2 (NPY2R) and α7-nicotinic acetylcholine receptor (α7-nAChR) (12–19).
Luteinizing hormone (LH) and its homolog, human chorionic gonadotropin (hCG), are heterodimeric glycoprotein hormones that bind to a common G-protein coupled receptor, often called LH/hCG receptor (LHR). Increased gonadal steroidogenesis is a hallmark endocrine function of LH and hCG. The synthesis and secretion of LH are subject to both positive and negative feedback regulation by gonadal steroids, estrogens and androgens. The major endocrine changes of the hypothalamic-pituitary-gonadal axis during aging are decreased sex steroid hormones due to aging of the ovary and testis. This results in chronic LH elevation (20).
Both hormonal alterations of the hypothalamic-pituitary-gonadal axis and the incidence of AD increase with age, suggesting that there may be a relationship between these processes. Estrogens have received considerable attention in the last decade for their effects on age-related neurodegenerative changes and cognitive decline (21, 22). Clinical trials, however, have not demonstrated that estrogens improve AD symptoms and whether there is a role for estrogens in the prevention of AD remains uncertain (23). There are several lines of circumstantial evidence suggesting that LH may be a factor relevant to the pathogenesis of AD. First, serum LH and hCG concentrations correlate with the incidence of AD. The increase in LH after menopause/andropause is higher and occurs earlier in women who are more susceptible to AD than men. There are 2-fold higher serum LH levels in individuals with AD than age-matched controls (24, 25). Second, several brain regions in various species, including humans, express functional LH/hCG receptors (26, 27). In particular, the hippocampus (HP) contains the highest density of LHR (26). Recent genetic analysis demonstrated that an Lhr variant reduced the risk of AD in male apolipoprotein E carriers (28). Third, LH and hCG can cross the blood-brain barrier from the peripheral circulation, and it is possible that they may be produced within the brain (27). Fourth, LH has been shown to alter APP processing towards the amyloidogenic pathway in vitro (29). Administration of hCG or pharmacological suppression of LH reduced Aβ accumulation in rat and mouse brains (30, 31). A recent study also demonstrated that serum LH levels were positively correlated with Aβ levels in elderly men (32).
Because Aβ is believed to be the key molecule that triggers many aspects of AD pathogenesis, we sought to establish an additional link between neuronal LH actions and AD. We investigated the effects of genetic ablation of Lhr can affect amyloidosis and the Aβ-linked biochemical and neurological alterations of AD in a mouse model. We provide in vivo evidence that genetic ablation of LH receptors from a human APP transgenic mouse results in a significant reduction of Aβ load in the brain and improvement of several Aβ relevant abnormalities, including increased phospho-tau protein (P-tau), astrogliosis and an imbalance of excitatory and inhibitory networks in the HP and cerebral cortex (CX).
MATERIALS AND METHODS
Animals
Bigenic animals were produced by crossbreeding Tg (HuAPP695.K670N-M671L) 2576 mice with LhrKO heterozygotes. Tg (HuAPP695.K670N-M671L) 2576 mouse is a well-characterized transgenic model that expresses human amyloid precursor protein (APP)-695 with the “Swedish” mutation and manifests age-dependent cognitive alterations and development of amyloid deposition, similar to human AD (33). In the present study, we adopted the name APPsw+, as described elsewhere for this mouse model (34). APPsw+ mice that were originally generated by Hsiao et al (33) were purchased from Taconic (Hudson, NY). LhrKO mice were previously established in our laboratory (35). The zygosities of these animals were determined by PCR of genomic DNA isolated from mouse-tails using the primer sets listed in Table 1. All animals were kept and maintained on a 12-hour light-dark cycle with food and water provided ad libitum as required under the NIH Guide for the Care and Use of Laboratory Animals. All animal studies were approved by the Animal Care and Use Committee at the University of Louisville.
Table 1.
Primer Sequences for Genotyping
| Gene | Primer sequence (5’ - 3’) |
|---|---|
| Lhr | F: TGACCTGTTCCTGGGGCTGCTG |
| R: AAATGGCCTCAACGGGTGTGCA | |
| Neo | F: CGGAAGCCCGGCATTCTGCA |
| R: ATTCAGGGCACCGGACAGGTCG | |
| APP | F: CTGACCACTCGACCAGGTTCTGGGT |
| R: GTGGATAACCCCTCCCCCAGCCTAGACCA | |
F, forward primer; R, reverse primer.
Animals of 3 different genotype were used in this study: 1) wild type mice containing no APPsw transgene with intact Lhr, designated as APPsw−/Lhr+/+; 2) transgenic mice expressing APPsw with intact Lhr, designated as APPsw+/Lhr+/+; and 3) bigenic animals expressing APPsw with Lhr knockout, designated as APPsw+/Lhr−/−. For each experiment, at 15 to 16 months of age, 3 to 6 male and female mice for each genotype were used. The animals were deeply anesthetized by i.p. injection of sodium phenobarbital (Henry Schein Co., Indianapolis, IN) and the brains were harvested. The hemibrains were fixed in 10% formalin (Fisher Scientific, Hanover Park, IL) for immunohistochemical staining. The other halves were dissected into HP and CX, and stored in RNAlater (Ambion, Inc., Austin, TX) at −80°C for RNA isolation or directly stored at –80°C for protein analysis.
Immunohistochemistry and Immunofluorescence
Six-µm-thick sagittal sections were cut from the paraffin-embedded blocks and were sequentially treated for 10 minutes with 88% formic acid (Fisher Scientific) for 1 hour with 2% blocking serum in phospate buffered saline. They were then incubated overnight at 4°C with 1:300 dilution of mouse anti-Aβ monoclonal antibody (Clone 4G8, which recognizes Aβx-42 including the P3 fragment [36] or 9C4, which recognizes Aβx-43, Millipore, Temecula, CA), 1:150 dilution of rabbit anti-NPY antibody (Immunostar, Hudson, WI), 1:150 dilution of rabbit anti-P-tau antibody (phosphothreonine 205, Millipore) and 1:300 dilution of rabbit anti-glial fibrillary acidic protein (GFAP) antibody (Sigma, St. Louis, MO), respectively. Immunostaining of Aβ was detected by an avidin-biotin immunoperoxidase complex method using a kit purchased from Vector Laboratories (Burlingame, CA).
Immunostaining of NPY was detected by incubation of 1:100 diluted fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG. The double immunofluorescent labeling was performed by incubation of the sections together with primary antibodies against Aβ and GFAP or Aβ and P-tau, and then together with both FITC-labeled goat anti-rabbit and texas red-labeled goat anti-mouse IgG (Jackson Immunoresearch, Inc., West Grove, PA). The slides were covered with Ultracruz mounting medium (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), which contains DAPI blue fluorescence for visualization of cell nuclei.
Quantification of Amyloid Plaque Load
The numbers of amyloid plaques in the entire areas of HP and CX were quantitatively analyzed using an Imaging Application Software, Microsuite Version 5 (Olympus Soft Imaging Solution Corp., Lakewood, CO). An average of 5 sections from each animal was used for counting. The results were expressed as number of amyloid plaques per µm2 of HP or CX. Based on the appearances of amyloid deposition, the plaques were classified as either ‘diffuse’ in which the immunostaining of the plaque does not have a clear border, or ‘cored,’, in which there was intense immunostaining and a relatively sharp border (37).
Semiquantitative RT-PCR
Total RNA of the HP and CX was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and 2 µg of isolated RNA was reverse-transcribed into cDNA using avian myeloblastosis virus reverse transcriptase (Promega Corp., Madison, WI) and random oligonucleotide hexamers (Invitrogen). The PCR primers listed in Table 2 were designed from the sequences obtained from Genbank using Vector NTI 9.0 program (InforMax, Frederick, MD) and synthesized by Operon Technologies (Alameda, CA). All primers were designed to amplify products that covered at least one or more exons. Then PCR reactions were performed with primer sets of a target gene and the ribosomal protein large subunit-19 (Rpl19), a housekeeping gene as an internal control for cDNA quantity and quality. Optimal conditions and PCR-cycle numbers for each target gene (Table 2) were predetermined to ensure that co-amplification was within the linear range. Each PCR-cycle consisted of denaturation for 45 seconds at 94°C, annealing for 1 minute at 57°C, and extension for 1 minute at 72°C. The amplified products were electrophoresed in 1.5% agarose gels, stained with ethidium bromide and analyzed using TotalLab Version 2.01 (Nonlinear USA, Durham, NC) image analysis software; ratios of target gene to housekeeping gene Rpl19 were then calculated. Representative pictures and the fold changes (mean ± SEM) calculated from 3 independent experiments are presented. All reactions were repeated 2 to 3 times for each genotype.
Table 2.
Primer Sequences for Semiquantitative RT-PCR
| Gene | Primer Sequence (5’-3’) | PCR Cycles |
|---|---|---|
| Neuropeptide Y | ||
| (Npy) | ||
| F: GCCCGCCACGATGCTAGGTAACAA | ||
| R: ATTGGTGGGACAGGCAGACTGG | 26 | |
| Neuropeptide Y receptor Y1 | ||
| (Npy1r) | ||
| F: AATGTGTCACTTGCGGCGTT | ||
| R: GGTGGCAATGATCTGGTGGT | ||
| 26 (HP) | ||
| 30 (CX) | ||
| Neuropeptide Y receptor Y2 | ||
| (Npy2r) | ||
| F: TCCTGCTTCTGATCTCACTT | ||
| R: TGGTGCTGTCTATGAGCTCC | ||
| 26 | ||
| Ribosomal protein large subunit19 | ||
| (Rpl19) | ||
| F: CTCAGGCTACAGAAGAGGCTT | ||
| R: GGACAGAGTCTTGATGATCTC | ||
| Co-amplified with the target gene | ||
F, Forward primer; R, Reverse primer; HP, hippocampus; CX, cerebral cortex.
Western Blotting
The HP and CX were homogenized for 30 seconds at 4°C in a lysis buffer (pH 7.4) composed of 50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 1 mM EDTA,1 mM Na3VO4, 1 mM NaF,150 µl/ml protease inhibitor mixture (Roche Applied Science, Indianapolis, IN). The protein concentrations in the homogenates were determined using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). The homogenates were separated into pellets and supernatants by centrifugation at 12,000 × g for 15 minutes at 4°C.
The pellets were extracted with 70% formic acid for determination of Aβ levels according to the procedure described by Kawarabayashi et al (38). Briefly, the formic acid fractions were evaporated using an N-EVAP nitrogen evaporator (Organomation Associates, Inc., Berlin, MA) and dissolved in dimethyl sulfoxide. The dissolved fractions were then separated on 16% Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and electrotransferred to nitrocellulose membranes (Bio-Rad). Membranes were then incubated overnight at 4°C with 1:1,000 dilution of 4G8 monoclonal antibody for detection of Aβ. For detections of P-tau, α7-nAChR, NPY1R, NPY2R and c-fos, an equal amount of proteins in the supernatants was loaded, separated on discontinuous 10% SDS-PAGE gels under reducing conditions and electrotransferred onto 0.4-µm polyvinylidene fluoride membranes (Millipore, Bedford, MA). The membranes were then incubated with antibodies against NPY1R (1:200 dilution, Santa Cruz Biotechnology, Inc), NPY2R (1:2,000 dilution, Abcam, Inc., Cambridge, MA), c-fos (1:1,000 dilution), α7-nAChR (1:1,500 dilution), and P-tau (phosphothreonine 205, 1:2,000 dilution, Millipore).
All the blots were probed by incubation of 1:2,000 dilution of horseradish peroxidase-labeled secondary antibody and visualized by an enhanced chemiluminescent detection system (Amersham Pharmacia Biotech, Inc., Piscataway, NJ). All membranes were re-blotted with either β-actin or β-tubulin antibody, which served as a loading control. The molecular sizes of the proteins were determined by running protein markers in an adjacent lane. Densitometric analysis of the specific bands using TotalLab Version 2.01 image analysis software normalized to β-actin or β-tubulin. Representative blots and the fold changes (mean ± SEM) calculated from 3 independent experiments are presented. All experiments were repeated 2 to 3 times for each genotype.
Statistical Analysis
All results were analyzed by one-way ANOVA and Student-Newman-Keuls using an Instat (Version 3.06 program (Graphpad Software, San Diego, CA). A p value <0.05 was considered significant.
RESULTS
Lhr KO Decreased the Aβ Burden in the Brain of APPsw+ Mice
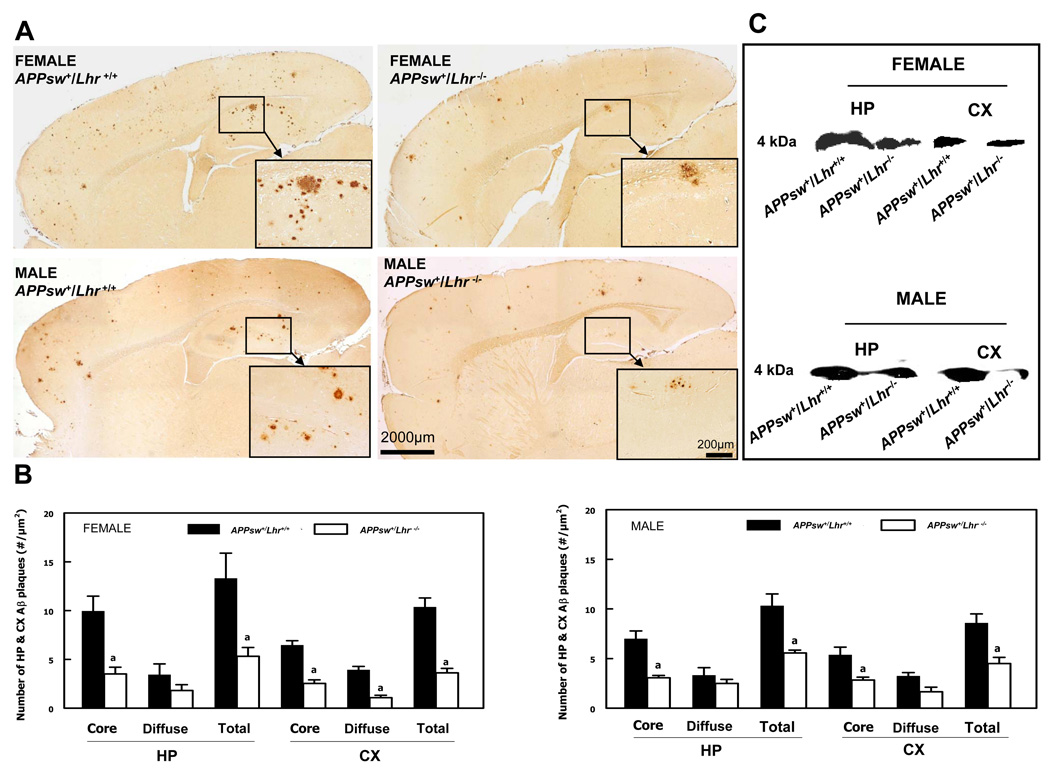
Immunohistochemistry demonstrated typical Aβ plaques in the HP and CX of APPsw+/Lhr+/+ and APPsw+/Lhr−/− mice of both genders. Both monoclonal antibodies, 4G8 and 9C4, gave the same pattern of immunostaining. Immunostaining of Aβ plaques was greater in female than in male mice and there were no plaques in either APPsw−/Lhr+/+ or APPsw−/Lhr−/− mice. The highest density of plaques was seen in the HP (Fig. 1A). LhrKO did not change the Aβ deposition pattern, but there were fewer Aβ plaques in the HP and CX of both female and male APPsw+/Lhr−/− mice compared to their APPsw+/Lhr+/+ littermates (Fig. 1A). The total plaque numbers in both regions were significantly reduced in APPsw+/Lhr−/− mice (Fig. 1B). When cored and diffuse plaques were counted separately, the majority of the plaques were cored and LhrKO led to a pronounced decrease in their numbers. The diffuse type plaques exhibited a decreased trend and they did not reach statistical significance except for those in the CX in female APPsw+/Lhr−/− mice (Fig. 1B). Western blot analysis indicated that HP and CX levels of 4 kDa Aβ protein were markedly decreased in both female and male APPsw+/Lhr−/− mice compared to APPsw+/Lhr+/+ littermates (Fig. 1C).
Figure 1.
Decreased amyloid deposition in APPsw+/Lhr−/− compared to APPsw+/Lhr+/+ mouse brains. (A) Immunohistochemical detection of amyloid plaques in the brains of female and male mice. The insets are the higher magnification of the hippocampal areas indicated by the small rectangles. Scale bars: 2,000 µm in all panels; 200 µm in all insets. (B) Quantitation of amyloid plaques in the hippocampus (HP) and cerebral cortex (CX) of female and male mice. The sum of cored and diffuse plaques is labeled as total. (a p < 0.05). (C) Western blotting analysis of Aβ protein levels in the HP and CX of female and male mice.
Lhr KO Alleviated Astrogliosis Surrounding Aβ Plaques in APPsw+ Mice
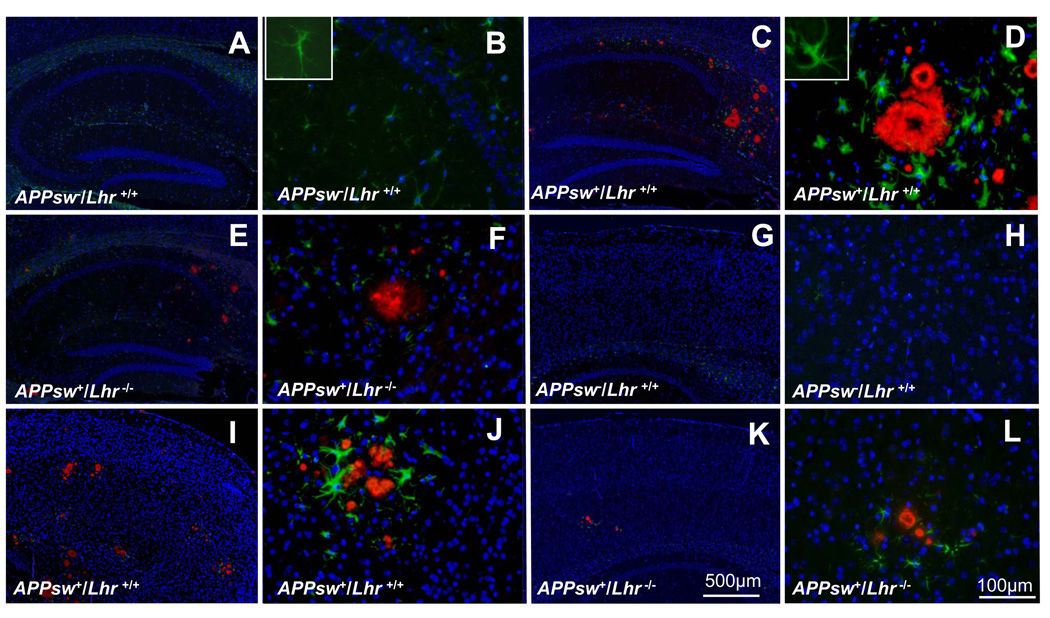
Double immunofluorescent staining for Aβ and GFAP, a marker of astrogliosis (39), demonstrated that both Aβ plaques and GFAP+ astrocytes were readily detectable in brain sections from APPsw+/Lhr+/+ (Fig. 2C, D, I, J). GFAP+ astrocytes, but not Aβ plaques, were detected in APPsw−/Lhr+/+ mice (Fig. 2A, B, G, H). Astrocytes surrounding cored amyloid plaques displayed thick processes and enlarged cell bodies. Astrogliosis of APPsw+/Lhr+/+ animals and of APPsw+/Lhr−/− animals was compared (Fig. 2C, D, I, J). The densities of Aβ plaques and astrogliosis in HP and CX were considerably reduced in APPsw+/Lhr−/− mice (Fig. 2E, F, K, L). Similar changes were observed in male and female mice (data not shown).
Figure 2.
Double-immunofluorescent labeling of Aβ to show amyloid plaques (red) and glial fibrillary acidic protein (GFAP) to detect astrocytes (green) in the hippocampus (A–F) and cerebral cortex (G–L) of female mice with the different indicated genotypes. DAPI staining (blue) labels cell nuclei. The inset in panel B is a normal astrocyte at higher magnification. The inset in panel D is a reactive astrocyte at higher magnification that displays thick processes and enlarged cell bodies. Scale bars: A, C, E, G, I, K = 500 µm; B, D, F, H, J, L = 100 µm.
Effects of Lhr Deficiency on Excitatory and Inhibitory Networks
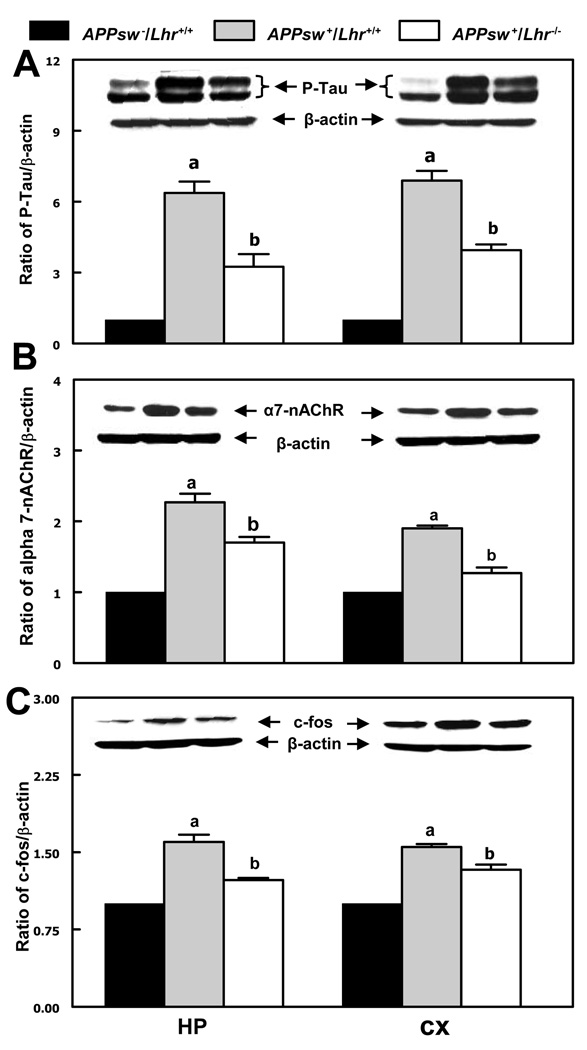
Western blots were performed to determine whether the change of Aβ accumulation in APPsw+/Lhr−/− mice affects the protein levels of phosphorylated-tau, α7-nAChR and c-fos in the HP and CX. Levels of P-tau (Fig. 3A), α7-nAChR (Fig. 3B) and c-fos (Fig. 3C) were remarkably elevated in both the HP and CX of APPsw+/Lhr+/+ mice compared to APPsw−/Lhr+/+ littermates. LhrKO resulted in a significant decrease although they did not completely return to the levels of APPsw−/Lhr+/+ mice. Male mice showed the same changes as female mice (not shown). In agreement with the Western blot results, double immunofluorescent staining of P-tau and Aβ also revealed a clear reduction of P-tau in APPsw+/Lhr−/− mice, especially in regions within and surrounding Aβ plaques (not shown).
Figure 3.
Western blotting analysis of protein levels of (A) phospho-tau protein (P-tau, 66 kDa and 55 kDa), (B) α7-nicotinic acetylcholine receptor (α7-nAChR, 56 kDa), and (C) c-fos (55 kDa) in the hippocampus (HP) and cerebral cortex (CX) of female mice of different genotypes. The relative protein levels were determined by the ratio of the protein of interest to β-actin. a & b p < 0.05; (a, vs. APPsw−/Lhr+/+; b, vs. APPsw+/Lhr+/+).
mRNA levels of Npy exhibited no marked alteration in the HP and CX among male and female mice of the different genotypes (Supplemental Fig. 1A). Consistent with the RT-PCR results, immunofluorescent analysis demonstrated that NPY protein was detected in the HP and CX. Abundant immunofluorescent staining was found particularly in the dentate gyrus and cornu ammonis 3 (CA3) of the HP but no significant differences were observed in the animals examined (Supplemental Fig. 1B).
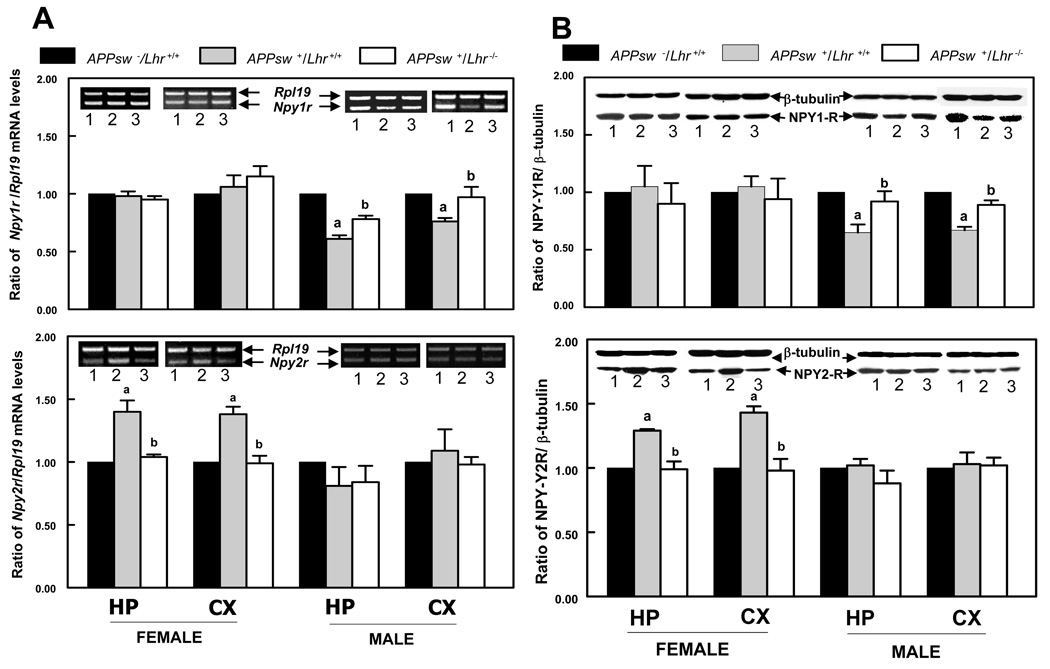
The mRNA levels of Npy1r in both the HP and CX of APPsw+/Lhr+/+ male mice were noticeably lower than in the APPsw−/Lhr+/+ counterparts (Fig. 4A), whereas female mice did not display significant changes among the genotypes. The decreased Npy1r mRNA levels in male mice were restored by genetic inactivation of Lhr (Fig. 4A). In contrast, the mRNA levels of Npy2r were markedly increased in APPsw+/Lhr+/+ female mice compared to APPsw−/Lhr+/+ littermates. This increase was completely reversed in APPsw+/Lhr−/− animals (Fig. 4A). Interestingly, this phenomenon was observed in the HP and CX of female but not male mice in any genotype (Fig. 4A). Western blot analysis also demonstrated that the NPY1R protein levels were reduced in the HP and CX of APPsw+/Lhr+/+ male mice. In Lhr-deficient male mice, the levels were comparable to those observed in APPsw−/Lhr+/+ male mice (Fig. 4B). The protein levels of NPY2R, on the other hand, were elevated in the HP and CX of APPsw+/Lhr+/+ female mice and returned to wild type levels in Lhr-deficient female mice (Fig. 4B).
Figure 4.
Neuropeptide Y receptor Y1 and neuropeptide Y receptor Y2 expression in mice of the indicated genotypes (1, APPsw−/Lhr+/+; 2, APPsw+/Lhr+/+; 3, APPsw+/Lhr−/−). (A) Semiquantitative RT-PCR for neuropeptide Y receptor Y1 (Npy1r) and neuropeptide Y receptor Y2 (Npy2r) in the hippocampus (HP) and cerebral cortex (CX). Relative mRNA levels are indicated as the ratio of target gene to Rpl19. a & b p < 0.05 (a, vs. APPsw−/Lhr+/+; b, vs. APPsw+/Lhr+/+). (B) Western blotting analysis of neuropeptide Y receptor Y1 (NPY1-R, 45 kDa) and neuropeptide Y receptor Y2 (NPY2-R, 40 kDa) in the HP and CX. Relative protein levels are indicated as the ratio of the protein of interest to β-tubulin. a & bp< 0.05; (a, vs. APPsw−/Lhr+/+; b, vs. APPsw+/Lhr+/+).
DISCUSSION
Transgenic mice that overexpress human APP display histopathological features that parallel those of AD (21, 40, 41). Since aging-associated LH dysregulation may be involved in AD pathogenesis, we sought to determine whether Aβ deposition was affected in LH-deficient human APP transgenic mice. We found that genetic inactivation of Lhr led to a reduction of Aβ plaques in the HP and CX with a much more pronounced reduction in female than in male mice. According to the Aβ-initiated hypothesis of AD pathogenesis, Aβ first induces aberrant hyperphosphorylation of tau protein and then formation of paired helical filaments that constitute NFTs (11, 42, 43). As in previous reports (33, 40), we did not observe typical NFTs in the brains of APPsw+ transgenic mice. We determined, however, that the phosphorylated tau content was markedly elevated over controls in APPsw+/Lhr+/+ mice and that a lack of LH was associated with a reduction of the increased phosphorylated tau level. Therefore, we postulate that abolishing LH signaling in the brain may alleviate not only the formation of Aβ plaques but also of NFTs and thus may improve cognitive impairment. Indeed, Casadesus et al demonstrated that although HP-associated cognitive test results of Lhr null mice was not distinguishable from that of wild type animals, transgenic mice with a chronic elevation of LH displayed poor cognitive performances (44). Moreover, a gonadotropin-releasing hormone agonist suppressed LH levels and markedly reduced Aβ burden in the HP with a concurrent improvement in HP-associated cognitive performance in APP transgenic mice (30).
The mechanism by which Lhr KO causes a dramatic reduction of Aβ accumulation in the brains of APPsw+ mice is not clear. Aβ is a product of the proteolytic processing of APP by β-secretase (BACE1) and γ-secretase (5, 45). γ-Secretase activity is mediated by a multi-protein complex consisting of presenilins (PS1 and PS2), anterior pharynx defective (APH) 1A homolog, APH1B, presenilin enhancer 2 homolog (Pen2) and nicastrin (3, 4). In addition, Ran-binding protein 9 and lipoprotein receptor-related protein (LRP) interact with APP and BACE1 to enhance Aβ generation (46). Degradation and clearance of Aβ in the brain are believed to depend primarily on insulin-degrading enzyme (IDE) and neprilysin (NEP) (47, 48). We found that the mRNA levels of BACE1, PS1, PS2, Pen2, LRP, IDE and NEP in the HP and CX did not differ significantly among APPsw−/Lhr+/+, APPsw+/Lhr+/+ and APPsw+/Lhr−/− mice (unpublished observations). Therefore, the mechanisms by which LH modulates Aβ production and catabolism in the brain require further investigation.
Astrogliosis is a prominent neuropathologic feature of AD, but it is not clear whether astrogliosis is induced by Aβ or if it precedes the appearance of Aβ plaque formation (12, 16, 41, 49–51). We found that reactive astrocytes were exclusively confined to the regions surrounding Aβ plaques. No reactive astrocytes were detected in areas without Aβ deposition or in the brains of wild type animals at the same age. The density of reactive astrocytes surrounding cored Aβ plaques in APPsw+/Lhr−/− mice decreased along with the reduction of Aβ plaques, suggesting that astrogliosis is a response to Aβ accumulation. Indeed, DeWitt et al reported that incubation of glial cells with Aβ triggered reactive astrogliosis in vitro (12). On the other hand, astrogliosis inhibits microglial phagocytosis thereby inhibiting Aβ removal (12, 52, 53). Our results raise the possibility that the decrease in cored plaques could be secondary to decreased astrogliosis, i.e. the reduced inhibition of the phagocytotic activity of microglia would result in increased removal of the plaques.
Neurons and astrocytes in the HP and CX contain c-fos and α7-nAChR (16, 54, 55) and there is in vitro and in vivo evidence that Aβ stimulates the expression of c-fos and α7-nAChR (13–15). Overexpression of c-fos may be a mechanism by which Aβ triggers programmed cell death in the brain (56). α7-nAChR has an extremely high affinity to Aβ; that is, cells that overexpress this receptor exhibited rapid binding, internalization and accumulation of exogenous Aβ (55). Therefore, accumulation of Aβ in the brain might be escalated by inducing overexpression of α7-nAChR, which eventually would exacerbate aberrant tau phosphorylation, brain cell death and memory impairment. Because amelioration of Aβ burden in APPsw+/Lhr−/− mice concomitantly lowered the elevated c-fos and α7-nAChR proteins, it is conceivable that blockade of LH actions in the brain might improve the aspects of brain pathophysiology in AD that are mediated through these molecules.
Disruption of the balance between excitatory and inhibitory neuronal activities is also characteristic of AD (17). NPY1R exerts a weak excitatory activity and NPY2R mediates a potent inhibitory activity of NPY (57). The NPY network is involved in learning and memory functions (58, 59), and imbalanced NPY network activity has also been implicated with an increased incidence of unprovoked seizures in AD (60, 61). We found that Npy expression was not significantly different among any of the 3 mouse genotypes studied, whereas NPY receptors exhibited gender-specific alterations. In females, NPY2R levels were elevated with no change in NPY1R. By contrast, NPY1R levels were reduced with no change in NPY2R in males. Nevertheless, both changes were attenuated in Lhr KO animals. Although it is not yet clear how Aβ perturbs NY1R and NPY2R differently in females and males, our results indicate that genetic removal of Lhr restored the altered pathophysiology. The differences between our study and previous reports showing Aβ-mediated diminishing of NPY and NPY1R and elevation of NPY2R might be due to the use of different APP transgenic animal models and/or different age and gender of the mice (18, 62).
In summary, the APPsw+/Lhr−/− animal model provides evidence that reduction of Aβ accumulation in the absence of neuronal LH action mitigates astrogliosis, corrects aberrant phospho-tau, c-fos and α7-nAChR protein levels, and appears to restore balance to the NPY network. These findings suggest that chronic elevation of LH levels may promote Aβ plaque formation and thus hasten AD progression. This bigenic APPsw+/Lhr−/− mouse model provides a useful tool to further our understanding on the role of LH-mediated events in AD pathogenesis as well as a potential means to test therapeutic interventions in AD.
ACKNOWLEDGMENT
We thank Leta Weedman for editing the manuscript.
This work is supported by research grants from the Kentucky Science and Engineering Foundation (KSEF-850-RDE-008) and the NIH (R01-HD057501).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Thal DR, Rub U, Orantes M, et al. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 3.Marlow L, Canet RM, Haugabook SJ, et al. APH1, PEN2, and Nicastrin increase Aβ levels and gamma-secretase activity. Biochem Biophys Res Commun. 2003;305:502–509. doi: 10.1016/s0006-291x(03)00797-6. [DOI] [PubMed] [Google Scholar]
- 4.Serneels L, Van Biervliet J, Craessaerts K, et al. Gamma-Secretase heterogeneity in the Aph1 subunit: Relevance for Alzheimer's disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling Y, Morgan K, Kalsheker N. Amyloid precursor protein (APP) and the biology of proteolytic processing: Relevance to Alzheimer's disease. Int J Biochem Cell Biol. 2003;35:1505–1535. doi: 10.1016/s1357-2725(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Deciphering the genesis and fate of amyloid β-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 8.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: A genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Samura E, Shoji M, Kawarabayashi T, et al. Enhanced accumulation of tau in doubly transgenic mice expressing mutant βAPP and presenilin-1. Brain Res. 2006;1094:192–199. doi: 10.1016/j.brainres.2005.12.134. [DOI] [PubMed] [Google Scholar]
- 12.DeWitt DA, Perry G, Cohen M, et al. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Exp Neurol. 1998;149:329–340. doi: 10.1006/exnr.1997.6738. [DOI] [PubMed] [Google Scholar]
- 13.Santiard-Baron D, Gosset P, Nicole A, et al. Identification of β-amyloid-responsive genes by RNA differential display: Early induction of a DNA damage-inducible gene, gadd45. Exp Neurol. 1999;158:206–213. doi: 10.1006/exnr.1999.7076. [DOI] [PubMed] [Google Scholar]
- 14.Kihiko ME, Tucker HM, Rydel RE, et al. c-Jun contributes to amyloid β-induced neuronal apoptosis but is not necessary for amyloid β-induced c-jun induction. J Neurochem. 1999;73:2609–2612. doi: 10.1046/j.1471-4159.1999.0732609.x. [DOI] [PubMed] [Google Scholar]
- 15.Dineley KT, Westerman M, Bui D, et al. β-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. J Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagele RG, Wegiel J, Venkataraman V, et al. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcock DM, Lewis MR, Van Nostrand WE, et al. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez JJ, Olabarria M, Chvatal A, et al. Astroglia in dementia and Alzheimer's disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- 20.Wise PM, Smith MJ, Dubal DB, et al. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20:243–248. doi: 10.1210/edrv.20.3.0364. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez De Aguilar JL, Rene F, Dupuis L, et al. Neuroendocrinology of neurodegenerative diseases. Insights from transgenic mouse models. Neuroendocrinology. 2003;78:244–252. doi: 10.1159/000074445. [DOI] [PubMed] [Google Scholar]
- 22.Norbury R, Cutter WJ, Compton J, et al. The neuroprotective effects of estrogen on the aging brain. Exp Gerontol. 2003;38:109–117. doi: 10.1016/s0531-5565(02)00166-3. [DOI] [PubMed] [Google Scholar]
- 23.Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. 2008;51:618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- 25.Short RA, Bowen RL, O'Brien PC, et al. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- 26.Lei ZM, Rao CV, Kornyei JL, et al. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- 27.Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Semin Reprod Med. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 28.Haasl RJ, Ahmadi MR, Meethal SV, et al. A luteinizing hormone receptor intronic variant is significantly associated with decreased risk of Alzheimer's disease in males carrying an apolipoprotein E epsilon4 allele. BMC Med Genet. 2008;9:37. doi: 10.1186/1471-2350-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen RL, Verdile G, Liu T, et al. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-β precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- 30.Casadesus G, Webber KM, Atwood CS, et al. Luteinizing hormone modulates cognition and amyloid-β deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Berry A, Tomidokoro Y, Ghiso J, et al. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-β levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdile G, Yeap BB, Clarnette RM, et al. Luteinizing hormone levels are positively correlated with plasma amyloid-β protein levels in elderly men. J Alzheimers Dis. 2008;14:201–208. doi: 10.3233/jad-2008-14208. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 34.Irizarry MC, McNamara M, Fedorchak K, et al. APPSw transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Lei ZM, Mishra S, Zou W, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 36.Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Sani S, Traul D, Klink A, et al. Distribution, progression and chemical composition of cortical amyloid-β deposits in aged rhesus monkeys: Similarities to the human. Acta Neuropathol. 2003;105:145–156. doi: 10.1007/s00401-002-0626-5. [DOI] [PubMed] [Google Scholar]
- 38.Kawarabayashi T, Younkin LH, Saido TC, et al. Age-dependent changes in brain, CSF, and plasma amyloid (β) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson JE, Ince PG, Lace G, et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Richardson JA, Burns DK. Mouse models of Alzheimer's disease: A quest for plaques and tangles. ILAR J. 2002;43:89–99. doi: 10.1093/ilar.43.2.89. [DOI] [PubMed] [Google Scholar]
- 41.Gotz J, Streffer JR, David D, et al. Transgenic animal models of Alzheimer's disease and related disorders: Histopathology, behavior and therapy. Mol Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 42.Tomidokoro Y, Harigaya Y, Matsubara E, et al. Brain Aβ amyloidosis in APPsw mice induces accumulation of presenilin-1 and tau. J Pathol. 2001;194:500–506. doi: 10.1002/path.897. [DOI] [PubMed] [Google Scholar]
- 43.Tomidokoro Y, Ishiguro K, Harigaya Y, et al. Aβ amyloidosis induces the initial stage of tau accumulation in APP(Sw) mice. Neurosci Lett. 2001;299:169–172. doi: 10.1016/s0304-3940(00)01767-5. [DOI] [PubMed] [Google Scholar]
- 44.Casadesus G, Milliken EL, Webber KM, et al. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Walsh DM, Klyubin I, Fadeeva JV, et al. Amyloid-β oligomers: Their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 46.Lakshmana MK, Yoon IS, Chen E, et al. Novel role of RanBP9 in BACE1 processing of amyloid precursor protein and amyloid-β peptide generation. J Biol Chem. 2009;284:11863–11872. doi: 10.1074/jbc.M807345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 48.Iwata N, Tsubuki S, Takaki Y, et al. Identification of the major Aβ 1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 49.Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci. 2003;23:5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassel JC, Mathis C, Majchrzak M, et al. Coexisting cholinergic and parahippocampal degeneration: A key to memory loss in dementia and a challenge for transgenic models? Neurodegen Dis. 2008;5:304–317. doi: 10.1159/000135615. [DOI] [PubMed] [Google Scholar]
- 51.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 52.Frackowiak J, Wisniewski HM, Wegiel J, et al. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce β-amyloid fibrils. Acta Neuropathol. 1992;84:225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- 53.el Hachimi KH, Foncin JF. Do microglial cells phagocyte the β/A4-amyloid senile plaque core of Alzheimer disease? C R Acad Sci III. 1994;317:445–451. [PubMed] [Google Scholar]
- 54.Anderson AJ, Cummings BJ, Cotman CW. Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer's disease: association with pathology. Exp Neurol. 1994;125:286–295. doi: 10.1006/exnr.1994.1031. [DOI] [PubMed] [Google Scholar]
- 55.Nagele RG, D'Andrea MR, Anderson WJ, et al. Intracellular accumulation of β-amyloid (1–42) in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer's disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 56.Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 57.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 58.Dumont Y, Fournier A, St-Pierre S, et al. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Redrobe JP, Dumont Y, St-Pierre JA, et al. Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res. 1999;848:153–166. doi: 10.1016/s0006-8993(99)02119-8. [DOI] [PubMed] [Google Scholar]
- 60.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 61.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng IH, Scearce-Levie K, Legleiter J, et al. Accelerating amyloid-β fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]