Abstract
To determine immunologic and epidemiologic correlates of acute Mycobacterium tuberculosis infection in household contacts of infectious tuberculosis cases, we performed a prospective, community-based cohort study of index cases and their household contacts in Kampala, Uganda. Contacts were evaluated for tuberculin skin test (TST) conversion over two years. Interferon-γ expression was measured using a whole blood assay after stimulating with M. tuberculosis culture-filtrate. In 222 contacts with a TST less than 5 mm at baseline, the one-year rate of TST conversion was 27%. The TST conversion was associated with the infectiousness of the index case and proximity of contact. Interferon-γ levels at baseline were greater among TST converters compared with those who did not convert. The risk of TST conversion increased four-fold as the baseline interferon-γ increased 10-fold, but only in contacts with BCG vaccination. In household contacts of tuberculosis, interferon-γ responses to non-specific mycobacterial antigens may be used to make an early diagnosis of tuberculosis infection, especially in resource-limited settings where bacille Calmette-Guérin vaccination is commonly used.
INTRODUCTION
Tuberculosis is an ancient disease. It has plagued humans throughout history and still poses a threat to personal and public health today. It is estimated that more than eight million new cases of tuberculosis occur each year and approximately two million people die of the disease.1 The force of mortality from tuberculosis hits hardest in developing countries where other conditions, such as infection with human immunodeficiency virus (HIV), malaria, and poverty, to name only a few, tax a limited infrastructure for public health and its capacity to find and treat cases of tuberculosis.
Our current tuberculosis control strategies have yielded only limited success. Although directly observed therapy is effective in controlling tuberculosis in some settings2,3, its global coverage is low and effectiveness is not universal4 because of poor adherence, logistical challenges, and cost. Moreover, the strategy of directly observed therapy does not eliminate transmission before the diagnosis is made, even when implemented under optimal conditions.5 Bacille Calmette-Guérin (BCG) vaccine is effective in preventing disseminated forms of disease, especially in young children,6 but has variable effectiveness in preventing pulmonary disease in adults,7 the most infectious form of disease. Population-based treatment of latent tuberculosis infection has not yet been shown to be an effective approach to the control of tuberculosis in disease-endemic countries. Each of these control strategies would be enhanced through more efficient and timely diagnosis of Mycobacterium tuberculosis infection and active disease. One way to enhance the diagnosis of M. tuberculosis infection and disease is to understand better the immune correlates of early infection.
The purpose of this study was to identify host immune responses that correlate with acute M. tuberculosis infection, as measured by tuberculin skin test (TST) conversion, in household contacts of tuberculosis cases. We chose to study acute infection because we could observe changes in host immunity before and after TST conversion, a marker for new infection in household contacts. We infer that these early changes reflect protective immune responses because most newly infected contacts of tuberculosis contain the infection and do not develop disease.8,9 Previous studies of household contacts describe risk factors for tuberculosis infection among contacts in cross-sectional designs but do not evaluate TST conversion.10-18 Most studies of TST conversion are surveys in populations19,20 or in high risk groups.21-23 In neither design are both the exposure explicitly identified and conversion observed after exposure.
In a prospective cohort study of infectious tuberculosis cases and their contacts, we systematically evaluated contacts with initial TST less than 5 mm to identify immunologic factors associated with TST conversion and thus with acute M. tuberculosis infection.
METHODS
Study population
Between October 1995 and February 1999, 302 tuberculosis cases and their household contacts were enrolled in a prospective cohort study and followed-up until the end of 2000.24 The study recruited sputum smear-positive pulmonary tuberculosis cases who were ≥ 18 years of age and had one or more household contacts living with them. Cases were identified at the Uganda National Tuberculosis and Leprosy Program treatment center at Old Mulago Hospital in Kampala, Uganda. During the initial home visit, made within two weeks of diagnosis in the index cases, home health visitors informed household contacts about the study and provided health education about tuberculosis. Active tuberculosis was treated with standard short-course therapy25 and latent tuberculosis infection was treated with isoniazid for six months in children ≤ 5 years of age, HIV-infected contacts, and TST converters.26 The study protocol was reviewed and approved by the AIDS Scientific Committee of Makerere University, The Uganda National Council on Science and Technology, and the institutional review board at University Hospitals of Cleveland. Written informed consent was obtained from the head of the household, all adults, and parents or guardians of children in the household less than 18 years of age.
An index case was defined as the first tuberculosis case identified in the household. A household contact was defined as an individual who had resided in the household for at least seven consecutive days during the three months prior to the diagnosis of tuberculosis in the index case.
Measurements
Household contacts were evaluated at baseline with standardized questionnaires about tuberculosis risk, a limited physical examination, ascertainment of a BCG scar, chest radiograph, HIV serology, and TST. The household environment was assessed for dwelling type, number of people in the household, number of habitable rooms, and number of windows in the house.
Based on this initial evaluation, household contacts without active tuberculosis were classified into two groups. Contacts with a TST reaction ≥ 5 mm were considered infected; contacts with a TST reaction < 5 mm were considered uninfected. Contacts with an initial TST reaction < 5 mm were retested at 3, 12, and 24 months. Conversion of the TST result was defined as 1) a baseline TST induration < 5 mm, 2) a TST reaction ≥ 5 mm upon subsequent testing, and 3) an increment in the size of the TST reaction ≥ 6 mm.27
The tuberculin skin test was done using the Mantoux method (0.1 mL of five tuberculin units of purified protein derivative, Tubersol; Cannaught Laboratories Limited, Willowdale, Ontario, Canada). The TST was administered on the volar aspect of the left forearm and read after 48–72 hours as the diameter (mm) of palpable induration. The same team of home health visitor did skin testing and reading; the intra-reader and inter-reader reliability was high (Pearson’s correlation coefficients = 0.99 and 0.97, respectively, P < 0.001). The BCG vaccination was ascertained through physical examination and review of medical records, if available. A contact was considered vaccinated if either a BCG scar was present or the medical records documented the vaccination. Enzyme-linked immunosorbent assay (ELISA) testing for HIV (Cambridge Bioscience, Worcester, MA) was performed with pre-test and post-test counseling on all consenting contacts. For children ≤ 5 years of age, HIV testing was done when the mother was seropositive or when the child was diagnosed with active tuberculosis; otherwise, a child was considered HIV seronegative if the mother tested negative. Except where shown, indeterminant HIV test results are classified as HIV seronegative.
Cytokine responses were measured at baseline on 181 of the contacts with a TST reaction < 5 mm using a whole-blood cytokine assay,28 on 34 contacts at the time of TST conversion, and 9 contacts without conversion on a second occasion. Blood samples were drawn before TST at baseline and approximately one week after TST conversion was identified. In this assay, blood cells were stimulated with M. tuberculosis culture filtrate (10 μg/mL) and incubated at 37°C. Culture supernatants were harvested at 18 hours for tumor necrosis factor-α and at 120 hours for interferon-γ and transforming growth factor-β. Supernatant cytokine concentrations (pg/ mL) were measured using commercial ELISAs for interferon-γ (Endogen, Rockford, IL) and for tumor necrosis factor-α and transforming growth factor-α (R&D Systems Inc., Minneapolis, MN).
Analytic strategy
The main objectives of the analysis were to estimate the rate of TST conversion and determine immunologic factors associated with conversion. The rate of TST conversion was estimated using the Kaplan-Meier method as the proportion (95% confidence intervals [CIs]) of contacts with a baseline TST reaction < 5 mm who met the definition of conversion. To identify factors associated with conversion, we performed a bivariate analysis between TST conversion with a set of epidemiologic, host characteristics of the household contact, and environmental factors. The variables that were associated with TST conversion in a univariate analysis (P < 0.15) were selected for a multivariable logistic regression analysis. A series of logistic regression models were fit using the generalized estimation equation method (GENMOD version 8.2; SAS Institute, Cary, NC) to adjust for within household correlations.29 Odds ratios (ORs) with 95% CIs derived from the coefficients of these models were used to estimate relative risk of conversion. A global test for interaction was performed and found to be significant at the 10% level; an interaction between BCG vaccination status and baseline interferon-γ levels accounted for this interaction. Separate models are presented for contacts with and without BCG vaccination.
RESULTS
Household description
Of the 302 index case households, at least one TST converter was identified in 46 households (15%). Most households were located in an urban (33, 72%) as opposed to a peri-urban or rural setting (13, 28%). The median number of household members in these homes was 7 (interquartile range = 2–15) and larger than the median number in homes without converters (5, range = 2–18). The median number of contacts per habitable room for households with cpnversions was 3 (inter-quartile range = 1–9). Most households were single-story, contiguous, small dwellings (25, 54%).
Of 1,206 household contacts, 965 contacts (80%) had a TST reaction ≥ 5 mm at the baseline evaluation and 241 contacts (20%) had a TST reaction < 5 mm (Figure 1). Of these 241 contacts, 222 (92%) had one or more TSTs done during follow-up to assess TST conversion. Of these 222 contacts, the mean duration of observation was 19.1 months; 95% were evaluated at 3 months, 82% at 12 months, and 62% at 24 months; follow-up of contacts with TST reactions < 5 mm was similar whether the contact converted or not. The initial TST reaction was 0 mm for 172 contacts and 0–4 mm for the remaining 50 contacts. The 19 contacts not included in the analysis were evaluated only at baseline; they were similar to contacts included in the analysis with regards to age, sex, and HIV serostatus but had a greater proportion without BCG vaccination.
Figure 1.
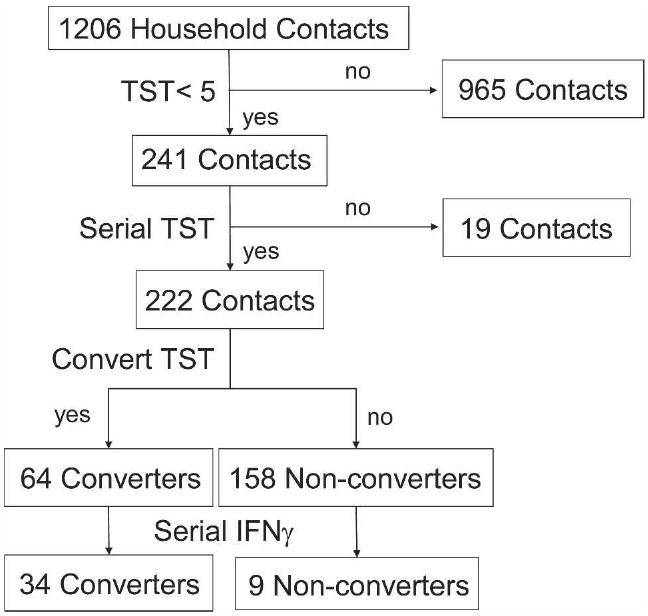
Study profile for the household contact study in Kampala, Uganda, 1995–1999. IFNγ = interferon-γ.
The mean ± SD age was 9.7 ± 10.6 years. Most contacts with TST reactions < 5 mm were younger than 16 years of age (81%, Table 1). BCG vaccination had been given to 175 (79%) subjects before contact evaluation. The HIV sero-prevalence was 9% and was similar to the prevalence in all household contacts and in the population at that time. Most contacts were first-degree relatives of the index case or spouses. Crowding was common and most contacts shared either a bed or bedroom with the index case. Most index cases had an acid-fast bacilli (AFB) smear of grade 3.
Table 1.
Baseline characteristics of 222 household contacts with tuberculin skin test (TST) reactions less than 5 mm*
| Characteristic | No. (%) |
|---|---|
| Age category (years) | |
| 0–5 | 97 (44) |
| 6–15 | 82 (37) |
| > 15 | 43 (19) |
| Sex | |
| Male | 113 (51) |
| Female | 109 (49) |
| BCG vaccination | |
| Yes | 175 (79) |
| No | 47 (21) |
| HIV result | |
| Positive | 20 (9) |
| Negative | 202 (91) |
| Spouse to index case | |
| Yes | 19 (9) |
| No | 203 (91) |
| Index case first-degree relative | |
| Yes | 137 (62) |
| No | 85 (38) |
| Share bed with index case | |
| Yes | 30 (14) |
| No | 192 (86) |
| Sleep same room as index case | |
| Yes | 130 (59) |
| No | 92 (41) |
| Care for index case | |
| Yes | 29 (13) |
| No | 193 (87) |
| Crowding (people/room) | |
| < 3 | 67 (30) |
| ≥ 3 | 155 (70) |
| Body mass wasting† | |
| Yes | 22 (10) |
| No | 199 (90) |
| Body mass index† (kg/m2) | |
| < 16.5 | 112 (51) |
| ≥ 16.5 | 109 (49) |
| Cavitary disease in index case | |
| Yes | 82 (37) |
| No | 140 (63) |
| AFB smear grade in index case | |
| 3+ | 149 (67) |
| 2+ | 44 (20) |
| 1+ | 29 (13) |
| TST converter | |
| Yes | 64 (29) |
| No | 158 (71) |
BCG = bacille Calmette-Guérin; HIV = human immunodeficiency virus; AFB = acid-fast bacilli.
Weight not available for one contact.
Among the 222 contacts, 64 contacts (29%, 95% CI = 26, 32%) converted the TST during follow-up. Of these converters, 34 contacts (53%, 95% CI = 42, 66%) had converted within three months, 57 (89%, 95% CI = 80, 95%) within 6 months, and 59 (92%, 95% CI = 86, 97%) within 12 months. The initial TST reaction was 0 mm for 54 converters (84%, Figure 2). The mean ± SD change in TST induration among converters was 13.3 ± 4.42 mm with 52 contacts (81%) experiencing a change ≥ 10 mm regardless of HIV status (Figure 2). Among the 158 contacts who did not convert, the TST did not change from baseline or showed only minimal fluctuations (< 3 mm) around the initial TST measurement.
Figure 2.
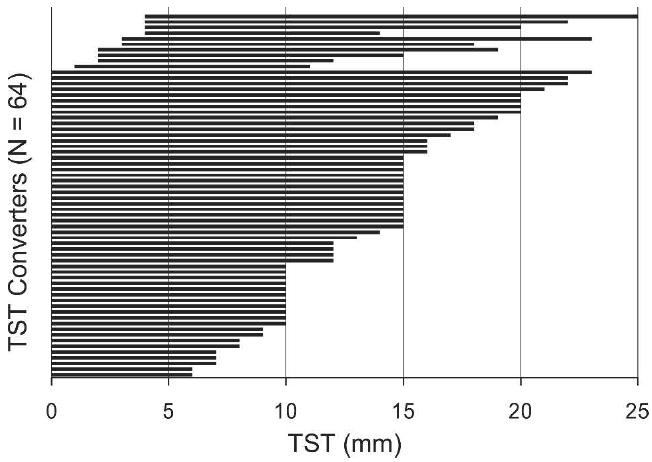
Change in tuberculin skin test (TST) in 64 household contacts in Kampala, Uganda, 1995–1999. Each horizontal bar represents a household contact who met the definition of converter. The left end of the bar marks the initial TST and the right end of the bar marks the TST at the time of conversion. The length of the bar represents the size of the TST conversion.
In a univariate analysis, BCG vaccination reduced the risk for TST conversion by 43% (Table 2). The odds of conversion were greater among spouses to index cases compared with other contacts, contacts who shared a bedroom with an index case, and contacts exposed to index cases with a smear grade of 3. Age, sex, relation to index case, HIV serostatus, and behavioral and environmental factors were not associated with TST conversion in this study population.
Table 2.
Baseline characteristics, proportion of tuberculin skin test (TST) conversion, and odds ratio for TST conversion*
| Characteristic | N | Converter no. (%) | Odds ratio | 95% confidence interval |
|---|---|---|---|---|
| Age category, years | ||||
| 0–5 | 97 | 29 (30) | 0.80 | 0.44, 1.46 |
| 6–15 | 82 | 21 (26) | 0.63 | 0.45, 1.28 |
| > 15 | 43 | 14 (33) | 1.00 | |
| BCG vaccination | ||||
| Yes | 175 | 45 (26) | 0.57 | 0.34, 0.98 |
| No | 47 | 19 (40) | 1.00 | |
| Sex | ||||
| Male | 113 | 36 (32) | 1.35 | 0.83, 2.19 |
| Female | 109 | 28 (26) | 1.00 | |
| HIV infection | ||||
| Yes | 20 | 7 (35) | 1.52 | 0.70, 3.33 |
| No | 202 | 51 (28) | 1.00 | |
| Spouse to index case | ||||
| Yes | 19 | 9 (47) | 2.42 | 0.99, 5.95 |
| No | 203 | 55 (27) | 1.00 | |
| First-degree relative | ||||
| Yes | 137 | 41 (30) | 1.07 | 0.65, 1.77 |
| No | 85 | 23 (27) | 1.00 | |
| Share bed with index case | ||||
| Yes | 30 | 10 (33) | 1.08 | 0.45, 2.57 |
| No | 192 | 54 (28) | 1.00 | |
| Share bedroom with index case | ||||
| Yes | 130 | 45 (35) | 2.03 | 0.99, 4.16 |
| No | 92 | 19 (21) | 1.00 | |
| Cares for index case | ||||
| Yes | 29 | 8 (28) | 0.90 | 0.37, 2.17 |
| No | 193 | 56 (29) | 1.00 | |
| Body mass index (kg/m2) | ||||
| < 16.5 | 112 | 32 (29) | 1.02 | 0.63, 1.63 |
| ≥ 16.5 | 109 | 32 (29) | 1.00 | |
| Cavitary disease in index case | ||||
| Yes | 82 | 26 (32) | 1.36 | 0.69, 2.70 |
| No | 140 | 38 (27) | 1.00 | |
| AFB smear grade | ||||
| 3+ | 149 | 51 (34) | 5.73 | 1.88, 17.43 |
| 2+ | 44 | 10 (23) | 2.92 | 0.77, 11.05 |
| 1+ | 29 | 3 (10) | 1.00 | |
| Crowding (people/room) | ||||
| < 3 | 67 | 16 (24) | 0.78 | 0.37, 1.65 |
| ≥ 3 | 155 | 48 (31) | 1.00 | |
For definitions of abbreviations, see Table 1.
Interferon-γ levels at baseline were greater among TST converters compared with those who did not convert (geometric means = 402 versus 103 pg/mL, respectively; P < 0.0001). Baseline levels were similar between converters and non-converters for tumor necrosis factor-α (geometric means: 969 versus 651 pg/ml) and transforming growth factor-β (geometric means = 2,444 versus 2,695 pg/mL). In a univariate logistic regression analysis, the odds of TST conversion increased two-fold (OR = 2.39, 95% CI = 1.49, 3.83) as the initial level of interferon-γ increased 10-fold. Neither tumor necrosis factor-α (OR = 1.35, 95% CI = 0.70, 2.59) nor transforming growth factor-α (OR = 0.44, 95% CI = 0.12, 1.53) were associated with TST conversion.
The interferon-γ levels at baseline were greater among the converters with BCG vaccination compared with those without BCG vaccination (geometric means = 617 versus 144 pg/mL, respectively; P = 0.001) (Table 3) and non-converters with (geometric mean = 95 pg/mL; P = 0.0001) and without BCG vaccination (geometric mean = 145 pg/mL; P = 0.0325).
Table 3.
Geometric mean of baseline IFN-γ levels by TST conversion and BCG vaccination among 222 household contacts of infectious tuberculosis cases*
| BCG vaccinated | BCG unvaccinated | |
|---|---|---|
| Geometric mean (95% confidence interval) | ||
| Converter | ||
| Yes | 617 (23, 16,395) | 144 (2, 9,524) |
| No | 95 (2, 6,142) | 145 (6, 3,810) |
IFN-γ = interferon-γ; TST = tuberculin skin test; BCG = bacille Calmette-Guérin
In a multivariable logistic regression analysis stratified by BCG vaccination and controlling for age, HIV serostatus, smear grade in the index case, and sharing a room with the index case at night, the baseline interferon-γ level remained associated with TST conversion (Table 4) in BCG-vaccinated contacts (P = 0.0001) but not in unvaccinated contacts (P = 0.80). Among the 175 BCG vaccinated contacts with TST reactions < 5 mm, the odds for conversion increased more than four times (OR = 4.30, 95% CI = 2.21, 8.35) for every 10-fold increase in interferon-γ at baseline. This relation was not observed in the 47 unvaccinated contacts where the odds of conversion did not change with interferon-γ levels. The results were similar for a multivariable analysis of TST conversion restricted to contacts who converted within six months of the household evaluation.
Table 4.
Risk factors associated with TST conversion among household contacts of tuberculosis cases in a multivariable logistic regression analysis stratified by BCG vaccination status and fit using generalized estimating equations*
| Characteristic | BCG vaccinated | BCG unvaccinated |
|---|---|---|
| Odds ratio (95% confidence interval) | ||
| Age (years) | 0.96 (0.89, 1.03) | 1.02 (0.97, 1.07) |
| HIV status | ||
| Positive | 3.75 (0.96, 14.68) | 2.77 (0.09, 82.7) |
| Indeterminant | 0.31 (0.06, 1.57) | 0.25 (0.03, 2.33) |
| Negative | 1.00 | 1.00 |
| Shares bedroom | ||
| Yes | 2.41 (0.95, 6.14) | 1.55 (0.37, 6.49) |
| No | 1.00 | 1.00 |
| Spouse to index case | ||
| Yes | 2.49 (0.27, 22.61) | 0.97 (0.11, 8.95) |
| No | 1.00 | 1.00 |
| AFB smear grade | ||
| 3+ | 7.65 (1.11, 52.50) | 1.39 (0.27, 7.21) |
| 2+ | 7.54 (0.75, 75.68) | 0.38 (0.05, 2.96) |
| 1+ | 1.00 | 1.00 |
| Log10 interferon-γ | 4.30 (2.21, 8.35) | 0.86 (0.27, 2.71) |
For definitions of abbreviations, see Table 1.
Among the 34 contacts who converted and had serial interferon-γ measurements, the interferon-γ level increased (median change = 353 pg/mL, inter-quartile range = −299, 2,337 pg/mL, P = 0.015, by signed rank test) from baseline within contacts. Although the initial interferon-γ levels were greater in BCG vaccinated contacts (median = 632 pg/mL) than in BCG-unvaccinated contacts (median = 297 pg/mL), the interferon-γ levels after conversion were similar (medians = 1,714 versus 1,662 pg/mL, respectively). The interferon-γ level increased from baseline to conversion in 23 of the contacts, whereas the interferon-γ level decreased in 11 contacts. The interferon-γ response depended on the starting level of interferon-γ (Figure 3). Among the 8 contacts with baseline interferon-γ levels less than the lowest quartile (115 pg/mL), the interferon-γ levels increased in six contacts. Among the 19 contacts in the inter-quartile range (115, 1,705 pg/mL), the interferon-γ levels increased in 15 contacts. Of the seven contacts in the upper quartile (> 1,705 pg/mL), only one contact experienced an increase in interferon-γ. In nine household contacts who did not convert, seven were vaccinated with BCG and had a baseline interferon-γ geometric mean of 173 pg/mL. The interferon-γ level did not change with time in these contacts (median change = −0.70 pg/mL, inter-quartile range = −2,001, 28 pg/mL, P = 0.59, by signed rank test).
Figure 3.
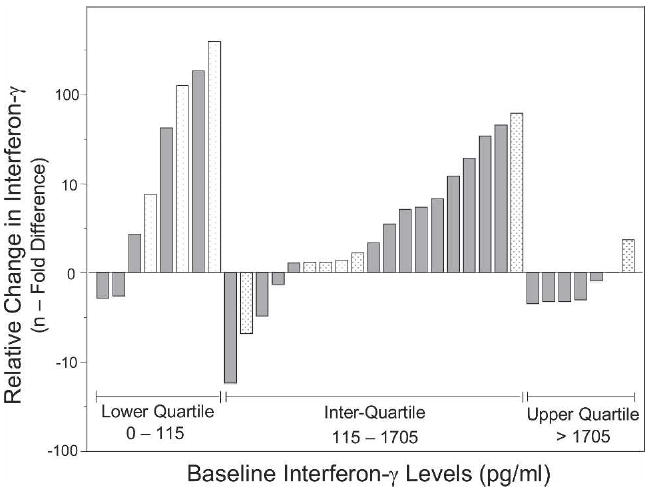
Changes in interferon-γ responses relative to baseline levels in 34 tuberculin skin test converters according to initial quartiles of interferon-γ (white bars) and bacille Calmette-Guérin vaccination status (gray bars).
DISCUSSION
In a prospective cohort study of tuberculosis cases and their household contacts, the observed rate of TST conversion was 27% within one year among contacts who had an initial TST reaction < 5 mm at the time of household evaluation. The TST conversion was associated with the AFB smear grade of the index case and with the nature and proximity of contact with the index case. Interferon-γ levels at the time of household investigation were associated with TST conversion but only in contacts with BCG vaccination. Since TST conversion in household contacts is a sign of recent infection with M. tuberculosis, this cohort of converters provides insight into early host immune responses to acute infection with M. tuberculosis.
We chose to study newly infected contacts for two reasons. First, since most individuals recently infected with M. tuberculosis contain the initial infection,9 the initial immune response may correlate with protective immune responses to M. tuberculosis. Second, a reactive TST at one point in time does not distinguish recent from remote infection. To reduce the potential for confounding introduced by the long and variable latent period of tuberculosis, we performed serial tuberculin skin testing in the context of known exposure to identify new infections.
Although the household contact design is efficient at identifying new infections with M. tuberculosis among contacts with TST reactions < 5 mm at the time of household evaluation, it is not possible with current technology to determine when infection occurred in those contacts with a TST reaction ≥ 5 mm. In the future, new infections may be detected among TST-positive contacts using in vitro interferon-γ assays that stimulate host cells with antigens specific for M. tuberculosis, such as early secretory antigen target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10). In household contacts, we cannot be certain that a conversion occurred directly as a result of contact with a particular index case because we cannot compare the strains of M. tuberculosis between the index case and the converter, unless the converter develops disease. Since most of the TST conversions were identified within six months, we infer that they resulted from exposure to the index case.
We used a standard definition of TST conversion that required a change in the TST reaction ≥ 6 mm to reduce the stochastic effects of measurement error.27 Misclassification of TST conversion, however, is possible because of inherent variability in the TST19 and the booster phenomenon.30 To minimize this misclassification, we included several features in our study design. The household contact design allows for direct observation of conversion during a defined time interval, thereby reducing some of the uncertainty arising from TST instability. Boosting of the skin test reaction may occur from repeated administration of purified protein derivative of M. tuberculosis.31,32 Although we do not have data regarding the booster phenomenon from Uganda at this time, we contend that the epidemiologic scenario of household exposure followed by a large increase in TST reaction most likely represents TST conversion and new infection with M. tuberculosis.
In this study, we found that TST conversion was associated with the AFB status of the index case and the proximity of the contact to that case. These are commonly found risk factors for the presence of tuberculosis infection in household contacts.10,12-14 We extend these observations by showing that the interferon-γ level measured at the time of household investigation was associated with TST conversion. The effect of interferon-γ, however, was seen only in contacts with previous BCG vaccination where the geometric mean for baseline interferon-γ was greater than other contacts and where the odds of conversion increased four-fold with a 10-fold increase in baseline interferon-γ levels. Thus, in the BCG-vaccinated contacts, the interferon-γ response appears to precede TST conversion. These findings raise the possibility that novel immune-based diagnostics33 may be most useful in individuals with an immune system primed by mycobacteria, through BCG vaccination or natural infection with non-tuberculous mycobacteria.
As seen in converters with serial interferon-γ measurements, the change in interferon-γ varied depending on the baseline interferon-γ levels. In converters with high initial interferon-γ responses, the levels decreased either because of an evolving immune response or regression toward the mean. In converters with low initial interferon-γ responses, most converters experienced a sharp increase in interferon-γ levels, consistent with recent infection. Despite differences in the initial interferon-γ level for BCG vaccinated and unvaccinated contacts, final levels of interferon-γ were similar.
The interferon-γ responses must be interpreted in light of the stimulus used in the whole blood assay. In this study, the stimulus was M. tuberculosis culture filtrate, a heterogeneous mixture of proteins from M. tuberculosis that preferentially stimulate CD4+ T cell responses. We infer from the interaction between BCG vaccination and interferon-γ responses among converters that CD4+ T cell production of interferon-γ is a recall response that depends on previous sensitization to BCG through vaccination. This finding is the epidemiologic correlate to the observation that recombinant vaccinia virus Ankara expressing 85A boosts BCG-primed immunity to M. tuberculosis.34,35 Because culture filtrate may contain antigens that cross-react with other mycobacteria, including M. bovis, our findings may not distinguish between recall responses to infection with mycobacteria other than M. tuberculosis. To determine whether observed immune responses are specific to M. tuberculosis infection, a panel of defined antigens, such as ESAT-6 and CFP-10, should be evaluated.36
In the current practice of contact tracing for tuberculosis, the TST is a central tool. Our study indicates in vitro interferon-γ responses to mycobacterial antigens in household contacts with an initial TST reaction < 5 mm may be a sign of recent infection with M. tuberculosis, even in a setting where BCG vaccination is common. Among BCG-vaccinated household contacts with an initial TST reaction < 5 mm, an interferon-γ level at that time may allow health workers to identify contacts at high risk of infection without the need to repeat the TST in three months. The QuantiFERON TB-Gold (Cellestis Limited, Carnegie, Victoria, Australia) and the T SPOT-TB (Oxford Immunotec, Oxford, United Kingdom) are two new immunologic field assays15,37,38 that could be used to evaluate interferon-γ responses in household contacts.39 A recent study of child contacts of tuberculosis cases shows the potential value of using antigens specific for M. tuberculosis as the basis of a diagnostic test for infection.17,18 With results from tests like these, treatment of latent tuberculosis infection could be started earlier after infection to reduce the risk of progressive primary disease8,40 and to preempt chronic infection. We did not evaluate this strategy in contacts at highest risk for progressive disease, such as children and HIV-infected contacts, because of sample size limitations.
In this study, we show that TST conversion is a marker of acute M. tuberculosis infection in household contacts with defined exposure to an infectious case. Our study also establishes the feasibility of using measures of immune function, such as interferon-γ levels after stimulation with mycobacterial antigens, to improve the timeliness in making the diagnosis of acute M. tuberculosis infection among TST-negative contacts, especially in contacts with previous BCG vaccination. These findings have direct relevance to sub-Saharan Africa where BCG vaccination is common and other strategies for tuberculosis control are failing. With the advent of immune-based diagnostics applied to household contacts of tuberculosis, new and more efficient strategies for contact tracing and prevention of tuberculosis can be designed and tested.
Acknowledgments
We acknowledge the invaluable contribution made by the study medical officers, health visitors and data personnel: Dr. David Guwatudde, Dr. Margaret Nakakeeto, Dr. Love Nakende, Mark Breda, Albert Maganda, Deborah Nsamba, Barbara Kyeyune, Margaret Nansumba, Faith Kintu, Gladys Mpalanyi, Philo Nassozi, and Augustine Banyanga. We thank Dr. Francis Adatu Engwau (Head of the Uganda National Tuberculosis and Leprosy Program) for his support of this project. We also acknowledge the medical officers, nurses, and counselors at the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study. We also thank Dr. Robert Wallis for providing culture filtrate used in these studies and Drs. Jerrold Ellner, Zahra Toossi, and Gilbert Bukenya for their advice and guidance with the conduct of the study.
Financial support: This study was supported by Tuberculosis Prevention and Control Research Unit (AI-45244-95383), the AIDS International Training and Research Program of the Fogarty International Center (TW-00011), and the Center for AIDS Research (AI 36219) at the National Institutes of Allergy and Infectious Diseases, the National Institutes of Health.
Footnotes
Disclosure: None of the authors has a conflict of interest with the publication of this research.
Contributor Information
CHRISTOPHER C. WHALEN, Department of Epidemiology and Biostatistics, Case Western Reserve University School of Medicine, Cleveland, OH 44106.
ALLAN CHIUNDA, Department of Medicine, Case Western Reserve University School of Medicine and University Hospitals of Cleveland, Cleveland, OH 44106.
SARAH ZALWANGO, Department of Medicine, Makerere University Medical School, Kampala Uganda.
LORNA NSHUTI, Department of Medicine, Makerere University Medical School, Kampala Uganda.
EDWARD JONES-LOPEZ, Department of Medicine, University of Medicine and Dentistry of New Jersey, Newark, NJ 07103.
ALPHONSE OKWERA, National Tuberculosis and Leprosy Control Programme, Kampala, Uganda.
CHRISTINA HIRSCH, Department of Medicine, Case Western Reserve University School of Medicine and University Hospitals of Cleveland, Cleveland, OH 44106.
PIERRE PETERS, Department of Medicine, Case Western Reserve University School of Medicine and University Hospitals of Cleveland, Cleveland, OH 44106.
W. HENRY BOOM, Department of Medicine, Case Western Reserve University School of Medicine and University Hospitals of Cleveland, Cleveland, OH 44106.
ROY D. MUGERWA, Department of Medicine, Makerere University Medical School, Kampala Uganda
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculosis. JAMA. 1995;274:945–951. [PubMed] [Google Scholar]
- 3.China Tuberculosis Control Collaboration. The effect of tuberculosis control in China. Lancet. 2004;364:417–422. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- 4.Whalen CC. Failure of directly observed treatment for tuberculosis in Africa: a call for new approaches. Clinical Infect Dis. 2006;432:1048–1050. doi: 10.1086/501022. [DOI] [PubMed] [Google Scholar]
- 5.China Tuberculosis Control Program. Results of directly observed short-course chemotherapy in 112,842 Chinese patients with smear-positive tuberculosis. Lancet. 1996;350:169–172. [PubMed] [Google Scholar]
- 6.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 7.Rodrigues LA, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 8.Ferebee SH, Mount FW. Tuberculosis morbidity in a controlled trial of the prophylactic use of isoniazid among household contacts. Am Rev Respir Dis. 1962;85:490–521. doi: 10.1164/arrd.1962.85.4.490. [DOI] [PubMed] [Google Scholar]
- 9.Comstock G. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 10.Espinal MA, Perez EN, Baez J, Henriquez L, Fernandez K, Lopez M, Olivo P, Reingold AL. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet. 2000;355:275–280. doi: 10.1016/S0140-6736(99)04402-5. [DOI] [PubMed] [Google Scholar]
- 11.Klausner JD, Ryder R, Baende E, Lelo U, Williame J, Ngamboli K, Perriens JH, Kaboto M, Prignot J. Mycobacterium tuberculosis in household contacts of human immunodeficiency virus type-1 seropositive patients with active pulmonary tuberculosis in Kinshasa, Zaire. J Infect Dis. 1993;168:106–111. doi: 10.1093/infdis/168.1.106. [DOI] [PubMed] [Google Scholar]
- 12.Reichler MR, Reves RR, Bur S, Thompson V, Mangura B, Ford J, Valway SE, Onorato IM. Contact Investigation Study Group. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002;287:991–995. doi: 10.1001/jama.287.8.991. [DOI] [PubMed] [Google Scholar]
- 13.Lienhardt C, Fielding K, Sillah J, Tunkara A, Donkor S, Manneh K, Warndorff D, McAdam KP, Bennett S. Risk factors for tuberculosis infection in sub-Saharan Africa: a contact study in The Gambia. Am J Respir Crit Care Med. 2003;168:448–455. doi: 10.1164/rccm.200212-1483OC. [DOI] [PubMed] [Google Scholar]
- 14.Bailey WC, Gerald LB, Kimerling ME, Redden D, Brook N, Bruce F, Tang S, Duncan S, Brooks CM, Dunlap ME. Predictive model to identify positive tuberculosis skin test results during contact investigations. JAMA. 2002;287:996–1002. doi: 10.1001/jama.287.8.996. [DOI] [PubMed] [Google Scholar]
- 15.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, Monk P, Lalvani A. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–1173. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 16.Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, Reece WH, Latif M, Pasvol G, Hill AV. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–2021. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 17.Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, Donkor SA, Hammond AS, Out JK, Corrah T, Adegbola RA, McAdam KP. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis. 2004;38:966–973. doi: 10.1086/382362. [DOI] [PubMed] [Google Scholar]
- 18.Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, Efe S, Staveley I, Ewer K, Lalvani A. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366:1443–1451. doi: 10.1016/S0140-6736(05)67534-4. [DOI] [PubMed] [Google Scholar]
- 19.Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–975. [PubMed] [Google Scholar]
- 20.Menzies R, Vissandjee B, Rocher I, St.Germain Y. The booster effect in two-step tuberculin testing among young adults in Montreal. Ann Intern Med. 1994;120:190–198. doi: 10.7326/0003-4819-120-3-199402010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Gordin FM, Perez-Stable EJ, Reid M, Schecter G, Cosgriff L, Flaherty D, Hopewell PC. Stability of positive tuberculin tests: are boosted reactions valid? Am Rev Respir Dis. 1991;144:560–563. doi: 10.1164/ajrccm/144.3_Pt_1.560. [DOI] [PubMed] [Google Scholar]
- 22.Louther J, Rivera P, Feldman J, Villa N, DeHovitz J, Sepkowitz KA. Risk of tuberculin conversion according to occupation among health care workers at a New York City hospital. Am J Respir Crit Care Med. 1997;156:201–205. doi: 10.1164/ajrccm.156.1.9611091. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg HM, Sotir M, Erwin M, Bachman R, Shulman JA. Risk of house staff tuberclin skin test conversion in an area with a high incidence of tuberculosis. Clin Infect Dis. 1998;27:826–833. doi: 10.1086/514963. [DOI] [PubMed] [Google Scholar]
- 24.Guwatudde D, Nakakeeto M, Jones-Lopez EC, Maganda A, Chiunda A, Mugerwa RD, Ellner JJ, Bukenya G, Whalen CC. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Treatment of tuberculosis, American Thoracic Society, CDC, and Infectious Diseases Society of America. MMWR Morb Mortal Wkly Rep. 2003;52:36–41. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Prevention and treatment of tuberculosis among patients infected with human immunodeficiency virus: principles of therapy and revised recommendations. MMWR Morb Mortal Wkly Rep. 1998;47:2–58. [PubMed] [Google Scholar]
- 27.Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15–21. doi: 10.1164/ajrccm.159.1.9801120. [DOI] [PubMed] [Google Scholar]
- 28.Hussain R, Kaleem A, Shahid F, Dojki M, Jamil B, Mehmood H, Dawood G, Dockrell HM. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J Immunol Methods. 2002;264:95–108. doi: 10.1016/s0022-1759(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 30.Thompson N, Glassroth J, Snider DE, Farer LS. The booster phenomenon in serial tuberculin testing. Am Rev Respir Dis. 1979;119:587–597. doi: 10.1164/arrd.1979.119.4.587. [DOI] [PubMed] [Google Scholar]
- 31.Gordin FM, Perez-Stable EJ, Flaherty D, Reid ME, Schecter G, Joe L, Slutkin G, Hopewell PC. Evaluation of a third sequential tuberculin skin test in a chronic care population. Am Rev Respir Dis. 1988;137:153–157. doi: 10.1164/ajrccm/137.1.153. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen M, Perry S, Parsonnet J. QuantiFERON-TB predicts tuberculin skin test boosting in U.S. foreign-born. Int J Tuberc Lung Dis. 2005;9:985–991. [PubMed] [Google Scholar]
- 33.Schlovinck E, Wilkinson KA, Whelan AO, Martineau AR, Levin M, Wilkinson RJ. Gamma interferon-based immunodiagnosis of tuberculosis: comparison between whole-blood and enzyme-linked immunospot methods. J Clin Microbiol. 2004;42:829–831. doi: 10.1128/JCM.42.2.829-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 35.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 36.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 37.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 38.Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR, Iademarco MF, Rothel JS. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740–1747. doi: 10.1001/jama.286.14.1740. [DOI] [PubMed] [Google Scholar]
- 39.Whalen CC. New diagnostic tests for latent tuberculosis infection: measure for measure. JAMA. 2005;293:2785–2787. doi: 10.1001/jama.293.22.2785. [DOI] [PubMed] [Google Scholar]
- 40.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Adv Tuberc Res. 1970;17:28–106. [PubMed] [Google Scholar]


