Abstract
Dietary protein malnutrition is manifested as amino acid deprivation of individual cells, which activates an amino acid response (AAR) that alters cellular functions, in part, by regulating transcriptional and posttranscriptional mechanisms. The AAR was activated in HepG2 human hepatoma cells, and the changes in mRNA content were analyzed by microarray expression profiling. The results documented that 1,507 genes were differentially regulated by P < 0.001 and by more than twofold in response to the AAR, 250 downregulated and 1,257 upregulated. The spectrum of altered genes reveals that amino acid deprivation has far-reaching implications for gene expression and cellular function. Among those cellular functions with the largest numbers of altered genes were cell growth and proliferation, cell cycle, gene expression, cell death, and development. Potential biological relationships between the differentially expressed genes were analyzed by computer software that generates gene networks. Proteins that were central to the most significant of these networks included c-myc, polycomb group proteins, transforming growth factor β1, nuclear factor (erythroid-derived 2)-like 2-related factor 2, FOS/JUN family members, and many members of the basic leucine zipper superfamily of transcription factors. Although most of these networks contained some genes that were known to be amino acid responsive, many new relationships were identified that underscored the broad impact that amino acid stress has on cellular function.
Keywords: microarray, nutrient sensing, starvation, liver gene expression
mammals have adaptive mechanisms to detect and respond to fluctuations in dietary nutrients. At the level of tissues and cells, dietary protein malnutrition is manifested as amino acid deprivation, which activates an amino acid response (AAR) (reviewed in Ref. 34). Amino acid limitation regulates nearly every step in gene expression (reviewed in Ref. 33), including transcription (4, 26, 33), posttranscriptional steps (37, 39, 52, 77), and translational processes (22, 76). Recent evidence also suggests that multiple signaling pathways are activated by amino acid limitation (8, 66). Several of the proteins that mediate the transcriptional component of the AAR have been identified and include members of the activating transcription factor (ATF) and CCAAT/enhancer binding protein (C/EBP) subfamilies of the basic region/leucine zipper (bZIP) transcription factor family. For the pathway most extensively studied, the general control nonderepressible 2 (GCN2) kinase acts as an intracellular amino acid concentration sensor by binding uncharged tRNA, leading to phosphorylation of the translation initiation factor eukaryotic initiation factor-2α (eIF2α) on serine 51 (62). The kinase activity of GCN2 is activated when the protein binds any one of the uncharged tRNA molecules, and so depletion of the cellular level of any individual amino acid can trigger the AAR (49). Gcn2 kinase-deficient mice have revealed that recognition of an amino acid-imbalanced diet by the brain, and the subsequent changes in feeding behavior, require uncharged tRNA sensing by Gcn2 (21, 46). The resulting phospho-eIF2α suppresses general protein synthesis but promotes a paradoxical increase in translation of selected mRNA species with upstream open reading frames, including ATF4 (42, 68). ATF4 triggers increased transcription from amino acid-responsive genes by binding to C/EBP-ATF response elements (CARE), so named because they are composed of a half-site for the C/EBP family and a half-site for the ATF family of transcription factors (18, 74). ATF4 is expressed in response to a variety of stress conditions by enhanced translation from preexisting ATF4 mRNA (42, 68). Amino acid deprivation (82), endoplasmic reticulum (ER) stress (23), the presence of dsRNA (81), and heme deficiency (80) each lead to eIF2α phosphorylation that, in turn, promotes increased ATF4 translation. Despite increased ATF4 expression by all four stress conditions, at the level of transcription specificity is achieved. For example, the SNAT2 amino acid transporter gene contains a C/EBP-ATF site that binds ATF4 in increased amounts after activation of either the AAR or ER stress, but transcription from the gene is only enhanced by the AAR (19). Consequently, the gene expression profile for each of these “ATF4-dependent” pathways needs to be established.
Amino acid homeostasis is highly dependent on dietary protein intake given the body's inability to synthesize approximately half of the amino acids, but few studies have screened the entire genome for the effect of protein or amino acid limitation. Before the availability of arrays covering the entire genome, microarray analysis of ATF4-deficient fibroblasts documented that ATF4 regulates a wide range of genes involved in amino acid transport, metabolism, oxidation status, and energy management (24). Endo et al. (17) used microarray analysis to show that total dietary protein deprivation of rats affected the hepatic expression of several classes of genes covering many cellular functions. Those results are consistent with data from yeast showing that amino acid limitation alters expression of 10–20% of the genome (reviewed in Ref. 29). More recently, Lee et al. (38) investigated the effect of cysteine deprivation on amino acid-sensitive and oxidative stress-responsive genes through microarray analysis. Those authors concluded that limiting cysteine induced the GCN2-eIF2α-ATF4 signaling pathway as expected, but most genes known to be activated by oxidative stress were unaffected. Deval et al. (15) screened the mouse genome after leucine starvation of mouse embryonic fibroblasts and discovered that 0.55% and 0.40% of the detected genes were up- and downregulated, respectively, using a cutoff value of 1.8-fold or greater. Dietary amino acid imbalance alters metabolism beyond that for amino acids. Induction of selected genes involved in cholesterol biosynthesis occurs after rats are fed a protein diet based on wheat gluten, which is deficient in lysine and threonine (17), whereas a protein-free diet results in a decrease in expression of these same genes. To understand the impact of amino acid-regulated metabolic and signaling pathways at the cellular and molecular levels, additional genomic scanning studies are needed to provide insight into the global control mechanisms.
The goal of the present study was to analyze the genomewide changes in gene expression after activation of the AAR in HepG2 human hepatoma cells. The data show that activation of the AAR by treatment of cells with the histidinyl tRNA synthetase inhibitor histidinol (HisOH) caused significant modification of the gene expression profile based on monitoring mRNA content by microarray analysis. The data were analyzed with Ingenuity Pathways Analysis software that illustrated the broad spectrum of cellular functions altered by the AAR and identified a large number of gene networks that revealed many functional interactions not previously known to be responsive to amino acid availability.
MATERIALS AND METHODS
Cell culture.
Human hepatoma cells (HepG2) were cultured in Dulbecco's modified Eagle's medium (DMEM), pH 7.4 (Mediatech, Herndon, VA) supplemented with 1× nonessential amino acids, 2 mM glutamine, 100 mg/ml streptomycin sulfate, 100 U/ml penicillin G, 0.25 mg/ml amphotericin B, and 10% (vol/vol) fetal bovine serum (FBS) and maintained at 37°C in an atmosphere of 5% CO2 and 95% air. The cells were cultured to ∼60–70% confluence so that the cells were still in a growth phase during the experiment. At 12 h before initiation of treatment, cells were given fresh medium and serum to ensure that no nutrient deprivation took place before the start of experimental incubations. To activate the amino acid response signal, four plates of cells were incubated in control DMEM and four plates of cells were incubated in DMEM supplemented with 5 mM HisOH for 4 h. HisOH blocks charging of histidine onto the corresponding tRNA and thus mimics histidine deprivation and triggers activation of the AAR (65). Analysis of eIF2α phosphorylation, ATF4 synthesis, and the transcriptional activation of several amino acid-responsive genes has shown that no difference can be detected between HisOH treatment and histidine deprivation.
RNA isolation and labeling.
Total RNA was isolated with the Qiagen RNeasy kit (Qiagen), including DNase I treatment before the final elution to eliminate any DNA contamination. The integrity of total RNA was monitored with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). A 200-ng aliquot of total RNA from four independent incubations for each condition (DMEM vs. DMEM + HisOH) was labeled with the Affymetrix GeneChip 3′ IVT Express Kit.
Microarray hybridization and scanning.
Biotin-labeled cRNA was fragmented for 35 min at 94°C, and then 0.05 μg/μl was mixed with the control oligonucleotide B2 (50 pM), 20× eukaryotic hybridization controls (BioB at 1.5 pM, BioC at 5 pM, BioDn at 25 pM, and CreX at 100 pM; Affymetrix 900454), herring sperm DNA (0.1 mg/ml; Promega), acetylated bovine serum albumin (50 mg/ml; GIBCO-BRL), and 2× hybridization buffer. The reaction was heated to 99°C for 5 min, then transferred to 45°C for 5 min, and finally centrifuged at 15,000 g in a microcentrifuge for 5 min. A 200-μl aliquot was injected into a Human Genome U133 Plus 2.0 Array (Affymetrix) representing over 47,000 mRNA species and 38,500 known human genes. Hybridization was completed in a 45°C oven for 16 h. The solution was removed, and the array was washed and stained with the Euk-WS2 fluidics protocol and streptavidin-phycoerythrin reagent (Affymetrix). The Genechips were scanned with an Affymetrix G7 scanner. The microarray data have been deposited in the NCBI Gene Expression Omnibus (GEO, accession no. GSE19495).
Microarray normalization and modeling.
Microarray data were normalized and a model-based expression matrix was derived with the perfect-match-only algorithms of dChip (40). For unsupervised analysis of the eight samples, probe sets for which the hybridization signal intensity varied across the data set with a coefficient of variation of >0.5 were identified and visualized by average linkage hierarchical clustering using the clustering algorithms in dChip. For the supervised analysis of the eight samples, leave-one-out cross-validation and Monte Carlo simulations were performed to determine the ability of significant probe sets to distinguish between control and HisOH-treated cells.
Ingenuity pathway analysis.
Gene function interpretation in the context of gene ontology, relevance to canonical pathways, and molecular networks was generated with the web-based software Ingenuity Pathways Analysis (IPA 7.1 software, Ingenuity Systems, http://www.ingenuity.com). Those genes that exhibited a statistically significant differential expression of P < 0.001 (5,027 genes) were considered altered in expression by activation of the AAR. To reduce the data set to a more reasonable number, of those 5,027 genes only the subset of 1,507 genes that exhibited a change of twofold or greater were subjected to further analysis. For the gene ontology analysis using the IPA database, the major categories of function were molecular and cellular functions, physiological system development and function, and diseases and disorders. Each of these function categories is composed of many subcategories, and each of the significantly altered genes was assigned to these subcategories based on a P value generated by Fisher's exact test that ranked the statistical significance. A P value of <0.05 indicates a statistically significant, nonrandom association between the AAR-dependent differentially expressed genes and the set of all genes associated with that functional subcategory in the IPA Knowledge Base.
The subset of 1,507 differentially expressed AAR-regulated genes was also used to identify potential interaction between genes and gene products, as reported in the literature and compiled in the IPA Knowledge Base. These data were assembled by the IPA software and are presented as molecular networks. Given that the size of these networks could grow to be quite large, the software caps the number of molecules in each network to 35 to represent the most relevant ones based on the number of connections. These “Network Eligible Molecules” are a subset of the differentially expressed genes analyzed. The software then assembles networks showing interactions between the Network Eligible Molecules and all other molecules in the Ingenuity Knowledge Base. Each network is scored based on the number of Network Eligible Molecules included. The higher the score, the lower the probability of finding the observed number of Network Eligible Molecules in a given network by random chance. The network score is the negative log of Fisher's exact test P value. Only networks with a score of at least 20 (a P value of 10−20 or greater) are included in the supplemental data for this article.1
Real-time quantitative PCR.
A 2-μg aliquot of total RNA was used to synthesize first-strand cDNA with the SuperScript III First-Strand Synthesis System Kit (Invitrogen, Carlsbad, CA). For real-time quantitative PCR (RT-qPCR), duplicate samples of 1–2 μl cDNA were mixed with 12.5 μl of SYBR Green master mixture and 6 pmol of forward and reverse primers in a total volume of 25 μl. The mixture was subjected to 35 cycles at 95°C for 30 s, 60°C for 30 s, and 68°C for 60 s. RT-qPCR was performed with a DNA Engine Opticon 3 system (Bio-Rad) and detection with SYBR Green. The primers used are listed in Supplemental Table S1. After PCR, melting curves were acquired by a stepwise increase of the temperature from 55°C to 95°C to ensure that a single product was amplified in the reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. All calculations were based on the ΔCt (where Ct is threshold cycle) of the analyzed gene relative to the GAPDH mRNA content in the same sample.
RESULTS AND DISCUSSION
Genes were up- or downregulated after HisOH treatment.
HepG2 hepatoma cells have become one of the most highly investigated in vitro models for human liver. Given the extensive literature that has been published on the metabolism and signaling pathways within these cells, we chose these cells to determine the genomewide response to amino acid limitation so that our data could be put into the context of these previously published studies. For these experiments, HepG2 cells were incubated for 4 h in control medium (DMEM) or medium containing 5 mM HisOH (n = 4 for each condition). RNA was collected and analyzed by microarray with the Affymetrix Human Genome U133 Plus 2.0 Array chip. As shown in Supplemental Fig. S1, with both unsupervised and supervised hierarchical clustering analysis the eight arrays were clearly separated into two groups that correspond to the two experimental conditions, cells treated with HisOH and those maintained in the control medium. The complete list of 5,027 genes for which mRNA content was significantly altered (P < 0.001) is given in Supplemental Table S2. Analysis of a subset of the 5,027 genes using an arbitrary value of twofold change or greater revealed 1,507 genes, 250 downregulated and 1,257 upregulated. Table 1 includes a partial listing of those genes that were upregulated; for space considerations, a value of ninefold or greater was arbitrarily chosen to illustrate the most highly induced genes. The affected gene products cover a wide range of functions, including proteins involved in cell signaling, stress response pathways, and transcriptional control. In particular, a number of kinases and phosphatases were observed, as were several proteins involved in chromatin structure and gene expression. Conversely, of the 250 genes for which the cellular mRNA content was downregulated, those that changed by threefold or greater are shown in Table 2. Among these genes was the heat shock protein HSP70, illustrating that amino acid limitation activates specific signal transduction mechanisms but does not elicit a response that would affect other stress pathways such as heat shock.
Table 1.
Genes increased in expression by ninefold or greater by AAR
| Probe Set | Gene Symbol | Description | Fold Change | P Value |
|---|---|---|---|---|
| 201693_s_at | EGR1 | Early growth response 1 | 41 | <1e-07 |
| 201694_s_at | EGR1 | Early growth response 1 | 33 | <1e-07 |
| 201473_at | JUNB | jun B protooncogene | 27 | <1e-07 |
| 210056_at | RND1 | Rho family GTPase 1 | 23 | <1e-07 |
| 209189_at | FOS | v-fos FBJ murine viral oncogene homolog | 19 | <1e-07 |
| 206115_at | EGR3 | Early growth response 3 | 17 | <1e-07 |
| 202672_s_at | ATF3 | Activating transcription factor 3 | 16 | <1e-07 |
| 207574_s_at | GADD45B | Growth arrest and DNA damage-inducible, β | 16 | <1e-07 |
| 213146_at | JMJD3 | Jumonji domain containing 3, histone demethylase | 16 | <1e-07 |
| 41386_i_at | JMJD3 | Jumonji domain containing 3, histone demethylase | 15 | <1e-07 |
| 223196_s_at | SESN2 | Sestrin 2 | 15 | <1e-07 |
| 206724_at | CBX4 | Chromobox homolog 4 (Pc homolog, Drosophila) | 15 | 7.00E-07 |
| 220987_s_at | NUAK2 | NUAK family, SNF1-like kinase, 2 | 15 | <1e-07 |
| 209304_x_at | GADD45B | Growth arrest and DNA damage-inducible, β | 14 | <1e-07 |
| 37028_at | PPP1R15A | Protein phosphatase 1, regulatory subunit 15A | 14 | <1e-07 |
| 215071_s_at | HIST1H2AC | Histone cluster 1, H2ac | 14 | <1e-07 |
| 210587_at | INHBE | Inhibin, βE | 14 | <1e-07 |
| 202014_at | PPP1R15A | Protein phosphatase 1, regulatory subunit 15A | 13 | <1e-07 |
| 209305_s_at | GADD45B | Growth arrest and DNA damage-inducible, β | 13 | 1.60E-06 |
| 227404_s_at | EGR1 | Early growth response 1 | 13 | <1e-07 |
| 201041_s_at | DUSP1 | Dual-specificity phosphatase 1 | 13 | <1e-07 |
| 218000_s_at | PHLDA1 | Pleckstrin homology-like domain, family A, member 1 | 12 | 5.20E-06 |
| 211965_at | ZFP36L1 | Zinc finger protein 36, C3H type-like 1 | 12 | <1e-07 |
| 210764_s_at | CYR61 | Cysteine-rich, angiogenic inducer, 61 | 11 | <1e-07 |
| 201465_s_at | JUN | jun oncogene | 11 | <1e-07 |
| 202768_at | FOSB | FBJ murine viral oncogene homolog B | 11 | <1e-07 |
| 218995_s_at | EDN1 | Endothelin 1 | 11 | <1e-07 |
| 201466_s_at | JUN | jun oncogene | 11 | <1e-07 |
| 207147_at | DLX2 | distal-less homeobox 2 | 11 | <1e-07 |
| 209383_at | DDIT3 | DNA damage-inducible transcript 3 | 10 | <1e-07 |
| 226267_at | JDP2 | Jun dimerization protein 2 | 9.8 | <1e-07 |
| 209774_x_at | CXCL2 | Chemokine (C-X-C motif) ligand 2 | 9.7 | <1e-07 |
| 212099_at | RHOB | Ras homolog gene family, member B | 9.5 | <1e-07 |
| 223195_s_at | SESN2 | Sestrin 2 | 9.4 | <1e-07 |
| 222802_at | EDN1 | Endothelin 1 | 9.4 | <1e-07 |
| 210023_s_at | PCGF1 | polycomb group ring finger 1 | 9.3 | 1.80E-06 |
| 201289_at | CYR61 | Cysteine-rich, angiogenic inducer, 61 | 9.3 | <1e-07 |
| 206924_at | IL11 | Interleukin-11 | 9.1 | <1e-07 |
Complete list of differentially expressed genes and additional details of analysis are included in Supplemental Table S2. AAR, amino acid response.
Table 2.
Genes decreased in expression by threefold or greater by AAR
| Probe Set | Gene Symbol | Description | Fold Change | P Value |
|---|---|---|---|---|
| 202973_x_at | FAM13A1 | Family with sequence similarity 13, member A1 | −8.8 | <1e-07 |
| 217047_s_at | FAM13A1 | Family with sequence similarity 13, member A1 | −8.8 | <1e-07 |
| 200800_s_at | HSPA1A | Heat shock 70-kDa protein 1A | −8.4 | <1e-07 |
| 208080_at | AURKA | aurora kinase A | −8.1 | 0.00085 |
| 202581_at | HSPA1B | Heat shock 70-kDa protein 1B | −8.0 | <1e-07 |
| 227337_at | ANKRD37 | Ankyrin repeat domain 37 | −5.7 | <1e-07 |
| 214842_s_at | ALB | Albumin | −5.4 | 0.00039 |
| 200799_at | HSPA1A | Heat shock 70-kDa protein 1A | −5.1 | <1e-07 |
| 201171_at | ATP6V0E1 | ATPase, H+ transporting, lysosomal V0 subunit 1 | −5.0 | 0.00091 |
| 231534_at | CDC2 | Cell division cycle 2, G1 to S and G2 to M | −4.7 | 3.43E-05 |
| 232861_at | PDP2 | Pyruvate dehydrogenase phosphatase isoenzyme 2 | −4.5 | 1.90E-06 |
| 211538_s_at | HSPA2 | Heat shock 70-kDa protein 2 | −4.2 | 3.14E-05 |
| 230060_at | CDCA7 | Cell division cycle associated 7 | −4.1 | <1e-07 |
| 209357_at | CITED2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 | −4.0 | <1e-07 |
| 225762_x_at | LOC284801 | Hypothetical protein LOC284801 | −3.8 | 6.50E-06 |
| 226643_s_at | NUDCD2 | NudC domain containing 2 | −3.8 | 0.00098 |
| 227345_at | TNFRSF10D | TNF receptor superfamily, member 10d, decoy with truncated death domain | −3.8 | <1e-07 |
| 244669_at | SNHG5 | Small nucleolar RNA gene 5 (nonprotein coding) | −3.4 | <1e-07 |
| 213349_at | TMCC1 | Transmembrane and coiled-coil domain family 1 | −3.4 | <1e-07 |
| 213051_at | ZC3HAV1 | Zinc finger CCCH-type, antiviral 1 | −3.4 | 3.00E-07 |
| 228990_at | C1orf79 | Chromosome 1 open reading frame 79 | −3.3 | <1e-07 |
| 209006_s_at | C1orf63 | Chromosome 1 open reading frame 63 | −3.3 | 0.00054 |
| 218507_at | HIG2 | Hypoxia-inducible protein 2 | −3.3 | <1e-07 |
| 1555826_at | EPR1 | Effector cell peptidase receptor 1 (noncoding) | −3.3 | 0.00032 |
| 230352_at | PRPS2 | Phosphoribosyl pyrophosphate synthetase 2 | −3.2 | 1.14E-05 |
| 243927_x_at | KIAA1429 | KIAA1429 | −3.1 | 1.00E-06 |
| 223925_s_at | LUZP6 | Leucine zipper protein 6 | −3.1 | 1.35E-05 |
| 229513_at | STRBP | Spermatid perinuclear RNA binding protein | −3.1 | 8.00E-06 |
| 225313_at | C20orf177 | Chromosome 20 open reading frame 177 | −3.4 | <1e-07 |
| 232441_at | KRR1 | KRR1, small subunit processome component | −3.3 | 3.13E-05 |
| 238003_at | HEPN1 | HEPACAM opposite strand 1 | −3.1 | 1.50E-06 |
Complete list of differentially expressed genes and additional details of analysis are included in Supplemental Table S2.
Confirmation of genes controlled by AAR.
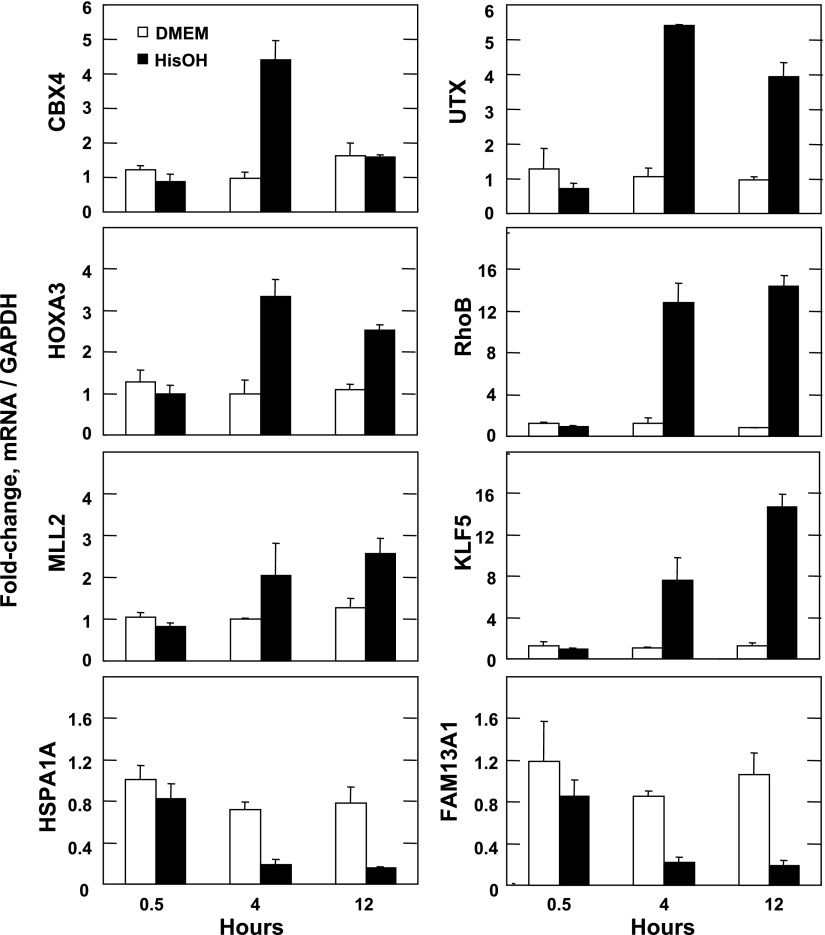
A number of genes have been documented to be transcriptionally activated by amino acid deprivation, and still others are induced through mRNA stabilization (33, 34). Some examples of genes for which elevated mRNA content has been documented by either Northern blotting or qPCR are listed in Table 3. The mRNA for each of these genes was shown to be induced by the microarray analysis, ranging from 2-fold to 27-fold. To further validate the results of the microarray, we randomly picked eight genes not previously reported to be targets of the AAR pathway for additional RT-qPCR analysis (Fig. 1). Six of these genes were upregulated [chromobox protein homolog 4 (CBX4), ubiquitously transcribed X chromosome tetratricopeptide (UTX), homeobox protein A3 (HOXA3), Ras homolog gene family-member B (RhoB), myeloid/lymphoid or mixed-lineage leukemia 2 (MLL2), and Kruppel-like factor 5 (KLF5)], and two of them [family with sequence similarity 13, member A1 (FAM13A1) and heat shock 70-kDa protein 1A (HSPA1A)] were downregulated based on the microarray data (Supplemental Table S2). As shown in Fig. 1, after activation of the AAR measurement of the steady-state cellular mRNA content by RT-qPCR revealed that each of the chosen genes is regulated in a manner consistent with the microarray data. Collectively, these results provide evidence that the microarray faithfully detected genes that are controlled by amino acid limitation of mammalian cells and that the results reflect the existing literature.
Table 3.
Known AAR-regulated genes previously confirmed by mRNA analysis
| Probe Set | Gene Symbol | Gene Description | Fold Change | P Value | References |
|---|---|---|---|---|---|
| 201473_at | JUNB | junB protooncogene | 27 | <1e-07 | 54 |
| 201465_s_at | JUN | jun oncogene | 11 | <1e-07 | 54 |
| 202672_s_at | ATF3 | Activating transcription factor 3 | 16 | <1e-07 | 51 |
| 209383_at | DDIT3 | DNA damage-inducible transcript 3 | 10 | <1e-07 | 5 |
| 226267_at | JDP2 | Jun dimerization protein 2 | 10 | <1e-07 | 11 |
| 212171_x_at | VEGFA | Vascular endothelial growth factor A | 3.5 | <1e-07 | 1 |
| 205047_s_at | ASNS | Asparagine synthetase | 3.4 | <1e-07 | 32 |
| 204039_at | CEBPA | CCAAT/enhancer binding protein, α | 3.2 | 9.70E-06 | 44 |
| 228463_at | FOXA3 | forkhead box A3 | 3.2 | <1e-07 | 64 |
| 212501_at | CEBPB | CCAAT/enhancer binding protein, β | 3.2 | <1e-07 | 9 |
| 214315_x_at | CALR | Calreticulin | 3.0 | 0.00035 | 28 |
| 204999_s_at | ATF5 | Activating transcription factor 5 | 2.4 | 0.00020 | 83 |
| 210103_s_at | FOXA2 | forkhead box A2 | 2.2 | 1.96E-05 | 64 |
| 1555788_a_at | TRIB3 | tribbles homolog 3 (Drosophila) | 2.1 | 0.00017 | 48 |
| 220924_s_at | SLC38A2 | Solute carrier family 38, member 2 | 2.0 | 0.00022 | 50 |
Complete list of differentially expressed genes and additional details of analysis are included in Supplemental Table S2.
Fig. 1.
Validation of differentially regulated genes in response to the amino acid response (AAR). Total RNA was isolated from HepG2 hepatoma cells incubated for 4 h in either control medium [Dulbecco's modified Eagle's medium (DMEM)] or in DMEM containing 5 mM histidinol (HisOH). After isolation of total RNA, real-time quantitative PCR (RT-qPCR) was used to measure the mRNA expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a negative control and 8 genes regulated by HisOH treatment. Data are expressed as a ratio to the GAPDH mRNA for each gene and plotted relative to the value for the DMEM control condition. Results are presented as averages ± SD of assays in triplicate. CBX4, chromobox protein homolog 4; UTX, ubiquitously transcribed X chromosome tetratricopeptide; HOXA3, homeobox A3; RhoB, Ras homolog gene family-member B; MLL2, myeloid/lymphoid or mixed-lineage leukemia 2; KLF5, Kruppel-like factor 5; HSPA1A, heat shock 70-kDa protein 1A; FAM13A1, family with sequence similarity 13 member A1.
Comparison of amino acid-responsive genes identified by independent studies.
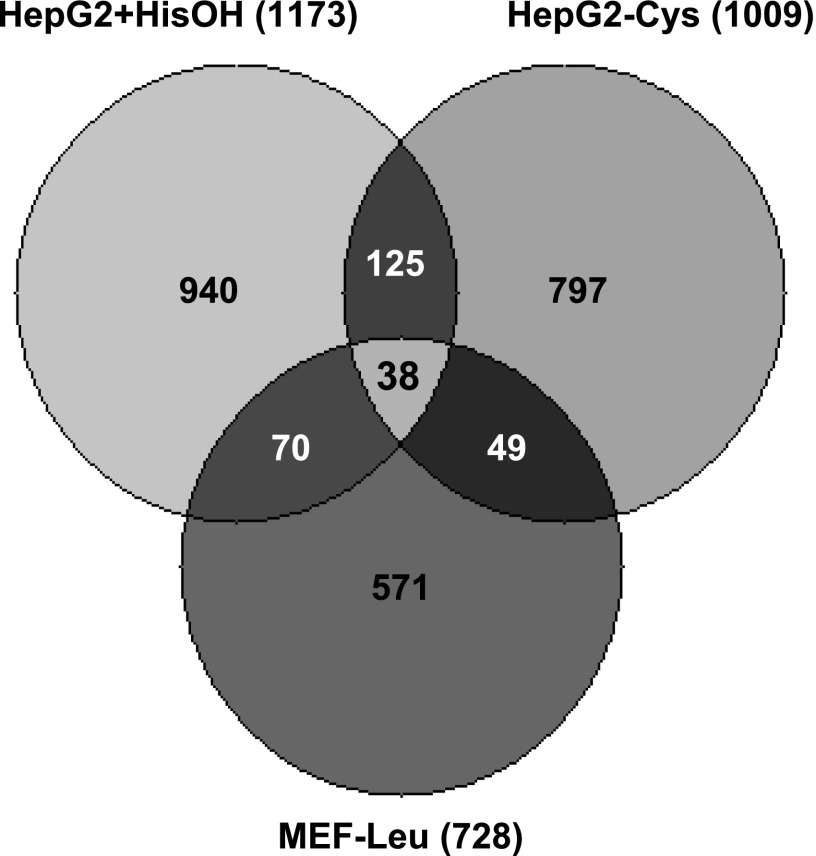
Two previous studies have investigated amino acid regulation by expression microarray analysis. Deval et al. (15) monitored the mouse genome after exposure of mouse embryonic fibroblasts to medium lacking leucine, whereas Lee et al. (38) incubated HepG2 hepatoma cells with medium lacking cysteine. Despite the fact that each study discovered from 700 to 1,200 regulated genes, only 38 genes were identified to be in common among all three studies (Fig. 2). This list of genes included those induced by up to 19-fold in the present study (FOS) as well as genes that were down-regulated by nearly 8-fold (HSP70) by HisOH (Supplemental Table S3). The 38 genes in common crossed all possible cellular functions. Comparison of the two investigations in the HepG2 cells revealed 125 genes in common that were not detected during the mouse fibroblast study, but liver-specific genes were not overly represented, and thus the basis for this list of unique genes is unclear.
Fig. 2.
Comparison of microarray data from 3 independent studies. Genes that were identified as amino acid responsive based on 3 independent investigations were evaluated for overlap, and data are presented as a Venn diagram. In addition to the results from the present study (HepG2+HisOH), the data from Lee et al. (38) (HepG2-Cys) and Deval et al. (15) (MEF-Leu) were included in the analysis. As indicated in parentheses, the number of total genes analyzed was 1,173 (present study), 1,009 (Lee et al.), and 728 (Deval et al). The 38 genes common to all 3 investigations are listed in Supplemental Table S3.
Functions of differentially expressed genes.
With the IPA software, a comprehensive analysis of the subset of genes differentially regulated by twofold or greater showed that the amino acid-responsive genes were associated with a broad range of cellular functions. Table 4 lists the number of regulated genes associated with specific areas of cell function or with known diseases. Although amino acid metabolism and amino acid transporters were well represented in the list of altered genes (Supplemental Table S2), the list of subcategories that contain large numbers of regulated genes reveals that activation of the AAR pathway has much broader implications for cellular function. Among the subcategories within the category of molecular and cellular functions, large numbers of genes were associated with cellular growth and proliferation, gene expression, cell death, cell development, and the cell cycle (Table 4). A more detailed analysis revealed that even though a topic such as cell signaling contains hundreds of genes, only 29 genes were affected by the AAR. This result is consistent with the fact that many of these proteins are involved in and regulated by posttranslational modification, but the result provides further evidence for the cellular specificity of the response to amino acid limitation. With a total of 322 genes, the category of cellular growth and proliferation contained the largest group of affected genes, including a number of known AAR target genes, such as ATF3, ATF5, and vascular endothelial growth factor (VEGF). A significant impact on this category of genes is consistent with the observation that amino acid limitation suppresses general protein synthesis and can induce a G1-S blockade of the cell cycle (20). In the second most common subcategory, gene expression, 259 genes were regulated, and of these 237 are related to transcription and transcriptional control. These transcription factors include those known amino acid response regulators, such as DNA damage inducible transcription-3 (DDIT3) or CHOP, JUN dimerization protein-2 (JDP2), tribbles homolog 3 (TRIB3), and ATF3, as well as other transcription factors, including JUN, JUN-B, JUN-D, FOS, FOS-B, early growth response-1, -2, and -4 (EGR1, EGR2, EGR4), GATA binding protein-2 and -4 (GATA2, GATA4), members of the B-cell leukemia/lymphoma family (BCL-3, BCL-6, BCL-2L1, BCL-9L), and members of the SMAD family (Mothers-against-decapentaplegic-homolog, SMAD2, SMAD3, SMAD6).
Table 4.
Selected examples of functions regulated by AAR
| Categories | Functions and Diseases | No. of Genes |
|---|---|---|
| Molecular and cellular functions | Cellular growth and proliferation | 322 |
| Gene expression | 259 | |
| Cell death | 228 | |
| Cellular development | 215 | |
| Cellular movement | 109 | |
| Antigen presentation | 94 | |
| Cell cycle | 92 | |
| Immune cell trafficking | 69 | |
| DNA replication, recombination, and repair | 54 | |
| Small molecule biochemistry | 52 | |
| Amino acid metabolism | 47 | |
| Posttranslational modification | 47 | |
| Molecular transport | 36 | |
| Cellular assembly and organization | 30 | |
| Cellular function and maintenance | 29 | |
| Cell signaling | 29 | |
| Vitamin and mineral metabolism | 29 | |
| Carbohydrate metabolism | 7 | |
| Lipid metabolism | 7 | |
| Physiological system development and function | Hematological system development and function | 141 |
| Tissue development | 130 | |
| Tissue morphology | 127 | |
| Connective tissue development and function | 112 | |
| Cell-mediated immune response | 107 | |
| Skeletal and muscular system function | 106 | |
| Hematopoiesis | 99 | |
| Humoral immune response | 90 | |
| Embryonic development | 47 | |
| Nervous system development and function | 47 | |
| Organismal survival | 42 | |
| Reproductive system development and function | 27 | |
| Behavior | 25 | |
| Organ development | 24 | |
| Organ morphology | 24 | |
| Lymphoid tissue structure and development | 19 | |
| Cardiovascular system development and function | 18 | |
| Organismal development | 10 | |
| Respiratory system development and function | 5 | |
| Hair and skin development and function | 3 | |
| Diseases and disorders | Cancer | 203 |
| Inflammatory response | 108 | |
| Inflammatory disease | 111 | |
| Immunological disease | 103 | |
| Hematological disease | 97 | |
| Cardiovascular disease | 96 | |
| Reproductive system disease | 85 | |
| Skeletal and muscular disorders | 58 | |
| Tumor morphology | 57 | |
| Renal and urological disease | 47 | |
| Dermatological diseases and conditions | 43 | |
| Organismal injury and abnormalities | 42 | |
| Gastrointestinal disease | 40 | |
| Hepatic system disease | 28 | |
| Genetic disorder | 18 | |
| Connective tissue disorders | 15 | |
| Renal and urological system function | 11 | |
| Neurological disease | 10 | |
| Respiratory disease | 3 |
Not surprisingly, several hundred differentially expressed genes were associated with the physiological system development and function category, implying that dietary protein limitation may affect tissue development and maintenance. Indeed, it is known that poor protein nutrition can lead to intrauterine growth retardation and low-birth-weight babies (14, 45). Significant numbers of differentially expressed genes were found in the hematological system and function (141), tissue development (130), and tissue morphology (127) subcategories (Table 4). A review of the AAR-regulated genes (Supplemental Table S2) revealed many genes transiently expressed during organ development, but genes encoding proteins associated with epigenetic mechanisms suggest that reduced dietary protein intake is likely to cause long-term developmental deficiencies as well. For example, among genes modulated by HisOH treatment were several HOX genes that are key transcription factors for body pattern and organ formation in early development. Also, there were many regulated genes encoding enzymes that mediate histone modifications, such as histone deacetylase 5 (HDAC5), histone acetyltransferase (monocytic leukemia) 3 (MYST3), and myeloid/lymphoid or mixed-lineage leukemia (MLL). Evidence suggests that maternal malnutrition, including protein deprivation, causes fetal epigenetic changes that alter gene expression after birth.
With regard to genes categorized in the diseases and disorders section, in the cancer subcategory there were 203 genes altered by AAR activation and among these were proteins involved in critical processes such as transformation, senescence, and apoptosis (Table 4). For example, cyclin-dependent kinase inhibitor 2B (CDKN2B), growth arrest and DNA damage 45A (GADD45A), Ras-associated proteins, transforming growth factor β1 (TGF-β1), DDIT3, forkhead box O3 (FOXO3), JUN/FOS family members, and SMAD7 were all activated, suggesting that amino acid limitation affects pathways involved in tumor development and maintenance. Given their critical roles in a range of cellular pathways, many AAR-activated genes were identified with several areas of cell function. For example, the transcription factor ATF3, induced in its expression by up to 16-fold based on the array analysis (Supplemental Table S2), has been reported to be involved in many aspects of cell function, and, accordingly, ATF3 was identified in the subcategories of cellular proliferation and oncogenesis, transcription, apoptosis, DNA repair, immune function and inflammation, and diabetes. Likewise, VEGF, upregulated by more than threefold, plays important roles in many signaling pathways, including promotion of angiogenesis related to solid tumor growth (25) and injury-induced angiogenesis in smooth muscle (43). Interestingly, the latter response was associated with ATF4-mediated signaling, consistent with the results of Abcouwer et al. (1), who first showed that VEGF is transcriptionally controlled by amino acid availability via an ATF4-dependent mechanism.
Signaling pathways affected by AAR.
The IPA software was also used to analyze the data for relationships between the AAR and canonical signaling pathways. This analysis identifies those pathways for which a significant number of members are differentially expressed, based on statistical analysis using Fisher's exact test (P < 0.05). With this approach, the five signaling pathways that were the most significantly affected by HisOH treatment were hepatic fibrosis/hepatic stellate cell activation, interleukin-6 (IL-6) signaling, retinol metabolism, innate immunity, and IL-10 signaling. Although the P values for many other pathways were not lower than the P < 0.05 threshold, activation of the AAR revealed differentially expressed genes in many other canonical metabolic and signaling pathways. These additional pathways included peroxisome proliferator-activated receptor (PPAR) signaling, oxidative phosphorylation, Wnt/β-catenin signaling, Toll-like receptors, NF-κB signaling, apoptosis, hypoxia-inducible factor (HIF)-1α signaling, and the ERK/MAPK pathway. Although the total number of amino acid-responsive genes within these pathways was not large enough to reach the statistical threshold, amino acid-dependent regulation of key individual members may result in significant modulation of the pathway. Indeed, the NF-κB and ERK/MAPK signaling pathways are known to be modulated by amino acid limitation, and subsequently they influence the regulation of other AAR target genes (30, 57, 66). Collectively, the results of the cellular function and signaling pathway analysis for the array data demonstrate that the impact of amino acid limitation is wide ranging and influences cellular functions well beyond protein and amino acid metabolism.
Networks assembled with differentially expressed molecules.
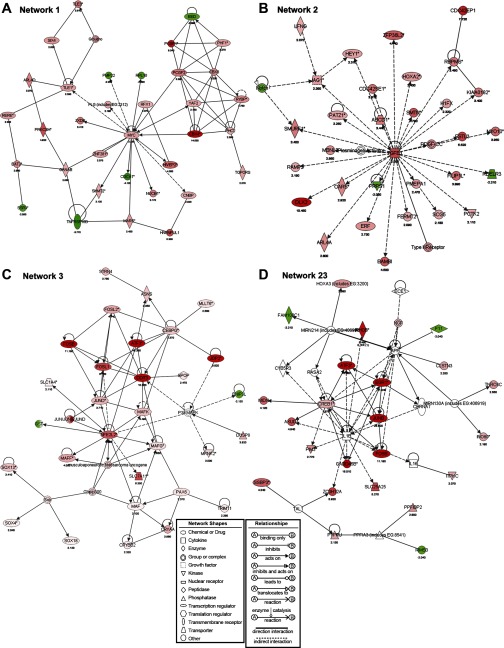
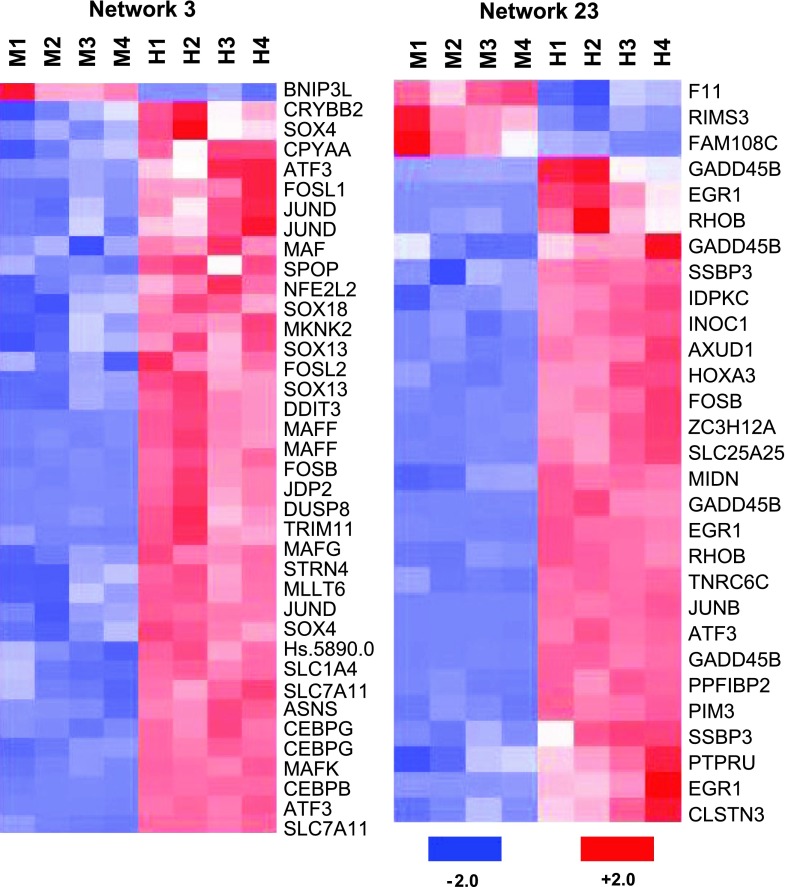
The biological relationship between the differentially-expressed AAR genes and “gene networks” were analyzed with the Ingenuity Knowledge Base. A gene network illustrates protein actions that involve protein-protein binding, posttranslational modification, or changes in gene expression. Combining the expression array data, which detect changes in mRNA, with a database capable of generating networks based on published information is useful to identify additional genes that may regulate cellular function without a change in mRNA but rather a change in activity, protein-protein interactions, or translational control or by posttranslational modification. Such networks may also provide the molecular basis for generating new hypotheses and identifying potential target genes for further analysis. Furthermore, the gene products that are located at the “hubs” of such networks are proposed to play a central role in related biological processes and may be targets for the development of therapeutic strategies.
Considering the entire set of 1,507 differentially expressed genes with a statistical difference of greater than P < 0.001 and more than a twofold change, there were 24 networks with a score >20 (a P value of <10−20) (Supplemental Table S4). The top three networks are illustrated in Fig. 3, along with network 23, which was chosen for more detailed discussion because it represents an example of a network that contains an illustrative combination of genes known to be associated with the AAR and genes not previously identified with this response. The remaining four of the top seven networks are included in Supplemental Fig. S2. To illustrate examples of the primary data from the eight individual RNA samples, heat maps for networks 3 and 23 are shown in Fig. 4. A network diagram contains “focus genes,” those included in the regulated data set, as well as nonregulated proteins added to provide connections between the focus genes. Network 1 contains the transcription factor c-myc (2.3-fold induced by HisOH) as a hub, which is linked to a constellation of AAR-activated genes that are members of (PCGF1, PCGF2, PHC2, EED, CBX4, and CBX8) or associated with (PHF1, YAF2, RYBP, TOPORS) the polycomb group protein (PcG) transcriptional repressive complexes PRC1 and PRC2. The PcG proteins were originally identified as repressors of the HOX family of developmentally associated genes. Interestingly, although most of these PcG genes exhibited enhanced expression in response to the AAR (Fig. 3A), several members of the HOX family were observed to be either increased or decreased by the AAR (Supplemental Table S2). The PRC2 complex methylates histone H3 on lysine 27 (H3K27me), which is associated with gene silencing, and the resulting H3K27me3 mark contributes to recruitment or stable binding of the PRC1 complex also associated with gene silencing (59). The presence of H3K27me3 near the transcription start site of a gene is correlated with transcriptional repression, even in the presence of H3K4me3, a mark considered to coincide with active transcription. Although initially associated with the HOX genes and other developmentally critical genes, it is now clear that PRC2 complexes are involved in the regulation of many genes linked to other functions such as X-inactivation, cell cycle control, and cell transformation (reviewed in Ref. 59). The polycomb-like PHF1 protein, which exhibited an enrichment of 2.3-fold after activation of the AAR, modulates the PRC2 complex to promote conversion of H3K27me2 to H3K27me3, which is a more effective mark for PRC1 recruitment. The expression of PRC2 member EED was the only PcG protein repressed (2-fold) by activation of the AAR pathway. EED can be expressed as one of four different isoforms that serve to activate PRC2 function to propagate the H3K27me3 mark, but EED can also lead to an unusual PRC2-mediated methylation of linker histone H1 instead of H3K27 methylation (reviewed in Ref. 61). Several PRC1 members were identified as induced genes by the array. CBX4 (14-fold) and CBX8 (3-fold) contain chromodomains, which allows binding of the PRC1 complex to the H3K27me3 mark, and PCGF1 (9.3-fold induction) and PCGF2 (3.4-fold) are RING finger proteins that serve as cofactors for E3 ubiquitin ligase and help to compact the chromatin in a repressed state. TOPORS, which was increased by 2.3-fold by the AAR, has been identified as a unique E3 ligase that has both ubiquitin and SUMO-1 ligase activity for chromatin-associated proteins and may play a role in PcG function (55).
Fig. 3.
Networks that contain significant numbers of genes associated with the AAR. The subset of 1,507 differentially expressed AAR-regulated genes (P < 0.001 and a 2-fold change or greater; Supplemental Table S2) was analyzed to identify potential interactions between gene products with the information compiled in the Ingenuity Knowledge Base. Each possible network was assigned a score that is the negative log of Fisher's exact test P value, and only networks with a score of at least 20 (P value of 10−20) were analyzed further (Supplemental Table S3). Networks 1 (A), 2 (B), and 3 (C) contained the greatest number of “focus genes,” while network 23 (D) is presented as an additional example of a network containing both known and newly identified genes associated with the AAR. Keys explain the symbols used and gene interactions shown. Intensity of pink-to-red symbols indicates degree of upregulation of gene expression and intensity of green symbols indicates downregulation in response to the AAR.
Fig. 4.
Heat maps of up- or downregulated genes involved in network 3 and network 23. Each column represents an individual sample incubated for 4 h in either control medium DMEM (M) or DMEM containing 5 mM HisOH (H). A probe set for each gene is depicted in each row, labeled with the gene name for that probe set. Gene expression level is depicted as intensity of red (upregulated) and blue (downregulated) boxes.
Although not considered part of the PRC1 or PRC2 core complexes, two PcG-associated proteins, YAF2 and RYBP (also known as YEAF1), were increased by the AAR by 2.2-fold and 2.8-fold, respectively. These proteins bind to the transcription factor YY1, which may play a role in the recruitment of the PRC2 complex (58, 61). It has been proposed that the PcG system not only mediates long-term transcriptional repression but also leads to dynamic regulation of some target genes (reviewed in Ref. 47). Consistent with the concept of dynamic regulation, two H3K27me3 demethylases, JMJD3 and UTX, were also among the genes for which increased expression was observed after activation of the AAR, with values of up to 15-fold and 3-fold, respectively (Supplemental Table S2). Four members of the Jumonji family of histone demethylases were also increased in their expression by activation of the AAR, and each of these demethylases removes histone methylation marks associated with transcriptional repression; thus increased gene expression would likely result. The AAR-regulated demethylating Jumonji members and the specific lysine residue that they demethylate are as follows: JMJD1 (H3K9), JMJD2 (H3K9 and H3K36), JMJD3 (H3K27), and UTX (H3K27). Demethylation of the repressive H3K27me3 mark is often associated with a concurrent trimethylation of H3K4 in the promoters of genes that are actively transcribed. Members of the mixed-lineage leukemia (MLL) family of proteins mediate this H3K4 methylation (3), and several were observed to be increased by the AAR based on the array data (MLL2, 3.4-fold; MLL3, 2.9-fold; and MLL4, 2.3-fold). Interestingly, MLL3 and MLL4 directly associate with JMJD3 and UTX (47). Although many questions remain to be addressed about the specific roles of these three families of epigenetic-related proteins during the AAR, such extensive regulation is consistent with the hypothesis that the AAR initiates a broad cellular response to the fundamental stress of amino acid limitation. Furthermore, there is evidence that PcG proteins preferentially repress genes encoding transcription factors (59). Consequently, AAR-regulated PcG action is likely to widely influence downstream signaling pathways, metabolism, and other critical cellular events.
Network 2 has 31 focus molecules centered around the hub protein of TGF-β1, for which the expression was increased by 5.5-fold after activation of the AAR pathway (Fig. 3B). Several differentially expressed proteins function downstream of TGF-β1, such as follistatin-like 3 (FSTL3), prostate transmembrane protein, androgen induced 1 (PMEPA1), and smoothelin (SMTN), which were elevated in expression by 5.5-fold, 2.5-fold, and 4.8-fold, respectively. Conversely, some of the activated genes, including bone morphogenic protein/activin membrane-bound inhibitor (BAMBI, 4.5-fold) and Smad ubiquitin regulatory factor-1 (SMURF-1, 3.4-fold), encode proteins associated with downregulation of TGF-β1 signaling, perhaps as a feedback inhibition mechanism. Extending the information of network 1 illustrating an effect of amino acid limitation on genes associated with development, among the induced genes in network 2 were HOXA2 (2.4-fold) and DLX2 (11-fold). The DLX genes constitute a subfamily of vertebrate homeobox genes that are structurally similar to the Drosophila distal-less gene, and six family members have been identified in mammals (63). The AAR-induced DLX2 has been previously identified as a downstream target of TGF-β and bone morphogenic protein 2 (BMP-2) signaling, whereas other DLX family members have been shown to inhibit transcription of TGF-β superfamily genes (12).
Network 3 has 29 focus genes, including 1 downregulated gene, BCL2/adenovirus E1B 19-kDa interacting protein 3-like (BNIP3L), and 28 upregulated genes (Fig. 3C). Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2), also known as NF-E2-related factor 2 (NRF2), was increased by sixfold and is one of the hubs in this network. NFE2L2/NRF2 is a basic leucine zipper transcription factor that plays a central role in the response to oxidative stress (75, 78) and forms heterodimers with a primary mediator of the AAR, ATF4 (27). NFE2L2/NRF2 regulates the transcription of numerous antioxidant enzyme genes by binding to the antioxidative response element (ARE) as a heterodimer with MAF (induced 2.1-fold) protein, which is one of several MAF genes (MAF-F, 4.1-fold; MAF-G, 2.7-fold; MAF-K, 3.5-fold) that were detected by the microarray as increased in expression (Fig. 3C and Supplemental Table S2). Many members of the FOS/JUN basic leucine zipper transcription factor family, cJUN (11-fold), JUN-B (27-fold), JUN-D (3.7-fold), cFOS (19-fold), and FOS-B (11-fold), were identified as highly upregulated based on the microarray. As shown in network 3 (Fig. 3C), these members of the FOS-JUN family interact directly or indirectly with NFE2L2/NRF2 and thereby modulate its control during oxidative stress (67). JUN dimerization protein 2 (JDP2) was identified by the microarray analysis (increased by 9.8-fold) and independently, the JDP2 gene has been documented to be induced by amino acid limitation (Awad K and Kilberg MS, unpublished results). JDP2 modulates the induction of the ATF3 in response to cellular stress (73), and it represses transcription of the amino acid-responsive CHOP gene (11). ATF3 exhibits an increased expression of >16-fold based on the array data (Supplemental Table S2) and is included in network 3 (Fig. 3C). ATF3 plays a critical role in both the AAR (51) and the unfolded protein response pathways (31) and serves as a repressor for the regulation of AAR target genes like asparagine synthetase (ASNS) (10, 51). ATF3 is reported to dimerize with NFE2L2/NRF2, and its expression is induced in response to the AAR pathway by both transcription (53) and mRNA stabilization (52). The ATF3-NFE2L2/NRF2 complex suppresses the transcription of oxidative response genes during oxidative stress (79). Cross talk between oxidative stress response pathways and the AAR has been documented previously, and ATF3 may function as one of the critical links between these two pathways (24, 31). Interestingly, ATF3 is also contained within another network, network 23, that was highly affected by amino acid limitation and features IL-1β as a hub protein (Fig. 3D). Evidence has implicated ATF3 in IL-1β-related immune regulation (60), and it is proposed that ATF3 functions as a negative regulator in proinflammatory chemokine production (13, 36).
Network 23, which has IL-1β as a central hub protein, has 22 focus genes, including 3 downregulated and 19 upregulated genes. IL-1β is a proinflammatory cytokine that plays a central role in inflammatory response and autoimmune disorders (55a). Like ATF3, the transcription factors FOS-B (11-fold increase) and JUN-B (27-fold increase) associated with network 23 provide a link between networks 3 and 23 (Fig. 3D). RhoB (enhanced by 9.5-fold), a member of network 23, is a component of the Rho family of GTPases that are molecular switches controlling a variety of signaling pathways (68a). RhoB further enhances transcription of nitric oxide synthase-2 induced by the cytokines INF-γ, IL-1β, and TNF-α (14a). The array analysis suggested that the mRNA level of network 23 member early growth response 1 (EGR1) was increased 41-fold by activation of the AAR (Fig. 3D, Supplemental Table S2). EGR1 is a zinc finger protein for which expression is induced by IL-1β and is also implicated in the regulation of IL-1β function (84) and thus provides another bridge between networks 3 and 23. Another IL-1β-linked protein included within network 23 is growth arrest and DNA damage-inducible 45β (GADD45β), for which the expression was increased by 16-fold. GADD45β is a member of the Gadd45 family that is involved in growth arrest, apoptosis, and DNA repair (40a). Although GADD45β was previously identified as a proapoptotic protein (53a), a recent report showed that GADD45β was selectively induced by IL-1β treatment of INS-1E pancreatic cells, and in this context GADD45β antagonized IL-1β-mediated beta cell apoptosis (37a). IL-1β is postulated to play a role in the destruction of pancreatic beta cells in diabetes (42a). These results suggest that the pro- or antiapoptotic function of GADD45β may depend on different stimuli or the cellular context. Although no significant change of IL-1β mRNA after HisOH treatment was detected on the microarray, the upregulation of these downstream effectors of IL-1β by amino acid limitation in this in vitro model suggests that protein malnutrition in vivo would significantly affect the IL-1β-mediated inflammatory response.
Analysis of identified networks for common transcription factors.
To test for transcriptional regulatory mechanisms that may contribute to a coordinated expression of the genes within a given network, “ModuleSearcher,” a routine in the web-based program TOUCAN (v.3.0.2, http://homes.esat.kuleuven.be/∼saerts/software/toucan.php), was used (2). Given that the transcriptional regulation of a metazoan gene often depends on the cooperative action of multiple transcription factors, this software defines a cis-regulatory module (CRM) as a region (200 bp was chosen for this analysis) that has multiple transcription binding sites. As an example, the genes in network 3 were analyzed for possible CRMs across the region spanning 2 kb upstream and 500 bp downstream from the transcription start site. For the genes in network 3, the program identified common clustering of regulatory elements for C/EBPα, glucocorticoid receptor (NR3C1), forkhead box L1 (FOXL1), GATA3, and bromodomain plant homeodomain transcription factor (BPTF) within the promoter region of the 30 genes analyzed. Interestingly, the genes encoding CEBPα (3.2-fold, probe set 204039_at), NR3C1 (2.4-fold, probe set 216321_s_at), and BPTF (1.9-fold, probe set 207186_s_at) were induced in expression after HisOH treatment (Supplemental Table S2). Regarding these factors, only C/EBPα has been previously linked to transcriptional regulation of amino acid-responsive genes. Of particular note among this collection of factors is BPTF, which is the human ortholog of Drosophila nucleosome remodeling factor NURF301, a subunit of the NURF chromatin remodeling complex. Thus far, no direct studies have linked chromatin remodeling complexes to an amino acid-regulated gene. Therefore, it is interesting to speculate that, in response to amino acid limitation, this collection of transcription factors may play a role in the induction of downstream target genes that form a particular network and thereby coordinately modulate specific physiological functions.
Conclusions.
The present report documents the changes in gene expression after activation of the AAR in human HepG2 hepatoma cells. The following novel observations were made. 1) When genes were selected with a statistical difference of P < 0.001 and the arbitrary value of twofold or greater change, >1,500 genes exhibited a significant change in mRNA content through either transcription or mRNA stability. 2) The functions for the differentially expressed genes ranged widely, covering most aspects of cell function. 3) Analysis of the AAR-regulated genes to generate potential gene networks revealed many interactions that had not previously been associated with dietary protein limitation in vivo or amino acid deprivation in cultured cells.
Extensive regulation of genes encoding proteins that mediate epigenetic changes in chromatin-associated proteins suggests that the AAR may have long-term effects. Furthermore, given the nature of epigenetic memory, the effect of AAR-induced changes may remain even if the protein/amino acid limitation is transient. Indeed, extensive data have emerged to support this concept. The 1944 Dutch famine is an example of the influence of in utero malnutrition on adult-onset chronic degenerative diseases in humans (reviewed in Ref. 56). Mothers who were exposed to famine during early gestation produced children who as adults had a higher risk of coronary heart disease, a higher body mass index, and a more atherogenic lipid profile. One possibility that would explain these data is that maternal malnutrition results in epigenetic changes in the placenta and/or fetus that, in turn, lead to stable, heritable changes in gene expression during adulthood (71). Animal studies reveal that even a limited period of maternal dietary deficiency can induce fetal epigenetic changes that manifest as changes in adult life (70). Mechanistically, protein restriction in utero causes genomewide changes in fetal hepatic DNA methylation, and those changes have the potential to alter the regulation of important genes in the offspring (41). Thus mounting evidence illustrates that maternal malnutrition, including protein limitation, causes fetal epigenetic changes that alter gene expression after birth. Understanding the regulation of the enzymes that mediate these epigenetic modifications represents an important area of investigation, and the present array studies underscore the breadth of these changes.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-59315 and DK-52064 to M. S. Kilberg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the other members of the Kilberg laboratory for helpful discussion and suggestions.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol Vis Sci 43: 2791–2798, 2002. [PubMed] [Google Scholar]
- 2. Aerts S, Thijs G, Coessens B, Staes M, Moreau Y, De Moor B. Toucan: deciphering the cis-regulatory logic of coregulated genes. Nucleic Acids Res 31: 1753–1764, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ansari KI, Mishra BP, Mandal SS. MLL histone methylases in gene expression, hormone signaling and cell cycle. Front Biosci 14: 3483–3495, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Bruhat A, Cherasse Y, Chaveroux C, Maurin AC, Jousse C, Fafournoux P. Amino acids as regulators of gene expression in mammals: molecular mechanisms. Biofactors 35: 249–257, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem 272: 17588–17593, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent l-asparaginase. J Biol Chem 284: 32742–32749, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang AC, Hook J, Lemckert FA, McDonald MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW, Reddel RR. The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology 149: 2403–2410, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Chaveroux C, Jousse C, Cherasse Y, Maurin AC, Parry L, Carraro V, Derijard B, Bruhat A, Fafournoux P. Identification of a novel amino acid response pathway triggering ATF2 phosphorylation in mammals. Mol Cell Biol 29: 6515–6526, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen C, Dudenhausen E, Chen H, Pan YX, Gjymishka A, Kilberg MS. Amino-acid limitation induces transcription from the human C/EBPbeta gene via an enhancer activity located downstream of the protein coding sequence. Biochem J 391: 649–658, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive bZIP transcription factors as well as localized histone acetylation. J Biol Chem 279: 50829–50839, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Cherasse Y, Chaveroux C, Jousse C, Maurin AC, Carraro V, Parry L, Fafournoux P, Bruhat A. Role of the repressor JDP2 in the amino acid-regulated transcription of CHOP. FEBS Lett 582: 1537–1541, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Chiba S, Takeshita K, Imai Y, Kumano K, Kurokawa M, Masuda S, Shimizu K, Nakamura S, Ruddle FH, Hirai H. Homeoprotein DLX-1 interacts with Smad4 and blocks a signaling pathway from activin A in hematopoietic cells. Proc Natl Acad Sci USA 100: 15577–15582, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cloos PA, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev 22: 1115–1140, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Boo HA, Harding JE. Protein metabolism in preterm infants with particular reference to intrauterine growth restriction. Arch Dis Child Fetal Neonatal Ed 92: F315–F319, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a. Delarue FL, Taylor BS, Sebti SM. Ras and RhoA suppress whereas RhoB enhances cytokine-induced transcription of nitric oxide synthase-2 in human normal liver AKN-1 cells and lung cancer A-549 cells. Oncogene 20: 6531–6537, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Deval C, Chaveroux C, Maurin AC, Cherasse Y, Parry L, Carraro V, Milenkovic D, Ferrara M, Bruhat A, Jousse C, Fafournoux P. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways. FEBS J 276: 707–718, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Eilers M, Eisenman RN. Myc's broad reach. Genes Dev 22: 2755–2766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Endo Y, Fu Z, Abe K, Arai S, Kato H. Dietary protein quantity and quality affect rat hepatic gene expression. J Nutr 132: 3632–3637, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339: 135–141, 1999. [PMC free article] [PubMed] [Google Scholar]
- 19. Gjymishka A, Palii SS, Shan J, Kilberg MS. Despite increased ATF4 binding at the C/EBP-ATF composite site following activation of the unfolded protein response, system A transporter 2 (SNAT2) transcription activity is repressed in HepG2 cells. J Biol Chem 283: 27736–27747, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gong SS, Basilico C. A mammalian temperature-sensitive mutation affecting G1 progression results from a single amino acid substitution in asparagine synthetase. Nucleic Acids Res 18: 3509–3513, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307: 1776–1778, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108, 2000. [DOI] [PubMed] [Google Scholar]
- 23. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904, 2000. [DOI] [PubMed] [Google Scholar]
- 24. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8: 880–887, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatzoglou M, Fernandez J, Yaman I, Closs EI. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr 24: 377–399, 2004. [DOI] [PubMed] [Google Scholar]
- 27. He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem 276: 20858–20865, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Heal R, McGivan JD. Induction of calreticulin expression in response to amino acid deprivation in Chinese hamster ovary cells. Biochem J 329: 389–394, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hinnebusch AG, Natarajan K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell 1: 22–32, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 23: 5651–5663, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24: 1365–1377, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kilberg MS, Barbosa-Tessmann IP. Genomic sequences necessary for transcriptional activation by amino acid deprivation of mammalian cells. J Nutr 132: 1801–1804, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr 25: 59–85, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through SER727 phosphorylation. J Biol Chem 284: 35425–35432, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kohler C, Villar CB. Programming of gene expression by Polycomb group proteins. Trends Cell Biol 18: 236–243, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Laine RO, Shay NF, Kilberg MS. Nuclear retention of the induced mRNA following amino acid-dependent transcriptional regulation of mammalian ribosomal proteins L17 and S25. J Biol Chem 269: 9693–9697, 1994. [PubMed] [Google Scholar]
- 37a. Larsen CM, Dossing MG, Papa S, Franzoso G, Billestrup N, Mandrup-Poulsen T. Growth arrest- and DNA-damage-inducible 45beta gene inhibits c-Jun N-terminal kinase and extracellular signal-regulated kinase and decreases IL-1beta-induced apoptosis in insulin-producing INS-1E cells. Diabetologia 49: 980–989, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Lee JI, Dominy JE, Jr, Sikalidis AK, Hirschberger LL, Wang W, Stipanuk MH. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol Genomics 33: 218–229, 2008. [DOI] [PubMed] [Google Scholar]
- 39. Leung-Pineda V, Pan Y, Chen H, Kilberg MS. Induction of p21 and p27 expression by amino acid deprivation of HepG2 human hepatoma cells involves mRNA stabilization. Biochem J 379: 79–88, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li C, Hung WW. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2: RESEARCH0032, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a. Liebermann DA, Hoffman B. Myeloid differentiation (MyD) primary response genes in hematopoiesis. Blood Cells Mol Dis 31: 213–228, 2003. [DOI] [PubMed] [Google Scholar]
- 41. Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135: 1382–1386, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 167: 27–33, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110: 851–860, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malabanan KP, Kanellakis P, Bobik A, Khachigian LM. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ Res 103: 378–387, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Marten NW, Burke EJ, Hayden JM, Straus DS. Effect of amino acid limitation on the expression of 19 genes in rat hepatoma cells. FASEB J 8: 538–544, 1994. [DOI] [PubMed] [Google Scholar]
- 45. Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med 261: 437–452, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab 1: 273–277, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Muller J, Verrijzer P. Biochemical mechanisms of gene regulation by polycomb group protein complexes. Curr Opin Genet Dev 19: 150–158, 2009. [DOI] [PubMed] [Google Scholar]
- 48. Ord D, Ord T. Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem Biophys Res Commun 330: 210–218, 2005. [DOI] [PubMed] [Google Scholar]
- 49. Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids 37: 79–88, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palii SS, Thiaville MM, Pan YX, Zhong C, Kilberg MS. Characterization of the amino acid response element within the human SNAT2 system A transporter gene. Biochem J 395: 517–527, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pan YX, Chen H, Siu F, Kilberg MS. Amino acid deprivation and endoplasmic reticulum stress induce expression of multiple ATF3 mRNA species which, when overexpressed in HepG2 cells, modulate transcription by the human asparagine synthetase promoter. J Biol Chem 278: 38402–38412, 2003. [DOI] [PubMed] [Google Scholar]
- 52. Pan YX, Chen H, Kilberg MS. Interaction of RNA-binding proteins HuR and AUF1 with the human ATF3 mRNA 3′-untranslated region regulates its amino acid limitation-induced stabilization. J Biol Chem 280: 34609–34616, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pan YX, Chen H, Thiaville MM, Kilberg MS. Activation of the ATF3 gene through a co-ordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J 401: 299–307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a. Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D'Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 6: 146–153, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Pohjanpelto P, Holtta E. Deprivation of a single amino acid induces protein synthesis-dependent increases in c-jun, c-myc, and ornithine decarboxylase mRNAs in Chinese hamster ovary cells. Mol Cell Biol 10: 5814–5821, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pungaliya P, Kulkarni D, Park HJ, Marshall H, Zheng H, Lackland H, Saleem A, Rubin EH. TOPORS functions as a SUMO-1 E3 ligase for chromatin-modifying proteins. J Proteome Res 6: 3918–3923, 2007. [DOI] [PubMed] [Google Scholar]
- 55a. Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev 60: 57–64, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol 185: 93–98, 2001. [DOI] [PubMed] [Google Scholar]
- 57. Rzymski T, Paantjens A, Bod J, Harris AL. Multiple pathways are involved in the anoxia response of SKIP3 including HuR-regulated RNA stability, NF-kappaB and ATF4. Oncogene 27: 4532–4543, 2008. [DOI] [PubMed] [Google Scholar]
- 58. Sawa C, Yoshikawa T, Matsuda-Suzuki F, Delehouzee S, Goto M, Watanabe H, Sawada J, Kataoka K, Handa H. YEAF1/RYBP and YAF-2 are functionally distinct members of a cofactor family for the YY1 and E4TF1/hGABP transcription factors. J Biol Chem 277: 22484–22490, 2002. [DOI] [PubMed] [Google Scholar]
- 59. Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 128: 735–745, 2007. [DOI] [PubMed] [Google Scholar]
- 60. Shin C, Manley JL. Cell signalling and the control of pre-mRNA splicing. Nat Rev Mol Cell Biol 5: 727–738, 2004. [DOI] [PubMed] [Google Scholar]
- 61. Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol 10: 697–708, 2009. [DOI] [PubMed] [Google Scholar]
- 62. Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α. Genetics 154: 787–801, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stock DW, Ellies DL, Zhao Z, Ekker M, Ruddle FH, Weiss KM. The evolution of the vertebrate Dlx gene family. Proc Natl Acad Sci USA 93: 10858–10863, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Su N, Thiaville MM, Awad K, Gjymishka A, Brant JO, Yang TP, Kilberg MS. Protein or amino acid deprivation differentially regulates the hepatic forkhead box protein A (FOXA) genes through an activating transcription factor-4-independent pathway. Hepatology 50: 282–290, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thiaville MM, Dudenhausen EE, Zhong C, Pan YX, Kilberg MS. Deprivation of protein or amino acid induces C/EBPbeta synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription. Biochem J 410: 473–484, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thiaville MM, Pan YX, Gjymishka A, Zhong C, Kaufman RJ, Kilberg MS. MEK signaling is required for phosphorylation of eIF2alpha following amino acid limitation of HepG2 human hepatoma cells. J Biol Chem 283: 10848–10857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Dam H, Castellazzi M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene 20: 2453–2464, 2001. [DOI] [PubMed] [Google Scholar]
- 68. Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101: 11269–11274, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68a. Vega FM, Ridley AJ. SnapShot: Rho family GTPases. Cell 129: 1430, 2007. [DOI] [PubMed] [Google Scholar]
- 69. Vesely PW, Staber PB, Hoefler G, Kenner L. Translational regulation mechanisms of AP-1 proteins. Mutat Res 22: 222–223, 2009. [DOI] [PubMed] [Google Scholar]
- 70. Waterland RA, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am J Clin Nutr 69: 179–197, 1999. [DOI] [PubMed] [Google Scholar]
- 71. Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 27: 363–388, 2007. [DOI] [PubMed] [Google Scholar]
- 72. Weidenfeld-Baranboim K, Bitton-Worms K, Aronheim A. TRE-dependent transcription activation by JDP2-CHOP10 association. Nucleic Acids Res 36: 3608–3619, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weidenfeld-Baranboim K, Hasin T, Darlyuk I, Heinrich R, Elhanani O, Pan J, Yokoyama KK, Aronheim A. The ubiquitously expressed bZIP inhibitor, JDP2, suppresses the transcription of its homologue immediate early gene counterpart, ATF3. Nucleic Acids Res 37: 2194–2203, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wolfgang CD, Chen BP, Martindale JL, Holbrook NJ, Hai T. gadd153/Chop10, a potential target gene of the transcriptional repressor ATF3. Mol Cell Biol 17: 6700–6707, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 11: 1473–1480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yaman I, Fernandez J, Liu H, Caprara M, Komar AA, Koromilas AE, Zhou L, Snider MD, Scheuner D, Kaufman RJ, Hatzoglou M. The zipper model of translational control. A small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 113: 519–531, 2003. [DOI] [PubMed] [Google Scholar]
- 77. Yaman I, Fernandez J, Sarkar B, Schneider RJ, Snider MD, Nagy LE, Hatzoglou M. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem 277: 41539–41546, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu S, Franceschi RT, Luo M, Fan J, Jiang D, Cao H, Kwon TG, Lai Y, Zhang J, Patrene K, Hankenson K, Roodman GD, Xiao G. Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PLoS One 4: e7583, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yuan Z, Gong S, Luo J, Zheng Z, Song B, Ma S, Guo J, Hu C, Thiel G, Vinson C, Hu CD, Wang Y, Li M. Opposing roles for ATF2 and c-Fos in c-Jun-mediated neuronal apoptosis. Mol Cell Biol 29: 2431–2442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhan K, Vattem KM, Bauer BN, Dever TE, Chen JJ, Wek RC. Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stresses. Mol Cell Biol 22: 7134–7146, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang F, Romano PR, Nagamura-Inoue T, Tian B, Dever TE, Mathews MB, Ozato K, Hinnebusch AG. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J Biol Chem 276: 24946–24958, 2001. [DOI] [PubMed] [Google Scholar]
- 82. Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem 283: 7064–7073, 2008. [DOI] [PubMed] [Google Scholar]
- 84. Zhu X, Lin Y, Bacanamwo M, Chang L, Chai R, Massud I, Zhang J, Garcia-Barrio T, Thompson WE, Chen YE. Interleukin-1 beta-induced Id2 gene expression is mediated by Egr-1 in vascular smooth muscle cells. Cardiovasc Res 76: 141–148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]