Abstract
Elevated alcohol consumption is associated with enhanced preference for sweet substances across species and may be mediated by oral alcohol-induced activation of neurobiological substrates for sweet taste. Here, we directly examined the contribution of the T1r3 receptor protein, important for sweet taste detection in mammals, to ethanol intake and preference and the neural processing of ethanol taste by measuring behavioral and central neurophysiological responses to oral alcohol in T1r3 receptor-deficient mice and their C57BL/6J background strain. T1r3 knockout and wild-type mice were tested in behavioral preference assays for long-term voluntary intake of a broad concentration range of ethanol, sucrose, and quinine. For neurophysiological experiments, separate groups of mice of each genotype were anesthetized, and taste responses to ethanol and stimuli of different taste qualities were electrophysiologically recorded from gustatory neurons in the nucleus of the solitary tract. Mice lacking the T1r3 receptor were behaviorally indifferent to alcohol (i.e., ∼50% preference values) at concentrations typically preferred by wild-type mice (5–15%). Central neural taste responses to ethanol in T1r3-deficient mice were significantly lower compared with C57BL/6J controls, a strain for which oral ethanol stimulation produced a concentration-dependent activation of sweet-responsive NTS gustatory neurons. An attenuated difference in ethanol preference between knockouts and controls at concentrations >15% indicated that other sensory and/or postingestive effects of ethanol compete with sweet taste input at high concentrations. As expected, T1r3 knockouts exhibited strongly suppressed behavioral and neural taste responses to sweeteners but did not differ from wild-type mice in responses to prototypic salt, acid, or bitter stimuli. These data implicate the T1r3 receptor in the sensory detection and transduction of ethanol taste.
Keywords: knockout mice, alcohol preference
a strong association exists between the ingestion of alcohol and sweet-tasting solutions. Enhanced consumption of sweet solutions is one of the most consistent phenotypic predictors of alcohol intake common across alcohol-preferring rodent lines/strains. Ethanol-preferring C57BL/6J mice (21, 44) and multiple independently selected lines of alcohol-preferring rats including P (ethanol-preferring; 56, 68), AA (Alko alcohol; 56), HAD (high alcohol drinking; 67, 71), and WHP (Warsaw high-preferring; 19) exhibit greater intake of both nutritive (sucrose) and nonnutritive (saccharin) sweeteners compared with their nonethanol-preferring counterparts [DBA/2J, NP (ethanol-nonpreferring), ANA (Alko nonalcohol), LAD (low alcohol drinking), and WLP (Warsaw low-preferring)]. Positive correlations between alcohol and sweetener intake have also been observed in randomly bred rats (32, 33), inbred and congenic mice (4, 7), and the F2 progeny derived from crosses of alcohol-preferring and -nonpreferring lines/strains (2, 50, 65, 66, 67). Human studies measuring preference for sucrose across a range of concentrations have shown that alcoholics prefer more highly concentrated sucrose solutions than nonalcoholic control subjects (29, 30, 34), and elevated sweet preference may be most clearly apparent in subtypes of alcoholism with a familial or genetic component (30, 72).
Although the mechanisms underlying covariation in alcohol and sweet intake are unknown, evidence from multiple species has indicated that alcohol possesses an appetitive sweet taste component. Conditioned taste aversions generalize between ethanol and sucrose in C57BL/6J mice (6, 7) and ethanol and sucrose mixtures in randomly bred rats (17, 37). Ethanol also elicits sweet taste sensations in humans (54, 70), and highly significant correlations exist between ratings of the sweetness of certain sucrose concentrations and ethanol taste intensity (54). Electrophysiological recordings from peripheral gustatory nerves in primates indicate that orally applied ethanol preferentially stimulates taste nerve fibers that respond most strongly to sweets relative to other kinds of tastants (26). More recently, it has been demonstrated in heterogeneous rats that oral alcohol stimulation directly activates central sweet-responsive gustatory neurons in the nucleus of the solitary tract (NTS) and that this effect is inhibited by peripheral pharmacological antagonism of sweet taste receptors (39). These findings indicate overlap in the gustatory receptor and central neural circuits that process alcohol and sweet taste. These sweet-responsive gustatory circuits are coupled downstream to limbic forebrain areas involved in regulating ingestive motivation and reinforcement (23, 24, 49), where ethanol taste input likely interacts in a complex manner with other sensory and postabsorptive influences of the drug to determine behavioral intake.
Taste transduction for a variety of sweet stimuli depends upon the presence of the T1r3 receptor protein, which combines with a related protein in the T1r family of receptors (T1r2) to form a functional sweet taste receptor (45, 47, 48). Mice with genetic deletion of the T1r3 receptor show large reductions in preference for sucrose and several artificial sweeteners (12, 74), although they do maintain some ability to perceptually detect and discriminate sucrose (16). Allelic variation in the Tas1r3 gene, encoding for the T1r3 protein, is also associated with differential sweet preference in mice (28, 52). This genetic locus (formerly Sac) encoding for the T1r3 taste receptor protein overlaps with a locus that strongly influences ethanol intake (Ap3q; 1). Here, we directly investigated the contribution of the T1r3 taste receptor to oral ethanol intake and preference and the processing of ethanol taste in NTS gustatory neurons by measuring behavioral and electrophysiological responses to oral ethanol in genetically manipulated mice lacking the T1r3 receptor protein and their C57BL/6J background strain.
EXPERIMENT 1: LONG-TERM INTAKE AND PREFERENCE
Materials and Methods
Animals.
Twenty-four naive adult male and female T1r3 knockout (KO) and age-matched C57BL/6J wild-type (WT) control mice (n = 12/genotype; n = 6/sex/genotype) were used. WT mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME) and T1r3 homozygous KO (−/−) mice from Mount Sinai School of Medicine. KO mice were generated by Damak and colleagues (12) by targeting of the T1r3 coding region in C57BL/6 embryonic stem cells and subsequent crossing to a C57BL/6J genetic background (12). All animals were reared at the University of Tennessee Health Science Center in a vivarium that maintained a 12-h light/dark cycle and an ambient temperature of ∼23°C. Food and water were available ad libitum. During the course of experimental procedures, animals were housed individually in standard shoebox cages (29.5 × 18.5 × 13 cm). Mean body weights at the start of the experiment were 24.08 g (± 1.66 SE) and 24.71 g (± 0.85 SE) for KO and WT mice, respectively. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center and were in accordance with National Institute of Health guidelines.
Intake tests.
Throughout the experiment, mice were provided continuous access to two 25 ml graduated drinking tubes in their home cages from which fluid intake volumes were measured to the nearest 0.1 ml and fluids replaced every 24 h. Food was available ad libitum at all times during the experiment. Mice were initially acclimated to individual housing conditions for 7 days prior to stimulus testing during which both tubes contained deionized water only and baseline levels of water intake were monitored. Following the acclimation period, mice were tested sequentially with an ascending concentration series of ethanol (3, 5, 10, 15, 25, and 40% vol/vol), sucrose (0.01, 0.03, 0.06, 0.1, 0.3, and 1 M), and quinine (0.003, 0.01, 0.03, 0.1, 0.3, and 1 mM) in a two-bottle choice procedure vs. deionized water. Each solution concentration was presented for a period of 4 days, previously shown to be an optimal test duration to differentiate stimulus preference across strains (69). The position of tastant and water bottles was rotated every 24 h to control for position preferences. A 1-wk break was interposed between testing of different tastants during which mice had access to deionized water only in both tubes. Body weights were measured on day 1 of the acclimation phase and at the start and end of each 4-day solution test period.
Stimuli.
Solutions were prepared fresh prior to testing using reagent grade chemicals (sucrose, quinine HCl-Sigma-Aldrich, St. Louis, MO; ethanol 95% stock-Pharmco Products, Brookfield, CT). All stimuli were dissolved in deionized water and were presented at room temperature. Ethanol, sucrose, and quinine HCl concentrations were selected to encompass a full behavioral orosensory response range based upon previous work (8, 20, 61, 63).
Data analysis.
For each mouse, average baseline daily water intake was calculated across all 7 days of the initial acclimation phase. Average 24-h fluid intake volumes for a given stimulus solution and for water were calculated across the 4 days of testing at that stimulus concentration. Fluid intake measures were corrected for individual differences in body size by dividing each subject's mean absolute intake volume of a given solution (ml) by that subject's average body weight (kg) determined from body weight measures taken immediately before and after testing of that solution. Relative preference for each stimulus concentration was calculated for each subject by dividing the mean absolute daily volume of stimulus consumed by the mean total daily volume of fluid (stimulus + water) consumed, and multiplying by 100. A preference score of 50% reflects equal volumes of water and stimulus consumed, with preference scores approaching 100% indicating increasingly higher intake of a stimulus relative to water, and scores approaching 0% indicating progressively lower stimulus intake relative to water.
To analyze for differences in baseline water intake prior to stimulus testing, a 2 (genotype) × 2 (sex) factorial analysis of variance (ANOVA) was conducted on the average daily ml/kg intake of water during the 7-day period of water acclimation. For each stimulus, mean daily ml/kg stimulus intake and preference score data as well as mean daily ml/kg total fluid intake were analyzed using 2 (genotype) × 2 (sex) × 6 (concentration) mixed ANOVAs, with genotype and sex as between-subject factors and concentration as a within-subject factor. Significant interactions from the overall ANOVAs were further analyzed using one-way ANOVAs to test for simple effects followed by Newman-Keuls test where appropriate. Alpha level for all statistical tests was 0.05.
Results
Baseline water intake.
T1r3 KO and WT mice did not differ in mean daily intake of water during the initial 7-day acclimation phase [nonsignificant (NS) effect of genotype: F1, 20 = 2.81, P = 0.11; NS genotype × sex interaction: F1, 20 = 0.15, P = 0.70]. Mean daily ml/kg intake of water during the acclimation period was 181.39 (± 12.20 SE) and 199.75 (± 13.80 SE) for controls and KOs, respectively. Females had significantly higher baseline ml/kg water intake than males (main effect of sex: F1, 20 = 42.12, P < 0.001). Mean daily ml/kg water consumption for females was 226.10 (± 9.24 SE) and for males was 155.05 (± 6.30 SE).
Ethanol intake and preference.
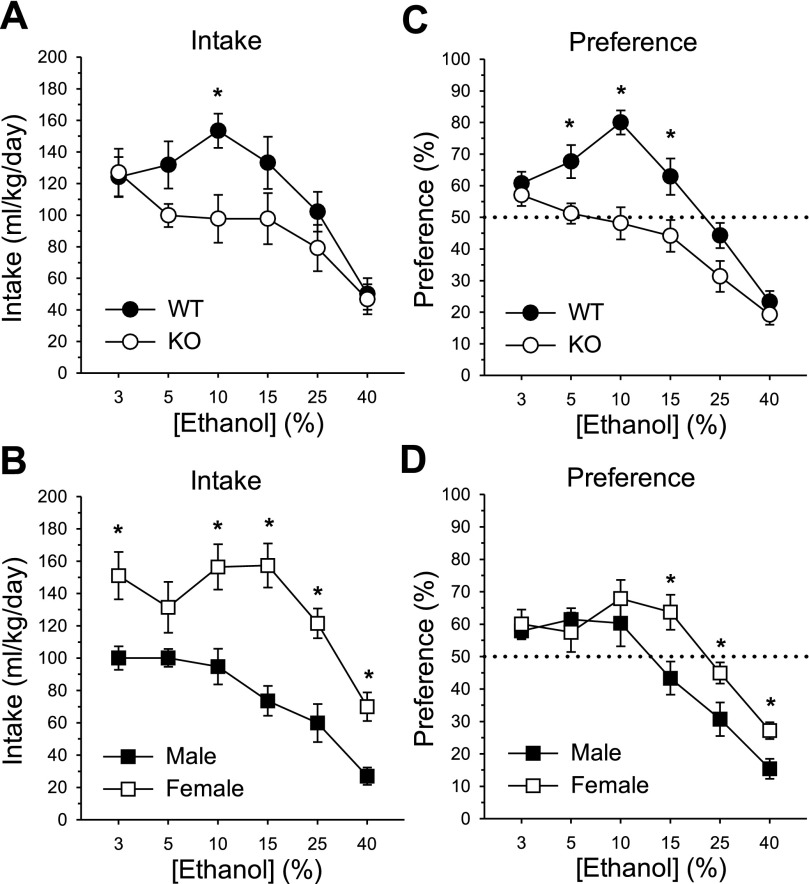
T1r3 KO mice displayed significantly lower ethanol intake than WT mice, which was dependent on ethanol concentration (main effect of genotype: F1, 20 = 5.41, P < 0.05; main effect of concentration: F5, 100 = 28.87, P < 0.001; genotype × concentration interaction: F5, 100 = 3.78, P < 0.01, Fig. 1A). One-way analyses to test for simple effects of genotype at each concentration indicated reduced ethanol intake in KOs at the 10% concentration (F1, 22 = 8.87, P < 0.01). Total fluid intake did not differ between KOs and controls [212.58 (± 14.65 SE) vs. 203.91 (± 11.94 SE), respectively; NS effect of genotype: F1, 20 = 0.75, P = 0.40; NS genotype × concentration interaction: F5, 100 = 1.51, P = 0.19]. Across-concentration analyses for each genotype indicated that ethanol intake by WT mice at 10% was greater than at 3, 25, and 40%; 3, 5, 15% > 25, 40%; and 25% > 40% (simple effect of concentration: F5, 55 = 22.70, P < 0.001, Newman-Keuls test, Ps < 0.05). In KOs, ethanol intake at 3% was greater than at 5, 25, 40% and 5–25% > 40% (simple effect of concentration: F5, 55 = 9.57, P < 0.001, Newman-Keuls test, Ps < 0.05). The overall ANOVA additionally yielded a main effect of sex (F1, 20 = 27.84, P < 0.001) and a sex × concentration interaction (F5, 100 = 2.64, P < 0.05). Females displayed greater ethanol intake than males for all concentrations except 5% (simple effects of sex: Fs1, 22 ≥ 9.67, Ps < 0.01; Fig. 1B). Overall ml/kg total fluid intake was also higher in females than in males [246.40 (± 9.21 SE) vs. 170.09 (± 3.33 SE), respectively; main effect of sex: F1, 20 = 57.79, P < 0.001]. Analyses across concentration for each sex indicated that among males, ethanol intake at 3–10% > 15–40% and 15, 25% > 40% (simple effect of concentration: F5, 55 = 20.64, P < 0.001, Newman-Keuls test, Ps < 0.05). In females, ethanol intake did not differ from 3–25%, and declined at 40% relative to lower concentrations (simple effect of concentration: F5, 55 = 11.36, P < 0.001, Newman-Keuls test, Ps < 0.001).
Fig. 1.
Voluntary intake (ml/kg/day) and percent preference for ethanol in a 2-bottle choice assay. A: mean ± SE ethanol intake as a function of concentration in T1r3 knockout (KO) and C57BL/6J wild-type (WT) mice; n = 12/genotype. B: mean ± SE ethanol intake by concentration in male and female mice; n = 12/sex. C: mean ± SE ethanol preference at each concentration in KO and WT mice; n = 12/genotype. D: mean ± SE ethanol preference by concentration in males and females; n = 12/sex. *Significant difference between KO and WT or male and female (P < 0.05).
Analysis of the ethanol preference data indicated that T1r3 KOs were indifferent to ethanol at concentrations preferred by WT mice (main effect of genotype: F1, 20 = 13.14, P < 0.01; main effect of concentration: F5, 100 = 56.83, P < 0.001; genotype × concentration interaction: F5, 100 = 5.82, P < 0.001, Fig. 1C). KOs exhibited significantly lower ethanol preference scores compared with controls at concentrations of 5–15% (simple effects of genotype: Fs1, 22 ≥ 6.05, Ps < 0.05). In WT mice, ethanol preference at 10% was greater than at all other concentrations; 3, 5, 15% > 25, 40%; and 25% > 40% (simple effect of concentration: F5, 55 = 34.51, P < 0.001, Newman-Keuls test, Ps < 0.05). In KOs, ethanol preference at 3% > 15–40%; 5–15% > 25, 40%; and 25% > 40% (simple effect of concentration: F5, 55 = 19.66, P < 0.001, Newman-Keuls test, Ps < 0.05). The overall analysis also revealed a main effect of sex (F1, 20 = 4.67, P < 0.05) and a sex × concentration interaction (F5, 100 = 4.00, P < 0.01). Preference for ethanol in females was elevated compared with males at the three highest ethanol concentrations (15–40%; simple effects of sex: Fs1, 22 ≥ 5.37, Ps < 0.05; Fig. 1D). Across concentration, ethanol preference in males did not differ from 3–10% and then declined with each successive concentration (simple effect of concentration: F5, 55 = 39.96, P < 0.001, Newman-Keuls test, Ps < 0.01). Among females, ethanol preference was constant from 3–15% and then declined from 15–25% and 25–40% (simple effect of concentration: F5, 55 = 15.81, P < 0.001, Newman-Keuls test, Ps < 0.05).
Sucrose intake and preference.
Sucrose consumption was substantially reduced in T1r3 KO mice from levels observed in WT controls, which varied with concentration (main effect of genotype: F1, 20 = 76.38, P < 0.001; main effect of concentration: F5, 100 = 79.90, P < 0.001; genotype × concentration interaction: F5, 100 = 25.17, P < 0.001, Fig. 2A). KOs consumed significantly less sucrose than controls from 0.03–0.3 M (simple effects of genotype: Fs1, 22 ≥ 17.43, Ps < 0.001) but did not differ from WT mice in sucrose intake at the 0.01 and 1 M concentrations (Fs ≤ 2.40, Ps ≥ 0.14). Lower sucrose intake among KOs at 0.03–0.3 M resulted in reduced total fluid intake in KOs compared with controls at these concentrations as well [0.03 M: 194.84 (± 12.72 SE) vs. 251.94 (± 22.73 SE); 0.06 M: 186.05 (± 15.26 SE) vs. 344.52 (± 35.60 SE); 0.1 M: 205.79 (± 22.50 SE) vs. 545.20 (± 59.93 SE); 0.3 M: 413.81 (± 74.71 SE) vs. 732.02 (± 37.73 SE), respectively; genotype × concentration interaction: F5, 100 = 22.40, P < 0.001, simple effects of genotype: Fs1, 22 ≥ 4.80, Ps < 0.05]. Across-concentration analyses indicated that sucrose intake in controls increased at each concentration up to 0.3 M, and then decreased at 1 M (simple effect of concentration: F5, 55 = 88.30, P < 0.001, Newman-Keuls test, Ps < 0.01). In KOs, sucrose intake did not differ from 0.01–0.1 M, increased at 0.3 M, and then declined at 1 M (simple effect of concentration: F5, 55 = 14.92, P < 0.001, Newman-Keuls test, Ps < 0.05). The overall ANOVA also yielded a main effect of sex (F1, 20 = 28.27, P < 0.001) and a sex × concentration interaction (F5, 100 = 4.14, P < 0.01). Females had significantly higher ml/kg sucrose intake than males for all concentrations except 0.1 M (simple effects of sex: Fs1, 22 ≥ 4.33, Ps < 0.05; Fig. 2B). Overall ml/kg total fluid intake was also higher in females than in males [384.60 (± 33.12 SE) vs. 254.88 (± 21.64 SE), respectively; main effect of sex: F1, 20 = 26.85, P < 0.001]. Among males, sucrose intake at 0.3 M was greater than at all other concentrations; 1 M > 0.01, 0.03 M; and 0.1 M > 0.01 M (simple effect of concentration: F5, 55 = 16.75, P < 0.001, Newman-Keuls test, Ps < 0.05). In females, sucrose intake at 0.3 M was also elevated compared with all other concentrations; 0.1 M > 0.01–0.06 M; and 0.06, 1 M > 0.01 M (simple effect of concentration: F5, 55 = 20.99, P < 0.001, Newman-Keuls test, Ps < 0.05).
Fig. 2.
Voluntary intake (ml/kg/day) and percent preference for sucrose in a 2-bottle choice assay. A: mean ± SE sucrose intake as a function of concentration in T1r3 KO and C57BL/6J WT mice; n = 12/genotype. B: mean ± SE sucrose intake by concentration in male and female mice; n = 12/sex. C: mean ± SE sucrose preference at each concentration in KO and WT mice; n = 12/genotype. D: mean + SE sucrose preference in KO and WT males and females collapsed across concentration; n = 6/sex/genotype. *Significant difference between KO and WT or male and female (P < 0.05).
Sucrose preference was significantly suppressed in KOs compared with controls at all except the highest sucrose concentration (main effect of genotype: F1, 20 = 338.02, P < 0.001; main effect of concentration: F5, 100 = 141.29, P < 0.001; genotype × concentration interaction: F5, 100 = 56.21, P < 0.001; Fig. 2C). T1r3 KO mice displayed lower preference scores than WT mice for 0.01–0.3 M sucrose (simple effects of genotype: Fs1, 22 ≥ 15.33, Ps < 0.001), whereas both genotypes exhibited near maximal preference for 1 M sucrose [mean % preference-knockouts: 96.46 (± 0.76 SE), controls: 98.46 (± 0.43 SE)]. Across concentration, sucrose preference in controls rose significantly from 0.01–0.03 M and 0.03–0.06 M, and then remained constant from 0.06–1 M (simple effect of concentration: F5, 55 = 125.59, P < 0.001, Newman-Keuls test, Ps < 0.05). In KOs, sucrose preference at 0.1 M was greater than at 0.01 M, and then further increased with each concentration from 0.1–1 M (simple effect of concentration: F5, 55 = 80.64, P < 0.001, Newman-Keuls test, Ps < 0.05). The overall analysis additionally revealed a main effect of sex (F1, 20 = 9.77, P < 0.01) and a genotype × sex interaction (F1, 20 = 9.63, P < 0.01). Elevated sucrose preference in females compared with males was observed only in T1r3 KOs (simple effect of sex: F1, 10 = 12.23, P < 0.01) but not in WT mice (F1, 10 = 0.00, P = 0.98; Fig. 2D). This interaction may have been influenced by a near ceiling effect in sucrose preference among WT mice [mean % preference-control males: 92.00 (± 0.64 SE), control females: 92.04 (± 1.24 SE)].
Quinine intake and preference.
T1r3 KO mice consumed more quinine than WT mice at the three lowest quinine concentrations (0.003–0.03 mM; main effect of genotype: F1, 20 = 9.55, P < 0.01; main effect of concentration: F5, 100 = 114.84, P < 0.001; genotype × concentration interaction: F5, 100 = 6.62, P < 0.001; simple effects of genotype: Fs1, 22 ≥ 4.78, Ps < 0.05; Fig. 3A). KOs also displayed higher overall total fluid intake than controls during quinine testing [238.08 (± 25.84 SE) vs. 166.34 (± 10.46 SE), respectively; main effect of genotype: F1, 20 = 13.91, P < 0.01]. Across concentration, quinine intake in WT mice declined with each concentration up to 0.3 mM, but failed to differ at 0.3 and 1 mM (simple effect of concentration: F5, 55 = 85.07, P < 0.001, Newman-Keuls test, Ps < 0.05). Quinine intake in knockouts decreased as a function of concentration from 0.01–0.3 mM, but did not differ at the two lowest (0.003 and 0.01 mM) or highest (0.3 and 1 mM) concentrations (simple effect of concentration: F5, 55 = 39.63, P < 0.001, Newman-Keuls test, Ps < 0.05). The overall ANOVA additionally revealed that females consumed significantly more quinine than males at 0.003 and 0.01 mM (main effect of sex: F1, 20 = 7.85, P < 0.05; sex × concentration interaction: F5, 100 = 6.72, P < 0.001; simple effects of sex: Fs1, 22 ≥ 6.36, Ps < 0.05; Fig. 3B). Total fluid intake during quinine testing was also greater in females than in males [248.40 (± 22.97 SE) vs. 156.02 (± 9.78 SE), respectively; main effect of sex: F1, 20 = 23.05, P < 0.001]. Across concentration, quinine intake among males at 0.003 mM > 0.01–1 mM; 0.01, 0.03 mM > 0.1–1 mM; and 0.1 mM > 0.3, 1 mM (simple effect of concentration: F5, 55 = 70.22, P < 0.001, Newman-Keuls test, Ps < 0.05). In females, quinine intake at 0.003, 0.01 mM > 0.03–1 mM; 0.03 mM > 0.1–1 mM; and 0.1 mM > 1 mM (simple effect of concentration: F5, 55 = 41.39, P < 0.001, Newman-Keuls test, Ps < 0.05). The greater quinine consumption observed in KOs relative to controls and in females compared with males at low concentrations was driven primarily by elevated quinine intake in KO females (genotype × sex interaction: F1, 20 = 4.70, P < 0.05). Simple effects analysis on the genotype × sex interaction indicated higher quinine intake in KO females compared with KO males (simple effect of sex: F1, 10 = 6.60, P < 0.05), while quinine consumption between male and female WT mice did not differ (F1, 10 = 1.56, P = 0.24; Fig. 3D).
Fig. 3.
Voluntary intake (ml/kg/day) and percent preference for quinine in a 2-bottle choice assay. A: mean ± SE quinine intake as a function of concentration in T1r3 KO and C57BL/6J WT mice; n = 12/genotype. B: mean ± SE quinine intake by concentration in male and female mice; n = 12/sex. C: mean ± SE quinine preference at each concentration in KO and WT mice; n = 12/genotype. D: mean + SE quinine intake in KO and WT males and females collapsed across concentration. n = 6/sex/genotype. *Significant difference between KO and WT or male and female (P < 0.05).
No difference existed in quinine preference between T1r3 KO and WT mice (NS effect of genotype: F1, 20 = 0.84, P = 0.37; NS genotype × concentration interaction: F5, 100 = 0.52, P = 0.76). Both genotypes displayed a monotonic decrease in quinine preference with each increasing concentration from 0.01–0.3 mM (main effect of concentration: F5, 100 = 184.25, P < 0.001, Newman-Keuls test, Ps < 0.001; Fig. 3C). Preference at the two lowest (0.003 and 0.01 mM) and highest (0.3 and 1 mM) quinine concentrations did not significantly differ (Ps > 0.06). There was no effect of sex (F1, 20 = 0.08, P = 0.78) or interaction of sex with other factors (Fs < 2.95, Ps > 0.10) for quinine preference.
EXPERIMENT 2: RESPONSES OF CENTRAL GUSTATORY NEURONS
Materials and Methods
Animals.
Naive adult male and female T1r3 KO and WT mice like those described in experiment 1 were used for the electrophysiological recording experiments. Twenty-six cells were sampled from 12 WT mice (7 males, 5 females) and 28 cells were recorded from 16 KO mice (11 males, 5 females). The numbers of neurons sampled by genotype and sex were: WT male: 15; WT female: 11; KO male: 17; KO female: 11. All neurons were used in a prior study that first described their taste response properties (41). The present experiment describes previously unreported sensitivities of these cells to oral application of ethanol. A portion of the previous taste sensitivity data from these neurons is shown here to characterize their response properties to ethanol relative to different taste stimuli. Performing concurrent experiments on these cells served to expedite completion of the research and reduce the number of mice used.
Single-unit electrophysiology.
Single-unit data were acquired from mouse NTS neurons using conventional extracellular electrophysiological recording techniques described previously in detail (39, 40, 41, 42). The preparation and technique are briefly outlined here. Mice were anesthetized using urethane (1 g/kg ip) followed by pentobarbital (0.05 g/kg ip). If necessary, urethane supplements (∼0.15 g/kg each ip) were administered during an experiment to maintain anesthesia. Each mouse was tracheotomized, which allowed for normal breathing during solution flow into the mouth, and situated in a nontraumatic head holder. A portion of the occipital bone and parts of the cerebellum were removed to expose the dorsal surface of the brain stem. This procedure allowed for vertical access to the rostral, gustatory zone of NTS, the first synapse in the brain for taste information processing. The region of NTS that harbored gustatory-responsive neurons was identified by a change in neural activity associated with oral application of various taste stimuli. Spikes generated by a single taste neuron were isolated using a waveform template-matching algorithm (Spike 2/Power 1401 acquisition system; CED, Cambridge, UK).
Taste stimuli.
Twenty taste chemicals and a concentration series of ethanol (Table 1) were used for testing. Stimuli were individually applied to the mouth using a funnel/gravity flow system. This device bathed the palate and much of the tongue including the anterior portion innervated by the VIIth nerve, which is believed to mediate perceptual taste discrimination in rodents (60, 64). Taste stimuli included sodium salts, acids (sour), bitter tastants, and an array of sweet-tasting stimuli. Stimulus concentrations were chosen based on those known to evoke effective integrated responses in peripheral taste nerves in WT mice (13, 22, 27, 28). Tastants were reagent grade (Sigma, St. Louis, MO; Fisher, Pittsburgh, PA; ethanol 95%, Pharmco, Brookfield, CT), dissolved in deionized water and presented at room temperature.
Table 1.
Taste stimuli, concentrations, and their abbreviations used in experiment 2
| Stimulus | Class | [M] or % (vol/vol) | Abbrev. |
|---|---|---|---|
| Ethanol | alcohol | 3, 5, 10, 15, 25, 40% | E |
| Standard stimuli | |||
| Glycine | sweet | 1 | G 1 |
| Sucrose | sweet | 0.5 | S 0.5 |
| NaCl | salt | 0.1 | N |
| HCl | acid | 0.01 | H |
| Quinine-HCl | bitter | 0.01 | Q |
| Other stimuli | |||
| Glycine | sweet | 0.5 | G 0.5 |
| Sucrose | sweet | 1 | S 1 |
| d-glucose | sweet | 1 | glu |
| d-fructose | sweet | 1 | fru |
| L-proline | sweet | 1 | pro |
| d-sorbitol | sweet | 1 | sor |
| Na+-saccharin | art. sweet | 0.01 | sac |
| Acesulfame-K+ | art. sweet | 0.1 | ace |
| L-glutamic acid (Na+) | glutamate | 0.5 | msg |
| Na+-acetate | salt | 0.1 | naa |
| Na+-nitrate | salt | 0.1 | nan |
| Potassium chloride | salt | 0.1 | kcl |
| Citric acid | acid | 0.01 | cit |
| Papaverine-HCl | bitter | 0.03 | pap |
| Denatonium benzoate | bitter | 0.01 | den |
Neurons were initially tested with a standard set of sweet, salty, acidic, and bitter stimuli (Table 1), presented in random order. Cells were subsequently presented with a randomized block of sweet stimuli, an ascending concentration series of ethanol and additional salt, acid and bitter tastants, randomly ordered. Stimulus trials were structured using a water → stimulus → water protocol. Deionized water was initially flowed over the oral epithelium to adapt neurons to any tactile features of oral stimulation. The solution was then switched to a taste stimulus 6 s into a trial by a computer-controlled solenoid valve. The stimulus was delivered for 6 s, after which solution flow returned to deionized water. One to two minutes were allowed to elapse between trials, which provided sufficient time for neurons to return to baseline levels of spontaneous firing.
Data analysis.
On each trial taste responses were quantified as the net number of action potentials evoked by a stimulus, defined as the number of spikes arising during the 6 s stimulus presentation minus the number of spikes that occurred during the 6 s period prior to stimulus onset. Taste responses were considered significant relative to baseline if the mean firing rate within a 500 ms sliding window of stimulus-evoked activity was greater than the mean prestimulus firing rate by 1.96 standard deviations (41). Where applicable, response data were analyzed using ANOVA. Significant interactions from the overall ANOVAs were further evaluated using one-way analyses to test for simple effects. The alpha level for all statistical tests was 0.05. For those stimuli tested more than once on a single neuron, net responses were averaged prior to analyses. A 2 (genotype) × 2 (sex) × 5 (stimulus) mixed ANOVA and a 2 (genotype) × 2 (sex) × 6 (concentration) mixed ANOVA were initially performed on taste responses to the standard stimuli (Table 1; sweet, salty, acid and bitter tastants) and to ethanol, respectively, to assess if sex influenced taste responses to these stimuli. Sex did not influence neural taste sensitivity to the standard stimuli (NS effect of sex: F1, 50 = 0.75, P = 0.39; NS sex × genotype interaction: F1, 50 = 0.08, P = 0.78; NS sex × stimulus interaction: F4, 200 = 0.84, P = 0.50; NS sex × genotype × stimulus interaction: F4, 200 = 0.28, P = 0.89) or to ethanol (NS effect of sex: F1, 50 = 3.04, P = 0.09; NS sex × genotype interaction: F1, 50 = 1.13, P = 0.30; NS sex × concentration interaction: F5, 250 = 1.05, P = 0.40; NS sex × genotype × concentration interaction: F5, 250 = 1.17, P = 0.30) and therefore was not included as a factor in subsequent analyses.
For WT and KO neurons, relationships among activity to ethanol and tastants were explored using correlations (Pearson's r) computed between across-neuron responses to different stimuli. An across-neuron response represents the pattern of activity evoked by a stimulus across all neurons sampled. This activity provides an estimate of a global, spatial response that a stimulus would evoke in NTS. Stimuli that are processed similarly evoke similar across-neuron patterns of response (55, 57, 58).
Results
Response properties of NTS gustatory neurons.
The breadth of responsiveness of each WT and KO NTS neuron was indexed by determining the number of prototype stimuli [(in M) 0.5 sucrose (sweet), 0.1 NaCl (salt), 0.01 HCl (acid) and 0.01 quinine (bitter)] that elicited a significant taste response. For WT cells, 1 cell (4%) responded to only 1 stimulus, 3 cells (12%) responded to 2 stimuli, 15 cells (58%) responded to 3 stimuli, and 7 cells (27%) responded to all 4 of the prototypes. Among KO neurons, 6 cells (21%) responded to 1 prototype, 4 cells (14%) responded to 2, 15 cells (54%) responded to 3, and 2 cells (7%) responded to all 4 prototypes. Although some cells from both genotypes displayed selectivity, the majority of neurons sampled were multisensitive: responding to stimuli of different taste qualities. Multisensitivity is a feature common to gustatory neurons in rodent NTS (62). The stimulus-response characteristics of a sample WT neuron are shown in Fig. 4. The relative sensitivities of all WT and KO neurons to (in M) 0.5 sucrose, 0.1 NaCl, 0.01 HCl, and 0.01 quinine are shown in Supplemental Fig. S1.1
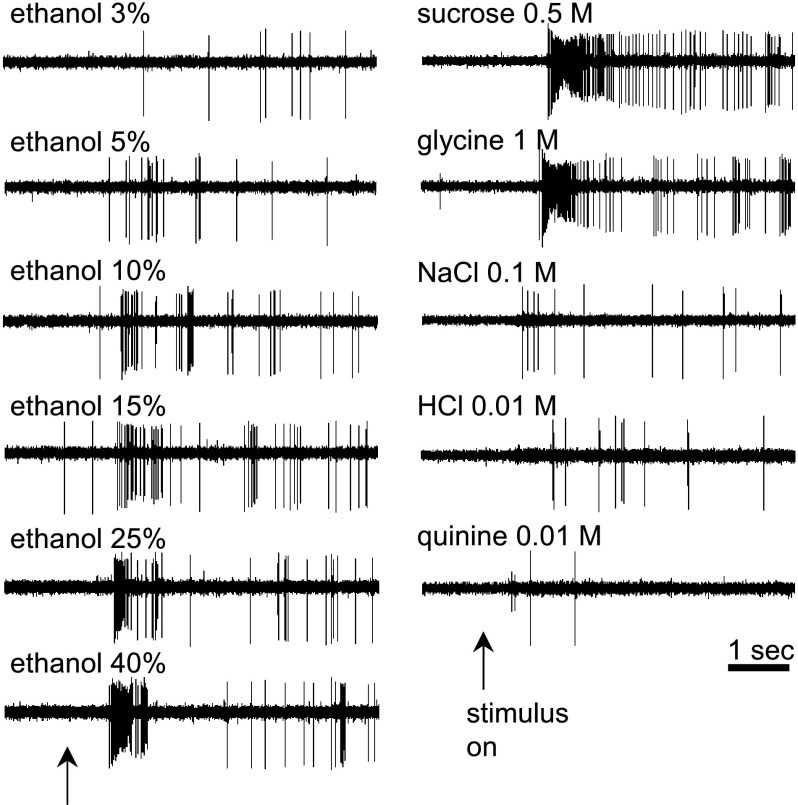
Fig. 4.
Digital oscilloscope records showing taste activity measured from a single nucleus of the solitary tract (NTS) neuron recorded from a C57BL/6J WT mouse. Taste-evoked trains of action potentials were elicited by oral application of various concentrations of ethanol and sweet (sucrose, glycine), salty (NaCl), acidic (HCl), and bitter (quinine) taste stimuli. ↑Stimulus onset.
Central gustatory responses to ethanol and standard taste stimuli.
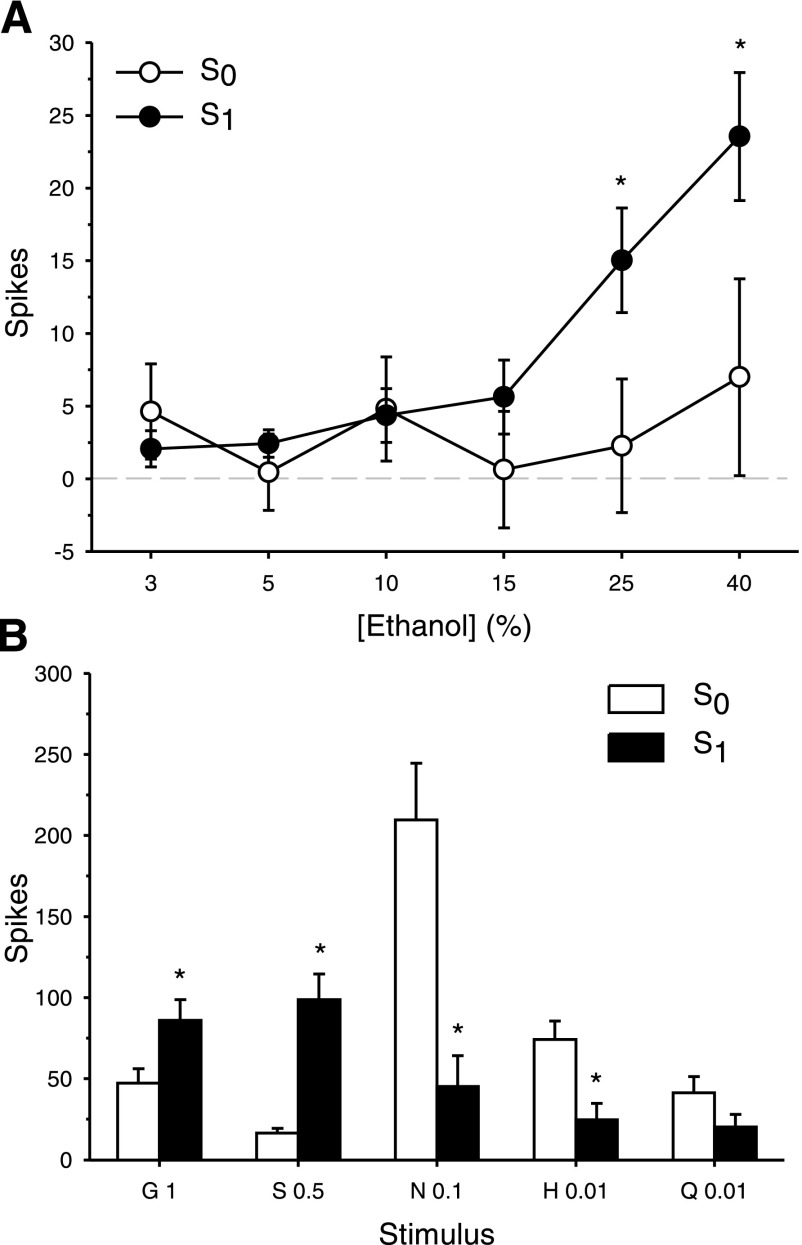
C57BL/6J WT mice consume larger amounts of sweet tasting substances and ethanol relative to other mouse strains (9, 21, 73). To determine in this strain the relationship between gustatory neural sensitivity to sweet stimuli and ethanol, WT neurons were classified as either significantly responsive to sucrose (S1, n = 15) or nonresponsive to sucrose (S0, n = 11) and taste responses to ethanol were compared between these neuron groups. Oral application of ethanol produced a concentration-dependent increase in the firing of WT NTS taste neurons (main effect of concentration: F5, 120 = 10.28, P < 0.0001). A significant interaction between sucrose responsiveness and ethanol concentration was found for WT cells (F5, 120 = 5.65, P = 0.0001, Fig. 5A). Analysis of simple effects revealed that taste responses to 25 and 40% ethanol were significantly larger in S1 neurons relative to S0 cells (Fs1, 24 ≥ 4.59, Ps = 0.04). Thus, in WT mice NTS taste neurons that significantly respond to sucrose are more strongly activated by high concentrations of ethanol than those cells unresponsive to sucrose. S1 and S0 cells also responded differentially to the standard taste stimuli (sucrose responsiveness × stimulus interaction: F4, 96 = 20.53, P < 0.0001), with S1 cells showing greater sensitivity, expectedly, to (in M) 1 glycine and 0.5 sucrose and S0 cells responding more strongly to 0.1 NaCl and 0.01 HCl (simple effects of sucrose responsiveness, Fs1, 24 ≥ 5.34, Ps ≤ 0.03, Fig. 5B).
Fig. 5.
Responses to ethanol and taste stimuli in sucrose responsive (S1, n = 15) and sucrose nonresponsive (S0, n = 11) NTS neurons recorded from C57BL/6J WT mice. A: mean ± SE responses in S0 and S1 cells to an ascending concentration (%, vol/vol) series of ethanol. B: mean + SE responses in S0 and S1 cells to glycine (G), sucrose (S), NaCl (N), HCl (H), and quinine (Q). Concentration ([M]) follows abbreviation. *Significant difference between S0 and S1 (P < 0.05).
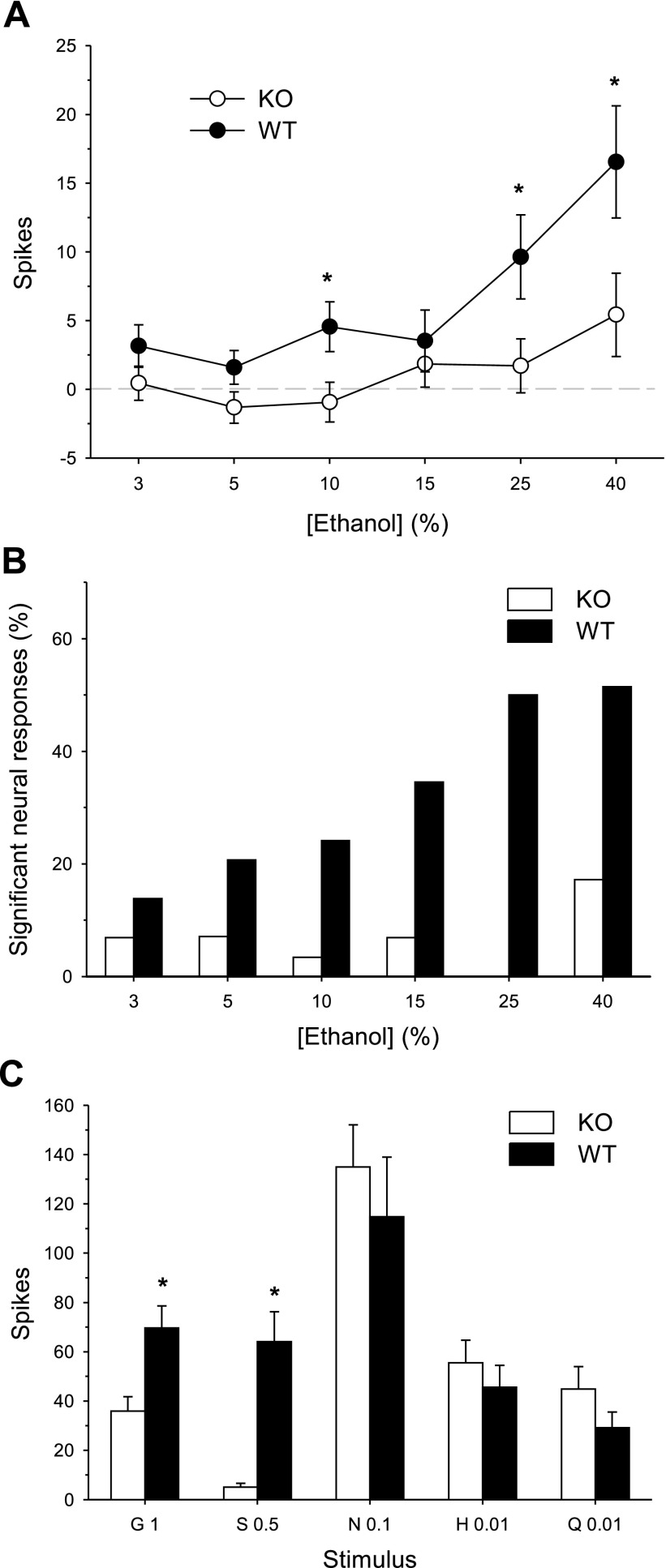
In experiment 1, T1r3 KO mice displayed significantly reduced ethanol intake and preference relative to WT controls (Fig. 1). To determine if the reduced behavioral preference for ethanol in T1r3 KO mice is accompanied by a reduction in the central neural processing of ethanol taste, we compared taste responses to ethanol and to the standard taste stimuli in NTS neurons sampled from KO and WT mice. A significant genotype × ethanol concentration interaction was observed (F5, 260 = 2.69, P = 0.02). Taste responses to 10, 25, and 40% ethanol were suppressed in KO neurons relative to WT cells (simple effects of genotype, Fs1, 52 ≥ 4.87, Ps ≤ 0.03, Fig. 6A). A simple effect of ethanol concentration on neural activity was observed for WT neurons (F5, 125 = 10.76, P < 0.001). The mean NTS response in WT cells to 25% ethanol was significantly greater than responses to 3, 5, 10, and 15% and the mean response to 40% ethanol was greater than responses to all lower concentrations (Newman-Keuls test, Ps < 0.04). NTS responses in WT cells to 3, 5, 10, and 15% ethanol did not differ from one another (Ps > 0.05). A simple effect of ethanol concentration on neural activity was also found for KO neurons (F5, 135 = 2.94, P = 0.01). NTS responses in KO cells to 40% ethanol were greater than those to 5 and 10% ethanol (Newman-Keuls test, Ps < 0.02). In KO neurons, responses to ethanol were significantly elevated from baseline activity on a small proportion of trials (Fig. 6B), with the highest frequency observed for 40% ethanol, which activated five of 28 KO neurons sampled (17.9%). Thus, ethanol produced some degree of activity in KO cells, particularly at the highest concentration tested. Compared with WT cells, however, there was a striking reduction in the proportion of trials for KO neurons where a significant taste response to ethanol was detected (Fig. 6B).
Fig. 6.
Responses to ethanol and taste stimuli in all NTS neurons (n = 28) sampled from T1r3 KO mice and all NTS neurons (n = 26) recorded from C57BL/6J WT mice. A: mean ± SE responses in KO and WT cells to an ascending concentration (%, vol/vol) series of ethanol. B: for each genotype, percentage of all sampled neural responses to each concentration of ethanol that were significantly elevated above baseline (prestimulus) activity. C: mean + SE responses in KO and WT cells to glycine (G), sucrose (S), NaCl (N), HCl (H), and quinine (Q). Concentration ([M]) follows abbreviation. *Significant difference between KO and WT (P < 0.05).
Genotype influenced NTS neural sensitivity to the standard stimuli (genotype × stimulus interaction: F4, 208 = 4.73, P = 0.001). Taste responses to (in M) 1 glycine and 0.5 sucrose were, expectedly, lower in KO neurons (simple effects of genotype, Fs1, 52 ≥ 10.27, Ps ≤ 0.002, Fig. 6C). Taste responses to 0.1 NaCl, 0.01 HCl and 0.01 quinine did not differ between genotypes (Fs1, 52 ≤ 1.95, Ps ≥ 0.17). Thus, NTS neurons in mice lacking T1r3 showed reduced sensitivity to ethanol and sweet stimuli, but not to stimuli from other taste classes.
Across-neuron correlations between responses to ethanol and taste stimuli.
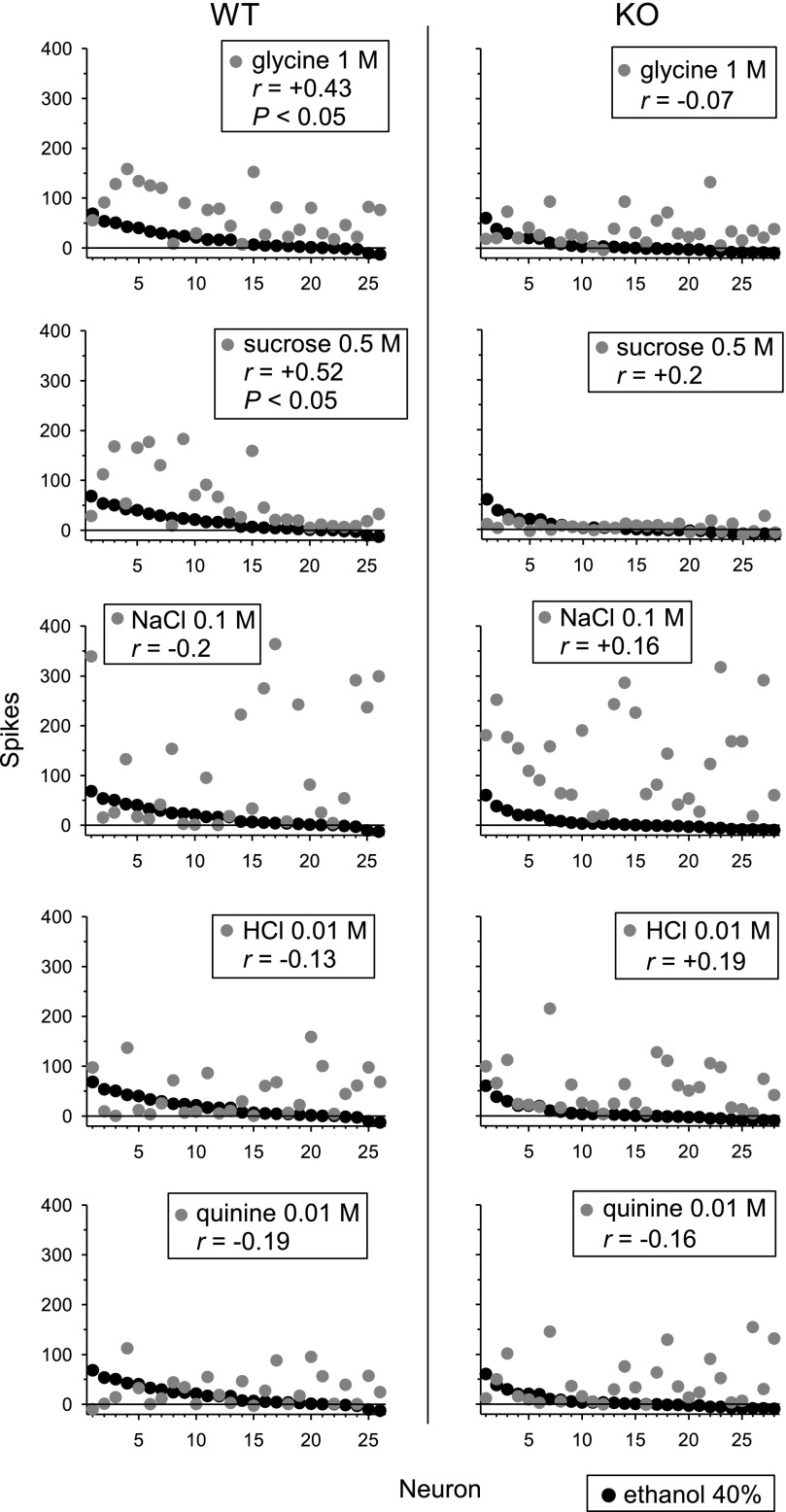
To examine the relationship between across-neuron responses to ethanol and other taste stimuli, we assessed correlations between responses to 40% ethanol, the concentration evoking the most salient activity in NTS, and responses to the standard stimuli (Table 1) across all WT and KO neurons. In WT neurons, across-neuron responses to ethanol positively correlated (P < 0.05) with those evoked by (in M) 1 glycine (r = +0.43) and 0.5 sucrose (r = +0.52) but showed no significant correlation (P > 0.05) with activity to 0.1 NaCl, 0.01 HCl, and 0.01 quinine (Fig. 7). Taste responses to ethanol in KO neurons showed no significant correlation with activity to any standard taste stimulus. Thus, among the standard stimuli, NTS taste activity to ethanol in WT mice selectively correlated with responses to sweet stimuli.
Fig. 7.
Across-neuron patterns of response to 40% ethanol and sweet (glycine, sucrose), salty (NaCl), acidic (HCl), and bitter (quinine) stimuli measured across NTS neurons (n = 26) recorded from C57BL/6J WT mice and NTS cells (n = 28) sampled from T1r3 KO mice. The Pearson coefficient of correlation (r) calculated between the response pattern to ethanol and each stimulus is given. P < 0.05 indicates that a correlation was significant.
A subset of WT (n = 24) and KO (n = 19) neurons were isolated long enough to allow for testing with all stimuli in Table 1. For these cells, correlations were computed within genotype among responses to each of the 6 concentrations of ethanol and all 20 taste stimuli (Table 2). In WT neurons, across-neuron responses to 25 and 40% ethanol, concentrations that evoked robust ethanol responses, positively correlated (P < 0.05) with activity evoked by several sweet stimuli (sucrose, fructose, glucose, glycine, sorbitol, and acesulfame-K) but showed no significant correlation (P > 0.05) with activity to any nonsweet tastant. Positive correlations were also observed between WT responses to 3% ethanol and certain Na+ stimuli. Such correlations, however, could potentially be spurious given that 3% ethanol consistently produced only very weak activity in NTS neurons (Figs. 4, 6) and correlations between WT activity to increasing concentrations of ethanol and Na+ stimuli did not systematically increase (Table 2). In KO neurons, responses to 25 and 40% ethanol showed no significant correlation (P > 0.05) with the response to any taste stimulus.
Table 2.
Pearson coefficients of correlation (r) among across-neuron responses to taste stimuli and ethanol in 24 WT and 19 KO NTS neurons
| WT |
KO |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol, % |
Ethanol, % |
|||||||||||
| 3 | 5 | 10 | 15 | 25 | 40 | 3 | 5 | 10 | 15 | 25 | 40 | |
| S 0.5 | −0.14 | 0.20 | 0.07 | 0.32 | 0.42 | 0.49 | 0.29 | −0.47 | 0.24 | 0.11 | 0.36 | 0.33 |
| S 1 | −0.22 | 0.18 | 0.08 | 0.31 | 0.44 | 0.47 | 0.16 | −0.04 | 0.03 | −0.28 | −0.02 | −0.07 |
| fru | −0.11 | 0.19 | 0.09 | 0.36 | 0.43 | 0.53 | 0.03 | −0.47 | −0.22 | −0.22 | −0.25 | −0.12 |
| glu | 0.00 | 0.20 | 0.21 | 0.36 | 0.41 | 0.53 | 0.10 | −0.56 | −0.09 | −0.01 | 0.00 | 0.12 |
| G 0.5 | −0.01 | 0.10 | −0.03 | 0.16 | 0.31 | 0.45 | −0.25 | −0.43 | −0.43 | −0.26 | −0.23 | −0.12 |
| G 1 | 0.02 | 0.03 | −0.01 | 0.10 | 0.25 | 0.39 | −0.12 | −0.44 | −0.31 | −0.24 | −0.15 | −0.08 |
| pro | −0.12 | −0.03 | −0.03 | 0.05 | 0.22 | 0.36 | −0.31 | −0.63 | −0.45 | −0.11 | −0.10 | −0.02 |
| sor | 0.02 | 0.16 | 0.15 | 0.22 | 0.27 | 0.52 | 0.24 | −0.48 | −0.31 | −0.27 | −0.15 | −0.13 |
| ace | −0.24 | 0.08 | 0.03 | 0.27 | 0.45 | 0.46 | −0.01 | −0.35 | −0.06 | 0.22 | −0.21 | 0.15 |
| sac | 0.19 | −0.06 | 0.26 | 0.04 | 0.00 | 0.10 | 0.02 | −0.15 | 0.04 | 0.42 | 0.17 | 0.30 |
| msg | 0.43 | −0.09 | 0.22 | −0.13 | −0.23 | −0.14 | −0.02 | −0.20 | −0.02 | 0.27 | −0.18 | 0.10 |
| N | 0.42 | −0.02 | 0.28 | −0.07 | −0.23 | −0.13 | 0.02 | −0.30 | 0.03 | 0.33 | −0.06 | 0.21 |
| nan | 0.38 | −0.09 | 0.22 | −0.14 | −0.27 | −0.18 | 0.06 | −0.27 | 0.14 | 0.45 | −0.03 | 0.31 |
| naa | 0.41 | −0.06 | 0.24 | −0.11 | −0.26 | −0.16 | 0.07 | −0.26 | 0.16 | 0.47 | −0.03 | 0.30 |
| kcl | 0.16 | −0.07 | −0.05 | −0.17 | −0.02 | −0.04 | −0.04 | −0.40 | 0.03 | 0.14 | 0.00 | 0.22 |
| H | 0.25 | −0.08 | 0.03 | −0.12 | −0.10 | −0.14 | −0.06 | −0.41 | 0.10 | 0.29 | −0.01 | 0.34 |
| cit | 0.03 | −0.27 | −0.24 | −0.28 | −0.21 | −0.28 | −0.18 | −0.32 | 0.11 | 0.20 | −0.07 | 0.22 |
| den | 0.26 | 0.01 | 0.10 | −0.06 | −0.18 | −0.15 | −0.16 | −0.53 | −0.02 | 0.29 | 0.23 | 0.36 |
| pap | 0.13 | −0.03 | −0.07 | −0.10 | −0.09 | −0.07 | −0.17 | −0.53 | −0.04 | 0.15 | −0.08 | 0.14 |
| Q | −0.01 | −0.40 | −0.28 | −0.37 | −0.26 | −0.25 | −0.24 | −0.54 | −0.19 | −0.02 | −0.15 | 0.03 |
| E 3% | 0.35 | 0.29 | 0.37 | 0.20 | 0.16 | 0.10 | 0.33 | 0.11 | 0.18 | 0.10 | ||
| E 5% | 0.81 | 0.83 | 0.74 | 0.76 | 0.20 | −0.06 | 0.08 | −0.09 | ||||
| E 10% | 0.80 | 0.70 | 0.72 | 0.62 | 0.69 | 0.70 | ||||||
| E 15% | 0.80 | 0.79 | 0.70 | 0.91 | ||||||||
| E 25% | 0.83 | 0.84 | ||||||||||
Coefficients in boldface are significant at P < 0.05. See Table 1 for abbreviations.
DISCUSSION
The present results demonstrate that the T1r3 taste receptor protein is mechanistically involved in oral ethanol intake and preference and the neurophysiological processing of ethanol taste in C57BL/6J mice. In long-term voluntary intake tests, mice lacking the T1r3 receptor exhibited indifference to alcohol at concentrations preferred by wild-type mice (5–15%). Furthermore, taste responses to ethanol recorded from NTS neurons in T1r3 KO mice were significantly reduced compared with C57BL/6J control mice, a strain for which oral ethanol stimulation produced a concentration-dependent activation of sweet-responsive NTS gustatory neurons and a pattern of activity in NTS taste circuits that correlated positively with that evoked by sweet stimuli. The T1r3 receptor subunit is known to be critically involved in taste detection and preference for several natural and artificial sweeteners, including sucrose, saccharin, sucralose, acesulfame K, SC45647, and others (12, 45, 47, 48, 74). The present data strongly suggest that the T1r3 receptor is also involved in the sensory transduction of ethanol taste and that ethanol's interaction with T1r3 may result in stimulation of appetitive gustatory circuitry that contributes to preference and intake of the drug in C57BL/6J mice.
Consistent with previous findings in heterogeneous rats (39), alcohol stimulation of the tongue and palate of C57BL/6J WT mice produced the most potent activation of sweet-responsive NTS gustatory neurons at high concentrations (25 and 40%) and neural taste responses to ethanol at these concentrations more robustly and significantly correlated with those to sucrose and other sweeteners than did taste responses at lower ethanol concentrations. This concentration-dependent increase in activation of sweet taste circuits by ethanol differs to some degree from the behavioral preference functions for ethanol in C57BL/6J mice in two-bottle choice procedures observed here and in other studies (3), where this strain displays an inverted U-shape function in ethanol preference, with peak preference at 10% and then a gradual decline in preference with rising ethanol concentration. Differences in the behavioral and physiological concentration-response functions are informative given that gustatory neural recordings more specifically isolate taste responses induced by ethanol, while preference patterns for ethanol in the two-bottle choice assay reflect the combined influence of gustatory, olfactory, somatosensory (i.e., trigeminal), and postabsorptive ethanol effects. Collectively, the present data indicate that the attenuated difference in ethanol preference between T1r3 KO and WT mice as ethanol concentration increased >15% was not due to a reduction in ethanol-induced sweet taste input in WT mice at higher concentrations but, rather, an increase in other sensory and/or postingestive influences resulting in a decline in overall ethanol preference at these concentrations. It has been demonstrated previously in heterogeneous rats that oral ethanol evoked activation of central trigeminal pathways increases at concentrations >15% (10), as does ethanol's burning and irritant sensations in humans (18, 70), which may serve to modulate ethanol's sweet taste properties at high concentrations. An aversive oral somatosensory component of ethanol and/or increased intoxicating effects at higher concentrations may therefore compete with sweet taste input, resulting in reduced differences in alcohol preference between T1r3 KO and WT mice that is not reflected in measurement of gustatory neural responses alone. That T1r3 receptor-mediated input is critical for behavioral ethanol preference in C57BL/6J mice at concentrations of 5–15% concurs with recent data of reduced ethanol preference in three mutant mouse strains lacking genes involved in sweet taste transduction (5). The number of significant neural taste responses evoked by ethanol at these and higher concentrations was also substantially reduced in T1r3 KO compared with WT mice (Fig. 6B). The present findings of suppressed NTS gustatory responses to ethanol in T1r3-deficient mice suggest that the absence of this receptor may inhibit the initial sensory transduction of ethanol taste and subsequent relaying of an appetitive ethanol taste message to the central nervous system.
NTS gustatory neurons receive ascending sensory input from afferent nerves and also descending centrifugal input from higher nuclei. In rats, taste responses in NTS neurons survive decerebration procedures where centrifugal input to the brain stem is removed (25), reflecting the prominent ascending oral sensory component to NTS gustatory activity. However, centrifugal inputs are known to modulate taste responses in NTS neurons. The central structures documented to influence processing in gustatory NTS include forebrain nuclei involved in affective responding and ingestive behaviors (11, 43). Although initiated by sensory input, taste responses measured from the NTS therefore represent network interactions between this nucleus and other central nuclei and not solely pure sensory messages from afferent nerves (59). Ascending and descending inputs likely contribute to NTS responses to orally applied ethanol, although the relative contribution of these inputs is not precisely known. Nevertheless, the present data suggest that ethanol and sucrose share attributes that are encoded by taste-responsive NTS neurons and that input from the T1r3 taste receptor partially mediates this overlap.
The maintenance of residual activity to ethanol in some KO cells indicates that mechanisms other than T1r3 may also contribute to ethanol taste activity in NTS neurons. Prior behavioral data have indicated that in addition to its sweet taste properties, ethanol may also possess a bitter gustatory component, as conditioned taste aversions to alcohol cross-generalize to mixtures of sucrose and quinine in rats (36, 37) and to either tastant alone in C57BL/6J mice (6). Nevertheless, the present study in C57BL/6J mice and other reports to date that have measured neurophysiological responses to ethanol and various tastants in gustatory circuits of outbred rats (39, 42) and nonhuman primates (26) have not found a relationship between neural taste responses elicited by ethanol and bitter stimuli. In both the chorda tympani and glossopharyngeal nerves of primates (innervating the anterior and posterior tongue, respectively), ethanol in mixtures with quinine has rather been shown to suppress bitter taste responses, consistent with the properties of a sweetener (14, 26). Given that cells sampled from T1r3 KOs did not display a significant correlation between responses to any bitter stimulus tested and that to 40% ethanol, the concentration at which residual ethanol-evoked activity was most pronounced, the source of the remaining activity to ethanol in a proportion of knockout cells remains to be determined.
As expected, sucrose consumption and preference were markedly reduced in T1r3 KOs compared with WT control mice, as were central neurophysiological taste responses to sucrose and other sweeteners. T1r3 receptor-deficient mice nevertheless maintained substantial preference for sucrose at concentrations of 0.3 M and above, with both KO and WT mice exhibiting near maximal preference for sucrose at the highest concentration tested (1 M). These data are consistent with those of Damak et al. (12), who found levels of sucrose preference in T1r3 null mice to begin to approach those of C57BL/6J WT mice at concentrations ≥0.16 M. Given that T1r3 KOs in the current study and that of Damak et al. (12) display strongly suppressed neural gustatory responses to sucrose at 0.5 M and that T1r3-deficient mice show only weak appetitive responses to 0.3 and 1 M sucrose in short-term lick tests (74), it is likely that the postingestive reinforcing effects (e.g., caloric density) of sucrose at high concentrations during long-term exposure mediate preference via mechanisms independent of T1r3. This assumption is in line with recent data that mice lacking another protein necessary for sweet taste signaling (TRPM5) can develop preference for highly concentrated sucrose based on its caloric content (15). T1r3 null mice may also retain some capacity to detect sucrose via other sensory receptors, as T1r3 knockouts have been shown to exhibit normal sucrose detection thresholds and only a partially diminished ability to discriminate sucrose in sensory discrimination tests (16). In contrast to strongly suppressed sweet responses, NTS cells from T1r3 KO and WT mice in the current study did not differ in responsiveness to prototypic salt, acid, and bitter stimuli, nor did T1r3 KO and control mice differ in behavioral preference for quinine. Although KOs consumed more quinine than WT mice at low concentrations (0.003–0.03 mM), this effect appeared to be a product of greater overall fluid intake among knockouts during quinine testing, as correction for variation in total fluid intake with the preference ratio measure resulted in comparable quinine preference scores for each genotype.
In addition to genotype differences in ethanol and sweetener responses, significant sex effects in alcohol preference were observed in the behavioral intake tests, with females displaying elevated preference relative to males at the highest ethanol concentrations (15–40%). These findings are consistent with other reports of greater alcohol consumption and preference in female than in male C57BL/6 mice (46) and rats (38, 53), particularly at high concentrations. An absence of sex differences in alcohol-elicited NTS taste responses in the current study suggests that the enhanced alcohol preference exhibited by female C57BL/6J mice may be mediated by sex differences in the drug's postabsorptive consequences vs. its immediate gustatory processing. Such factors may include metabolic differences resulting in reduced acetaldehyde accumulation in females compared with males (51). Female mice also displayed modestly higher overall sucrose preference than did male mice; however, this effect was observed only among T1r3 KOs and thus may be mediated by mechanisms other than those producing sex differences in alcohol preference. While KO females in the present study also consumed more quinine overall per unit body weight than males, adjustment for variation in total fluid intake yielded no sex differences in quinine preference. Furthermore, no differences in the magnitude of gustatory neural activity evoked by standard concentrations of sucrose, glycine, quinine, NaCl, or HCl were found between males and females of either genotype, indicating no obvious influence of sex on basic taste responses in C57BL/6J mice at the level of the NTS.
Implications
The present findings that the T1r3 taste receptor protein is directly involved in oral ethanol intake and preference in C57BL/6J mice are in agreement with prior genetic mapping data that the Sac/Tas1r3 locus (encoding for the T1r3 protein) corresponds to a genetic locus that strongly influences 10% ethanol intake in F2 hybrids from the B6 × 129 strains (Ap3q; 1). While a direct role for sweet taste substrates in alcohol consumption in other species remains to be determined, previous data in outbred rats has shown that pharmacological antagonism of oral sweet receptors significantly inhibits the ability of oral alcohol to activate central sweet-responsive gustatory pathways (39). Furthermore, several independently derived lines of genetically ethanol-preferring rats, including those used as animal models of alcoholism, are known to display elevated sweetener intake (19, 56, 67, 68, 71) and elevated sweet preference may also serve as a phenotypic marker of genetic risk for alcoholism in humans (31, 35).
There is known allelic variation in the Tas1r3 taste receptor gene that is associated with differential sweetener preference in mice. Tas1r3 polymorphisms correlate with saccharin preference across multiple inbred mouse strains (52) and impact behavioral and neural taste responses to several sweeteners in B6 × 129 F2 hybrids (28) and 129.B6-Tas1r3 congenic mice (27). The present data predict that genetic variation in Tas1r3 may also be associated with individual and/or strain differences in ethanol preference, by influencing detection of an appetitive ethanol taste signal mediated via the T1r3 receptor at early stages of gustatory processing. Variation in oral ethanol processing at the taste receptor level would be expected to have downstream consequences in central reinforcement pathways previously shown to be activated by sweet taste inputs. For example, oral sucrose stimulation results in an immediate concentration-dependent increase in dopamine release in the nucleus accumbens (24), which is attenuated by selective damage to limbic, but not thalamocortical, taste projections (23, 49).
In summary, these findings support overlap in the gustatory receptor mechanisms responsible for processing alcohol and sweet taste. Specifically, the T1r3 taste receptor, important for mammalian sweet taste transduction, is essential for the expression of oral ethanol preference in C57BL/6J mice, and suppression of neural taste responses to ethanol in mice lacking this receptor suggests that the T1r3 receptor contributes to the initial sensory detection and transduction of ethanol taste. These data warrant further examination of the importance of gustatory substrates and associated ingestive circuits linked to this sensory system in alcohol consumption.
GRANTS
This research was supported by National Institutes of Health Grants AA-015741 (S. M. Brasser) and DC-008194 (C. H. Lemon).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Robert Margolskee and Kristin Hamre for providing us with the T1r3 KO and C57BL/6J WT mice, respectively, and Drs. Thomas Scott and Edward Riley for valuable comments on an earlier version of the manuscript. We remember Dr. David Smith for his past support of this work.
A portion of these data were presented at the 2006 meeting of the Society for Neuroscience, Atlanta, GA, and the 2008 meeting of the Research Society on Alcoholism, Washington, DC.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Bachmanov AA, Reed DR, Li X, Li S, Beauchamp GK, Tordoff MG. Voluntary ethanol consumption by mice: genome-wide analysis of quantitative trait loci and their interactions in a C57BL/6ByJ × 129P3/J F2 intercross. Genome Res 12: 1257–1268, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet 26: 563–573, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res 20: 201–206, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 112: 503–510, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 7: 1–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet 37: 146–159, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res 24: 253–258, 2000. [PubMed] [Google Scholar]
- 8. Brasser SM, Mozhui K, Smith DV. Differential covariation in taste responsiveness to bitter stimuli in rats. Chem Senses 30: 793–799, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Capeless CG, Whitney G. The genetic basis of preference for sweet substances among inbred strains of mice: preference ratio phenotypes and the alleles of the Sac and dpa loci. Chem Senses 20: 291–298, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol 80: 465–492, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Cho YK, Li CS, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses 28: 155–171, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Danilova V, Hellekant G. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci 4: 5, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Danilova V, Hellekant G. The taste of ethanol in a primate model. II. Glossopharyngeal nerve response in Macaca mulatta. Alcohol 21: 259–269, 2000. [DOI] [PubMed] [Google Scholar]
- 15. De Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron 57: 930–941, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1r3 knockout mice. Chem Senses 31: 351–357, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol 3: 55–61, 1986. [DOI] [PubMed] [Google Scholar]
- 18. Diamant H, Funakoshi M, Strom L, Zotterman Y. Electrophysiological studies on human taste nerves. In: Olfaction and Taste I, edited by Zotterman Y. New York: Pergamon, 1963. [Google Scholar]
- 19. Dyr W, Kostowski W. Animal model of ethanol abuse: rats selectively bred for high and low voluntary alcohol intake. Acta Pol Pharm 57 Suppl: 90–92, 2000. [PubMed] [Google Scholar]
- 20. Ellingson JM, Silbaugh BC, Brasser SM. Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet 39: 62–72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuller JL. Single-locus control of saccharin preference in mice. J Hered 65: 33–36, 1974. [DOI] [PubMed] [Google Scholar]
- 22. Gannon KS, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol Behav 57: 231–239, 1995. [DOI] [PubMed] [Google Scholar]
- 23. Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286: R31–R37, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Hayama T, Ito S, Ogawa H. Responses of solitary tract nucleus neurons to taste and mechanical stimulation of the oral cavity in decerebrate rats. Exp Brain Res 60: 235–242, 1985. [DOI] [PubMed] [Google Scholar]
- 26. Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model: I. Chorda tympani nerve response in Macaca mulatta. Alcohol 14: 473–484, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics 32: 82–94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci 24: 2296–2303, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 154: 269–270, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Kampov-Polevoy AB, Garbutt JC, Davis CE, Janowsky DS. Preference for higher sugar concentrations and Tridimensional Personality Questionnaire scores in alcoholic and nonalcoholic men. Alcohol Clin Exp Res 22: 610–614, 1998. [DOI] [PubMed] [Google Scholar]
- 31. Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res 27: 1743–1749, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol 7: 83–85, 1990. [DOI] [PubMed] [Google Scholar]
- 33. Kampov-Polevoy AB, Overstreet DH, Crosby RD, Rezvani AH, Janowsky DS, Halikas JA. Saccharin-induced polydipsia as a predictor of voluntary alcohol intake in Wistar rats. In: Biological Basis of Individual Sensitivity to Psychotropic Drugs, edited by Seredenin SB, Longo V, Gaviraghi G. Edinburgh: Graffham, 1994. [Google Scholar]
- 34. Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol 36: 165–170, 2001. [DOI] [PubMed] [Google Scholar]
- 35. Kampov-Polevoy AB, Ziedonis D, Steinberg ML, Pinsky I, Krejci J, Eick C, Boland G, Khalitov E, Crews FT. Association between sweet preference and paternal history of alcoholism in psychiatric and substance abuse patients. Alcohol Clin Exp Res 27: 1929–1936, 2003. [DOI] [PubMed] [Google Scholar]
- 36. Kiefer SW, Lawrence GJ. The sweet-bitter taste of alcohol: aversion generalization to various sweet-quinine mixtures in the rat. Chem Senses 13: 633–641, 1988. [Google Scholar]
- 37. Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses 18: 509–522, 1993. [Google Scholar]
- 38. Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol 9: 415–420, 1992. [DOI] [PubMed] [Google Scholar]
- 39. Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol 92: 536–544, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Lemon CH, Imoto T, Smith DV. Differential gurmarin suppression of sweet taste responses in rat solitary nucleus neurons. J Neurophysiol 90: 911–923, 2003. [DOI] [PubMed] [Google Scholar]
- 41. Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101: 2459–2471, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol 94: 3719–3729, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Li CS, Cho YK, Smith DV. Taste responses of neurons in the hamster solitary nucleus are modulated by the central nucleus of the amygdala. J Neurophysiol 88: 2979–2992, 2002. [DOI] [PubMed] [Google Scholar]
- 44. Lush IE. The genetics of tasting in mice VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res 53: 95–99, 1989. [DOI] [PubMed] [Google Scholar]
- 45. Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001. [DOI] [PubMed] [Google Scholar]
- 46. Middaugh LD, Kelley BM, Bandy AE, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol 17: 175–183, 1999. [DOI] [PubMed] [Google Scholar]
- 47. Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–498, 2001. [DOI] [PubMed] [Google Scholar]
- 48. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. [DOI] [PubMed] [Google Scholar]
- 49. Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 89: 531–535, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res 17: 366–369, 1993. [DOI] [PubMed] [Google Scholar]
- 51. Peterson CM, Scott BK, McLaughlin SD. Studies of whole blood associated acetaldehyde as a marker for alcohol intake: effect of gender in mice. Alcohol 8: 35–38, 1991. [DOI] [PubMed] [Google Scholar]
- 52. Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci 24: 938–946, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Russell KE, Stern MH. Sex and strain as factors in voluntary alcohol intake. Physiol Behav 10: 641–642, 1973. [DOI] [PubMed] [Google Scholar]
- 54. Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend 60: 199–206, 2000. [DOI] [PubMed] [Google Scholar]
- 55. Scott TR, Giza BK. Issues of gustatory neural coding: where they stand today. Physiol Behav 69: 65–76, 2000. [DOI] [PubMed] [Google Scholar]
- 56. Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol 9: 155–160, 1992. [DOI] [PubMed] [Google Scholar]
- 57. Smith DV, Scott TR. Gustatory neural coding. In: Handbook of Olfaction and Gustation (2nd ed.), edited by Doty RL. New York: Marcel Dekker, 2003. [Google Scholar]
- 58. Smith DV, St. John SJ. Neural coding of gustatory information. Cur Opin Neurobiol 9: 427–435, 1999. [DOI] [PubMed] [Google Scholar]
- 59. Smith DV, Ye MK, Li CS. Medullary taste responses are modulated by the bed nucleus of the stria terminalis. Chem Senses 30: 421–434, 2005. [DOI] [PubMed] [Google Scholar]
- 60. Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol Regul Integr Comp Physiol 263: R169–R176, 1992. [DOI] [PubMed] [Google Scholar]
- 61. Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110: 1096–1109, 1996. [PubMed] [Google Scholar]
- 62. Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005. [DOI] [PubMed] [Google Scholar]
- 63. St John SJ, Garcea M, Spector AC. Combined, but not single, gustatory nerve transection substantially alters taste-guided licking behavior to quinine in rats. Behav Neurosci 108: 131–140, 1994. [DOI] [PubMed] [Google Scholar]
- 64. St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci 18: 4353–4362, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stewart RB, Bice P, Foroud T, Lumeng L, Li TK, Carr LG. Correlation of saccharin and ethanol intake in the F2 progeny of HAD2 and LAD2 crosses. Alcohol Clin Exp Res 27 Suppl: 49A, 2003. [Google Scholar]
- 66. Stewart RB, Murphy JM, Lumeng L, Li TK. Sweet preference and spontaneous motor activity are correlated with alcohol intake in the F2 progeny of an alcohol-preferring (P) and -nonpreferring (NP) rat cross. Alcohol Clin Exp Res 21 Suppl: 16A, 1997. [Google Scholar]
- 67. Stewart RB, Murphy JM, Lumeng L, Li TK. Intake of saccharin solution in selectively-bred high and low alcohol drinking (HAD and LAD) lines of rats and in the F2 progeny of HAD and LAD rat crosses. Alcohol Clin Exp Res 22 Suppl: 55A, 1998. [Google Scholar]
- 68. Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res 18: 375–381, 1994. [DOI] [PubMed] [Google Scholar]
- 69. Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses 27: 759–768, 2002. [DOI] [PubMed] [Google Scholar]
- 70. Wilson CW, O'Brien C, MacAirt JG. The effect of metronidazole on the human taste threshold to alcohol. Br J Addict Alcohol Other Drugs 68: 99–110, 1973. [DOI] [PubMed] [Google Scholar]
- 71. Woods JE, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, Lewis MJ, June HL. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res 27: 926–936, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Wronski M, Skrok-Wolska D, Samochowiec J, Ziolkowski M, Swiecicki L, Bienkowski P, Korkosz A, Zatorski P, Kukwa W, Scinska A. Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol Alcohol 42: 75–79, 2007. [DOI] [PubMed] [Google Scholar]
- 73. Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42: 149–160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.