Abstract
Tuberculosis remains a serious threat to public health, especially in sub-Saharan Africa. To determine the host and environmental factors responsible for tuberculosis in African households, the authors performed a prospective cohort study of 1,206 household contacts of 302 index cases with tuberculosis enrolled in Uganda between 1995 and 1999. All contacts were systematically evaluated for active tuberculosis and risk factors for active disease. Among the 1,206 household contacts, 76 secondary cases (6%) of tuberculosis were identified. Of these cases, 51 were identified in the baseline evaluation, and 25 developed during follow-up. Compared with index cases, secondary cases presented more often with minimal disease. The risk for secondary tuberculosis was greater among young children than adults (10% vs. 1.9%) and among human immunodeficiency virus-seropositive than -seronegative contacts (23% vs. 3.3%). Host risk factors could not be completely separated from the effects of environmental risk factors, suggesting that a household may represent a complex system of interacting risks for tuberculosis.
Keywords: cohort studies, disease transmission, risk, risk factors, tuberculosis
Since the discovery of the tubercle bacillus by Robert Koch in 1882, the study of household contacts of infectious tuberculosis cases has contributed substantially to our current understanding of tuberculosis and its transmission. In the era before effective antituberculous chemotherapy, studies of household contacts established that tuberculosis cases were most infectious when acid-fast bacilli were present in sputum (1–4). In these studies, young children were at great risk for developing tuberculosis, and the clustering of cases within families hinted at a familial susceptibility (5, 6). After the advent of effective antituberculous therapy, household studies demonstrated that proper treatment of tuberculosis was as effective as physical isolation in limiting further infection or disease in the household (7). Household studies were an effective way to estimate the efficacy of Bacillus Calmette-Guérin (BCG) vaccination (8, 9) and chemoprohylaxis of tuberculosis infection (10, 11). Since the emergence of the human immunodeficiency virus (HIV) pandemic, studies from Africa of household contacts have assessed the effect of HIV on transmission of Mycobacterium tuberculosis with variable results (12–15).
Previous studies of tuberculosis have shown that comorbidities such as HIV infection, lymphoma, and malnutrition increase the risk for active tuberculosis, yet except for HIV disease these conditions do not account for the burden of tuberculosis worldwide. Other studies have shown that environmental characteristics such as crowding and social factors (16, 17), including poverty, are associated with tuberculosis, but a causal link between environment and host factors for susceptibility has not been explicitly made. Finally, little is known about the virulence of different M. tuberculosis strains and whether it affects transmission or disease. These issues are rendered more complex where transmission of M. tuberculosis is high and recurrent exposure and reinfection may occur.
The current study was performed to examine the relative contribution of host and environmental factors to the risk of tuberculosis in African households. The household is a natural setting in which to study tuberculosis because the epidemiology of infection and disease can be characterized. In this article, we describe in detail the epidemiology of tuberculosis disease and factors associated with it in Ugandan households.
MATERIALS AND METHODS
Study population
Between October 1995 and February 1999, tuberculosis cases and their household contacts were enrolled in a prospective cohort study. The study recruited sputum smear-positive pulmonary tuberculosis cases who were aged 18 or more years and who had one or more household contacts living with them. Cases were identified at the Uganda National Tuberculosis and Leprosy Program treatment center at Old Mulago Hospital in Kampala, Uganda. On the initial home visit made within 2 weeks of diagnosis for index cases, home health visitors informed household contacts about the study and provided health education about tuberculosis. Written informed consent was obtained from the head of the household, all adults, and parents or guardians of children in the household aged less than 18 years. The study was approved by institutional review boards in both the United States and Uganda.
A household was defined as a group of people living within one residence who share meals together and identified a head of family who made decisions for the household. An index case was defined as the first tuberculosis case identified in the household. A household contact was defined as an individual who had resided in the household for at least 7 consecutive days during the 3 months prior to the diagnosis of tuberculosis in the index case. Secondary cases were defined as tuberculosis cases among household contacts of the index case. Secondary cases were classified as “coprevalent” if active tuberculosis was present at the time of the baseline household investigation or as “incident” if active tuberculosis was absent at the time of the baseline household investigation and developed during follow-up.
Measurements
Index cases were evaluated with a medical history and physical examination performed by Ugandan medical officers. Sputum samples were collected for microscopic examination and mycobacterial culture. Chest radiographs were taken, and HIV serology and tuberculin skin testing were performed. Index cases were treated with standard self-administered, short-course tuberculosis chemotherapy (18). Home visitors evaluated eligible household contacts at baseline with standardized questionnaires, a limited physical examination including height and weight (19), and tuberculin skin testing.
Children aged 5 years or less, HIV-seropositive contacts older than 5 years, and contacts with signs or symptoms of tuberculosis were considered tuberculosis suspects. These suspects were evaluated for active tuberculosis with a medical examination, specimen microscopy, mycobacterial culture, and chest radiograph. Children aged 5 years or less and HIV-seropositive adults without active tuberculosis were offered a 6-month course of isoniazid treatment. All confirmed secondary tuberculosis cases were treated with short-course therapy (18).
After the baseline evaluation, contacts were evaluated at 3, 12, and 24 months for active tuberculosis. Study participants were instructed to attend the study clinic at any time in case of illness. Patients not attending scheduled appointments were traced by home visitors to enhance follow-up and to determine their vital status.
Tuberculin skin testing was performed on all study subjects by placing 0.1 ml of 5 tuberculin units of purified protein derivative (Tubersol; Connaught Laboratories, Limited, Toronto, Canada) on the left forearm using the Mantoux method. After 48–72 hours, the diameter of palpable induration was recorded by home visitors using standardized procedures (20). A tuberculin skin test was considered positive if it was 5 mm or greater (21).
A posteroanterior chest radiograph was taken on all study subjects at baseline and on tuberculosis suspects. The extent of disease on these radiographs was graded on a four-category ordinal scale by experienced physicians (22).
Three sputum samples were collected from index cases and tuberculosis suspects. A sputum smear grade of acid-fast bacilli was recorded on a four-point ordinal scale (23). In children and patients unable to produce a sputum sample, gastric lavage was performed. Specimens were cultured on Lowenstein-Jensen slants at 37°C and examined weekly for 8 weeks.
HIV testing by enzyme-linked immunosorbent assay (Cambridge BiosScience, Worcester, Massachusetts) was performed with counseling on all consenting study subjects. For children 5 years of age or younger, HIV testing was done when the mother was seropositive or when the child was diagnosed with active tuberculosis; otherwise, a child was considered HIV seronegative if the mother tested negative.
The household environment was assessed for dwelling type, number of people in the household, number of habitable rooms, and number of windows in the house. A house was classified as a “muzigo” if it was a multifamily housing unit on the same building block, usually with one or two rooms per family.
Tuberculosis was classified as definite, probable, and possible tuberculosis (24) by investigators using clinical, radiographic, and microbiologic findings in tuberculosis suspects. Response to therapy was defined by improvement of radiographic abnormalities and weight gain with treatment. For this analysis, active tuberculosis was defined as definite or probable tuberculosis. In three incident cases, review of baseline chest radiographs indicated subtle abnormalities in the area of subsequent disease; these cases were reclassified as coprevalent instead of incident cases in this analysis.
Analytical strategy
The goal of the analysis was to determine whether characteristics of the index case, household contact, and environment were associated with active tuberculosis in household contacts. The main outcome was active tuberculosis in a household contact; separate analyses were performed for coprevalent and incident tuberculosis. To determine factors associated with active tuberculosis in contacts, we performed a univariate analysis with secondary tuberculosis as the outcome. Predictor variables included clinical and epidemiologic characteristics of the index case and household contacts, as well as environmental factors. Variables associated with tuberculosis in the univariate analysis (p < 0.15) were retained for the multivariable analysis. A series of logistic regression models were fit using the generalized estimation equation method (SAS version 8.0, PROC GENMOD; SAS Institute, Inc., Cary, North Carolina) to adjust for household correlations (25). No two-way interaction effects were found. Crude and adjusted odds ratios and 95 percent confidence intervals are reported.
RESULTS
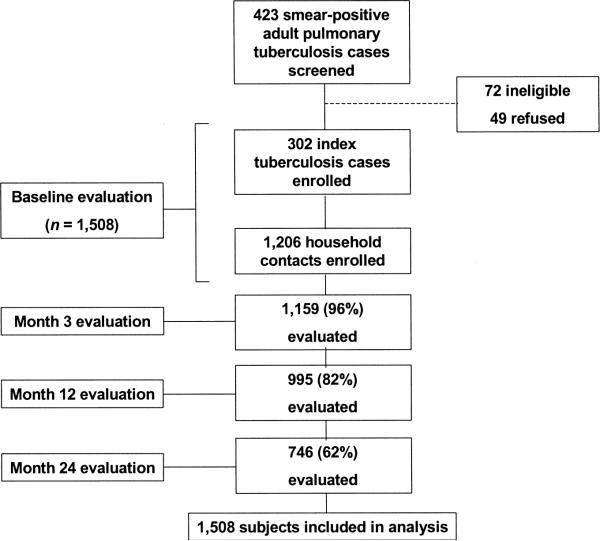
Between October 1995 and February 1999, 423 consecutive cases of smear-positive pulmonary tuberculosis patients were screened at the study center. Of these, 302 were enrolled as index cases into the study. Patients who were not enrolled were either ineligible (n = 72) or refused participation in the study (n = 49). Among the 302 index cases, 1,206 household contacts were eligible and enrolled into the study. Follow-up proportions at 3, 12, and 24 months were 96 percent, 82 percent, and 62 percent, respectively (figure 1).
FIGURE 1.
Study profile, Uganda, 1995–2001.
The median family size was five, but one third of households had six or more members (table 1). The study population was stable, as 259 (86 percent) households had not experienced changes in family composition in the 3 months before the baseline study evaluation. Most households were located in rural or semiurban areas of Kampala and were part of multifamily housing units called muzigos. Households were crowded with a median of three persons per room. Ventilation was minimal in most houses as there was typically only one window per house; 67 houses (22 percent) had no windows.
TABLE 1.
Baseline demographic and environmental characteristics of households, Uganda, 1995–1999
| Characteristic | Summary measure |
|
|---|---|---|
| No. | %* | |
| No. of households | 302 | |
| Household member | 1,508 | 100 |
| Index tuberculosis cases | 302 | 20 |
| Household contacts | 1,206 | 80 |
| Median family size (range, 2–18) | 5 | |
| Household size distribution | ||
| 2–3 persons | 89 | 30 |
| 4–5 persons | 113 | 37 |
| ≥6 persons | 100 | 33 |
| Household composition (last 3 months) | ||
| Unchanged | 259 | 86 |
| Changed | 43 | 14 |
| New member | 11 | 4 |
| Member who moved away | 28 | 9 |
| Recent death | 4 | 1 |
| Household characteristics | ||
| Geographic location of household | ||
| Urban | 3 | 1 |
| Trading center near paved road | 10 | 3 |
| Trading center near dirt road | 31 | 10 |
| Rural | 60 | 20 |
| Semiurban/suburb | 114 | 38 |
| Slum | 84 | 28 |
| Type of residence | ||
| Muzigo with walls not reaching ceiling | 40 | 13 |
| Muzigo with walls reaching ceiling | 135 | 45 |
| Single family house | 120 | 40 |
| Other | 7 | 2 |
| Inside cooking | ||
| Firewood/charcoal | 62 | 21 |
| Electricity | 14 | 5 |
| Household amenities | ||
| Potable water inside household | 155 | 51 |
| Electricity inside household | 117 | 39 |
| Family use of own toilet or latrine | 78 | 26 |
| Indicators of crowding | ||
| Median no. of habitable rooms per household (range, 1–7) | 2 | |
| Median no. of outside windows per house (range, 0–8) | 1 | |
| Median no. of persons per habitable room (range, 0–9) | 3 | |
Percentages were rounded to the nearest integer so they may not add to 100%.
Household contacts
Among the 1,206 household contacts, the median age was 11 years, including 335 (28 percent) children aged 5 years or less (table 2). Among the contacts, 672 (56 percent) were women, and 885 (73 percent) were from the Ganda tribe. Most adult contacts were self-employed or held multiple jobs, and most school-age children attended school. First-degree relatives accounted for 59 percent of the contacts living with index cases. Contacts often shared either the same bedroom or the same bed with the index case. Slim body build was present in 86 children younger than 18 years (11 percent) and in 40 adults (10 percent).
TABLE 2.
Baseline demographic and clinical characteristics of household contacts, Uganda, 1995–1999
| Characteristic | Summary measure |
|
|---|---|---|
| No. | % | |
| No. of subjects | 1,206 | |
| Median age in years (range, 0–70)* | 11 | |
| Age distribution in years | ||
| 0–5 | 335 | 28 |
| 6–15 | 439 | 36 |
| 16–25 | 226 | 19 |
| 26–35 | 119 | 10 |
| >35 | 87 | 7 |
| Female sex | 672 | 56 |
| Marital status in 460 adults (≥15 years) | ||
| Never married | 193 | 42 |
| Married in monogamous relationship | 201 | 44 |
| Other | 66 | 14 |
| Religion | ||
| Catholic | 465 | 39 |
| Anglican | 441 | 37 |
| Muslim | 266 | 22 |
| Other | 34 | 2 |
| Educational level | ||
| None/preprimary | 432 | 36 |
| Primary school | 549 | 46 |
| Secondary school | 209 | 17 |
| Degree or more | 12 | 1 |
| Ethnicity (tribe) | ||
| Ganda | 885 | 73 |
| Soga | 60 | 5 |
| Nyankole | 40 | 3 |
| Other | 221 | 19 |
| Biologic relationship to index case | ||
| Spouse | 181 | 15 |
| Parent | 34 | 3 |
| Son/daughter | 579 | 48 |
| Sibling | 91 | 8 |
| Unrelated | 123 | 10 |
| Other | 198 | 16 |
| Intimacy of contacts with index case | ||
| Share same bed | 213 | 18 |
| Share bedroom but not bed | 522 | 43 |
| Different bedroom | 456 | 38 |
| Unknown | 15 | 1 |
| z scores for 814 subjects aged <18 years | ||
| Weight for age and height for age > −2 | 634 | 78 |
| Weight for age < −2 | 27 | 3 |
| Height for age < −2 | 95 | 12 |
| Both | 58 | 7 |
| Body mass index (kg/m2) for 386 subjects aged ≥18 years† | ||
| Median (range, 15–43) | 22 | |
| Body build | ||
| Contacts aged <18 years with weight-for-age z score of <−2.0 | 86 | 11 |
| Contacts aged ≥18 years with body mass index of <19kg/m2 | 40 | 10 |
| Previous history of tuberculosis | 7 | 1 |
| Exposure to other known tuberculosis case | 86 | 7 |
| BCG‡ vaccination | ||
| Scar present | 878 | 73 |
| Vaccination record available | 363 | 30 |
| Vaccination recorded, scar absent | 20 | 6 |
| PPD‡ skin test | ||
| Positive (≥5 mm) | 963 | 80 |
| Positive (≥10 mm) | 801 | 66 |
| Positive (≥15 mm) | 544 | 45 |
| Median induration in mm (range, 0–40) | 13 | |
| HIV‡ status | ||
| Positive | 123 | 10 |
| Negative | 840 | 70 |
| Unknown | 243 | 20 |
| Treatment of latent tuberculosis infection | ||
| Children aged ≤5 years | ||
| Eligible subjects§ | 272 | |
| Accepted | 209 | 77 |
| Completed (5 or 6 months) | 163 | 78 |
| HIV-infected contacts | ||
| Eligible for treatment§ | 105 | |
| Accepted treatment | 27 | 26 |
| Completed treatment | 22 | 81 |
The youngest contact was 4 days of age.
Body mass index was calculated in contacts aged 18 years or more, and “weight-for-age” z scores were used in contacts aged less than 18 years. Six children did not have information to calculate z scores.
BCG, Bacillus Calmette-Guérin; PPD, purified protein derivative; HIV, human immunodeficiency virus.
Not all patients were eligible for preventive therapy (i.e., suspected or confirmed active tuberculosis).
BCG vaccination was evident in 898 (79 percent) of the contacts; 878 contacts (73 percent) had a characteristic scar, whereas 20 contacts (6 percent) had no discernible scar but BCG vaccination was recorded on the medical record. BCG vaccination varied with age: 622 (83 percent) children aged less than 15 years had a scar or record, whereas 276 (60 percent) adults had evidence of vaccination. Tuberculin skin test reactions of 5 mm or more were present in 963 contacts (80 percent) and of 10 mm or more in 801 contacts (66 percent). Of 963 contacts who consented to HIV testing, 123 contacts were HIV seropositive; 243 contacts were not tested. Of the 123 HIV-seropositive contacts, 27 (22 percent) were younger than 15 years.
Tuberculosis patients
As shown in table 3, among the 302 index cases, there was a 1:1 male:female ratio, only 54 percent had BCG scars, and 49 percent were HIV seropositive. Cough was present in all the cases, and it had lasted more than 90 days in half of the patients. The chest radiograph at diagnosis indicated either moderately advanced or far advanced disease in 86 percent of the index cases. All index cases were smear positive, most with high smear grades and positive mycobacterial cultures.
TABLE 3.
Clinical characteristics of tuberculosis cases, Uganda, 1995–2001*
| Characteristic | Index cases | Coprevalent cases |
Incident cases | |
|---|---|---|---|---|
| ≤5 years | >5 years | |||
| Demographic | ||||
| No. of cases | 302 | 34 | 17 | 25 |
| Age in years | ||||
| Median | 30 | 2 | 24 | 31 |
| Range | 18–60 | 0–5 | 7–40 | 1–59 |
| Age distribution (years) | ||||
| 0–5 | 34 (100) | 3 (12) | ||
| 6–15 | 3 (18) | 2 (8) | ||
| 16–25 | 81 (27) | 6 (35) | 5 (20) | |
| 26–35 | 123 (41) | 7 (41) | 8 (32) | |
| >35 | 98 (32) | 1 (6) | 7 (28) | |
| Male sex | 158 (52) | 19 (56) | 6 (35) | 13 (52) |
| Biologic relationship to index case | ||||
| Spouse | 8 (47) | 14 (56) | ||
| First-degree relative | 31 (91) | 4 (24) | 11 (44) | |
| Other relative | 3 (9) | 3 (18) | 0 (0) | |
| Unrelated | 0 (0) | 2 (12) | 0 (0) | |
| Clinical | ||||
| BCG† vaccination | 164 (54) | 23 (68) | 9 (53) | 13 (52) |
| PPD† skin test | ||||
| ≥5 mm | 269 (89) | 27 (79) | 12 (71) | 23 (92) |
| ≥10 mm | 240 (79) | 23 (68) | 10 (59) | 20 (80) |
| Induration in mm | ||||
| Median | 15 | 13 | 15 | 15 |
| Range | 0–30 | 0–22 | 0–23 | 0–27 |
| Nutritional status | ||||
| Mean weight-for-age z score | −1.13 (1.24)‡ | −1.69 (0.18)‡ | ||
| Mean body mass index (kg/m2) | 19.2 (2.7)‡ | 22 (4.7)‡ | 22 (3.7)‡ | |
| Comorbid illnesses | 18 (6) | 0 (0) | 0 (0) | 1 (4) |
| Symptoms | ||||
| Cough present for >3 weeks | 302 (100) | 25 (74) | 13 (76) | 16 (64) |
| Duration of cough in days | ||||
| Median | 90 | 14 | 45 | 30 |
| Range | 1–730 | 3–365 | 4–300 | 7–252 |
| Weight loss | 264 (87) | 7 (21) | 9 (53) | 8 (32) |
| Fever | 205 (68) | 12 (35) | 9 (54) | 8 (32) |
| Chest radiograph findings§ | ||||
| Normal | 11 (4) | 1 (3) | 3 (18) | 8 (32) |
| Minimal | 28 (9) | 18 (53) | 10 (59) | 6 (24) |
| Moderately advanced | 98 (32) | 15 (44) | 2 (12) | 8 (32) |
| Far advanced | 164 (54) | 0 (0) | 2 (12) | 3 (12) |
| HIV† status | ||||
| Positive | 147 (49) | 4 (12) | 11 (65) | 13 (52) |
| Negative | 155 (51) | 30 (88) | 6 (35) | 12 (48) |
| Unknown/indeterminate | 0 | 0 | 0 | 0 |
| Diagnostic microbiology | ||||
| Criteria | ||||
| Definite | 299 (99) | 9 (26) | 13 (76) | 18 (72) |
| Probable | 3 (1) | 25 (74) | 4 (24) | 7 (28) |
| Specimen results | ||||
| AFB† positive and culture positive | 299 (99) | 2 (6) | 11 (65) | 12 (48) |
| AFB negative and culture positive | 0 (0) | 7 (21) | 2 (12) | 6 (24) |
| AFB positive and culture negative or unknown | 3 (1) | 7 (21) | 2 (12) | 5 (20) |
| AFB negative and culture negative | 0 (0) | 14 (41) | 2 (12) | 1 (4) |
| Missing information | 0 (0) | 4 (12) | 0 (0) | 1 (4) |
| Sputum AFB grade | ||||
| Negative | 21 (62) | 4 (24) | 7 (28) | |
| Scanty | 0 (0) | 5 (15) | 1 (6) | 3 (12) |
| 1 | 23 (8) | 4 (12) | 4 (24) | 6 (24) |
| 2 | 30 (10) | 0 (0) | 3 (18) | 4 (16) |
| 3 | 249 (82) | 0 (0) | 5 (29) | 4 (16) |
| Missing information | 0 | 4 (12) | 0 | 1 (4) |
Values are no. and % unless indicated otherwise.
BCG, Bacillus Calmette-Guérin; PPD, purified protein derivative; HIV, human immunodeficiency virus; AFB, acid-fast bacilli.
Values in designated parentheses, standard deviation.
One chest radiograph from an index case not reviewed.
Secondary tuberculosis cases
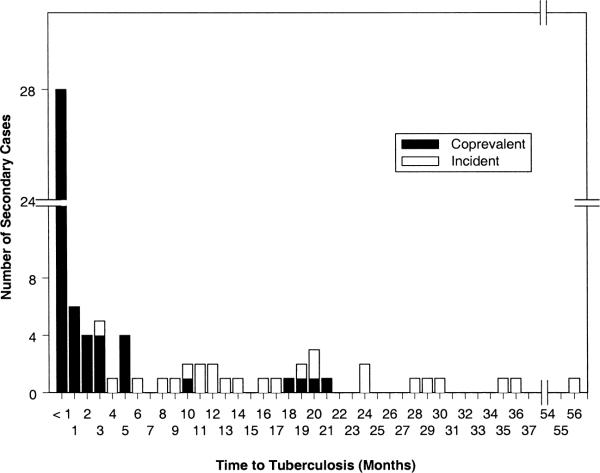
A total of 76 secondary tuberculosis cases were identified among 530 household contacts suspected of active tuberculosis. Among the 1,206 contacts, 51 contacts (4.2 percent) had coprevalent tuberculosis, and 25 contacts (2.1 percent) had incident tuberculosis. In most coprevalent cases, the diagnosis was made within 3 months of the baseline household evaluation, but in nine coprevalent cases (18 percent), the diagnosis was made between 5 and 21 months after their baseline evaluation (figure 2). The diagnosis in these contacts was delayed because they initially presented with nonspecific symptoms and subtle radiographic findings that were appreciated only after active disease had developed. Except for three cases, incident tuberculosis occurred after 6 months of observation. The median time to diagnosis of coprevalent tuberculosis was 27 days, while the median time to diagnosis of incident tuberculosis was 16 months.
FIGURE 2.
Timing of diagnosis of coprevalent and incident tuberculosis cases following diagnosis in the index case, Uganda, 1995–2001.
Of the 51 coprevalent cases (table 3), 34 were found among 335 contact children aged 5 years or less (10.1 percent). Children with coprevalent disease were more likely to have received BCG vaccination than were adult coprevalent or index cases (68 percent vs. 53 percent, p = 0.09). These children presented with short-lived disease compared with index cases, as chronic cough was less common in children than in index cases (74 percent vs. 100 percent, p = 0.0001). Minimal disease, or normal chest radiograph, was more common among children (56 percent vs. 13 percent, p = 0.0001). Among 83 children with suspected tuberculosis, 216 gastric aspirates were performed (range, 1–3 gastric aspirates per child). Of the 34 children with coprevalent disease, nine (27 percent) had positive mycobacterial cultures, all obtained through gastric aspirate, and seven (21 percent) had positive microscopy with negative cultures. The diagnosis of tuberculosis was based solely on clinical presentation in 18 children.
Coprevalent cases aged more than 5 years were more likely to be female or HIV infected when compared with index cases. Like children with coprevalent disease, older coprevalent cases had a shorter duration of symptoms and less advanced disease on chest radiograph than did index cases. Unlike children, 13 of 17 coprevalent cases aged more than 5 years (77 percent) were confirmed through culture.
Children aged 5 years or less and HIV-seropositive contacts carried the highest risk for active tuberculosis (table 4). In this logistic regression model, age was the only confounder of the association between HIV infection and tuberculosis. In stratified analyses, the proportion of tuberculosis cases was lower in BCG-vaccinated versus nonvaccinated contacts, among not only children (8 percent vs. 19 percent; odds ratio (OR) = 0.41, 95 percent confidence interval (CI): 0.19, 0.89) but also older contacts (3.5 percent vs. 7.2 percent; OR = 0.46, 95 percent CI: 0.25, 0.86). A similar association between BCG vaccination and disease was seen in HIV-seropositive (17 percent vs. 34 percent; OR = 0.45, 95 percent CI: 0.18, 1.09) and HIV-seronegative (9 percent vs. 12 percent; OR = 0.62, 95 percent CI: 0.33, 1.15) contacts. Cavitary disease in the index case, prolonged contact with an index case (>18 hours/day), and muzigo residence were independently associated with coprevalent disease (table 4). Other host factors were not significantly associated with active tuberculosis in the contacts.
TABLE 4.
Crude and adjusted odds ratios for characteristics associated with coprevalent tuberculosis in household contacts of adult infectious index cases with tuberculosis, Uganda, 1995–1999
| Characteristic | No. | Active tuberculosis |
Crude odds ratio | 95% confidence interval | Adjusted odds ratio* | 95% confidence interval | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| HIV serostatus of contact | |||||||
| Positive | 123 | 15 | 12.2 | 4.03 | 2.16, 7.53 | 6.40 | 3.14, 13.03 |
| Negative | 1,083 | 36 | 3.3 | 1.00 | 1.00 | ||
| Age | |||||||
| ≤5 years | 335 | 34 | 10.1 | 5.80 | 3.16, 10.65 | 6.88 | 3.61, 13.14 |
| >5 years | 871 | 17 | 1.9 | 1.00 | 1.00 | ||
| BCG vaccination | |||||||
| Yes | 878 | 32 | 3.6 | 0.61 | 0.34, 1.12 | 0.51 | 0.27, 0.96 |
| No | 327 | 19 | 5.8 | 1.00 | 1.00 | ||
| Contact with index case | |||||||
| ≥18 hours/day | 105 | 14 | 13.3 | 4.42 | 2.29, 8.52 | 2.39 | 1.23, 4.63 |
| <18 hours/day | 1,101 | 37 | 3.4 | 1.00 | 1.00 | ||
| House type | |||||||
| Muzigo | 567 | 36 | 6.3 | 2.85 | 1.58, 5.15 | 2.12 | 1.13, 3.95 |
| Other | 639 | 15 | 2.3 | 1.00 | 1.00 | ||
| Chest radiograph of index case | |||||||
| Cavitary | 670 | 36 | 5.4 | 1.97 | 1.05, 3.71 | 2.23 | 1.14, 4.35 |
| Noncavitary | 536 | 15 | 2.8 | 1.00 | 1.00 | ||
Logistic regression analysis using generalized estimating equations adjusting for Bacillus Calmette-Guérin (BCG) vaccination, human immunodeficiency virus (HIV) serostatus in the household contact, age less than 5 years, duration of daily contact with the index case, type of house, and presence of cavitary disease in the index case.
Of the 25 incident tuberculosis cases, 80 percent were aged 15 or more years, and 52 percent were HIV infected (table 3). Incident cases were either the spouse of the index case or a first-degree relative. At baseline, 23 incident cases (92 percent) had purified protein derivative reactions of 5 mm or more, and 20 cases (80 percent) had reactions of 10 mm or more. When compared with index cases, incident cases were identified at an early stage of disease as indicated by the short duration of symptoms, preserved body mass (19.2 vs. 22 kg/m2, p = 0.007), and minimal disease or normal chest radiographs at presentation (13 percent vs. 56 percent, p = 0.0001). Sixteen incident cases (64 percent) presented with negative, scanty, or grade 1 acid-fast bacilli smears, and 18 incident cases (72 percent) were culture positive.
At the time of baseline household investigation, HIV infection and the presence of chronic cough in the contact were associated with the development of incident tuberculosis (table 5). The rate of tuberculosis tended to be lower in 236 contacts treated with isoniazid compared with 925 contacts not treated (1.7 percent vs. 2.2 percent; OR = 0.75, 95 percent CI: 0.26, 2.24). Of the 236 contacts that received isoniazid treatment, 185 contacts completed 5–6 months of treatment, and tuberculosis occurred in three of these contacts.
TABLE 5.
Characteristics associated with incident tuberculosis in household contacts of adult infectious index cases of tuberculosis, Uganda, 1995–2001
| Characteristic | No. | Active tuberculosis |
Crude odds ratio | 95% confidence interval | Adjusted odds ratio* | 95% confidence interval | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| HIV serostatus of contact | |||||||
| Positive | 123 | 13 | 10.6 | 11.87 | 5.25, 26.83 | 4.43 | 1.55, 12.64 |
| Negative or unknown | 1,083 | 12 | 1.1 | 1.00 | 1.00 | ||
| Chronic cough | |||||||
| Present | 166 | 16 | 9.4 | 16.17 | 6.84, 38.23 | 15.47 | 5.21, 45.95 |
| Absent | 1,038 | 9 | 0.9 | 1.00 | 1.00 | ||
Logistic regression analysis using generalized estimating equations adjusting for age, human immunodeficiency virus (HIV) serostatus in the household contact, and presence of cough for more than 3 weeks at the time of initial evaluation.
Among the host characteristics for tuberculosis, the age of 5 years or less was associated with all other risk factors except cavitary disease in the index case (table 6). Children were more likely than adults to be vaccinated with BCG, to be a first-degree relative of the index case, and to be thin, but they were less likely to be HIV seropositive. Children lived in crowded settings, often in muzigos, and had prolonged daily exposure to the index case.
TABLE 6.
Relations among host and environmental characteristics associated with tuberculosis among household contacts, Uganda, 1995–1999*
| Characteristic associated with tuberculosis present (yes) or absent (no) | No. of contacts in each group | Host characteristics† |
Environmental characteristics† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age ≤5 years | HIV+ | BCG‡ | Slim body build | First-degree relative | Cavity in index | Contact > 18 hours/day | Crowded household | Muzigo house | ||
| Age ≤5 years | ||||||||||
| Yes | 335 | 5§ | 81§ | 14§ | 74§ | 58 | 19§ | 42§ | 56§ | |
| No | 871 | 12 | 70 | 9 | 52 | 55 | 5 | 25 | 44 | |
| HIV+ | ||||||||||
| Yes | 123 | 15§ | 11 | 31§ | 49 | 11 | 29 | 61§ | ||
| No | 1,083 | 29 | 10 | 62 | 56 | 8 | 30 | 45 | ||
| BCG vaccination | ||||||||||
| Yes | 878 | 10 | 60∥ | 56 | 7§ | 30 | 49∥ | |||
| No | 327 | 11 | 54 | 55 | 12 | 29 | 42 | |||
| Slim body build | ||||||||||
| Yes | 126 | 60 | 63 | 13∥ | 37 | 46 | ||||
| No | 1,079 | 58 | 55 | 8 | 29 | 47 | ||||
| First-degree relative | ||||||||||
| Yes | 704 | 54 | 10 | 34§ | 50§ | |||||
| No | 502 | 57 | 8 | 25 | 43 | |||||
| Cavity in index | ||||||||||
| Yes | 670 | 8 | 30 | 45 | ||||||
| No | 536 | 9 | 29 | 50 | ||||||
| Contact > 18 hours/day | ||||||||||
| Yes | 105 | 42§ | 58∥ | |||||||
| No | 1,101 | 28 | 46 | |||||||
| Crowded household | ||||||||||
| Yes | 360 | 86§ | ||||||||
| No | 846 | 30 | ||||||||
Values are the % with the characteristic.
The columns labeled “Host characteristics” and “Environmental characteristics” indicate the proportions of contacts with those characteristics. For example, among 335 contacts of age ≤5 years, 5% were human immunodeficiency virus seropositive (HIV+), whereas among 871 contacts of age >5 years, 12% were HIV seropositive.
BCG, Bacillus Calmette-Guérin.
p ≤ 0.01 (chi-square test).
p ≤ 0.05 (chi-square test).
DISCUSSION
In this household contact study from Kampala, Uganda, active tuberculosis was common among household contacts of infectious index cases, occurring in 6 percent of contacts. Most secondary cases were present at the time of baseline household evaluation, although some incident disease did occur despite treatment of latent tuberculous infection in children and HIV-infected adults.
The presentation of the secondary tuberculosis cases differed from that of the index cases and posed challenges to the diagnosis of tuberculosis in the household contacts. These challenges were imposed partly by the young age of the contacts and the inherent difficulty in making the diagnosis of active disease in children (26, 27). The diagnostic criteria for pediatric tuberculosis used in the study were conservative, requiring either culture or smear evidence of disease and weight gain or improvement in chest radiograph appearance with treatment. Children with minimal extent of disease on chest radiograph were not often considered to have disease unless additional signs or symptoms of tuberculosis were present.
The challenge of diagnosis was also imposed by the early stage of tuberculosis at presentation. Although no formal staging system for active tuberculosis exists, many of the secondary cases presented with pauci-bacillary, minimal disease. This presentation differed markedly from that of the index cases, who often had advanced signs and symptoms, moderate or advanced disease on chest radiograph, and positive sputum microscopy or mycobacterial culture. Further, many incident cases had a chronic cough at the time of the baseline household evaluation but no other evidence of active tuberculosis despite a detailed clinical evaluation, sputum examination and culture, and chest radiography. If these persons with cough at baseline had subclinical, active disease and were misclassified, then conventional methods for diagnosis were inadequate. Finally, the presentation of secondary cases may have been affected by the prevalence of HIV infection in the population (approximately 10 percent) and the atypical presentation of tuberculosis seen in HIV-infected persons. The methods for diagnosis of tuberculosis in advanced disease are, for the most part, satisfactory, but the findings from our study emphasize the need for diagnostic tools that identify tuberculosis in its early stages.
Because secondary cases often present with subtle manifestations of disease, one may ask how best to identify these cases in the context of household investigation. The risk of transmission of M. tuberculosis has been correlated with the clinical characteristics of the index case (4, 28, 29) and behaviors that promote contact with infectious cases (16, 29). Once infection has occurred, the risk for disease is attributed to the duration of infection (30) and intrinsic characteristics such as age (31), body build (32), BCG vaccination (33), HIV infection (34), and host genetic susceptibility (35, 36).
In the current study, we found that young age and HIV infection were associated with increased risk for active tuberculosis at the time of contact evaluation, whereas BCG vaccination reduced the risk. Ten percent of children aged less than 5 years had active tuberculosis at the time of the contact investigation. The findings in children must be interpreted with caution because culture-confirmed cases were few (26 percent), giving rise to the possibility of misclassification and overestimation of disease status in the young children. Of the 123 HIV-seropositive contacts, 23 percent had or developed active tuberculosis, indicating the high risk for tuberculosis faced by these individuals, as seen in other studies (12, 13, 37, 38).
BCG vaccination was the only factor that reduced the risk for tuberculosis among contacts of all ages, even in this setting of acute transmission. For contact cases, the magnitude of protection based on the adjusted logistic regression model was 49 percent and is consonant with published studies (33, 39). These findings support the continued use of BCG vaccination in Uganda where transmission of M. tuberculosis is high and where HIV infection is endemic. Isoniazid treatment of latent tuberculosis infection reduced the risk of incident tuberculosis, but the magnitude of protective effect, 25 percent, was less than the protective effect of 77 percent seen in clinical trials (11). This attenuated effect may result from incomplete adherence with treatment, as it was self-administered, or from the short duration of treatment. Revised guidelines for the treatment of tuberculosis infection recommend 9 instead of 6 months of treatment (40). The moderate effect observed with the shorter regimen provides support for longer periods of isoniazid treatment in this setting.
In this study, host characteristics were not the only factors associated with secondary tuberculosis in households. Cavitary disease in the index case, duration of daily contact with the index case, and type of housing were also associated with the risk for disease. In previous household contact studies, the presence of acid-fast bacilli in the sputum of the index case was a consistent predictor of active disease in household contacts (3, 4, 6, 28). Because we enrolled only patients with smear-positive tuberculosis, it was not possible to examine the effect of smear-negative disease on tuberculosis in the household. Among smear-positive patients, the presence of cavitary disease in the index case was found to be important. Cavitary disease has been associated with a greater burden of organisms and greater grade of acid-fast bacilli (41). These two clinical measures were highly correlated in our study, suggesting that patients with cavitary disease had a high burden of organisms and were highly infectious.
Among the many environmental factors explored, only the duration of contact with the index case and the muzigo type of housing were associated with disease. These factors likely measured the nature of contact with the index case. Of the variables included to assess the intensity of contact with the index case, contact of more than 18 hours per day was associated with the presence of active tuberculosis. In many instances, prolonged contact occurred when the caregiver of a dependent child had active tuberculosis, most often the mother. A muzigo is a building with multiple rooms that often share air space. These dwellings have limited ventilation, usually with only one door or window, and are often crowded, so they are an efficient type of setting for transmission of tuberculosis.
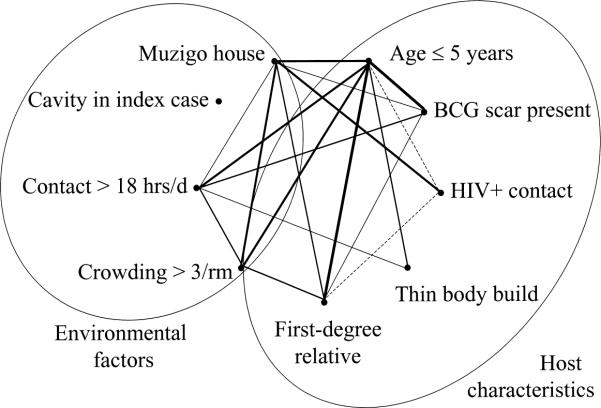
Why would these environmental factors, usually associated with transmission of M. tuberculosis and tuberculous infection, be associated with development of disease? The results of the study are consistent with at least two possible explanations. First, recent infection with M. tuberculosis confers a high risk for developing progressive primary tuberculosis (30). Second, environmental features could be markers for or determinants of risk for tuberculosis (16, 29). This explanation is likely because the environmental factors and host characteristics were correlated in this study. Indeed, there was a web of connectedness among the many factors associated with tuberculosis (figure 3), consistent with a multicausal model for tuberculosis (42–44). In this web, young children appear most vulnerable. For example, although young children were more often vaccinated with BCG, they more often lived in crowded muzigos and had more contact-hours with infectious cases. The only factor not linked with the other risk factors was cavitary disease in the index case. Although each factor was independently associated with the risk of tuberculosis, thereby conferring unique information about the risk for disease, the web of connections between these host and environmental factors suggests that the household may function more like a complex system of risk than a collection of individuals at risk.
FIGURE 3.
Interrelations among environmental and host factors associated with tuberculosis, Uganda, 1995–1999. These interrelations are consistent with a multicausal model of disease. Lines connecting points represent a statistically significant association. The thickness of the line represents the strength of the association between two points. Solid lines represent factors with direct correlation; dashed lines represent inverse correlation. BCG, Bacillus Calmette-Guérin; HIV+, human immunodeficiency virus seropositive; rm, room; hrs/d, hours/day.
In Uganda, many household contacts were related to the index case, but in a culture of extended families, the contact network extends beyond the nuclear family. The current study focuses attention only on the well-defined social network of a household and does not delineate contact networks outside the household. Transmission of tuberculosis does occur in the community (45), but the balance of tuberculosis transmission—whether it occurs mostly in households or in the community at large—is not known and may differ from region to region depending on the prevalence of tuberculosis and mixing patterns of infectious cases. Knowing where tuberculosis is transmitted can have important implications for tuberculosis control strategies.
In conclusion, tuberculosis is common among household contacts of index cases in Africa, especially among young children and HIV-infected contacts. Host risk factors cannot, however, be completely distinguished from the effects of environmental risk factors, suggesting that a household may represent a complex system of interacting risks for disease. Household evaluation of contacts is hindered by shortcomings of current diagnostic methods for early or minimal disease. New approaches to household contact tracing will require improved diagnostic methods and assessment of household environmental risks. Further efforts to improve living conditions and to control HIV infection are essential in reducing household risk for tuberculosis in Africa.
ACKNOWLEDGMENTS
This study was supported by the Tuberculosis Prevention and Control Research Unit (AI-45244-95383), the AIDS International Training and Research Program of the Fogarty International Center (TW-00011), and the Center for AIDS Research (AI 36219) at the National Institute of Allergy and Infectious Diseases, the National Institutes of Health.
The authors would like to acknowledge the invaluable contribution made by the study medical officers, health visitors, and data clerk: Dr. Sarah Zalwango, Dr. Love Nakende, Barbara Kyeyune, Margaret Nansumba, Faith Kintu, Gladys Mpalanyi, and Philo Nassozi. They would like to acknowledge and thank Dr. Francis Adatu Engwau, Head of the Uganda National Tuberculosis and Leprosy Program, for his expert advice and support of this project. They would like to acknowledge Dr. Alphonse Okwera and the staff of the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program, and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, and thank them for their contributions to this study.
Abbreviations
- BCG
Bacillus Calmette-Guérin
- CI
confidence interval
- HIV
human immunodeficiency virus
- OR
odds ratio
REFERENCES
- 1.McPhedran FM, Opie EL. The spread of tuberculosis in families. Am J Hyg. 1935;22:565–643. [Google Scholar]
- 2.Loudon RG, Spohn SK. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1969;99:109–11. doi: 10.1164/arrd.1969.99.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc. 1954;69:724–32. doi: 10.1164/art.1954.69.5.724. [DOI] [PubMed] [Google Scholar]
- 4.Rose CE, Zerbe GO, Lantz SO, et al. Establishing priority during investigation of tuberculosis contacts. Am Rev Respir Dis. 1979;119:603–9. doi: 10.1164/arrd.1979.119.4.603. [DOI] [PubMed] [Google Scholar]
- 5.Puffer RR, Zeidberg LD, Dillon A, et al. Tuberculosis attack and death rates of household associates. Am Rev Tuberc. 1952;65:111–27. doi: 10.1164/art.1952.65.2.111. [DOI] [PubMed] [Google Scholar]
- 6.Brailey M. A study of tuberculous infection and mortality in the children of tuberculous households. Am J Hyg. 1940;31:1–43. doi: 10.2105/ajph.30.7.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamat SR, Dawson JJY, Devadata S, et al. A controlled trial of the influence of segregation of tuberculosis patients for one-year on the attack rate of tuberculosis in a 5-year period in close family contacts in South India. Bull World Health Organ. 1966;34:517–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal SR, Loewinsohn E, Graham ML, et al. BCG vaccination in tuberculous households. Am Rev Respir Dis. 1961;84:690–704. doi: 10.1164/arrd.1961.84.5P1.690. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal SR, Loewinsohn E, Graham ML, et al. BCG vaccination against tuberculosis in Chicago. Pediatrics. 1961;28:622–41. [PubMed] [Google Scholar]
- 10.Egsmose T, Ang'awa JOW, Poti SJ. The use of isoniazid among household contacts of open cases of pulmonary tuberculosis. Bull World Health Organ. 1965;33:419–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferebee SH, Mount FW. Tuberculosis morbidity in a controlled trial of the prophylactic use of isoniazid among household contacts. Am Rev Respir Dis. 1962;85:490–521. doi: 10.1164/arrd.1962.85.4.490. [DOI] [PubMed] [Google Scholar]
- 12.Elliott AM, Hayes RJ, Haleiindi B, et al. The impact of HIV on infectiousness of pulmonary tuberculosis: a community study in Zambia. AIDS. 1993;7:981–7. doi: 10.1097/00002030-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Nunn PP, Mungai M, Nyamwaya J, et al. The effect of human immunodeficiency virus type-1 on the infectiousness of tuberculosis. Tuber Lung Dis. 1994;75:25–32. doi: 10.1016/0962-8479(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 14.Klausner JD, Ryder R, Baende E, et al. Mycobacterium tuberculosis in household contacts of human immunodeficiency virus type-1 seropositive patients with active pulmonary tuberculosis in Kinshasa, Zaire. J Infect Dis. 1993;168:106–11. doi: 10.1093/infdis/168.1.106. [DOI] [PubMed] [Google Scholar]
- 15.Cruciani M, Malena M, Bosco O, et al. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis. 2001;33:1922–30. doi: 10.1086/324352. [DOI] [PubMed] [Google Scholar]
- 16.Chapman JS, Dyerly MD. Social and other factors in intrafamilial transmission of tuberculosis. Am Rev Respir Dis. 1964;90:48–60. doi: 10.1164/arrd.1964.90.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Rubel AJ, Garro LC. Social and cultural factors in the successful control of tuberculosis. Public Health Rep. 1992;107:626–36. [PMC free article] [PubMed] [Google Scholar]
- 18.Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149:1359–74. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan KM, Gorstein J. ANTHRO software for calculating anthropometry. Version 1.02. Centers for Disease Control and Prevention; Atlanta, GA: 1999. Y2K compliant. [Google Scholar]
- 20.Huebner RE, Schein MF, Bass JB. The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 21.Dunlap NE, Bass JB, Fujiwara P, et al. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 22.Falk A, O'Connor JB, Pratt C. Diagnostic standards and classification of tuberculosis. National Tuberculosis and Respiratory Disease Association; New York, NY: 1969. Classification of pulmonary tuberculosis; pp. 68–76. [Google Scholar]
- 23.International Union against Tuberculosis and Lung Disease Technical guide for sputum examination for tuberculosis by direct microscopy. Bull Int Union Tuberc Lung Dis. 1986;61:1–16. [Google Scholar]
- 24.Whalen CC, Johnson JL, Okwera A, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N Engl J Med. 1997;337:801–8. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. Erratum in: Biometrics 1989;45:347. [PubMed] [Google Scholar]
- 26.Starke JR. Diagnosis of tuberculosis in children. Pediatr Infect Dis J. 2000;19:1095–6. doi: 10.1097/00006454-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int J Tuberc Lung Dis. 2001;5:594–603. [PubMed] [Google Scholar]
- 28.Loudon RG, Williamson J, Johnson JM. An analysis of 3,485 tuberculosis contacts in the city of Edinburgh during 1954–1955. Am Rev Tuberc. 1958;77:623–43. doi: 10.1164/artpd.1958.77.4.623. [DOI] [PubMed] [Google Scholar]
- 29.Dow DJ, Lloyd WE. The incidence of tuberculous infection and its relation to contagion in children under 15. Br Med J. 1931;2:183–6. doi: 10.1136/bmj.2.3682.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Adv Tuberc Res. 1970;17:28–106. [PubMed] [Google Scholar]
- 31.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–8. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 32.Edwards LD, Livesay VT, Aquaviva FA, et al. Height, weight, tuberculous infection, tuberculous disease. Arch Environ Health. 1971;22:106–12. doi: 10.1080/00039896.1971.10665820. [DOI] [PubMed] [Google Scholar]
- 33.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 34.O'Brien RJ, Perriens JH. Preventive therapy for tuberculosis in HIV infection: the promise and the reality. AIDS. 1995;9:665–73. doi: 10.1097/00002030-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Bellamy RJ, Hill AV. Host genetic susceptibility to human tuberculosis. Novartis Found Symp. 1998;217:3–13. doi: 10.1002/0470846526.ch2. [DOI] [PubMed] [Google Scholar]
- 36.Comstock G. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 37.Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. N Engl J Med. 1992;326:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 38.Espinal MA, Perez EN, Baez J, et al. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet. 2000;355:275–80. doi: 10.1016/S0140-6736(99)04402-5. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues LA, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–8. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 40.Targeted tuberculin testing and treatment of latent tuberculosis infection American Thoracic Society. MMWR Recomm Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 41.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis. 1965;92:687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 42.MacMahon B, Pugh TF, Ipsen J. Epidemiologic methods. Little, Brown and Company; Boston, MA: 1960. Epidemiologic concepts of cause; pp. 11–22. [Google Scholar]
- 43.Jaramillo E. Encompassing treatment with prevention: the path for a lasting control of tuberculosis. Soc Sci Med. 1999;49:393–404. doi: 10.1016/s0277-9536(99)00114-8. [DOI] [PubMed] [Google Scholar]
- 44.Lienhardt C. From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev. 2001;23:288–301. doi: 10.1093/oxfordjournals.epirev.a000807. [DOI] [PubMed] [Google Scholar]
- 45.van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]