Abstract
Bacteria and matrix are essential for the development of biofilms, and assays should therefore target both components. The current European guidelines for biocidal efficacy testing are not adequate for sessile microorganisms; hence, alternative discriminatory test protocols should be used. The activities of a broad range of biocides on Staphylococcus aureus and Pseudomonas aeruginosa biofilms were evaluated using such in vitro assays. Nearly all selected biocides showed a significant decrease in S. aureus biofilm viability, with sodium hypochlorite and peracetic acid as the most active biocides. Only hydrogen peroxide and sodium hypochlorite showed some inhibitory effect on the matrix. Treatment of P. aeruginosa biofilms was roughly comparable to that of S. aureus biofilms. Peracetic acid was the most active on viable mass within 1 min of contact. Isopropanol ensured a greater than 99.999% reduction of P. aeruginosa viability after at least 30 min of contact. Comparable to results with S. aureus, sodium hypochlorite and hydrogen peroxide markedly reduced the P. aeruginosa matrix. This study clearly demonstrated that despite their aspecific mechanisms of action, most biocides were active only against biofilm bacteria, leaving the matrix undisturbed. Only hydrogen peroxide and sodium hypochlorite were active on both the biofilm matrix and the viable mass, making them the better antibiofilm agents. In addition, this study emphasizes the need for updated and standardized guidelines for biofilm susceptibility testing of biocides.
Microbial communities irreversibly attached to a surface and encapsulated in a self-produced polymeric matrix are known as biofilms. A particular characteristic is their extreme resistance to antimicrobial treatment (6). This resistance is mediated by several mechanisms that can act together: (i) poor penetration or inactivation of antimicrobials in the matrix, (ii) an altered bacterial metabolic state, (iii) the formation of persister cells, and (iv) resistance induced by the antimicrobial itself following the use of sublethal concentrations and the upregulation of efflux pumps (2, 7). Hence, biofilms are hard to eradicate and are claimed to be responsible for up to 60% of all infections in humans (1, 5). Staphylococcus aureus and Pseudomonas aeruginosa are notorious biofilm producers, the first being nosocomial and responsible mainly for medical device-associated infections (13, 28) and the latter being an opportunistic pathogen causing life-threatening infections mainly in cystic fibrosis patients (13, 19).
Looking at the high biofilm-related morbidity and mortality, the antibiofilm properties of antimicrobials have been studied extensively, with a main focus on the activity of antibiotics (1, 17, 20, 26). However, a recent study indicated that the prospect of using solely antibiotics to achieve complete biofilm destruction is limited, since the biofilm matrix persists (25). As their mechanisms of action are not limited to the bacterial metabolism, biocides should also be considered as valuable candidates for antibiofilm treatment of material surfaces and human mucosa (8, 11).
Whereas antibiotic susceptibility assays are based mostly on MIC values, biocides must kill in a short period of time (10, 23), and standard growth inhibition assays are therefore not suitable. In that respect, different European guidelines regarding biocides, which consider different microorganisms (bacteria, viruses, and fungi) and environments (medical and industrial settings), have been issued (3). However, these guidelines are not adequate for sessile microorganisms, endorsing the need for a more specific assay for biocidal efficacy on biofilms. For example, the plate count challenge test (PCCT), also called the European suspension test, determines basal bactericidal activity based on susceptibilities of planktonic cells only. Any new protocol should closely resemble the current European guidelines, adopting the same reaction conditions (e.g., standardized contact times and temperatures) to allow comparison with other studies (10). Briefly, the mixture of test product and challenge microorganism must be neutralized after a specified contact time, and reduction factors (RFs) are calculated using the viable plate count method. To avoid protracted antimicrobial action of the test product during microbial quantification, selective neutralization of the biocide under study after the desired contact time is essential, endorsing the need to determine the efficacies and toxicities of neutralizers before initiating antimicrobial testing.
For S. aureus and P. aeruginosa, a biocide is considered effective if a ≥5-log10 reduction in viability is obtained within 60 min at 20°C (3). However, for the study of adherent communities, viability tests alone are insufficient (24). The biofilm matrix can be recolonized, and assays should therefore target viable mass and matrix in a discriminatory way (9, 12, 25). For example, a recently developed S. aureus model uses dimethyl methylene blue (DMMB) for detecting the matrix, while resazurin is used as a viability indicator (24). A similar test model with crystal violet and resazurin was also implemented for P. aeruginosa. These microtiter plate models allow high-capacity screening and can easily be adapted for the evaluation of biocidal activity by inserting the neutralization step. The redox indicator resazurin is preferential to the viable plate count method, as it is very hard to recover all surviving adherent bacteria as single cells (16). Moreover, resazurin is nontoxic and does not affect the cells, leaving the biofilm ultrastructure intact after viability testing and allowing further analysis (14).
The aim of the present study was to test the efficacies of biocides representing different classes and mechanisms of action (i) against planktonically growing S. aureus and P. aeruginosa by using the European suspension test (3) and (ii) against the same microorganisms adopting an adherent growth pattern by using the above-mentioned discriminatory biofilm assays. Dose-response relationships were determined for the biocides active on both biofilm matrices and viable masses.
MATERIALS AND METHODS
Bacterial strains.
One biofilm-producing S. aureus strain (ATCC 6538), originating from a human lesion, and one adherent environmental P. aeruginosa isolate (ATCC 700928) were included. Both strains were subcultured in tryptic soy broth (TSB) (Lab M, International Medical, Brussels, Belgium) at 37°C and stored at −80°C in aliquots containing 1.5 × 108 to 5.0 × 108 CFU/ml.
Test products.
Twelve biocides that represent different classes and mechanisms of action were selected. As sessile bacteria are more resistant than their planktonic counterparts, biocides were tested at their highest-use concentrations (Table 1). All dilutions were aseptically and freshly prepared in sterile distilled water (AD). Due to an additional in-test dilution (see below), the concentration of the stock solution was 1.25 times the required concentration. The ready-to-use products povidone-iodine (Isobetadine), benzalkonium chloride (Cedium), and nitrofurazone (Furacine) were therefore tested at 80% of the required concentrations.
TABLE 1.
Biocides used in this study
| Class and biocide(s) | Active constituent | Test concn (%) | Manufacturer |
|---|---|---|---|
| Alcohols | |||
| Ethanol | Ethanol | 70 (vol/vol) | Acros Organics, Geel, Belgium |
| Isopropanol | Isopropanol | 70 (vol/vol) | Acros Organics, Geel, Belgium |
| Biguanide | |||
| Hibitane | Chlorhexidine-digluconate | 1 (wt/vol) | Regent Medical Limited, Manchester, England |
| Halogens | |||
| Chloramine-T | Sodium-tosyl-chloramide | 1.25 (wt/vol) | Sigma-Aldrich, Bornem, Belgium |
| Isobetadinea | Povidone-iodine | 10 (wt/vol) | Mundipharma, Basel, Switzerland |
| Sodium hypochlorite | Hypochlorite | 1 (wt/vol) | Sigma-Aldrich, Bornem, Belgium |
| Peroxygens | |||
| Hydrogen peroxide | Hydrogen peroxide | 5 (vol/vol) | Merck, VWR International, Haasrode, Belgium |
| Peracetic acid | Peracetic acid | 0.3 (vol/vol) | Sigma-Aldrich, Bornem, Belgium |
| Phenol | |||
| Dettol | Chloroxylenol | 0.25 (wt/vol) | Reckitt Benckiser, Brussels, Belgium |
| QACs | |||
| Cediuma | Benzalkonium chloride | 0.1 (wt/vol) | Qualiphar, Bornem, Belgium |
| Cetrimide | Cetrimide | 1 (wt/vol) | Sigma-Aldrich, Bornem, Belgium |
| Other | |||
| Furacinea | Nitrofurazone | 0.2 (wt/vol) | Norgine, Heverlee, Belgium |
Due to in-test dilution, these commercially available products were tested at 80% of the mentioned dose.
Neutralizers.
The efficacies and toxicities of several neutralizers (Table 2) were evaluated prior to antibacterial testing. All neutralizers were freshly aseptically prepared. Components were purchased from Sigma-Aldrich unless stated otherwise. Lecithin originated from soybean, except for that in N10, which was derived from egg yolk.
TABLE 2.
Compositions of tested neutralizers
| Neutralizer | Composition |
|---|---|
| N1 | 5 g/liter sodium thiosulfate (Merck, VWR International) in AD |
| N2 | 30 g/liter polysorbate 80 + 30 g/liter saponin + 1 g/liter l-histidine + 1 g/liter cysteine in AD |
| N3 | 50 ml/liter bovine catalase in TSB (Lab M, International Medical) |
| N4 | 3 g/liter lecithin + 30 g/liter polysorbate 80 in AD |
| N5 | 3 g/liter lecithin + 30 g/liter polysorbate 80 + 5 g/liter sodium thiosulfate + 1 g/liter l-histidine + 30 g/liter saponin (from Quillaja bark) in AD |
| N6 | 10 g/liter lecithin + 20 g/liter polysorbate 80 in AD |
| N7 | 15 g/liter lecithin + 50 g/liter polysorbate 80 in AD |
| N8 | 30 g/liter lecithin + 100 g/liter polysorbate 80 in AD |
| N9 | 3 g/liter lecithin + 30 g/liter polysorbate 80 + 4 g/liter SDS in AD |
| N10 | 3 g/liter lecithin (from egg yolk) + 30 g/liter polysorbate 80 + 4 g/liter SDS in AD |
| N11 | 5 g/liter sodium thiosulfate + 26 g/liter potassium dihydrogen phosphate (Merck, VWR International) + 5 g/liter sodium hydroxide (Merck, VWR International) + 2 ml/liter bovine catalase in TSB |
Neutralization test.
Since in the European challenge test the reaction between the test product and the microorganisms must be neutralized after the desired contact time, the efficacies and toxicities of the neutralizing agents must be determined for each bacterial strain (3). Cryopreserved microorganisms were thawed at 37°C and diluted to contain 1 × 103 CFU/ml. All reagents (test solutions, AD, neutralizers, and bacterial suspensions) were equilibrated to the test temperature at 20°C. The efficacy of the neutralizer was evaluated by mixing 100 μl test product and 800 μl neutralizer in a microtube (Eppendorf; VWR International, Haasrode, Belgium) for 5 min at 20°C, followed by addition of 100 μl diluted microbial inoculum. After 30 min of incubation at 20°C, 100 μl of this test mixture was homogenously spread onto a tryptic soy agar (TSA) plate (Lab M, International Medical, Brussels, Belgium). After 24 h of incubation, colonies were counted and CFU/ml was calculated. About 10 colonies were counted for the control sample. Neutralizer toxicity was tested by adding 100 μl of AD to 800 μl neutralizer in a microtube. After 5 min of incubation at 20°C, 100 μl diluted microbial suspension was added. After 30 min, the test mixture was treated as described above. Control samples were prepared in both tests by replacing either the neutralizer or the test product with AD. At least two independent replicates were carried out.
Antibacterial susceptibility testing.
Prior to the study of biocides on biofilms, their efficacies on planktonically growing microorganisms were determined according to the European guidelines, with some modifications (3). In brief, cryopreserved bacteria were rapidly thawed at 37°C. All reagents (test solutions, AD, neutralizers, and bacterial suspensions) were equilibrated to the test temperature at 20°C. Biocidal activity was evaluated by mixing 800 μl of test product with 100 μl AD and 100 μl diluted microbial suspension (test mixture). After the desired contact times (1 min, 5 min, 15 min, 30 min, and 60 min), 100 μl of the test mixture was added to 800 μl neutralizer and 100 μl AD. After a further 5 min of incubation, the neutralized test mixture was 10-fold serially diluted in TSB, and 100 μl of the test mixture or 100 μl of each dilution was homogenously spread on a TSA plate. Colonies were counted after 24 h, and CFU/ml was calculated. The number of viable microorganisms in a control sample was determined by replacing the test product and the neutralizer with AD.
Biofilm growth and biocide testing.
Cryopreserved S. aureus or P. aeruginosa samples were thawed and diluted in TSB to 106 CFU/ml. Bacterial suspensions (100 μl) were added to 96-well plates (Greiner, Wemmel, Belgium) and incubated on a horizontal shaking plate (Schüttelmaschine RO20; Gerhardt, Bonn, Germany) at 37°C. Noninoculated TSB was included as a control sample. S. aureus was incubated for 72 h, with growth medium being changed every 24 h, and mature P. aeruginosa biofilms were obtained after 24 h. Before the addition of 100 μl of biocide, growth medium was discarded and biofilms were washed twice with phosphate-buffered saline (PBS). After the desired contact times (1 min, 5 min, 15 min, 30 min, and 60 min), the test product was discarded and treated biofilms were washed with PBS. Next, 100 μl of neutralizer was added for 5 min. After removal of the neutralizer, biofilms were washed twice with PBS. Untreated biofilms were obtained by the addition of 100 μl of sterile AD instead of biocide.
Determination of dose-response relationships.
Dose-response relationships were determined for biocides active on both biofilm matrix and viable mass. Selected compounds were 2-fold serially diluted starting from the initial test concentration. The antibiofilm efficacies of these dilutions were tested as mentioned above, at a single contact time of 5 min.
Detection of treated S. aureus biofilms. (i) Preparation of decomplexation solution.
A 500-ml portion of a 50 mM sodium acetate (Merck) buffer (pH 6.8), added to 50 ml of 1-propanol (Acros Organics, Geel, Belgium), was used to dissolve guanidine hydrochloride (Sigma-Aldrich, Bornem, Belgium) to a final concentration of 4 M. The solution is stable for at least 6 months when stored at room temperature.
(ii) Quantification of matrix and viable microbial burden.
The activities of biocides on the S. aureus matrix and the viable microbial burdens were assessed using parallel cultures as previously described (24). In brief, 200 μl DMMB solution per well was added, and the mixture was incubated for 30 min while protected from light. The dye was discarded after centrifugation at 2,800 × g for 20 min. Following a washing step with AD to remove all unbound DMMB, 250 μl decomplexation solution per well was added to resolubilize the biofilm-bound DMMB. After 30 min of incubation, the optical density was measured at 650 nm (Multiskan microplate reader; VWR International, Haasrode, Belgium). To evaluate the S. aureus viable microbial burden, rinsed wells were filled with 200 μl TSB and 10 μl resazurin (0.5 μg/well). After 30 min of incubation, fluorescence was measured (excitation wavelength [λex] of 550 nm and emission wavelength [λem] of 590 nm) using a GENios microplate reader (Tecan, Mechelen, Belgium).
Detection of treated P. aeruginosa biofilms.
The biocidal activity on P. aeruginosa biofilms was determined using parallel cultures as described previously (25). After removal of the neutralizer and subsequent rinsing with PBS, treated adherent populations were fixed using 100 μl 99% (vol/vol) methanol per well. After 15 min of incubation, methanol was removed and plates were air dried. Next, 200 μl of a 0.7% (wt/vol) crystal violet solution (Sigma-Aldrich, Bornem, Belgium) was added, and the mixture was incubated for 5 min, followed by a wash under running tap water. Plates were air dried, and 250 μl of 33% (vol/vol) glacial acetic acid per well was added to resolubilize the biofilm-bound crystal violet. After 15 min of incubation, the optical density was measured at 570 nm (Multiskan microplate reader). To assess P. aeruginosa cell viability, 0.5 μg resazurin per well was added. After 4 h of incubation, fluorescence was measured (λex of 550 nm and λem of 590 nm) using a GENios microplate reader.
Statistical analysis.
All tests were performed on two different days, with at least 8 replicates per variable per day. Results were statistically analyzed by one-way analysis of variance (ANOVA), followed by Dunnett's multiple-comparison test. P values of less than 0.05 were considered significant.
RESULTS
Neutralization test.
Prior to antimicrobial testing, the efficacies and toxicities of the different neutralizers were assessed (Table 3). When exposed to in-test bacterial loads as proposed in the European guidelines (6 × 101 to 3 × 102 CFU/ml), efficacious neutralizers show no significant difference between the control sample (100%, no test product, and no neutralizer) and samples treated with neutralized test product (test product was neutralized before addition of microorganisms). In our study, an appropriate neutralizer inhibited the activity of the test product in the presence of both S. aureus and P. aeruginosa. For example, N5 was determined to be the optimal neutralizer for chloramine-T, since N1 was not able to neutralize its activity in the presence of P. aeruginosa. Practical considerations, such as ease of preparation, also determined the final choice of the neutralizer. Looking at S. aureus, alcohols and halogens could be neutralized using solely sodium thiosulfate (N1), while phenolics and quaternary ammonium compounds (QACs) required more-complex mixtures of polysorbate and lecithin (N4 to N10). The activity of chlorhexidine-digluconate (Hibitane) was the most difficult to inhibit, since only lecithin derived from egg yolk in combination with polysorbate and sodium dodecyl sulfate (SDS) achieved complete neutralization (N10). Alcohols were the easiest to neutralize, as a 1/10 dilution was already sufficient to inhibit their activity (data not shown). However, to ensure complete neutralization and to increase the uniformity between the different tests, N1 was used as the neutralizer for ethanol and isopropanol. Hydrogen peroxide was easily neutralized by catalase. Due to the prior knowledge regarding S. aureus, fewer efficacy tests were carried out with P. aeruginosa. Test mixtures containing the latter were generally easier to neutralize (e.g., chlorhexidine-digluconate and cetrimide). Peracetic acid was the exception as it became neutralized only with the combination of catalase, sodium thiosulfate, and sodium hydroxide (N11).
TABLE 3.
Results of neutralizer efficacy tests for S. aureus and P. aeruginosa
| Organism and neutralizer | Microbial loada (log10 CFU/ml) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ETH | IP | HIB | CHT | IB | HYP | H2O2 | PAA | DET | CED | CET | FUR | |
| Staphylococcus aureus | |||||||||||||
| N1 | 2.12 | 2.13* | 2.10* | 0 | 2.12* | 2.12* | 2.13* | NT | 1.80 | 0 | 0 | 0 | 1.30 |
| N2 | 2.11 | 1.98* | 2.11* | 1.13 | NT | 0 | 0 | 0 | NT | 2.12* | 1.00 | 0 | 1.45 |
| N3 | 2.12 | 2.11* | 2.11* | 0 | NT | 0 | 0 | 2.13* | NT | 0 | 0 | 0 | 2.11* |
| N4 | 2.12 | 2.11* | 2.13* | 0 | NT | 0 | 0 | 0 | NT | 2.14* | 1.35 | 0 | 1.31 |
| N5 | 1.80 | NT | NT | 0 | 1.99* | NT | NT | NT | 1.98* | NT | NT | NT | NT |
| N6 | 2.11 | NT | NT | 0 | NT | NT | NT | NT | NT | NT | 2.13* | 0 | NT |
| N7 | 1.98 | NT | NT | 0 | 0 | NT | NT | NT | 1.20 | 2.00* | 1.93* | 1.20 | 2.10* |
| N8 | 1.90 | NT | NT | 1.28 | 0 | NT | NT | NT | NT | 2.11* | 2.22 | 1.20 | 1.80* |
| N9 | 2.11 | NT | NT | 0 | 0 | NT | NT | NT | 0 | NT | NT | 2.10* | NT |
| N10 | 2.18 | NT | NT | 2.18* | NT | NT | NT | NT | 0 | NT | NT | NT | NT |
| N11 | 1.95 | NT | NT | NT | NT | NT | NT | NT | 2.10* | NT | NT | NT | NT |
| Pseudomonas aeruginosa | |||||||||||||
| N1 | 1.70 | 1.80* | 1.70* | NT | 0 | 2.11* | 1.75* | NT | 0 | NT | NT | NT | NT |
| N3 | 1.70 | NT | NT | NT | NT | NT | NT | 1.93* | NT | NT | NT | NT | 1.85* |
| N5 | 2.00 | NT | NT | 2.13* | 2.13* | NT | NT | NT | 0 | 2.00* | 2.12* | 2.12* | 2.13* |
| N7 | 1.65 | NT | NT | 1.80* | 0 | NT | NT | NT | 0 | 1.80* | 1.83* | 1.70* | 1.80* |
| N8 | 1.75 | NT | NT | 1.90* | 0 | NT | NT | NT | 0 | 1.85* | 2.14 | 2.16 | 2.11* |
| N9 | 2.11 | NT | NT | 2.12* | 0 | NT | NT | NT | 0 | 2.13* | 1.90* | 2.14* | 2.12* |
| N10 | 1.85 | NT | NT | 1.90* | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| N11 | 1.90 | NT | NT | NT | NT | NT | NT | NT | 2.00* | NT | NT | NT | NT |
| Conclusionb | N1 | N1 | N10 | N5 | N1 | N1 | N3 | N11 | N7 | N7 | N9 | N3 | |
The microbial load was calculated by multiplying the number of colonies by the dilution factor of 10, and results are expressed as averages from at least two independent replicates. ETH, ethanol; IP, isopropanol; HIB, chlorhexidine-digluconate (Hibitane); CHT, chloramine-T; IB, povidone-iodine (Isobetadine); HYP, sodium hypochlorite; H2O2, hydrogen peroxide; PAA, peracetic acid; DET, chloroxylenol (Dettol); CED, benzalkonium chloride (Cedium); CET, cetrimide; FUR, nitrofurazone (Furacine). NT, not tested; *, no significant difference from the control sample (i.e., the neutralizer is fully effective).
Conclusion, neutralizer selected for antimicrobial testing. The compositions of the neutralizers are listed in Table 2.
Antibacterial susceptibility testing.
The antibacterial effects of the different biocides on planktonic S. aureus and P. aeruginosa were evaluated first (Table 4). In view of the high concentrations used, all test products, with the exception of povidone-iodine, hydrogen peroxide, and nitrofurazone, eradicated S. aureus with at least a 5-log reduction of viability, thereby passing the European challenge test after 1 min of contact (Table 4). Povidone-iodine and hydrogen peroxide met these criteria after contact times of 5 min and 15 min, respectively. Even after 60 min of contact time, nitrofurazone hardly reduced S. aureus viability.
TABLE 4.
Biocides which do not pass the European challenge test for planktonic S. aureus and P. aeruginosa
| Organism and biocide | Concn (%) | Bacterial level (log10 CFU/ml) for untreated samplea | Biocidal activity (log10 RF) after contact time of: |
||||
|---|---|---|---|---|---|---|---|
| 1 min | 5 min | 15 min | 30 min | 60 min | |||
| Staphylococcus aureus | |||||||
| Povidone-iodineb | 10 | 6.45 | 4.62 | ≥5.45 | ≥5.45 | ≥5.45 | ≥5.45 |
| Hydrogen peroxide | 5 | 6.40 | 0.64 | 4.65 | ≥5.40 | ≥5.40 | ≥5.40 |
| Nitrofurazoneb | 0.2 | 6.48 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| Pseudomonas aeruginosa | |||||||
| Hydrogen peroxide | 5 | 6.21 | 3.69 | ≥5.21 | ≥5.21 | ≥5.21 | ≥5.21 |
| Nitrofurazoneb | 0.2 | 5.83 | 0.28 | 0.41 | 1.02 | 1.92 | 2.83 |
Results for untreated samples represent the final bacterial levels after dilution with the test product and AD and after neutralization.
Due to in-test dilution, these commercially available products were tested at 80% of the mentioned dose.
The planktonic susceptibility of P. aeruginosa was comparable to that of S. aureus. Most biocides, including povidone-iodine, easily met the European criteria after 1 min of contact time (Table 4). Hydrogen peroxide was highly effective against P. aeruginosa after 5 min of contact. Nitrofurazone showed a more gradual action on P. aeruginosa viability, achieving a reduction factor (RF) of 2.83 after 60 min of contact time. However, this was not enough to pass the European challenge test criteria.
Antibiofilm susceptibility testing.
The activities of biocides on established S. aureus and P. aeruginosa biofilms were studied using the DMMB-resazurin and crystal violet-resazurin assays, respectively. Both protocols can discriminate between activity on viable microorganisms and that on the biofilm matrix. In addition, since the biocide was discarded and biofilms were washed twice with PBS, use of a neutralizer sometimes became unnecessary as no differences were observed with and without neutralizer (data not shown). In these cases, the neutralizer was omitted from the test protocol (Table 5, “Neutralizer” column), further increasing the reproducibility of the assay, as biofilm integrity becomes affected by extensive manipulations.
TABLE 5.
Effects of biocidal treatments on matrices and viable bacteria of S. aureus and P. aeruginosa biofilms
| Organism and test product | Concn (%) | Neutralizer | % reduction in biofilmb (avg ± SD) after contact time of: |
Categoryc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 min |
5 min |
15 min |
30 min |
60 min |
|||||||||
| Matrix | Bacteria | Matrix | Bacteria | Matrix | Bacteria | Matrix | Bacteria | Matrix | Bacteria | ||||
| Staphylococcus aureus | |||||||||||||
| Ethanol | 70 | No Na | — | 89 ± 4** | — | 96 ± 4** | — | 96 ± 0.1** | — | 96 ± 0.1** | — | 99 ± 0.4** | C |
| Isopropanol | 70 | No N | — | 94 ± 0.8** | — | 96 ± 0.1** | — | 96 ± 0.4** | — | 97 ± 0.3** | — | 98 ± 0.6** | C |
| Chlorhexidine-digluconate | 1 | N10 | — | 84 ± 2** | — | 95 ± 1** | — | 95 ± 0.2** | — | 95 ± 0.7** | — | 97 ± 0.2** | C |
| Chloramine-T | 1.25 | No N | — | 93 ± 7** | — | 96 ± 4** | — | 99 ± 0.1** | — | 99 ± 0.5** | — | 99 ± 0.1** | C |
| Povidone-iodine | 10 | N1 | — | 77 ± 0.5** | — | 79 ± 3** | — | 86 ± 6** | — | 90 ± 4** | — | 94 ± 5* | C |
| Sodium hypochlorite | 1 | No N | — | 99 ± 0.5** | 21 ± 8* | 99 ± 0.3** | 45 ± 1** | 99 ± 0.8** | 54 ± 6** | 99 ± 0.6** | 55 ± 3** | 99 ± 0.5** | D |
| Hydrogen peroxide | 5 | No N | 89 ± 3** | 84 ± 0.4** | 85 ± 8** | 83 ± 2** | 83 ± 9** | 82 ± 6** | 87 ± 3** | 82 ± 4** | 84 ± 7** | 80 ± 0.3** | D |
| Peracetic acid | 0.3 | No N | — | 98 ± 0.2** | — | 99 ± 0.4** | — | 99 ± 0.4** | — | 99 ± 0.1** | — | 99 ± 0.2** | C |
| Chloroxylenol | 0.25 | N7 | — | 82 ± 8** | — | 87 ± 6** | — | 91 ± 7** | — | 94 ± 2** | — | 94 ± 2** | C |
| Benzalkonium chloride | 0.1 | N7 | — | 53 ± 9** | — | 72 ± 9** | — | 86 ± 3** | — | 87 ± 4** | — | 84 ± 6** | C |
| Cetrimide | 1 | N9 | — | 78 ± 8** | — | 93 ± 1** | — | 92 ± 2** | — | 91 ± 2** | — | 91 ± 3** | C |
| Nitrofurazone | 0.2 | No N | — | — | — | — | — | — | — | — | — | — | A |
| Pseudomonas aeruginosa | |||||||||||||
| Ethanol | 70 | No N | — | 89 ± 7** | — | 90 ± 2** | — | 96 ± 4** | — | 97 ± 3** | — | 96 ± 1** | C |
| Isopropanol | 70 | No N | — | 79 ± 1** | — | 96 ± 1** | — | 99 ± 2** | — | >99.999** | — | >99.999** | C |
| Chlorhexidine-digluconate | 1 | N10 | — | — | — | — | — | — | — | 40 ± 7** | — | 57 ± 7** | C |
| Chloramine-T | 1.25 | No N | — | 95 ± 0.1** | — | 95 ± 1** | — | 94 ± 1** | — | 94 ± 1** | — | 94 ± 2** | C |
| Povidone-iodine | 10 | N1 | — | 94 ± 0.1** | — | 94 ± 1** | — | 91 ± 4** | — | 91 ± 5** | — | 96 ± 1** | C |
| Sodium hypochlorite | 1 | No N | 66 ± 3** | 92 ± 9** | 76 ± 9** | 93 ± 8** | 91 ± 2** | 94 ± 6** | 85 ± 2** | 95 ± 7** | 92 ± 1** | 94 ± 7** | D |
| Hydrogen peroxide | 5 | No N | 68 ± 4** | 48 ± 8** | 75 ± 7** | 80 ± 7** | 78 ± 5** | 97 ± 4** | 78 ± 7** | 98 ± 3** | 85 ± 6** | 99 ± 1** | D |
| Peracetic acid | 0.3 | No N | — | 99 ± 0.3** | — | 99 ± 0.1** | — | 98 ± 0.8** | — | 98 ± 0.4** | — | 99 ± 0.1** | C |
| Chloroxylenol | 0.25 | N7 | — | 77 ± 5** | — | 86 ± 4** | 51 ± 5* | 84 ± 3** | 55 ± 23* | 82 ± 8** | 66 ± 24* | 85 ± 7** | D |
| Benzalkonium chloride | 0.1 | N7 | — | 72 ± 7** | — | 69 ± 6** | — | 71 ± 7** | — | 75 ± 4** | — | 86 ± 5** | C |
| Cetrimide | 1 | N9 | — | 69 ± 3** | — | 70 ± 5** | — | 67 ± 8** | — | 77 ± 8** | — | 88 ± 4** | C |
| Nitrofurazone | 0.2 | No N | — | — | — | — | — | 24 ± 6* | — | 32 ± 4** | — | 33 ± 5** | C |
No N, no neutralizer.
Levels of significance, as determined by ANOVA, are indicated as follows: —, P > 0.05 (no significant activity); *, P < 0.05; **, P < 0.01.
Biocides can be classified according to their activities on matrices and bacteria. See Table 6 for descriptions of categories.
Biocidal activity on S. aureus biofilms.
By use of the DMMB-resazurin assay, nearly all biocides resulted in a significant decrease in biofilm viability (ANOVA, P < 0.05) (Table 5). Sodium hypochlorite and peracetic acid were the most active, with an almost complete eradication of biofilm bacteria within 1 min of contact time. Longer contact times generally increased the antibiofilm activity (e.g., for ethanol and chlorhexidine-digluconate), but a stable bacterial reduction was reached in most cases after 15 min. For hydrogen peroxide, the reduction in viability seemed independent of contact time, with 84% of S. aureus bacteria being killed from 1 min of contact onwards. On the other hand, hydrogen peroxide was highly effective against the biofilm matrix, reaching an 89% reduction after 1 min of contact. Here again, hydrogen peroxide activity was not proportional to contact time. The opposite was true for sodium hypochlorite, for which the antimatrix activity increased from 0 to 55% within the time span of 0 to 60 min. Similarly to the biocidal activity on planktonic S. aureus, nitrofurazone did not show any significant activity on either component of the adherent population.
A system comparable to the classification system suggested for antibiotics (25) was used for the classification of biocides according to their activities on biofilm matrices and viable bacteria (Tables 5 and 6). The majority of biocides belong to category C, inhibiting only biofilm viable mass. Category D is clinically the most relevant and comprises biocides affecting both matrices and bacteria. Only nitrofurazone is listed in category A, with no significant activity on either biofilm component. No biocides were included in category B, affecting only the matrix, or in category E, giving rise to biofilm stimulation.
TABLE 6.
Classification of biocides into 5 categories according to their activities on biofilm matrices and viable bacteria
| Category | Effect on bacteriab | Effect on matrixb | Sample biocide (organism[s])c |
|---|---|---|---|
| A | 0 | 0 | Nitrofurazone (Sa) |
| B | 0 | ↓ | |
| C | ↓ | 0 | Isopropanol (Sa and Pa) |
| D | ↓ | ↓ | H2O2 (Sa and Pa) |
| Ea | ↓ (↑) | ↓ |
Category E includes biocides with an inhibitory activity on the viable microbial burden at high concentrations and a stimulatory effect at low concentrations.
0, no activity; ↓, inhibitory activity; ↑, stimulatory activity.
Sa, Staphylococcus aureus; Pa, Pseudomonas aeruginosa.
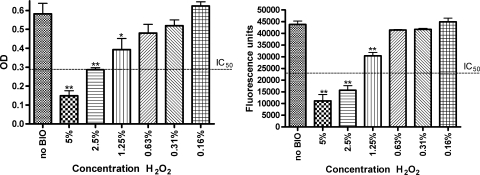
The category D biocides hydrogen peroxide and sodium hypochlorite were also dose titrated after 5 min of contact. As biocides are generally used for only a short period of time, a contact time of 5 min is often chosen as the standard time point in challenge testing (15). A clear dose response was observed for both test products. The 50% inhibitory concentration (IC50) of hydrogen peroxide on the matrix was 2.5%, while a 50% reduction in viability was obtained at about 1.7% (Fig. 1). For sodium hypochlorite, the IC50 on the biofilm matrix was 1%, with a corresponding IC50 below 0.031% against viable mass (data not shown).
FIG. 1.
After a contact time of 5 min, a clear dose response could be observed for hydrogen peroxide on the S. aureus biofilm matrix (left) and viable mass (right). The matrix was measured spectrophotometrically at 650 nm using DMMB, and viable mass was fluorimetrically measured with resazurin (λex of 550 nm and λem of 590 nm). Results are classified as highly significant (**, P < 0.01), significant (*, P < 0.05), or not significant (no asterisk, P > 0.05). BIO, biocide; OD, optical density.
Biocidal activity on P. aeruginosa biofilms.
Treatment of P. aeruginosa biofilms was roughly comparable to that of S. aureus biofilms, and most test products markedly reduced bacterial viability starting from 1 min of contact time, with a stable reduction being reached within 15 min (Table 5). Analogous to planktonic susceptibility, P. aeruginosa was more amenable to treatment with povidone-iodine and nitrofurazone. The activity of the first amounted to 94% after 1 min of contact, which is about 20% more than that for S. aureus. While nitrofurazone did not have any effect on S. aureus biofilms, a 25 to 35% reduction in P. aeruginosa viability was obtained after a contact time of 15 min or more. In contrast, chlorhexidine-digluconate was far less active, since 30 min of contact resulted in only a 40% decrease in P. aeruginosa viability, compared to an 84% decrease in S. aureus viability after 1 min. Hydrogen peroxide resulted in a 48% reduction in P. aeruginosa viability within 1 min, with a further reduction proportional to the contact time. After 60 min, an almost complete eradication of P. aeruginosa bacteria was achieved. Again, peracetic acid was the most active against viable mass (99% reduction after 1 min). Isopropanol ensured a greater than 99.999% reduction of P. aeruginosa viability after at least 30 min of contact time.
Fully comparable to the results for S. aureus, sodium hypochlorite and hydrogen peroxide also markedly reduced the P. aeruginosa matrix. Sodium hypochlorite caused a 66% matrix reduction after only 1 min, increasing to 91% after 15 min. Hydrogen peroxide antimatrix activity increased over time from 68% to 85%. Chloroxylenol (Dettol) showed some matrix-reducing activity, but standard deviations were quite high.
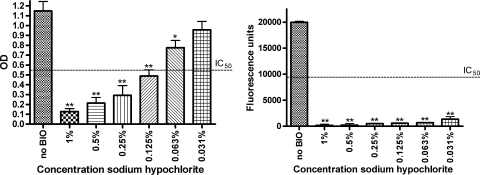
As for S. aureus, the different biocides were categorized according to their P. aeruginosa antibiofilm activities (Tables 5 and 6). Dose-response relationships were obtained for sodium hypochlorite and hydrogen peroxide after 5 min of contact. The antimatrix effect increased with increasing sodium hypochlorite concentrations (IC50 of about 0.09%) (Fig. 2). Low concentrations of sodium hypochlorite greatly affected P. aeruginosa viability (IC50 and 90% inhibitory concentration [IC90] of <0.031%). Similar trends could be observed after 5 min of hydrogen peroxide treatment; however, they were less pronounced, i.e., IC50s for the biofilm matrix and viable mass were around 3.2% and 0.21%, respectively (data not shown).
FIG. 2.
A clear dose response on the P. aeruginosa matrix was observed after 5 min of treatment with sodium hypochlorite (left). The effect on P. aeruginosa viable mass was more pronounced, with an IC50 below 0.031% (right). The matrix was measured spectrophotometrically at 570 nm using crystal violet, and viable mass was fluorimetrically measured with resazurin (λex of 550 nm and λem of 590 nm). Results are classified as highly significant (**, P < 0.01), significant (*, P < 0.05), or not significant (no asterisk, P > 0.05). BIO, biocide; OD, optical density.
DISCUSSION
The aim of this study was to evaluate the efficacies of a range of biocides on S. aureus and P. aeruginosa biofilms by adapting previously described models that discriminate between matrix and viable microbial burden (25). As both biofilm constituents can rapidly generate a new adherent community when left behind after treatment, eradication of both is essential for successful antibiofilm therapy. Moreover, unraveling the discriminatory actions of test products may be very useful in designing combinations of compounds that can reduce the viability of adherent bacteria and agents that have antimatrix activity.
Biocide assays should also meet the criteria of the European guidelines regarding, e.g., contact time between test products and microorganisms, test temperature, and neutralization of test compounds after treatment (3). As biocides need to kill microorganisms within a short period of time, MIC values used to describe the activities of antibiotics are not of great value and may even lead to inappropriate conclusions (18). Instead, the reduction of viable mass is evaluated after a specified contact time between the test product and the microorganism. An effective and nontoxic neutralizer is required to prevent further antimicrobial action of the test product during microbial detection and quantification. In our study, an appropriate neutralizer was found for every test product in the presence of both S. aureus and P. aeruginosa. The neutralization of test products was generally more difficult for S. aureus than for P. aeruginosa (Table 5), which may be linked to the fact that Gram-positive bacteria are intrinsically more susceptible to biocides than their Gram-negative counterparts (27).
In contrast to planktonic bacterial cultures, biofilms adhere to a surface, allowing removal of the test product after treatment. As such, the neutralization step could sometimes be omitted. However, this was largely dependent on the test product under study and cannot be generalized. The necessity of a neutralizer should therefore always be evaluated prior to biofilm susceptibility testing. To improve the reproducibility and accuracy of the assay, a single large seeding stock of cryopreserved bacteria with a pretitrated viable load of test microorganisms was used instead of freshly grown cultures for all experiments, as proposed by the European standard EN 1040 (3). The cryopreserved bacteria had sensitivities to antimicrobial agents that were comparable to those of freshly grown cultures (data not shown).
Against planktonic cultures, all biocides except povidone-iodine (only for S. aureus), nitrofurazone, and hydrogen peroxide passed the European challenge test after 1 min of contact time, causing at least a 5-log reduction in viable mass. Due to in-test dilution, the undiluted povidone-iodine and nitrofurazone solutions were tested at only 80% of their normal-use dose, which may explain their reduced efficacies. P. aeruginosa was more susceptible to hydrogen peroxide treatment than S. aureus.
Differences were striking when planktonic and biofilm populations were compared. No complete reduction of the adherent populations was achieved after 60 min of contact, whereas isopropanol showed a >99.999% reduction of P. aeruginosa. Sodium hypochlorite and hydrogen peroxide were the most promising antibiofilm agents, since they were active on both the viable mass and the matrix.
For the chlorine-releasing agents, sodium hypochlorite acted well on the P. aeruginosa biofilm matrix, reaching a 91% reduction after 15 min, while chloramine-T was totally inactive on the biofilm slime layer. During treatment, sodium hypochlorite decomposes to sodium hydroxide and hypochlorite, which is a strong oxidizing agent. Chloramine-T gives rise to an imine group and free chlorine, which are intrinsically less active. Peracetic acid, commonly used in antibiofilm treatment, eliminated 98% and 99% of viable S. aureus and P. aeruginosa bacteria, respectively, after only 1 min of contact. However, the biofilm matrix was left undisturbed.
Chlorhexidine-digluconate was far less active on P. aeruginosa biofilm bacteria than on S. aureus biofilm bacteria, which is likely due to differences in cell wall composition (22). Moreover, chlorhexidine-resistant P. aeruginosa strains have frequently been reported (18). Resistance to biocides has gained an increasing interest, as numerous studies have reported biocide-antibiotic cross-resistance (21, 27). When antibacterial targets are shared between biocides and antibiotics, selection pressure of the first can provoke resistance to the latter (8, 18). Sublethal stress caused by biocides can induce general microbial defense mechanisms, such as activation of efflux pumps (4). While the effectiveness of the biocide is usually not impaired, this can be sufficient to create multidrug-resistant microorganisms, as antibiotics are pumped out at the same time. For example, the extensive use of chlorhexidine in urinary-catheter management resulted in patients suffering from urinary tract infections with not only chlorhexidine-resistant but also multidrug-resistant bacteria (21).
In conclusion, most biocides were active only on S. aureus and P. aeruginosa biofilm cells, leaving the matrix undisturbed. Only the strong oxidizers hydrogen peroxide and sodium hypochlorite were active on both the biofilm matrix and the viable mass, making them very valuable antibiofilm agents. Future research should focus on synergistic combinations further exploiting the antibiofilm properties of these two biocides. This study also emphasizes the need for standardized European guidelines for biofilm susceptibility testing of biocides. Looking at the striking differences in biocidal efficacy between planktonic and adherent cultures, it is obvious that the current European suspension test does not satisfy the need.
Acknowledgments
Paul Cos is a postdoctoral researcher for the Fund for Scientific Research (FWO-Flanders).
Footnotes
Published ahead of print on 2 April 2010.
REFERENCES
- 1.Abdi-Ali, A., M. Mohammadi-Mehr, and Y. A. Alaei. 2006. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 27:196-200. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. G., and G. A. O'Toole. 2008. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 322:85-105. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1997. Chemical disinfectants and antiseptics—basic bactericidal activity—test method and requirements (phase 1). European standard EN 1040. Belgisch Instituut voor Normalisatie (BIN), Brussels, Belgium.
- 4.Chuanchuen, R., K. Beinlich, T. T. Hoang, A. Becher, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 45:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., L. Montanaro, and C. R. Arciola. 2005. Biofilm in implant infections: its production and regulation. Int. J. Artif. Organs 28:1062-1068. [DOI] [PubMed] [Google Scholar]
- 6.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert, P., A. J. McBain, and A. H. Rickard. 2003. Formation of microbial biofilm in hygienic situations: a problem of control. Int. Biodeterior. Biodegradation 51:245-248. [Google Scholar]
- 8.Gilbert, P., D. G. Allison, and A. J. McBain. 2002. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 92:98S-110S. [PubMed] [Google Scholar]
- 9.Liaqat, I., and A. N. Sabri. 2008. Effect of biocides on biofilm bacteria from dental unit water lines. Curr. Microbiol. 56:619-624. [DOI] [PubMed] [Google Scholar]
- 10.Luppens, S. B., M. W. Reij, R. W. van der Heijden, F. M. Rombouts, and T. Abee. 2002. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 68:4194-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meiller, T. F., L. G. Depaola, J. I. Kelley, A. A. Baqui, B. F. Turng, and W. A. Falkler. 1999. Dental unit waterlines: biofilms, disinfection and recurrence. J. Am. Dent. Assoc. 130:65-72. [DOI] [PubMed] [Google Scholar]
- 13.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller. 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 14.O'Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell toxicity. Eur. J. Biochem. 267:5421-5426. [DOI] [PubMed] [Google Scholar]
- 15.Payne, D. N., J. R. Babb, and C. R. Bradley. 1999. An evaluation of the suitability of the European suspension test to reflect in vitro activity of antiseptics against clinically significant organisms. Lett. Appl. Microbiol. 28:7-12. [DOI] [PubMed] [Google Scholar]
- 16.Pettit, R. K., C. A. Weber, M. J. Kean, H. Hoffmann, G. R. Pettit, R. Tan, K. S. Franks, and M. L. Horton. 2005. Microplate alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 49:2612-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus aureus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell, A. D. 2003. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 3:794-803. [DOI] [PubMed] [Google Scholar]
- 19.Ryder, C., M. Byrd, and D. J. Wozniak. 2007. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 10:644-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, K., A. Perez, G. Ramage, C. G. Gemmell, and S. Lang. 2009. Comparison of biofilm-associated cell survival following in vitro exposure of methicillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int. J. Antimicrob. Agents 33:374-378. [DOI] [PubMed] [Google Scholar]
- 21.Stickler, D. J. 2002. Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J. Appl. Microbiol. 92:163S-170S. [PubMed] [Google Scholar]
- 22.Suller, M. T., and A. D. Russell. 1999. Antibiotic and biocide resistance in methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus. J. Hosp. Infect. 43:281-291. [DOI] [PubMed] [Google Scholar]
- 23.Thomas, L., A. D. Russell, and J. Y. Maillard. 2005. Antimicrobial activity of chlorhexidine diacetate and benzalkonium chloride against Pseudomonas aeruginosa and its response to biocide residues. J. Appl. Microbiol. 98:533-543. [DOI] [PubMed] [Google Scholar]
- 24.Toté, K., D. Vanden Berghe, L. Maes, and P. Cos. 2008. A new colorimetric microtitre model for the detection of Staphylococcus aureus biofilms. Lett. Appl. Microbiol. 46:249-254. [DOI] [PubMed] [Google Scholar]
- 25.Toté, K., D. Vanden Berghe, M. Deschacht, K. de Wit, L. Maes, and P. Cos. 2009. Inhibitory efficacy of various antibiotics on matrix and viable mass of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Int. J. Antimicrob. Agents 33:525-531. [DOI] [PubMed] [Google Scholar]
- 26.Tré-Hardy, M., F. Vanderbist, H. Traore, and M. J. Devleeschouwer. 2008. In vitro activity of antibiotic combinations against Pseudomonas aeruginosa biofilm and planktonic cultures. Int. J. Antimicrob. Agents 31:329-336. [DOI] [PubMed] [Google Scholar]
- 27.Tumah, H. N. 2009. Bacterial biocide resistance. J. Chemother. 21:5-15. [PubMed] [Google Scholar]
- 28.Vinogradov, E., I. Sadovskaya, J. J. Li, and S. Jabbouri. 2006. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr. Res. 341:738-743. [DOI] [PubMed] [Google Scholar]