Abstract
Efficacy of chlorine dioxide (CD) gas generated by two distinct generation systems, Sabre (wet system with gas generated in water) and ClorDiSys (dry system with gas generated in air), was evaluated for inactivation of Bacillus anthracis spores on six building interior surfaces. The six building materials included carpet, acoustic ceiling tile, unpainted cinder block, painted I-beam steel, painted wallboard, and unpainted pinewood. There was no statistically significant difference in the data due to the CD generation technology at a 95% confidence level. Note that a common method of CD gas measurement was used for both wet and dry CD generation types. Doses generated by combinations of different concentrations of CD gas (500, 1,000, 1,500, or 3,000 parts per million of volume [ppmv]) and exposure times (ranging between 0.5 and 12 h) were used to evaluate the relative role of fumigant exposure period and total dose in the decontamination of building surfaces. The results showed that the time required to achieve at least a 6-log reduction in viable spores is clearly a function of the material type on which the spores are inoculated. The wood and cinder block coupons required a longer exposure time to achieve a 6-log reduction. The only material showing a clear statistical difference in rate of decay of viable spores as a function of concentration was cinder block. For all other materials, the profile of spore kill (i.e., change in number of viable spores with exposure time) was not dependent upon fumigant concentration (500 to 3,000 ppmv). The CD dose required for complete spore kill on biological indicators (typically, 1E6 spores of Bacillus atrophaeus on stainless steel) was significantly less than that required for decontamination of most of the building materials tested.
In the fall of 2001, dissemination of virulent spores of Bacillus anthracis (the causative agent for anthrax) via letters sent through the U.S. Postal Service resulted in contamination of numerous buildings either directly (e.g., handling of the contaminated letters) or secondarily (cross-contamination). Contaminated buildings included media offices, postal facilities, Capitol Hill, the Department of State and Department of Justice mail facilities, and residences (4, 5). Several of these facilities were determined to have minimal contamination, likely due to cross-contamination, based upon the low numbers of surface samples positive for the presence of B. anthracis spores. Such cross-contaminated facilities were remediated using surface treatment methods, such as bleach and/or liquid chlorine dioxide (CD), with disposal of contaminated items. However, the buildings that were directly contaminated, where workers developed symptoms of inhalation anthrax, were subjected to complex, time-consuming, and costly cleanups using fumigation as a principal remedial tool. Two key fumigants, vaporous hydrogen peroxide (VHP) and CD gas, were used in decontamination; CD gas was used in the cleanup of the Hart Senate Office Building, Brentwood Mail Processing and Distribution Center, Trenton Processing and Distribution Center, and the American Media, Inc., building in Boca Raton, FL (4).
CD gas has been shown to be virucidal (6, 28), bactericidal, and sporicidal (3, 10, 13, 22). However, no pesticide was registered through the Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) specifically for use against B. anthracis spores at the time of the remediation. Furthermore, use of a sporicide at the scale required was unprecedented. Based upon a limited set of data (generated in trial experiments prior to building decontamination) (23), conditions for building cleanup via fumigation with CD gas were required to be a dose of 9,000 parts per million by volume per hour ([ppmv-h] calculated as the concentration multiplied by exposure time [CT]) sustained throughout the building by maintaining 750 ppmv of CD gas for 12 h at a minimum temperature of 75°F, with a relative humidity (RH) of 75% (2). Even though there is no direct data available on the role of temperature and RH, hydration of spores by ≥75% RH appears to be a prerequisite for high sporicidal action of CD gas. Success of a fumigation was indirectly determined via the use of biological indicators (BIs) or spore strips; effective kill of all BIs (no growth on analysis) was required (4, 23). Ultimately, successful remediation of the facility was concluded based on no growth being observed upon environmental surface sampling (4, 26).
On-site generation of CD gas has been achieved by wet or dry processes. For the wet process, hydrochloric acid is reacted with sodium hypochlorite to generate chlorine, which immediately reacts with sodium hypochlorite to produce CD that can be stripped out of solution for fumigation (the method of Sabre Technical Services, LLC). In the dry process, chlorine gas is passed over a bed of sodium hypochlorite, resulting in generation of CD gas (ClorDiSys Technology, ClorDiSys Solution, Inc. [CSI]). Vendors providing both technologies claim generation of “pure” CD gas with minimal or no by-product impurities. The wet process of CD generation has been used in all CD gas building fumigations for B. anthracis to date. In principle, either process could be used. However, differences in generation capacity to achieve the target fumigation requirements should be considered a primary factor. For laboratory use on a small chamber, the engineering controls of the good manufacturing practice (GMP)-quality dry CD gas generation system provided automatic control of concentration, temperature, and RH, resulting in an increased ease of use. Furthermore, comparative sporicidal efficacy of the CD gas generated by the two methods has not been fully investigated in the context of building interior cleanup (18).
BIs have been extensively used in all fumigations for B. anthracis to assess effective distribution of CD gas across three-dimensional space and to perform a primary determination of the success of the fumigation (4, 5). BIs are made with paper or steel discs, which are inoculated with spores and encased in a gas-permeable envelope. Bacillus atrophaeus spores (1E6 spores/disc) are assumed to have the same degree of resistance to CD gas as B. anthracis Ames (23, 27). After fumigation, an increase in turbidity of a nutrient broth due to growth of one or more viable spore(s) remaining on the BI is construed as unsuccessful fumigation in the area in which the BI was spatially located. Two factors confound the use of BIs in drawing such conclusions: qualitative results fail to provide estimates of the number of viable spores remaining on a BI, and no correlation between the inactivation kinetics of BI spores and spores on building surfaces has been established. A relatively low dose of CD gas, 1,000 to 2,000 ppmv-h, has been reported to result in total spore kill on BIs (23). This dose is far lower than the dose (9,000 ppmv-h) required as the minimum target for building fumigations with CD gas. Hence, successful inactivation of BI spores does not indicate that target building fumigation conditions were met. A comparative study for spore kill on BIs versus building interior materials is lacking.
The primary objective of this study was to investigate relative efficacy of CD gas generated by the two distinct methods described above for the decontamination of six building interior surfaces contaminated with avirulent anthrax spores. These materials included carpet, ceiling tile, painted wallboard, unpainted cinder block, painted I-beam steel, and unpainted pinewood. A secondary objective was to evaluate the relative effectiveness of the CD doses (CT) generated by different combinations of concentration and exposure time in the decontamination of building interior surfaces. Additionally, the spore kill profiles of BIs relative to the building interior surfaces were also determined in this study. The body of scientific information generated here is expected to provide critical information for future building cleanup efforts in two fundamental areas: (i) the scientific data to support the selection of target fumigation parameters to obtain maximal sporicidal effect while keeping the collateral damage (building and its contents) to a minimum and (ii) the usefulness and limitations of BIs for predicting the success of the fumigation process for building decontamination, obviating the need for costly and time-consuming building interior sampling.
MATERIALS AND METHODS
Building material sources, coupon preparation, and handling.
Bulk quantities of four of six building interior materials (carpet, ceiling tile, pinewood, and wallboard) were procured from a high-volume retail store (Home Depot, Aberdeen, MD). Cinder blocks were procured from York Building Materials (Aberdeen, MD). The structural steel was procured from Specialized Metals (Coral Springs, FL). Pieces (1.3 by 1.3 cm) of each material (henceforth referred to as coupons) were cut from the interior sections of the bulk materials; the cinder block coupons were 2 to 2.5 by 1.3 cm. The wallboard coupons were painted with Glidden's Speed-Wall Interior PVA Primer (Home Depot, Aberdeen, MD), followed by a topcoat of Glidden Evermore Interior latex paint (Home Depot, Aberdeen, MD). The I-beam coupons were painted with TT-P-636 Red Oxide Primer (35-147; Colorado Paint). Dried painted I-beam steel coupons were rinsed in 70% ethanol, washed thoroughly in distilled water, and dried completely before use. Cinder block coupons were washed in water (to remove loose residues resulting from the cutting process) and dried completely before use. Prior to testing, all coupons were sterilized in large glass petri dishes by autoclaving for 45 min using a dry cycle.
Bacterial strain, culture, and spore preparation.
A plasmid-free strain of B. anthracis (NNR1Δ1, derived from NNR1; received from USAMRIID, Fort Detrick, MD) (8) was used in this study. Lack of both plasmids was confirmed by PCR amplification of tox and cap loci (results not shown). Broth cultures were grown in tryptic soy broth (TSB), and titer enumerations were performed using tryptic soy agar (TSA) plates after incubation for 22 ± 2 h at 37°C. Spores were prepared on Lemko agar plates and then harvested as previously described (18). The spore titer in the suspension was enumerated by making 10-fold serial dilutions in 0.5% buffered peptone water (BPW) (Becton, Dickinson, and Co., Sparks, MD) and plating an aliquot of 0.1 ml between 10−5 and 10−8 dilutions in triplicate on TSA plates. Equal volumes of spore suspension and 1% sterile fetal bovine serum solution were mixed to obtain a working stock with a titer of ∼2E8 spores/ml containing 0.5% fetal bovine serum protein. All working stocks were stored at 4°C in sterile tubes and used within 30 days of their preparation.
Coupon inoculation.
Sterile coupons were inoculated with an aliquot of 50 μl of spore suspension containing approximately 1E7 spores as seven droplets of 7.1 μl each over the entire surface area. The painted surfaces of I-beam steel, wallboard, and ceiling tile were inoculated. The spores were dried for 16 ± 2 h at 22 ± 2°C in a biosafety level 2 (BSL-2) hood. Both the test and the positive-control coupons were inoculated at the same time.
Spore extraction from coupons and enumeration.
Each coupon was transferred into individual 50-ml disposable sterile tubes containing 10 ml of BPW containing 0.05% Tween 80 surfactant. The tubes were then sonicated for 10 min and vortexed for 2 min to dislodge the spores from the coupon surface. Sonication was performed using a Bransonic tabletop ultrasonic cleaner (40 kHz frequency; Bransonic Ultrasonic, Danbury, CT).
Following extraction, 10-fold dilutions were performed as needed, and 0.1-ml aliquots were spread in triplicate on TSA plates (spread plating). The plates were incubated for 18 to 24 h at 37°C. The number of CFU was read using a QCount colony counter (Spiral Biotech Inc., Norwood, MA) after 22 ± 2 h of incubation. For fumigated test samples with very low numbers of viable spores (<10 per plate), 1-ml aliquots were transferred from the zero-dilution tube in triplicate (3 ml total) to each of three petri dishes, followed by the addition of 20 to 25 ml of liquefied TSA (prewarmed to 55°C) to each plate, and samples were mixed by gentle rotational swirling (pour plating). Following 2 to 3 h of solidification at room temperature, the plates were inverted and incubated at 37°C. CFU counts were read using a QCount colony counter after 2 and 6 days of incubation.
CD gas generator and coupon fumigation.
Two commercial processes and generators for the production of CD were used in this study. In the first process, chlorine gas (2% with 98% nitrogen) was passed over sodium hypochlorite solid to generate pure CD (CSI, Lebanon, NJ). The Cloridox-GMP (CSI), a portable CD gas generator system, was used for generating this type of CD. The other generator was procured from Sabre Technical Services, LLC (Albany, NY). In this process, CD was produced on demand by mixing controlled amounts of 25% sodium chlorite, 12.5% sodium hypochlorite, and 15% hydrochloric acid under vacuum. The CD gas was stripped from this chlorite-rich aqueous solution of CD in a counter-current air stripper with a reported approximate efficiency of 80 to 90% (of the CD dissolved in the liquid).
For both CD gas studies, an 8-ft3 test chamber (2 ft by 2 ft by 2 ft) constructed by CSI with 316-grade stainless steel was used. The test chamber was equipped with temperature, RH, pressure, and CD sensors. Additionally, the test chamber contained five antechambers for easy removal of one or more petri dishes containing the fumigated coupons without altering the process parameters. Each antechamber had an inner and an outer airlock door. Three circulation fans installed in the chamber ensured that the chamber atmosphere was well mixed and uniform. Air circulation in the chamber ensures even distribution of the fumigant over the exposed surfaces. The CD gas was measured in real time with a spectrophotometer contained within the Cloridox-GMP unit. For the Cloridox-GMP, the CD concentration in the chamber was controlled automatically via programmed logic control (PLC) valves. The regulation of concentration in the chamber during the fumigation cycle with the Sabre generator was done manually via monitoring of the real-time concentration in the chamber and manual injection of CD gas as needed. The Cloridox-GMP was used to control the chamber temperature and RH regardless of the CD generation system used. Typically, a cycle was programmed in the Cloridox-GMP with preconditioning (RH ramps to 75% ± 5%), conditioning (30 min at 75% ± 5% RH), charging (CD concentration ramps to the set point ranging between 1.4 to 8.4 mg/liter or 500 to 3,000 ppmv), and exposure or sterilization (holding time during which the RH was maintained at 75% ± 5% and CD gas concentration was at the target value ± 10%), followed by an aeration phase. For the Sabre generator, the conditioning phase was extended until the end of the cycle (not to advance to the next phase), and the set temperature and RH were maintained to allow the experiment (charging and exposure) to be run manually before proceeding to aeration via abortion of the cycle. Because of the relatively small size (8 ft3) of the fumigation chamber and the use of three fans to circulate the gas, CD concentration was assumed to be relatively uniform across the inside of the chamber. Irrespective of the generation method used, confirmation of the CD gas concentration in the chamber was performed every 60 to 120 min throughout the sterilization phase using Standard Method 4500-ClO2 E, Amperometric Method II (1).
Test coupons and uninoculated control coupons were placed in petri plates (15-mm size). Each plate contained five replicate test coupons and one blank of each material type. Test coupons and positive controls were inoculated with the target spores, as discussed previously, and blank coupons (not inoculated) were used to monitor for cross-contamination. Petri plates were placed on the floor of the test chamber prior to the start of a fumigation run. After fumigation for the specified time, one petri plate was placed in one of the antechambers by opening and closing the inner door, and it was removed after the antechamber was vented to remove residual CD gas. One of the five antechambers was used for each of the five samples drawn during the fumigation cycle. The dose was calculated as the average concentration (in ppmv) of CD in the chamber during the sterilization phase multiplied by the exposure time (in hours) of the coupons during that phase and is reported in units of ppmv-h.
Spore enumeration, log reduction, and data handling.
The samples were either spread plated or pour plated within 2 to 3 h of spore extraction. Samples were stored at 4°C until plates were counted. The plates with CFU counts between 10 and 300 (averaged from three replicate plates) were included in the enumeration calculation. Samples with CFU values outside this range were replated at appropriate dilutions. Since only a small fraction (1/30th) of the sample extract volume (0.3 out of 10 ml) was analyzed by spread plating, samples with CFU counts of 0 on the lowest dilution were further analyzed by screening a one-third fraction of the sample volume via pour plating (1 ml/plate on three plates). This analysis was done within 24 h of sample storage at 4°C. This procedure enabled a significant improvement in the level of detection of the viable spores, providing for the capability to detect the presence of as few as one to five viable spores in each sample. For all materials, if a sample contained >3 to 5 spores/10 ml, at least 1 CFU/3 ml would be expected to be observed on plates. The log values from the total number of CFU/coupon were determined for each of the five replicates, and the average and standard deviations (SDs) were computed from these five replicate values. Samples with 0 CFU on all three pour plates were assigned a value of 1 to obtain a log value of 0. Log reduction was computed at each time point within a fumigation cycle by subtracting the average log CFU value of the fumigated replicate samples from the average CFU value of all of the replicate positive-control samples for each material type. The SDs were computed through this calculation as the square root of the sum of the squares of the SDs for the positive controls and fumigated samples at each time point.
A one-way analysis of variance (ANOVA) was performed to answer the question of whether the time response was the same for each treatment. That is, is there a common slope (decrease in number of viable spores with time) or a different slope depending upon treatment?
Experimental test matrix.
A total of 19 of experiments (fumigation cycles) were conducted in this study. Five runs were conducted with the CSI generation system, and 14 were done with the Sabre system. The specific experiments, indicating the CD generation technology, target chamber concentration, the average temperature and RH throughout the sterilization phase, and the times at which five replicate coupons of each material type and one BI were withdrawn from the chamber during fumigation cycles are summarized in Table 1.
TABLE 1.
Experimental test matrixa
| CD generation type (run no.) | CD concn (ppmv) | Average RH (%)b | Coupon and BI removal times (h)c |
|---|---|---|---|
| Sabre (SB05001001) | 500 | 80 | 0.5, 1, 3, 5, 10 |
| Sabre (SB05001002) | 500 | 77 | 0.5, 1, 3, 5, 10 |
| Sabre (SB10000701) | 1,000 | 78 | 0.5, 1, 3, 5, 7 |
| Sabre (SB10000801) | 1,000 | 79 | 0.5, 1, 3, 5, 8 |
| Sabre (SB10000802) | 1,000 | 78 | 0.5, 1, 3, 5, 8 |
| Sabre (SB10000901) | 1,000 | 76 | 1, 3, 5, 7, 9 |
| Sabre (SB15000501) | 1,500 | 80 | 0.5, 1, 2, 3, 5 |
| Sabre (SB15000502) | 1,500 | 81 | 0.5, 1, 2, 3, 5 |
| Sabre (SB15000601) | 1,500 | 77 | 0.5, 1, 2, 3, 6 |
| Sabre (SB30000701) | 3,000 | 74 | 0.5, 1, 2, 3, 7 |
| Sabre (SB30000702) | 3,000 | 75 | 0.5, 1, 2, 3, 7 |
| Sabre (SB30000703) | 3,000 | 75 | 0.5, 1, 2, 3, 5 |
| Sabre (SB30000901) | 3,000 | 76 | 1, 3, 5, 7, 9 |
| Sabre (SB30000902) | 3,000 | 75 | 1, 3, 5, 7, 9 |
| CSI (CSI05001003) | 500 | 80 | 0.5, 1, 3, 5, 10 |
| CSI (CSI05001004) | 500 | 78 | 0.5, 1, 3, 5, 10 |
| CSI (CSI05001005) | 500 | 78 | 0.5, 1, 3, 5, 10 |
| CSI (CSI30000901) | 3,000 | 75 | 1, 3, 5, 7, 9 |
| CSI (CSI30000902) | 3,000 | 75 | 1, 3, 5, 7, 9 |
The chamber temperature was set at 25°C.
Standard deviations for mean temperature and RH were <1°C and <3%, respectively.
Time zero is defined as the start of the sterilization phase of the fumigation.
RESULTS
Comparative spore kill by the two CD generation systems.
The sporicidal efficacy of CD gas generated by the two different methods could potentially be different because of the distinct methods used for their generation and the possible presence of different amounts of contaminants (7; A. R. Pitochelli, presented at the Third International Symposium, Chlorine Dioxide: Drinking Water, Process Water, and Wastewater Issues, New Orleans, LA, 1995). Fumigation experiments were performed with both technologies at a constant concentration of 500 ppmv of CD gas during the sterilization phase of the decontamination cycle. The average log reduction values (± SDs) from these experiments with Sabre and CSI generation technologies are summarized in Table 2. The results shown are the average and standard deviation of two tests with the Sabre technology and three with CSI, with each test having five replicates of each coupon type per time point. The Fisher's least significant difference (LSD) posthoc means test (15) showed that there was no statistically significant difference in the data due to the CD generation technology at a 95% confidence level. This test was used to separate the different treatments into groups whose means were not significantly different from one another. Conversely, this test finds treatments whose means are also different from one another. A similar analysis was performed for the two replicate runs with the CSI and five total runs with the Sabre technology (three runs for up to 7 h and two runs for up to 9 h) at a CD concentration of 3,000 ppmv (Table 3). Again, no statistically significant difference resulting from the CD generation technology used was discerned. In addition to the comparison of results as a function of CD generation, the difference in log reduction values due to material type is also shown in Tables 2 and 3. The wood and cinder block coupons required a longer exposure to achieve a 6-log reduction.
TABLE 2.
Summary of log reduction in viable spores as a function of exposure to 500 ppmv of CD gas
| CT (ppmv-h) | Log reduction (avg ± SD) in no. of viable spores by material typea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carpet |
Ceiling tile |
Cinder block |
I-beam steel |
Wallboard |
Wood |
|||||||
| CSI | Sabre | CSI | Sabre | CSI | Sabre | CSI | Sabre | CSI | Sabre | CSI | Sabre | |
| 250 | 3.3 ± 1.1 | 2.7 ± 0.5 | 2.7 ± 0.5 | 2.5 ± 0.5 | 2.0 ± 0.7 | 1.5 ± 1.0 | 2.4 ± 1.1 | 2.4 ± 0.5 | 2.1 ± 0.9 | 1.2 ± 0.5 | 1.4 ± 0.4 | 1.0 ± 0.3 |
| 500 | 4.3 ± 1.5 | 3.5 ± 0.9 | 5.7 ± 1.1 | 4.5 ± 1.0 | 2.4 ± 1.3 | 1.9 ± 0.8 | 4.6 ± 1.6 | 3.0 ± 0.6 | 4.7 ± 2.3 | 2.3 ± 0.6 | 2.2 ± 0.7 | 1.6 ± 0.6 |
| 1,500 | 6.9 ± 0.2 | 5.5 ± 1.2 | 6.9 ± 0.4 | 6.8 ± 0.5 | 4.1 ± 2.2 | 4.2 ± 2.2 | 5.9 ± 1.3 | 4.9 ± 1.8 | 6.1 ± 1.5 | 5.6 ± 1.4 | 3.1 ± 0.7 | 3.4 ± 1.2 |
| 2,500 | 6.9 ± 0.2 | 6.6 ± 0.2 | 7.2 ± 0.1 | 6.8 ± 0.4 | 4.4 ± 2.1 | 4.1 ± 1.3 | 5.9 ± 1.1 | 5.3 ± 1.1 | 6.4 ± 1.4 | 5.6 ± 1.4 | 4.3 ± 1.2 | 3.3 ± 0.7 |
| 5,000 | 6.9 ± 0.2 | 6.6 ± 0.2 | 7.2 ± 0.1 | 7.0 ± 0.1 | 4.2 ± 1.7 | 5.3 ± 1.5 | 6.7 ± 0.7 | 6.4 ± 0.8 | 7.2 ± 0.2 | 6.9 ± 0.2 | 6.3 ± 1.1 | 4.8 ± 1.6 |
| PCb | 6.9 ± 0.2 | 6.6 ± 0.2 | 7.2 ± 0.1 | 7.0 ± 0.1 | 7.1 ± 0.2 | 6.9 ± 0.2 | 7.1 ± 0.2 | 7.0 ± 0.1 | 7.2 ± 0.1 | 6.9 ± 0.2 | 7.1 ± 0.2 | 7.0 ± 0.1 |
Inoculated coupons were exposed to either CSI or Sabre CD gas at 500 ppmv for a period of 0.5, 1, 3, 5 or 10 h, and viable spores were extracted from building materials as detailed in the Materials and Methods section. Values highlighted in boldface indicate complete kill, based on pour-plating data of the one-third fraction (detection limit of approximately 5 to 10 viable spores per sample).
PC, positive control. Values represent the average (with SD) of the log number of recovered spores from all positive controls of each material type. This value is used to calculate the log reduction, by subtracting the average log number of viable spores recovered from the replicate coupons of each material type at each CT value.
TABLE 3.
Summary of log reduction in viable spores as a function of exposure to 3,000 ppmv of CD gas
| CT (ppmv-h) | Log reduction (avg ± SD) in no. of viable spores by material typea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carpet |
Ceiling tile |
Cinder block |
I-beam steel |
Wallboard |
Wood |
|||||||
| CSI | Sabre | CSI | Sabre | CSI | Sabre | CSI | Sabre | CSI | Sabre | CSI | Sabre | |
| 1,500 | NT | 5.6 ± 1.3 | NT | 2.6 ± 0.4 | NT | 5.2 ± 1.7 | NT | 3.4 ± 1.2 | NT | 2.7 ± 0.6 | NT | 1.7 ± 0.5 |
| 3,000 | 6.8 ± 0.5 | 6.6 ± 0.5 | 3.6 ± 0.3 | 3.7 ± 0.9 | 6.8 ± 0.2 | 5.7 ± 1.1 | 6.2 ± 1.1 | 5.1 ± 1.4 | 5.4 ± 1.3 | 3.7 ± 1.3 | 2.9 ± 0.8 | 2.0 ± 0.6 |
| 6,000 | NT | 6.7 ± 0.3 | NT | 4.5 ± 0.4 | NT | 6.1 ± 0.9 | NT | 5.9 ± 1.0 | NT | 5.6 ± 1.0 | NT | 3.2 ± 0.7 |
| 9,000 | 7.0 ± 0.2 | 6.7 ± 0.3 | 6.6 ± 0.7 | 5.5 ± 1.1 | 6.8 ± 0.2 | 6.3 ± 0.6 | 7.1 ± 0.2 | 5.7 ± 1.3 | 7.0 ± 0.4 | 5.8 ± 1.1 | 5.0 ± 1.6 | 4.1 ± 1.6 |
| 15,000 | 7.0 ± 0.2 | 6.7 ± 0.3 | 7.2 ± 0.2 | 6.5 ± 0.6 | 6.7 ± 0.3 | 6.4 ± 0.4 | 7.1 ± 0.3 | 6.7 ± 0.5 | 7.1 ± 0.3 | 6.2 ± 0.7 | 6.9 ± 0.5 | 4.9 ± 1.6 |
| 21,000 | 7.0 ± 0.2 | 6.7 ± 0.3 | 7.2 ± 0.2 | 6.7 ± 0.5 | 6.8 ± 0.2 | 6.4 ± 0.4 | 7.1 ± 0.2 | 6.9 ± 0.3 | 6.7 ± 0.8 | 6.3 ± 0.6 | 7.0 ± 0.2 | 5.9 ± 1.2 |
| 27,000 | 7.0 ± 0.2 | 6.7 ± 0.3 | 7.2 ± 0.2 | 6.9 ± 0.2 | 6.8 ± 0.2 | 6.4 ± 0.4 | 6.9 ± 0.8 | 6.9 ± 0.3 | 6.8 ± 0.8 | 6.1 ± 0.9 | 7.0 ± 0.2 | 6.6 ± 0.4 |
| PCb | 7.0 ± 0.2 | 6.7 ± 0.3 | 7.2 ± 0.2 | 6.9 ± 0.2 | 6.8 ± 0.2 | 6.4 ± 0.4 | 7.1 ± 0.2 | 6.9 ± 0.3 | 7.1 ± 0.3 | 6.5 ± 0.5 | 7.0 ± 0.2 | 6.6 ± 0.4 |
Inoculated coupons were exposed to either CSI or Sabre CD gas at 3,000 ppmv for a period of 0.5, 1, 2, 3, 5, 7, or 9 h and viable spores were extracted from building materials as detailed in Materials and Methods. Values highlighted in boldface indicate complete kill, based on pour-plating data of the one-third fraction (detection limit of approximately 5 to 10 viable spores per sample). NT, not tested.
PC, positive control. Values represent the average (with SD) of the log number of recovered spores from all positive controls of each material type. This value is used to calculate the log reduction by subtracting the average log number of viable spores recovered from the replicate coupons of each material type at each CT value.
It should be noted that the values highlighted in boldface in Tables 2 and 3 indicate that no viable spores were detected in any samples from that material fumigated at the indicated dose. The maximum attainable log reduction for each material type is that of the log number of viable spores recovered from the positive controls. This value may differ for each material since the recovery of spores is material dependent. Differences in highlighted values between materials (e.g., 6.6 for carpet with Sabre and 7.2 for ceiling tile with CSI) (Table 2), hence, should not be construed as differences in effectiveness.
Correlation between CD gas CT values and spore kill.
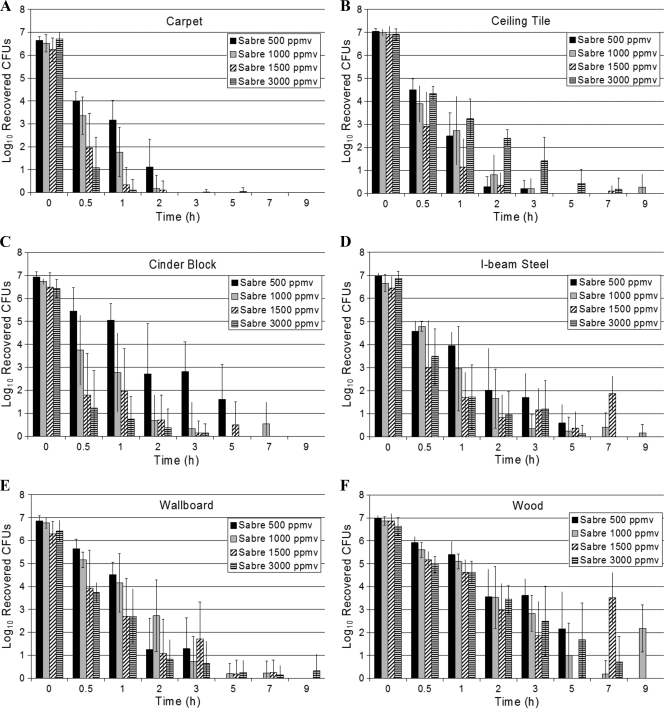
The log recovered spores as number of CFU were plotted as a function of fumigation time for each material type. Results from these experiments for the cycles performed with 500, 1,000, 1,500, and 3,000 ppmv of Sabre CD gas are shown in Fig. 1. The plots show the relationship between the logs of viable spores recovered and exposure times for each of the four target CD concentrations. The time zero data shown are the recovered viable spores from the positive controls, averaged for all experiments. The nonzero time point data shown are the average (and SD) for all common experiments. For example, the results shown for 1 h at 1,000 ppmv reflect the average of the five replicate coupons of a particular material type for all four runs at that concentration with a coupon removal at that time point, hence, a total of 20 data points (n = 20).
FIG. 1.
Spore kill profiles as a function of fumigation time with 500, 1,000, 1,500, and 3,000 ppmv of Sabre CD gas.
In general, a trend in reduction in the number of viable spores recovered is observed with increasing exposure time at each of the four CD concentrations. Hence, the dose-response relationship observed does exemplify the sensitivity of the bacterial spore to the cumulative dose of CD gas rather than just the exposure time or the CD concentration. The time required to reduce the number of recovered viable spores by 6 logs or more (e.g., no detectable viable spores) is a function of the CT. For carpet, cinder block, I-beam steel, and wallboard, the time required to achieve at least a 6-log reduction is generally reduced at higher concentrations. For ceiling tile and pinewood, this trend is less clear, especially with respect to the data reported for the duplicate runs performed at 3,000 ppmv of CD gas.
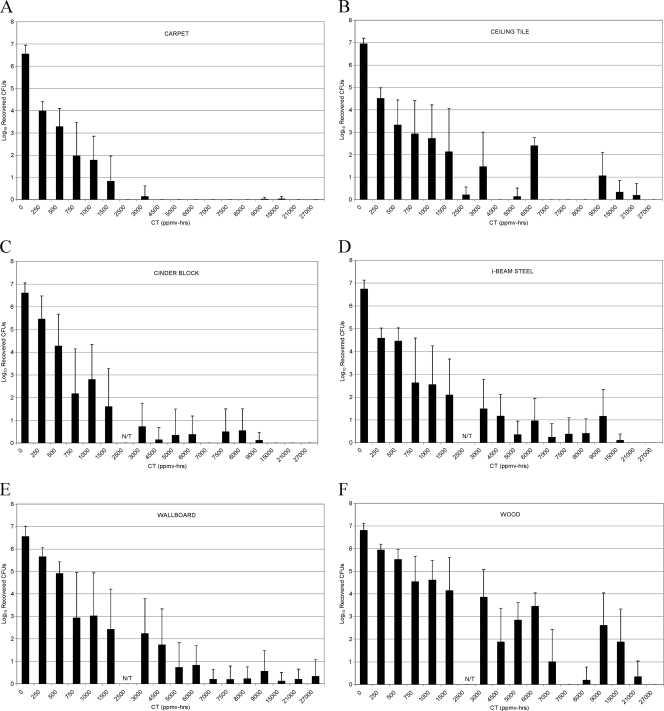
The time required to achieve at least a 6-log reduction in viable spores is clearly a function of the material type on which the spores are inoculated. Unequivocally, the raw pinewood posed the greatest challenge. Figure 2 shows the same data plotted as a function of CT to clarify this result. For a 6-log reduction, the decontamination profiles appear to fall into three categories: (i) carpet, cinder block, and ceiling tile, requiring 3,000 to 6,000 ppmv-h; (ii) I-beam steel and wallboard, requiring a dose between 6,000 to 9,000 ppmv-h; and (iii) pinewood, requiring >9,000 ppmv-h.
FIG. 2.
Spore kill profiles as a function of fumigation time with 500, 1,000, 1,500, and 3,000 ppmv of ClorDiSys CD gas.
Further analysis was performed on the data set to determine (i) whether the apparent differences with respect to the time required for reducing the number of viable spores on each material type were statistically significant and (ii) whether a statistically significant difference exists for each material as a function of each concentration. The time response curves for each treatment combination were best fit by an exponential decay function. For linearization, X (or time) was transformed to log (X + 0.1), where 0.1 was the minimum nonzero response value. The data were reduced to the analysis of slopes since (i) the time responses were repeated measurements from the same decontamination chamber (i.e., the time responses are correlated and not independent observations), and (ii) the times at which responses were measured were not the same for each experiment (i.e., times were adjusted based upon the results of preceding cycles). The rate of decay (slope) was then compared using one-way completely randomized analysis of variance (ANOVA) to determine statistical significance of measured differences. The null hypothesis was then that a common slope existed for all treatments (hence, the time for “successful” decontamination was independent of concentration). The term treatment is used here to mean the cycle parameters (i.e., fumigation conditions) to which the materials were exposed. The alternate hypothesis then followed that this time was dependent upon the concentration. The only material showing a clear statistical difference in rate of decay of viable spores as a function of concentration was cinder block. For all other materials, the profile of spore kill (i.e., change in number of viable spores with fumigation time, or slope) was not dependent upon fumigant concentration (500 to 3,000 ppmv).
CD dose required for inactivation of BIs.
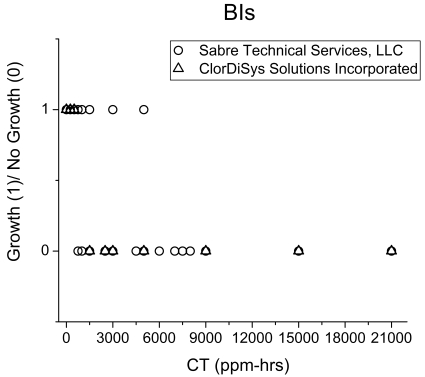
Since BIs have been extensively used in all building cleanups related to B. anthracis contamination to date to assess the success of the fumigation (4), it was of interest to compare the spore inactivation on the BIs relative to inactivation on the building materials. Inactivation of BIs was first assessed qualitatively by observing the turbidity resulting from a viable spore(s) recovered from a fumigated BI. As seen in the results summarized in Fig. 3, complete inactivation of BIs required a dose of ∼3,000 ppmv-h. Based on the CD dose required, the spore kill profile on BIs appears to correlate with spore kill only on carpet and ceiling tile surfaces. The BIs do not provide an indication of successful inactivation of B. anthracis spores on the other four materials, i.e., cinder block, wallboard, pinewood, and I-beam steel.
FIG. 3.
Number of BIs testing positive (represented as 1) or negative (represented as 0) based on turbidity as a function of CD gas dose.
In addition, a quantitative experiment was performed to contrast the CD dose required for inactivation of spores on BIs relative to those on pinewood. The results are summarized in Fig. 4. As expected, complete spore kill was observed on BIs with a dose of 1,500 to 3,000 ppmv-h of CD gas. However, only 2- to 3-log reductions in values were observed for spore kill on wood surface with comparable CD gas doses.
FIG. 4.
Log reduction of viable spores on BIs and wood coupons with 500 ppmv of CSI CD gas.
DISCUSSION
Compared to glass or steel, two of the most commonly used materials for sporicidal efficacy studies (14, 25), building interior materials used in this study represent a range of structural complexities and possess different porosities. Due to this fact, variability (among five replicates within a sample and between experimental repeats) in log reduction values was expected. The variability could be further exacerbated by four additional factors: (i) different degrees of uniformity and heterogeneous structural composition of the materials; (ii) different interactions of fumigant with the building materials; (iii) dissolution of ceiling tile and wallboard, making spore enumeration in these samples difficult; and (iv) exposure to sublethal fumigant doses resulting in partial spore inactivation. Additionally, the microscopic differences (i.e., lack of uniformity) of the replicate coupons from complex material types may result in various degrees of spore and/or gas penetration.
In an attempt to minimize the variability and increase the ability to detect statistically significant differences, several steps unique to this study were adopted. These steps included the following: (i) use of five replicate coupons per data point as opposed to the three that are typically used (20, 21, 24); (ii) use of three replicate plates per dilution as opposed to one or two; (iii) analysis of a one-third fraction of the total extracted volume recovered in samples with fewer than 10 viable spores, thus ensuring low (1 to 5 CFU) detection limits; (iv) multiple experimental repeats; and (v) control of key process parameters (i.e., RH, temperature, and fumigant concentration within 10% of the set values). Based on other sporicidal efficacy studies at sublethal treatment levels, standard deviation values of 0.5 to 1.5 log CFU were expected (13, 22, 24).
The efficacy relationship between the two CD generation types as measured in terms of spore kill on six building surfaces was investigated in this study. The reduction in the number of viable spores compared to the positive controls (noted as CT values of 0 in the tables and figures) was used as the indicator for sporicidal efficacy of CD gas, with a target reduction of at least 6 logs deemed as effective or successful kill. Based upon the statistical analysis of the data presented in Table 2, the log reduction for each material was observed not to be dependent upon the two generation methods used in this study. Note that a common method of CD gas measurement was used for both generation methods. The measurement of CD gas in the chamber is a critical parameter to ensure that the target effective dose (i.e., CT) has been achieved. Hence, a CD gas technology is then dependent upon both its generation system and its reliable measurement. No standardized method currently exists for the determination of CD gas in an atmosphere at the concentrations used/required for fumigation. An incorrect measurement method can severely skew the observed efficacy.
Five of the six materials were highly porous and complex in their composition. These characteristics of the materials may contribute to the significant differences in the dose of CD gas required to achieve similar levels of sporicidal activity. The longest exposure time and highest dose (CT) were required for pinewood. This observation might be explained by the high CD demand (amount of CD gas fed in a closed space to maintain its concentration to a set point in the presence of reactive material such as pinewood). Of all the materials included in this study, wood and ceiling tile had the highest demand for CD (2, 11). Hence, at the wood-spore-gas interface, the local concentration of CD might be lower at the spore surface due to interaction with the cellulosic and lignin components within the pinewood. Reaction of CD molecules with the wood may decrease the effective concentration of gas such that spores embedded within the wood might not be exposed. The microscopic pores and structural nonuniformity of the wood (i.e., differing pore sizes) may lead to the high level of variability observed at the long exposure periods nearing complete inactivation (Fig. 1F).
Although there appears to be an increase in log reduction as a function of concentration at the same time points, the difference was determined to be statistically significant only for cinder block. Hence, for all of the other five materials, the time required to achieve successful fumigation was determined to be independent of the fumigant concentrations used (500, 1,000, 1,500, and 3,000 ppmv). The results (i.e., reduced effectiveness) at 3,000 ppmv for ceiling tile cannot be explained at this time. Since the data for the other materials in these 3,000-ppmv runs seem consistent with the expected results, a systematic error in the fumigation cycle can be excluded as the cause of the unexpected results for ceiling tile.
These results can potentially have a major impact on the practical application of CD gas in remediating building interior surfaces contaminated with anthrax spores. Past use has required a minimum target concentration to be maintained until the specified fumigation time and CT have been achieved, with the clock paused if the CD gas concentration fell below the minimum. This work shows that exposure time is more critical than CT as long as the concentration is maintained within a target range. Additionally, in future building cleanup efforts, lower concentrations may be favored in many instances to avoid issues with material or equipment compatibility (2, 23).
Extensive numbers of BIs have been used in past building cleanup events to make assessments of fumigant distribution and CT values maintained during a cycle. BIs were used to provide an indication of the success of the fumigation process (9, 19, 26). The BIs were comprised of a surrogate spore possessing resistance to the chemical treatment at least equal to B. anthracis Ames. The surrogate spores were placed on either paper or stainless steel at a loading of nominally 1E6 spores/BI. Each BI was typically wrapped individually in a gas-permeable envelope such as Tyvek. This work clearly shows that such BIs fail to indicate that the target CT of 9,000 ppmv-h (4) required for all past fumigation events related to B. anthracis Ames contamination was achieved. The CT values required for spore kill on BIs were found to be significantly less (1,500 to 3,000 ppmv-h) than those required for spore kill on building materials. Additionally, the traditional steel or glass BIs were completely inactivated at much shorter fumigation times and lower CT values than the B. anthracis spores on all building materials included in this study. Thus, the spore kill on BIs does not indicate that successful decontamination of the building interior surfaces has been achieved. All past remediation events for B. anthracis contamination have also relied on environmental sampling before such a facility was cleared for reoccupation.
Collectively, these findings have a significant impact on requirements for future remedial use of CD gas in building cleanup. The materials upon which the spores are deposited have a substantial impact on CT values required for spore inactivation. Hence, BI use should not be justified based upon the fact that the spores alone (e.g., B. atrophaeus) are of comparable resistance to the sporicidal chemical as the target spore. The backing material has a critical impact on doses of CD gas required for complete spore kill. BIs need to be developed specifically for the intended purpose or surface types to be decontaminated. For example, for facility decontamination the use of a common material found in the facility (or a material “hard to decontaminate”) might be more appropriate, with the selection based upon work such as described in this paper. Additional efforts are needed to develop a more “realistic” BI.
Some of the recent work on the efficacy of two types of CD gas generation is conflicting because a dose of 9,000-ppmv-h Sabre gas was reported to be more effective than 12,000-ppmv-h CDG-type CD gas (19, 21) in the inactivation of anthrax spores on carpet, bare wood, glass, and decorative laminate. However, in the two studies cited, significantly different methods were used to measure CD gas concentration in the fumigation chambers. While the method used in the evaluation of the Sabre Technology (19) was consistent with the method used in our study (i.e., Standard Method 4500-ClO2 E, Amperometric Method II), the method used for the evaluation of the CDG Technology (21) was not validated or checked against a reference method. The results of the Sabre CD testing (12) are consistent with the findings in our current study.
Finally, CD gas is a very strong oxidant and behaves as a free radical because of the presence of one unpaired electron in its molecular orbital (12, 25). The antimicrobial activity of CD gas is due to protein denaturation, some of which may be important for spore viability and/or integrity (12, 16, 24). Recently, Ogata and Shibata (17) demonstrated model peptide denaturation (derived from influenza A virus) via modification of tyrosine and tryptophan residues. Future work in confirming modification of the same residues in the spore coat or cortical proteins and DNA of B. anthracis and possible correlation with spore viability is required to demonstrate the mode of action of CD gas. A clear understanding of the mode of action of CD gas and the dosage required in conjunction with different environmental surfaces will significantly aid in designing improved run conditions for cleanup of anthrax-contaminated buildings while minimizing the collateral damage to the structural components of the building.
Acknowledgments
We thank John Mason (Sabre Technologies, LLC) for his generous help in providing training on the use of a laboratory-scale generator. Additionally, we acknowledge the statistical support provided by Jim Heltshe of Computer Sciences Corporation and Ross Leadbetter of the University of North Carolina at Chapel Hill, both through independent contract vehicles with the U.S. EPA.
The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and collaborated with the Department of the Army, Edgewood Chemical Biological Center, in the research described herein under Interagency Agreement DW-21-93991701. It has been subject to an administrative review but does not necessarily reflect the views of the Agency. No official endorsement should be inferred. EPA does not endorse the purchase or sale of any commercial products or services.
Footnotes
Published ahead of print on 19 March 2010.
REFERENCES
- 1.Aieta, E. M., P. V. Roberts, and M. Hernandez. 1984. Determination of chlorine dioxide, chlorine, chlorite, and chlorate in water. J. Am. Water Works Assoc. 76:64-70. [Google Scholar]
- 2.Bartram, P. W., J. T. Lynn, L. P. Reif, M. D. Brickhouse, T. A. Lalain, S. Ryan, B. Martin, and D. Stark. 2008. Material demand studies: interaction of chlorine dioxide gas with building materials EPA/600/R-08/091. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/NHSRC/pubs/600r08091.pdf.
- 3.Beuchat, L. R., C. A. Pettigrew, M. E. Tremblay, B. J. Roselle, and A. J. Scouten. 2004. Lethality of chlorine, chlorine dioxide, and a commercial fruit and vegetable sanitizer to vegetative cells and spores of Bacillus cereus and spores of Bacillus thuringiensis. J. Food Prot. 67:1702-1708. [DOI] [PubMed] [Google Scholar]
- 4.Canter, D. A. 2005. Remediating anthrax-contaminated sites: learning from the past to protect the future. Chem. Health Safety 12:13-19. [Google Scholar]
- 5.Canter, D. A., D. Gunning, P. Rodgers, L. O'Connor, C. Traunero, and C. J. Kempter. 2005. Remediation of Bacillus anthracis contamination in the U.S. Department of Justice mail facility. Biosecur. Bioterror. 3:119-127. [DOI] [PubMed] [Google Scholar]
- 6.Fukayama, M. Y., H. Tan, W. B. Wheeler, and C. I. Wei. 1986. Reactions of aqueous chlorine and chlorine dioxide with model food compounds. Environ. Health Perspect. 69:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon, G., R. G. Kieffer, and D. H. Rosenblatt. 1972. The chemistry of chlorine dioxide, p. 201-286. In S. J. Lippard (ed.), Progress in inorganic chemistry, vol. 15. Wiley InterScience, New York, NY. [Google Scholar]
- 8.Green, B. D., L. Battisti, T. M. Koehler, C. B. Thorne, and B. E. Ivins. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall, L., J. A. Otter, J. Chewins, and N. L. Wengenack. 2007. Use of hydrogen peroxide vapor for deactivation of Mycobacterium tuberculosis is a biological safety cabinet and a room. J. Clin. Microbiol. 45:810-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han, Y., B. Applegate, R. H. Linton, and P. E. Nelson. 2003. Decontamination of Bacillus thuringiensis spores on selected surfaces by chlorine dioxide gas. J. Environ. Health 66:16-21. [PubMed] [Google Scholar]
- 11.Hubbard, H. B., D. Poppendieck, and R. L. Corsi. 2009. Chlorine dioxide reactions with indoor materials during building disinfection: surface uptake. Environ. Sci. Technol. 43:1329-1335. [DOI] [PubMed] [Google Scholar]
- 12.Jeng, D. K., and A. G. Woodworth. 1990. Chlorine dioxide gas sterilization under square-wave conditions. Appl. Environ. Microbiol. 56:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreske, A. C., J. H. Ryu, and L. R. Beuchat. 2006. Evaluation of chlorine, chlorine dioxide and a peroxyacetic acid-based sanitizer for effectiveness in killing Bacillus cereus and Bacillus thuringiensis spores in suspensions, on the surface of stainless steel and on apples. J. Food Prot. 69:1892-1903. [DOI] [PubMed] [Google Scholar]
- 14.Majcher, M. R., K. A. Bernard, and S. A. Sattar. 2008. Identification by quantitative carrier test of surrogate spore-forming bacteria to assess sporicidal chemicals for use against Bacillus anthracis. Appl. Environ. Microbiol. 74:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muth, J. E. D. 2006. Basic statistics and pharmaceutical statistical applications, 2nd ed. Chapman and Hall/CRC, New York, NY.
- 16.Ogata, N. 2007. Denaturation of protein by chlorine dioxide: oxidative modifications of tryptophan and tyrosine residues. Biochemistry 46:4898-4911. [DOI] [PubMed] [Google Scholar]
- 17.Ogata, N., and T. Shibata. 2008. Protective effect of low concentration chlorine dioxide gas against influenza a virus infection. J. Gen. Virol. 89:60-67. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi, V. K., L. Wallace, S. Smith, S. P. Ryan, and B. Martin. 2009. Quantitative method to determine sporicidal decontamination of building surfaces by gaseous fumigants and issues related to laboratory-scale studies. Appl. Environ. Microbiol. 75:3688-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers, J. V., W. R. Richter, Y. W. Choi, J. D. Waugh, M. L. Taylor, K. B. Riggs, H. J. Stone, Z. J. Willenberg, R. T. Krile, and J. P. Wood. 2006. Technology evaluation report: evaluation of sporicidal decontamination technology, SabreTechnology services chlorine dioxide gas generator. EPA 600-R-06. U.S. Environmental Protection Agency, Washington, DC.
- 20.Rogers, J. V., W. R. Richter, M. Q. Shaw, and Y. W. Choi. 2008. Vapor-phase hydrogen peroxide inactivates Yersinia pestis dried on polymers, steel, and glass surfaces. Lett. Appl. Microbiol. 47:279-285. [DOI] [PubMed] [Google Scholar]
- 21.Rogers, J. V., C. L. Sabourin, M. L. Taylor, K. B. Riggs, Y. W. Choi, W. R. Richter, D. C. Rudnicki, and H. J. Stone. 2004. Environmental technology verification report: CDG research corporation bench-scale chlorine dioxide gas:solid generator. U.S. Environmental Protection Agency, Washington, DC.
- 22.Sagripanti, J.-L., M. Carrera, J. Insalaco, M. Ziemski, and J. R. R. Zandomeni. 2007. Virulent spores of Bacillus anthracis and other Bacillus sp. deposited on solid surfaces have similar sensitivity to chemical decontaminants. J. Appl. Microbiol. 102:11-21. [DOI] [PubMed] [Google Scholar]
- 23.Science Applications International Corp. 2005. Compilation of available data on building decontamination alternatives. EPA 600-R-05-036. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/NHSRC/pubs/600r05036.pdf.
- 24.Tilt, N., and M. A. Hamilton. 1999. Repeatability and reproducibility of germicide tests: a literature review. J. AOAC Int. 82:384-389. [PubMed] [Google Scholar]
- 25.Tomasino, S. F., and M. A. Hamilton. 2007. Comparative evaluation of two quantitative test methods for determining the efficacy of liquid sporicides and sterilants on a hard surface. J. AOAC Int. 90:456-464. [PubMed] [Google Scholar]
- 26.U.S. National Response Team. 2005. Technical assistance for anthrax response, interim-final draft. U.S. National Response Team, Washington, DC.
- 27.West Dugway Test Center. 2002. Abbreviated test report for the validation of chlorine dioxide decontamination, test project no. 8-CO-210-000-084. Document WDTC-TR-02-059. West Dugway Test Center, U.S. Environmental Protection Agency, Denver, CO.
- 28.Young, S. B., and P. Setlow. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 95:54-67. [DOI] [PubMed] [Google Scholar]