Abstract
Extracellular DNA (eDNA) is an important component of the biofilm matrix. We show that removal of eDNA from Gram-positive bacteria reduces initial adhesion to and aggregation of bacteria on surfaces. Thermodynamic analyses indicated that eDNA introduces favorable acid-base interactions, explaining the effect of eDNA on aggregation and adhesion to the surface.
Extracellular polymeric substances in bacterial biofilms are composed of polysaccharides, proteins, and extracellular DNA (eDNA) (6). eDNA released by autolysis (2, 3, 10, 11, 12) acts as an adhesive (13) and strengthens biofilms (14). In Staphylococcus epidermidis 1457, autolysin E (encoded by atlE) induces production of eDNA, and a strain lacking AtlE (a ΔatlE mutant) formed significantly less biofilm (11).
Bacterial adhesion and aggregation are mediated by nonspecific long-range attractive Lifshitz-Van der Waals forces and electrostatic and acid-base interactions, as well as by protein-specific interactions, as a localized corollary of the above-mentioned forces (1, 8).
Initial adhesion to substratum surfaces and aggregation of bacteria are important steps in biofilm formation, but the role of eDNA in these processes is unclear. Therefore, we investigated the effect of naturally occurring eDNA on the initial adhesion and surface aggregation of several Gram-positive bacteria. The mechanism by which eDNA affects the adhesion and surface aggregation of two model strains (S. epidermidis 1457 and the ΔatlE mutant) was analyzed by a surface thermodynamic approach.
The strains listed in Table 1 were grown on blood agar at 37°C, except Streptococcus mutans LT11, which was grown on brain heart infusion (BHI) agar and incubated in 5% CO2 at 37°C. Ten-milliliter precultures in tryptone soya broth for staphylococci, or BHI for S. mutans LT11, were used to inoculate 200-ml main cultures in the same media. After 16 h of growth, cultures were washed in phosphate-buffered saline (PBS: 150 mM NaCl-10 mM potassium phosphate, pH 6.8) and sonicated on ice for 3 × 10 s at 30 W (5) to remove aggregates. Finally, bacteria were resuspended in PBS to a density of 3 × 108 ml−1. To remove eDNA, bacterial suspensions were treated with DNase I in the presence of 10 mM MgCl2 for 45 min at 37°C and subsequently washed twice with PBS.
TABLE 1.
Initial bacterial j0 and total numbers of adhering bacteria after 60 min to a hydrophilic or hydrophobic surface after 60 min (N60 min) in the presence and absence of naturally occurring eDNAa
| Strain and DNase I treatment | j0 (cm−2 s−1) |
N60 min (106 cm−2) |
||
|---|---|---|---|---|
| Hydrophilic surface | Hydrophobic surface | Hydrophilic surface | Hydrophobic surface | |
| S. epidermidis 1457 | ||||
| No | 3,700 (3,820) | 4,460 | 10.6 (11.0) | 10.3 |
| Yes | 2,570 | 2,940 | 6.1 | 7.1 |
| S. epidermidis 1457 ΔatlE mutant | ||||
| No | 2,150 (1,810) | 2,600 | 6.0 (6.1) | 6.9 |
| Yes | 2,057 | 2,370 | 6.0 | 6.3 |
| S. epidermidis HBH 276 | ||||
| No | 2,000 (2,230) | 2,070 | 7.3 (7.7) | 7.6 |
| Yes | 1,790 | 2,130 | 6.9 | 7.5 |
| S. aureus ATCC 12600 | ||||
| No | 1,360 (1,200) | 1,440 | 5.3 (4.6) | 5.1 |
| Yes | 1,170 | 1,170 | 4.2 | 4.3 |
| S. mutans LT11 | ||||
| No | 1,630 (1,900) | 1,740 | 6.5 (6.7) | 6.1 |
| Yes | 1,160 | 1,360 | 3.8 | 4.4 |
Data within parentheses refer to bacteria treated with heat-inactivated DNase I as a control. The standard deviations of j0 and N60 min amounted to 250 cm−2 s−1 and 0.5 × 106 cm−2, respectively, for all of the bacterial strains, as averaged over three experiments with separately grown bacteria. Data in bold indicate a statistically significant difference between data obtained in the presence and absence of eDNA (P < 0.05).
Glass or dimethyldichlorosilane (DDS)-coated glass microscope slides, possessing a hydrophilic or hydrophobic surface, respectively, were placed in the bottom of a parallel-plate flow chamber (5). Bacterial adhesion and surface aggregation at a shear rate of 16 s−1 was monitored for 60 min by microscopy. Photographic images were used to calculate the initial deposition rate (j0), the total number of bacteria adhering per unit area at time t, and the degree of surface aggregation. The area, in terms of pixel number, occupied by a single attached bacterium was different for each strain and determined by image analysis in order to calculate the number of bacteria present in an aggregate. The percentage of adhering bacteria in large aggregates (>5 bacteria) was calculated by dividing the total number of bacteria in large aggregates by the total number of adhering bacteria.
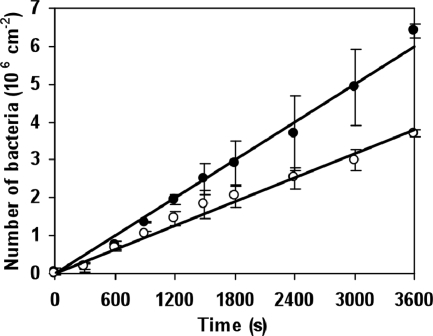
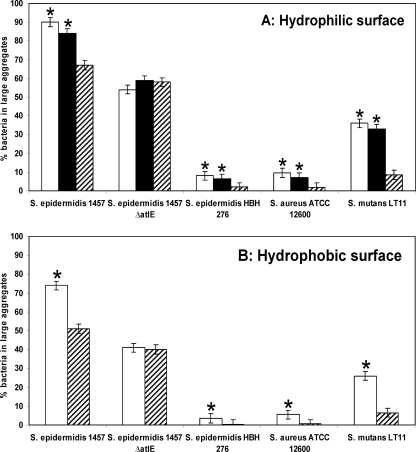
Bacterial deposition rates increased almost linearly during the duration of an experiment (Fig. 1) and were similar for hydrophilic and hydrophobic surfaces. In the presence of eDNA, bacteria adhered faster (j0) and in higher numbers (N60 min), although differences were only statistically significant for S. epidermidis 1457 and S. mutans LT11 (Table 1). The ΔatlE mutant strain's adhesion characteristics resembled those of DNase I-treated samples, further illustrating the effect of eDNA. Removal (DNase I treatment) or absence (ΔatlE mutant) of eDNA prior to adhesion significantly reduced the percentage of adhering bacteria involved in large aggregates. On hydrophilic surfaces, the presence of eDNA increased the percentage of bacteria in large aggregates (although this was only significant for S. epidermidis 1457 and S. mutans LT11) compared to that on hydrophobic surfaces (Fig. 2A and B).
FIG. 1.
Example of the adhesion kinetics of S. mutans LT11 on a hydrophilic surface for 60 min in the presence (closed symbols) or absence (open symbols) of naturally occurring eDNA. The error bars denote the standard deviations over three experiments with separately grown bacteria. The lines indicate the initial deposition rates (j0) calculated by linear least-square fitting.
FIG. 2.
Percentages of adhering bacteria involved in large aggregates 60 min after deposition onto a hydrophilic (panel A) or a hydrophobic DDS-coated (panel B) glass surface in the presence (white) or absence (striped) of naturally occurring eDNA. For the hydrophilic surface, control experiments were conducted with heat-inactivated DNase I (black), with results similar to those obtained with untreated samples. The error bars denote the standard deviations over three experiments with separately grown bacteria. Asterisks indicate statistically significant difference between data obtained in the presence or absence of eDNA (P < 0.05).
Contact angles with water, formamide, methylene iodide, and α-bromonaphthalene were measured for substrata and staphylococcal lawns prior to and after DNase I treatment (5) for surface free-energy calculations based on the concept of Lifshitz-Van der Waals/acid-base components (5). Subsequently, free energies of adhesion (bacterium-surface) and aggregation (bacterium-bacterium) were calculated, assuming interaction in an aqueous phase (8).
Upon the removal of eDNA, the hydrophobicity of S. epidermidis 1457 decreased significantly, while that of the ΔatlE mutant remained unaffected (Table 2). Accordingly, favorable (negative) interaction energies in the presence of eDNA became unfavorable (positive) due to changes in acid-base interaction energies. Lifshitz-Van der Waals energies were not affected by the presence or absence of eDNA.
TABLE 2.
Contact angles measured on bacterial lawns and on surfaces with water, formamide, α-bromonaphthalene, and methylene iodidea
| Strain and DNase I treatment or surface | Avg contact angle (degrees) ± SD |
|||
|---|---|---|---|---|
| Water | Formamide | α-Bromonaphthalene | Methylene iodide | |
| S. epidermidis 1457 | ||||
| No | 85 ± 6 | 69 ± 1 | 44 ± 2 | 23 ± 4 |
| Yes | 44 ± 5 | 40 ± 3 | 53 ± 2 | 49 ± 3 |
| S. epidermidis 1457 ΔatlE mutant | ||||
| No | 39 ± 4 | 35 ± 2 | 59 ± 2 | 44 ± 2 |
| Yes | 36 ± 2 | 36 ± 3 | 54 ± 2 | 40 ± 2 |
| Clean glass | 4 ± 8 | 4 ± 9 | 40 ± 1 | 22 ± 3 |
| DDS-coated glass | 104 ± 8 | 92 ± 2 | 72 ± 2 | 64 ± 3 |
The results shown are from three experiments with separately grown bacteria or different surfaces. Bold values indicate a statistically significant difference between data obtained in the presence and absence of naturally occurring eDNA (P < 0.05).
Initial adhesion and surface aggregation of bacteria have great implications for the adhesive (to a surface) and cohesive (bacterium-bacterium) strength of biofilm and its structure (7, 9). Bacteria present in larger aggregates are protected against environmental challenges (4) but can experience slow growth rates due to lack of nutrition (9).
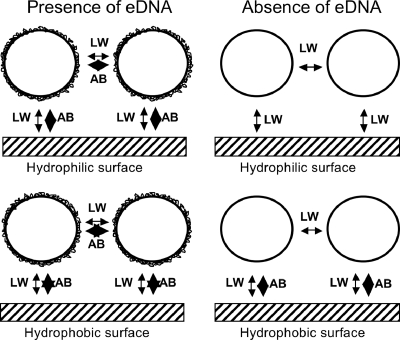
Physicochemical theories often only partly explain microbial interaction phenomena because they neglect micro- or nanometer structures on bacterial surfaces (8). For that reason, we selected S. epidermidis 1457 and a ΔatlE mutant deficient in eDNA release for thermodynamic analysis. The presence of eDNA created a highly hydrophobic bacterial cell surface. As a result, S. epidermidis 1457 had a strong thermodynamic preference for adhesion to a hydrophobic surface and to each other. However, initial adhesion of S. epidermidis 1457 in the presence of eDNA was similar on both hydrophilic and hydrophobic surfaces (compare Tables 1 and 3), but the percentage of large aggregates was significantly higher on hydrophilic surfaces (Fig. 2). It is likely that its adhesion to hydrophobic surfaces is equally driven by adhesion and surface aggregation, whereas its adhesion to hydrophilic surfaces is predominantly driven by surface aggregation. Single staphylococci, the foci of surface aggregation, adhere to hydrophilic surfaces through weak attractive Lifshitz-Van der Waals forces only and not by highly attractive acid-base interactions. Similarly, surface aggregation in the absence of attractive acid-base interactions will be weak and a result of the ubiquitously present attractive Lifshitz-Van der Waals interactions. Note from Fig. 2 that S. epidermidis 1457 forms more extensive aggregates in the presence of eDNA than does the ΔatlE mutant. The interplay between adhesion and surface aggregation in initial bacterial adhesion to a surface and the role of surface energetics is shown in Fig. 3.
TABLE 3.
Lifshitz-Van der Waals (ΔGLW) and acid-base (ΔGAB) free energies of interaction for adhesion and surface aggregation of S. epidermidis 1457 and a ΔatlE mutant in the presence or absence of eDNA
| Strain and DNase I treatment | ΔGLW/ΔGAB (mJ/m2) |
||
|---|---|---|---|
| Hydrophilic surface adhesion | Hydrophobic surface adhesion | Surface aggregation | |
| S. epidermidis 1457 | |||
| No | −5/−10 | 0/−58 | −5/−49 |
| Yes | −3/+15 | 0/−15 | −2/+17 |
| S. epidermidis 1457 ΔatlE mutant | |||
| No | −3/+16 | 0/−12 | −2/+19 |
| Yes | −4/+20 | 0/−10 | −2/+26 |
FIG. 3.
Lifshitz-Van der Waals (LW) and acid-base (AB) interactions involved in staphylococcal adhesion and surface aggregation to a hydrophobic and hydrophilic surface in the presence or absence of eDNA. The thickness of the arrows referring to the different interactions indicates their relative importance.
In conclusion, the presence of eDNA on bacterial cell surfaces enhances adhesion and surface aggregation due to the involvement of acid-base interactions. eDNA also creates thermodynamically favorable conditions for bacterial adhesion to hydrophobic surfaces, whereas adhesion to a hydrophilic surface is mediated predominantly by thermodynamically favorable conditions for surface aggregation of adhering bacteria.
Acknowledgments
We thank S. Molin for providing us with S. epidermidis 1457 and the S. epidermidis 1457 ΔatlE mutant. We sincerely thank Hans Kaper for technical assistance.
Footnotes
Published ahead of print on 2 April 2010.
REFERENCES
- 1.Abu-Lail, N. I., and T. A. Camesano. 2006. Specific and nonspecific interaction forces between Escherichia coli and silicon nitride, determined by Poisson statistical analysis. Langmuir 22:7296-7301. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, S. J., and R. A. Burne. 2006. The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis. J. Bacteriol. 188:6877-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allesen-Holm, M., K. B. Barken, L. Yang, M. Klausen, J. S. Webb, S. Kjelleberg, S. Molin, M. Givskov, and T. Tolker-Nielsen. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114-1128. [DOI] [PubMed] [Google Scholar]
- 4.Battin, T. J., W. T. Sloan, S. Kjelleberg, H. Daims, I. M. Head, T. P. Curtis, and L. Eberl. 2007. Microbial landscapes: new paths to biofilm research. Nat. Rev. Microbiol. 5:76-81. [DOI] [PubMed] [Google Scholar]
- 5.Boks, N. P., W. Norde, H. C. van der Mei, and H. J. Busscher. 2008. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 154:3122-3133. [DOI] [PubMed] [Google Scholar]
- 6.Costerton, J. W. 1995. Overview of microbial biofilms. J. Ind. Microbiol. 15:137-140. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Hermansson, M. 1999. The DLVO theory in microbial adhesion. Colloids Surf. B Biointerfaces 14:105-119. [Google Scholar]
- 9.Johnson, L. R. 2008. Microcolony and biofilm formation as a survival strategy for bacteria. J. Theor. Biol. 251:24-34. [DOI] [PubMed] [Google Scholar]
- 10.Petersen, F. C., L. Tao, and A. A. Scheie. 2005. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 187:4392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin, Z. Q., Y. Z. Ou, L. A. Yang, Y. L. Zhu, T. Tolker-Nielsen, S. Molin, and D. Qu. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083-2092. [DOI] [PubMed] [Google Scholar]
- 12.Steinberger, R. E., and P. A. Holden. 2005. Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl. Environ. Microbiol. 71:5404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilain, S., J. M. Pretorius, J. Theron, and V. S. Brozel. 2009. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl. Environ. Microbiol. 75:2861-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitchurch, C. B., T. Tolker-Nielsen, P. C. Ragas, and J. S. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]