Abstract
A number of studies have suggested that resistance of target cells to natural killing (NK) may be correlated with their level of expression of major histocompatibility complex (MHC) class I antigens. To examine this hypothesis directly, a NK-sensitive class I-deficient human B-cell line was transfected with MHC class I genes. The expression of transfected HLA, but not H-2, class I gene products resulted in loss of susceptibility to human NK-mediated conjugation and cytolysis. Furthermore, this protection did not extend to cytotoxicity mediated by interleukin 2-stimulated human NK effector cells.
Full text
PDF
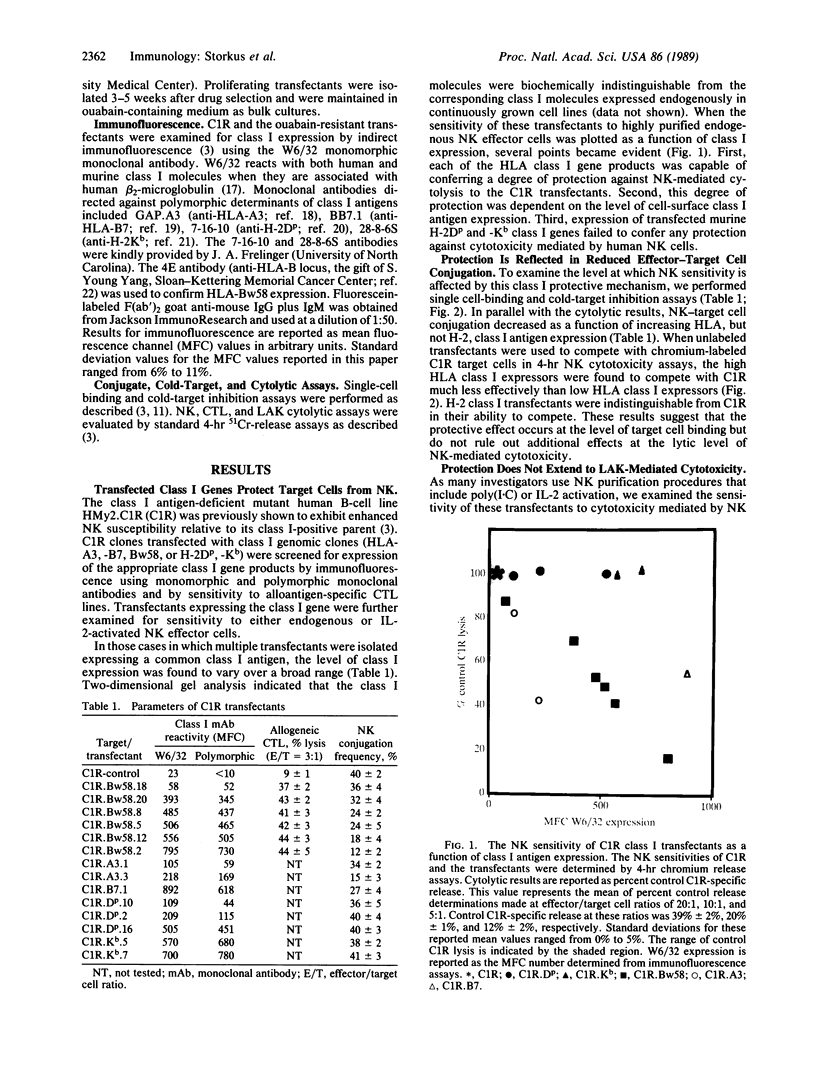
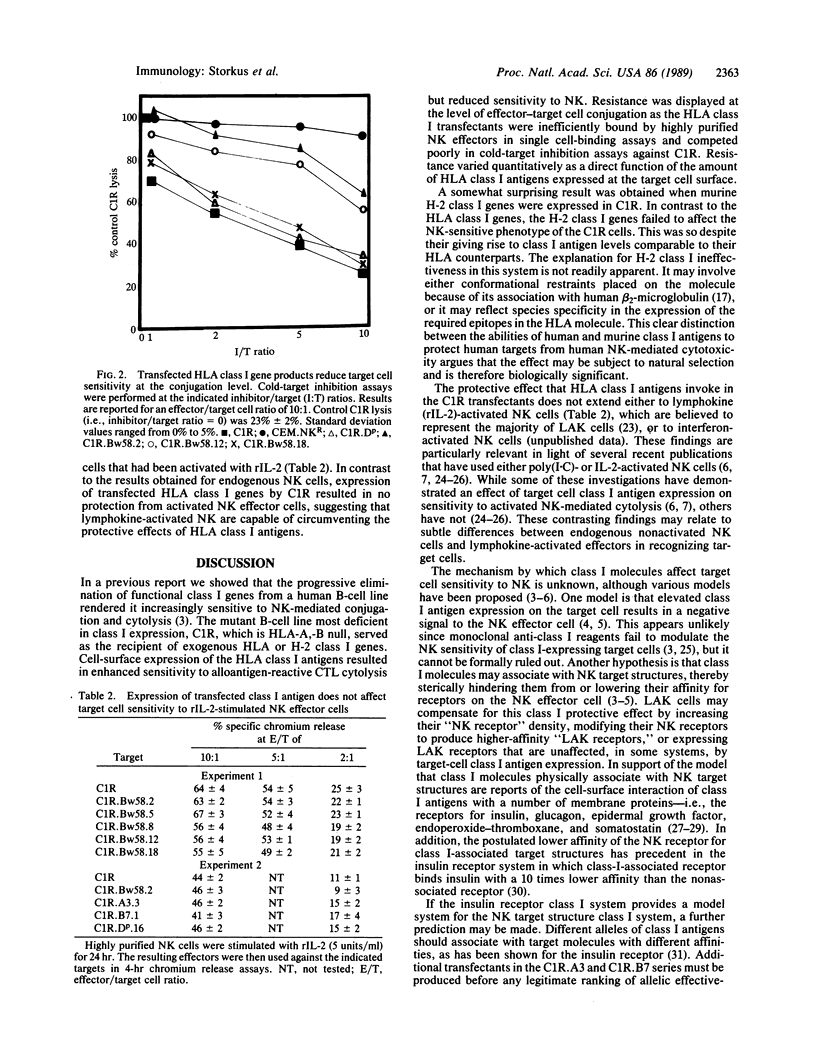

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Chervenak R., Wolcott R. M. Target cell expression of MHC antigens is not (always) a turn-off signal to natural killer cells. J Immunol. 1988 Jun 1;140(11):3712–3716. [PubMed] [Google Scholar]
- Claas F. H., van Ree J. M., Verhoeven W. M., van der Poel J. J., Verduyn W., de Wied D., van Rood J. J. The interaction between gamma-type endorphins and HLA class I antigens. Hum Immunol. 1986 Apr;15(4):347–356. doi: 10.1016/0198-8859(86)90011-x. [DOI] [PubMed] [Google Scholar]
- Dennert G., Landon C., Lord E. M., Bahler D. W., Frelinger J. G. Lysis of a lung carcinoma by poly I:C-induced natural killer cells is independent of the expression of class I histocompatibility antigens. J Immunol. 1988 Apr 1;140(7):2472–2475. [PubMed] [Google Scholar]
- Edwards P. A., Smith C. M., Neville A. M., O'Hare M. J. A human-hybridoma system based on a fast-growing mutant of the ARH-77 plasma cell leukemia-derived line. Eur J Immunol. 1982 Aug;12(8):641–648. doi: 10.1002/eji.1830120804. [DOI] [PubMed] [Google Scholar]
- Engelhard V. H., Yannelli J. R., Evans G. A., Walk S. F., Holterman M. J. Construction of novel class I histocompatibility antigens by interspecies exon shuffling. J Immunol. 1985 Jun;134(6):4218–4225. [PubMed] [Google Scholar]
- Gorelik E., Gunji Y., Herberman R. B. H-2 antigen expression and sensitivity of BL6 melanoma cells to natural killer cell cytotoxicity. J Immunol. 1988 Mar 15;140(6):2096–2102. [PubMed] [Google Scholar]
- Hanna N., Burton R. C. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981 Nov;127(5):1754–1758. [PubMed] [Google Scholar]
- Harel-Bellan A., Quillet A., Marchiol C., DeMars R., Tursz T., Fradelizi D. Natural killer susceptibility of human cells may be regulated by genes in the HLA region on chromosome 6. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5688–5692. doi: 10.1073/pnas.83.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon R. C., Stein N., Frelinger J. A. Monoclonal antibodies reactive with H-2 determinants. Immunogenetics. 1983;18(5):541–545. doi: 10.1007/BF00364395. [DOI] [PubMed] [Google Scholar]
- Herrera V. L., Emanuel J. R., Ruiz-Opazo N., Levenson R., Nadal-Ginard B. Three differentially expressed Na,K-ATPase alpha subunit isoforms: structural and functional implications. J Cell Biol. 1987 Oct;105(4):1855–1865. doi: 10.1083/jcb.105.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D. N., Andreotti P. E., Dawson J. R., Cresswell P. Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J Immunol. 1985 Feb;134(2):971–976. [PubMed] [Google Scholar]
- Kavathas P., Bach F. H., DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Taniguchi K., Toshitani A., Nomoto K. Synergistic defense system by cooperative natural effectors against metastasis of B16 melanoma cells in H-2-associated control: different behavior of H-2+ and H-2- cells in metastatic processes. J Immunol. 1986 Jun 15;136(12):4729–4734. [PubMed] [Google Scholar]
- Kievits F., Ivanyi P. Monomorphic anti-HLA monoclonal antibody (W6/32) recognizes polymorphic H-2 heavy-chain determinants exposed by association with bovine or human but not murine beta 2-microglobulin. Hum Immunol. 1987 Oct;20(2):115–126. doi: 10.1016/0198-8859(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Kittur D., Shimizu Y., DeMars R., Edidin M. Insulin binding to human B lymphoblasts is a function of HLA haplotype. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1351–1355. doi: 10.1073/pnas.84.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lafuse W., Edidin M. Influence of the mouse major histocompatibility complex, H-2, on liver adenylate cyclase activity and on glucagon binding to liver cell membranes. Biochemistry. 1980 Jan 8;19(1):49–54. doi: 10.1021/bi00542a008. [DOI] [PubMed] [Google Scholar]
- Murray R., Hutchison C. A., 3rd, Frelinger J. A. Saturation mutagenesis of a major histocompatibility complex protein domain: identification of a single conserved amino acid important for allorecognition. Proc Natl Acad Sci U S A. 1988 May;85(10):3535–3539. doi: 10.1073/pnas.85.10.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Quillet A., Presse F., Marchiol-Fournigault C., Harel-Bellan A., Benbunan M., Ploegh H., Fradelizi D. Increased resistance to non-MHC-restricted cytotoxicity related to HLA A, B expression. Direct demonstration using beta 2-microglobulin-transfected Daudi cells. J Immunol. 1988 Jul 1;141(1):17–20. [PubMed] [Google Scholar]
- Schold M., Colombero A., Reyes A. A., Wallace R. B. Oligonucleotide-directed mutagenesis using plasmid DNA templates and two primers. DNA. 1984 Dec;3(6):469–477. doi: 10.1089/dna.1.1984.3.469. [DOI] [PubMed] [Google Scholar]
- Simonsen M., Skjødt K., Crone M., Sanderson A., Fujita-Yamaguchi Y., Due C., Rønne E., Linnet K., Olsson L. Compound receptors in the cell membrane: ruminations from the borderland of immunology and physiology. Prog Allergy. 1985;36:151–176. [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Howell D. N., Salter R. D., Dawson J. R., Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987 Mar 15;138(6):1657–1659. [PubMed] [Google Scholar]
- Ways J. P., Coppin H. L., Parham P. The complete primary structure of HLA-Bw58. J Biol Chem. 1985 Oct 5;260(22):11924–11933. [PubMed] [Google Scholar]
- Yang S. Y., Morishima Y., Collins N. H., Alton T., Pollack M. S., Yunis E. J., Dupont B. Comparison of one-dimensional IEF patterns for serologically detectable HLA-A and B allotypes. Immunogenetics. 1984;19(3):217–231. doi: 10.1007/BF00364765. [DOI] [PubMed] [Google Scholar]