Abstract
Examination of Listeria monocytogenes prevalence among ready-to-eat foods in Japan revealed frequent (5.7 to 12.1%) contamination of minced tuna and fish roe products, and the isolates had the same virulence levels as clinical isolates in terms of invasion efficiency and infectivity in cell cultures and a murine infection model, respectively. Premature stop codons in inlA were infrequent (1 out of 39 isolates). Cell numbers of L. monocytogenes in minced tuna and salmon roe increased rapidly under inappropriate storage temperatures (from a most probable number [MPN] of 100 to 101/g to an MPN of 103 to 104/g over the course of 2 days at 10°C). Thus, regulatory guidelines are needed for acceptable levels of L. monocytogenes in these foods.
Listeria monocytogenes causes listeriosis in humans mainly through consumption of ready-to-eat (RTE) foods. In Japan, the first reported food-borne listeriosis outbreak occurred in 2001, caused by contaminated cheese (16). Interestingly, this outbreak was detected from routine monitoring in the cheese manufacturing plant. Since this cheese was contaminated with L. monocytogenes at a most probable number (MPN) of 107/g (16), individuals who had consumed cheese made in the plant were retrospectively examined and were found to have been infected. This was the first and only reported food-borne outbreak in Japan; however, we are unsure if previous or subsequent listeriosis outbreaks have occurred, as there are no official statistics on the incidence of listeriosis, due to the lack of a mandatory notification system (20). On the other hand, a questionnaire-based nationwide surveillance of hospitals estimated that an average of 83 listeriosis cases occur every year, which is equivalent to 0.65 per million inhabitants in Japan (20). Moreover, the pathogen has been detected in surveys of RTE foods at rates similar to those of other industrialized countries (21).
Japan has a unique diet, comprising large quantities of raw RTE seafood, such as sashimi and sushi. Our previous study on L. monocytogenes contamination in such foods (11) revealed that minced tuna and fish roe products had high contamination rates (14.3% for minced tuna and 10.0 to 11.4% for fish roe products). In this study, we investigated L. monocytogenes prevalence in such RTE foods further, using a larger number of raw RTE seafood and other RTE food products. We also investigated the virulence potential of isolates in invasion efficiency and in a mouse model and determined whether each product type could support the growth of the pathogen. These results can provide baseline data for regulatory guidelines necessary for the safety of such products.
Seafood products and other RTE foods were purchased from 229 different grocery stores and delicatessens located around Tokyo, Japan, between October 2004 and July 2008. By following a two-step enrichment procedure (11), five colonies on Palcam agars (Merck, Darmstadt, Germany) from each enrichment were randomly picked. Serotype was determined by the agglutination method using commercial Listeria antiserum (Denka Seiken, Tokyo, Japan). Each isolate was considered to be a different strain if it had a different serotype or multilocus sequence type (MLST), based on six virulence genes (prfA, inlB, inlC, dal, clpP, and lisR) as described previously (30) (data not shown). Similar to what was found in our previous study, minced tuna and fish roe products were highly contaminated with L. monocytogenes, as determined by a mini-VIDAS LMO screening test (bioMérieux Vitek, Marcy l'Etoile, France) (Table 1).
TABLE 1.
Prevalence of L. monocytogenes in RTE foods available at retail outlets in Japana
| Sample type | No. of samples tested | No. of positive samples by mini-VIDAS LMO (%) |
|---|---|---|
| Seafood | ||
| Tuna | ||
| Minced tuna | 116 | 14 (12.1) |
| Tuna block | 38 | 1 (2.6) |
| Fish roe | ||
| Salmon roe | 123 | 7 (5.7) |
| Cod roe | 164 | 15 (9.1) |
| Sushi | 36 | 0 (0.0) |
| Smoked salmonb | 33 | 1 (3.0) |
| Dried seafood | 16 | 0 (0.0) |
| Other RTE food | ||
| Natural cheeseb | 65 | 0 (0.0) |
| Salad | 61 | 0 (0.0) |
| Deli sandwich | 32 | 0 (0.0) |
| Hamb | 17 | 0 (0.0) |
| Total | 701 | 38 (5.4) |
Food samples were purchased from 229 different grocery stores and delicatessens located in and around Tokyo, Japan, from October 2004 to July 2008. Screening of L. monocytogenes in foods was performed by mini-VIDAS LMO.
Previously, 2.4% (33/1,387 samples) of imported natural cheese, 0% (0/15 samples) of ham, and 5.4% (5/92 samples) of smoked salmon retailed in Japan were reported to be contaminated (21).
Food processing plants have been found to be the most frequent source of L. monocytogenes contamination in many types of foods, including RTE seafood (1, 3, 18, 19, 26, 28). As minced tuna and fish roe products require more processing than other raw seafood products, there is a greater possibility of cross-contamination in such food processing plants. In fact, the contamination rates of minced tuna and fish roe products in Japan were relatively high compared to rates determined in the United States and Europe for other products, such as dairy products (14), vegetables (4), smoked seafood, and meat products (10).
Virulence potential, which differs among L. monocytogenes isolates (2, 23, 24), is another important factor in listeriosis risk. Out of 13 known serotypes, three (1/2a, 1/2b, and 4b) are known to be responsible for >90% of human listeriosis cases (17). In this study, approximately 79% (31/39) of the isolates from 36 RTE seafood products comprised these three serotypes (Table 2). The serotypes of the remaining isolates included 3a (7 isolates that were obtained from 7 different cod roe samples purchased from 6 different stores) and 3b (1 isolate obtained from cod roe), which are rarely isolated from human clinical cases (6, 15). Furthermore, almost all (38/39) of the raw RTE seafood isolates were found to encode full-length InlA, a protein required in invasion of host cells (12) (DNA Data Bank of Japan accession numbers AB276379 to AB276437 and AB522784 to AB522794); one serotype 1/2a isolate had a truncated InlA. Even though the number of isolates sequenced was relatively small (n = 39), the scarcity of inlA with premature stop codons was in marked contrast to results from other studies (13, 22, 23, 27). This indicates that raw RTE seafood isolates have been through environments where full-length InlA may be required, unlike the other food isolates.
TABLE 2.
Serotype distribution of L. monocytogenes isolates from minced tuna and fish roe samplesa
| Sample type | No. of isolates |
|||||
|---|---|---|---|---|---|---|
| Total | By serotype |
|||||
| 1/2a | 3a | 1/2b | 3b | 4b | ||
| Minced tuna | 15 | 12 | 2 | 1 | ||
| Salmon roe | 6 | 4 | 2 | |||
| Cod roe | 18 | 5 | 7 | 3 | 1 | 2 |
| Total | 39 | 21 | 7 | 7 | 1 | 3 |
From 36 L. monocytogenes-positive samples (14 minced tuna, 7 salmon roe, and 15 cod roe samples) determined by mini-VIDAS LMO (Table 1), 39 isolates were obtained, with 3 food samples producing no isolates. Two isolates with different subtypes (serotypes and/or MLSTs) were obtained from six food samples.
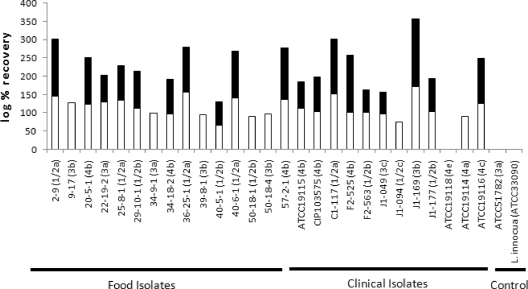
The virulence potential of the seafood isolates was assessed based on in vivo bioassays using a mouse model (Table 3 and Fig. 1). Three days after intravenous inoculation with 103 to 104 CFU, recovery of L. monocytogenes from livers and spleens was detected for all the isolates tested, except for one serotype 4e strain (Fig. 1). No recovery was detected in some isolates when homogenized livers were directly plated, but colonies were recovered after enrichment from these liver samples. Statistical analysis (Student's t test) revealed no significant differences in infectivity in liver (P = 0.691), spleen (P = 0.274), or both (P = 0.882) between raw RTE seafood and clinical isolates. These data suggest similar levels of virulence between raw RTE seafood isolates and clinical isolates in this animal model. The one raw RTE seafood isolate with truncated InlA was highly infective in liver and spleen, indicating that full-length InlA is not essential in infecting these organs.
TABLE 3.
L. monocytogenes isolates used in the mouse assay
| Strain | Serotype | Sampling date | Origin | Reference or source |
|---|---|---|---|---|
| Food isolates | ||||
| 2-9 | 1/2a | 19 November 2002 | Salmon roe | 13 |
| 25-8-1 | 1/2a | 9 December 2004 | Minced tuna | 13 |
| 36-25-1 | 1/2a | 2 June 2005 | Cod roe | 13 |
| 40-6-1 | 1/2a | 26 July 2005 | Minced tuna | 13 |
| 22-19-2 | 3a | 16 November 2004 | Cod roe | 13 |
| 34-9-1 | 3a | 28 April 2005 | Cod roe | 13 |
| 29-10-1 | 1/2b | 17 February 2005 | Minced tuna | 13 |
| 40-5-1 | 1/2b | 26 July 2005 | Salmon roe | 13 |
| 50-18-1 | 1/2b | 13 April 2006 | Cod roe | This study |
| 9-17 | 3b | 2 February 2003 | Salmon roe | 13 |
| 39-8-1 | 3b | 21 July 2005 | Salmon roe | 13 |
| 50-18-4 | 3b | 13 April 2006 | Cod roe | This study |
| 20-5-1 | 4b | 28 October 2004 | Cod roe | 13 |
| 34-18-2 | 4b | 28 April 2005 | Cod roe | 13 |
| 57-2-1 | 4b | 20 July 2006 | Minced tuna | This study |
| Clinical isolates | ||||
| C1-117 | 1/2a | Human | Pathogen Trackera | |
| F2-563 | 1/2b | Human | Pathogen Tracker | |
| J1-177 | 1/2b | Human | Pathogen Tracker | |
| J1-169 | 3b | Human | Pathogen Tracker | |
| J1-094 | 1/2c | Human | Pathogen Tracker | |
| J1-049 | 3c | Human | Pathogen Tracker | |
| ATCC 19114 | 4a | Animal | ||
| ATCC 19115 | 4b | Human | ||
| CIP103575 | 4b | Milk | ||
| F2-525 | 4b | Human | Pathogen Tracker | |
| ATCC 19116 | 4c | Animal | ||
| ATCC 19118 | 4e | Animal | ||
| Controls | ||||
| ATCC 51782 | 3a | Cheese | ||
| ATCC 33090 (L. innocua) | 6a | Animal |
Available at http://www.pathogentracker.net/.
FIG. 1.
Virulence of L. monocytogenes isolates from RTE seafood in the mouse model. Seven-week-old female BALB/cCrSlc mice were infected via intravenous inoculation at 103 to 104 CFU. Bacteria were enumerated from the liver (black columns) and spleen (white columns) 3 days after infection. The rate of recovery was determined using the following formula: log (number of cells recovered)/log (number of cells inoculated) × 100. L. monocytogenes ATCC 51782, which has attenuated virulence due to the K220T substitution in PrfA (25), and L. innocua ATCC 33090 were used as negative controls.
We also investigated the possibility that large amounts of L. monocytogenes could be ingested through the consumption of contaminated RTE seafood products. To investigate whether raw RTE seafood supports pathogenic growth, we inoculated foods with L. monocytogenes (2 strains of serotype 1/2a and 4b, both isolated from fish roe products) and examined them under temperature conditions that could exist during distribution and prior to consumption. A portion (25 g) of each minced tuna and salmon roe sample was inoculated with L. monocytogenes at an MPN of 100 to 101/g and then incubated at 22°C for 6 h and at 5°C or 10°C for 7 days. Although most minced tuna and fish roe products in Japan have a shelf life of less than 3 days, it is possible that they may be consumed after the expiration date. Moreover, certain fish roe products have a 7-day shelf life. Results at room temperature (22°C) were examined to reflect situations such as those in sushi restaurants or small home parties, in which food might remain unrefrigerated for extended periods. Minced tuna and fish roe, which are popular ingredients of sushi, allowed minimal growth of the pathogen, with an increase in cell number to an MPN of 102/g, even at room temperature for 6 h (data not shown), while refrigeration (5°C) resulted in an MPN of 102/g following 3 and 2 days of incubation in minced tuna and salmon roe, respectively. After a 7-day incubation at 5°C, L. monocytogenes cell numbers reached an MPN of 103 to 104/g. However, increasing the temperature to 10°C resulted in increases of L. monocytogenes to an MPN of 103 to 104/g following only 2 days of incubation and an MPN of 107/g after 7 days of incubation. The appearance and odor of all samples were assessed by a panel of five judges to determine the extent of spoilage. At day 2, all products were judged to be unspoiled and safe to eat.
These data raise the concern that raw RTE seafood products available at retail outlets in Japan are at risk for food-borne listeriosis and raise the possibility that these products have already been the cause of illness in the past. Considering the contamination level of RTE foods that have caused listeriosis outbreaks in the past (mostly ≥104 CFU/g) (5), the level determined in this study was quite low (Table 4), indicating that the samples were relatively safe in terms of contamination level at the time of purchase. However, this is the case only if they are consumed immediately after purchase. In foods that support growth, cell number is expected to increase at the time of consumption, especially when the food is not properly maintained under refrigeration. The United States also retains a zero-tolerance policy for RTE foods that support growth (9). In the European Union (EU), regulatory guidelines set different tolerance levels of L. monocytogenes contamination depending on whether the food supports growth, with zero tolerance for foods that support growth “before the food has left the immediate control of the food business operator, who has produced it” and a tolerance level of 100 CFU/g for “products placed on the market during their shelf-life” and for foods that do not support growth throughout the shelf life (8). Although most minced tuna and salmon roe products have a short shelf life, they should be categorized as foods that support pathogenic growth. In fact, the U.S. Food and Drug Administration categorizes raw seafood as RTE foods that support L. monocytogenes growth (9).
TABLE 4.
Numbers of L. monocytogenes cells in minced tuna and fish roe products purchased from retail stores in Japan, as determined by the MPN methoda
| Sample type | No. of samples tested | MPN/g |
|||
|---|---|---|---|---|---|
| <0.3 | 0.3-0.94 | 1.1-9.3 | 12-15 | ||
| Minced tuna | 14 | 10 | 3 | 1 | |
| Salmon roe | 7 | 4 | 2 | 1 | |
| Cod roe | 15 | 6 | 6 | 3 | |
| Total | 36 | 20 | 9 | 6 | 1 |
See reference 7.
In conclusion, our data suggest that raw RTE seafood may pose risks for food-borne listeriosis. In the United States, preventative regulatory guidelines have been effective in reducing the listeriosis incidence (29), while in Japan, regulation of L. monocytogenes is currently limited to dairy products and RTE meat products. Our data will be useful for establishing regulations for microbial food safety that include RTE seafood.
Acknowledgments
This work was partly supported by a grant from the Food Safety Commission of Japan (0605), by a research project ensuring food safety from farm to table (FV-7208 and FP-6104), which was funded by the Ministry of Agriculture, Forestry and Fisheries (MAFF) of Japan, and by a grant-in-aid for Scientific Research (B 20380121) from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Published ahead of print on 26 March 2010.
REFERENCES
- 1.Autio, T., S. Hielm, M. Miettinen, A.-M. Sjöberg, K. Aarnisalo, J. Björkroth, T. Mattila-Sandholm, and H. Korkeala. 1999. Sources of Listeria monocytogenes contamination in a cold-smoked rainbow trout processing plant detected by pulsed-field gel electrophoresis typing. Appl. Environ. Microbiol. 65:150-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrang, M. E., R. J. Meinersmann, J. K. Korthcutt, and D. P. Smith. 2002. Molecular characterization of Listeria monocytogenes isolated from a poultry further processing facility and from fully cooked product. J. Food Prot. 65:1574-1579. [DOI] [PubMed] [Google Scholar]
- 4.Beuchat, L. R. 1996. Listeria monocytogenes incidence on vegetables. Food Control 7:223-228. [Google Scholar]
- 5.Center for Food Safety and Applied Nutrition, Food and Drug Administration; Food Safety and Inspection Service, U.S. Department of Agriculture; and Centers for Disease Control and Prevention. 2003. Quantitative assessment of the relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. http://www.fda.gov/Food/ScienceResearch/ResearchAreas/RiskAssessmentSafetyAssessment/ucm183966.htm.
- 6.Cheng, Y., R. M. Siletzky, and S. Kathariou. 2008. Genomic division/lineages, epidemic clones, and population structure, p. 337-358. In D. Liu (ed.), Handbook of Listeria monocytogenes. CRC Press, Boca Raton, FL.
- 7.Cochran, W. G. 1950. Estimation of bacterial densities by means of the “most probable number.” Biometrics 6:105-116. [PubMed] [Google Scholar]
- 8.European Commission. 2005. Commission regulation (EC) no. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union L 338:1-26. [Google Scholar]
- 9.Food and Drug Administration. 2008. Draft Compliance Policy Guide Sec. 555.320. Listeria monocytogenes. http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm136694.htm.
- 10.Gombas, D. E., Y. Chen, R. S. Clavero, and V. N. Scott. 2003. Survey of Listeria monocytogenes in ready-to-eat foods. J. Food Prot. 66:559-569. [DOI] [PubMed] [Google Scholar]
- 11.Handa, S., B. Kimura, H. Takahashi, T. Koda, K. Hisa, and T. Fujii. 2005. Incidence of Listeria monocytogenes in raw seafood products in Japanese retail stores. J. Food Prot. 68:411-415. [DOI] [PubMed] [Google Scholar]
- 12.Handa-Miya, S., B. Kimura, H. Takahashi, M. Sato, T. Ishikawa, K. Igarashi, and T. Fujii. 2007. Nonsense-mutated inlA and prfA not widely distributed in Listeria monocytogenes isolates from ready-to-eat seafood products in Japan. Int. J. Food Microbiol. 117:312-318. [DOI] [PubMed] [Google Scholar]
- 13.Jacquet, C., M. Doumith, J. I. Gordon, P. M. V. Martin, P. Cossart, and M. Lecuit. 2004. A molecula rmarker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. J. Infect. Dis. 189:2094-2100. [DOI] [PubMed] [Google Scholar]
- 14.Kozak, J., T. Balmer, R. Byrne, and K. Fisher. 1996. Prevalence of Listeria monocytogenes in foods: incidence in dairy products. Food Control 7:215-221. [Google Scholar]
- 15.Lyytikäinen, O., T. Autio, R. Maijala, P. Ruutu, T. Honkanen-Buzalski, M. Miettinen, M. Hatakka, J. Mikkola, V.-J. Anttila, T. Johansson, L. Rantala, T. Aalto, H. Korkeala, and A. Siitonen. 2000. An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J. Infect. Dis. 181:1838-1841. [DOI] [PubMed] [Google Scholar]
- 16.Makino, S.-I., K. Kawamoto, K. Takeshi, Y. Okada, M. Yamasaki, S. Yamamoto, and S. Igimi. 2005. An outbreak of food-borne listeriosis due to cheese in Japan, during 2001. Int. J. Food Microbiol. 104:189-196. [DOI] [PubMed] [Google Scholar]
- 17.McLauchlin, J. 1990. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. Eur. J. Clin. Microbiol. Infect. Dis. 9:210-213. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen, M. K., K. J. Björkroth, and H. J. Korkeala. 1999. Characterization of Listeria monocytogenes from an ice cream plant by serotyping and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 46:187-192. [DOI] [PubMed] [Google Scholar]
- 19.Nesbakken, T., G. Kapperud, and D. A. Caugant. 1996. Pathways of Listeria monocytogenes contamination in the meat processing industry. Int. J. Food Microbiol. 31:161-171. [DOI] [PubMed] [Google Scholar]
- 20.Okutani, A., Y. Okada, S. Yamamoto, and S. Igimi. 2004. Nationwide survey of human Listeria monocytogenes infection in Japan. Epidemiol. Infect. 132:769-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okutani, A., Y. Okada, S. Yamamoto, and S. Igimi. 2004. Overview of Listeria monocytogenes contamination in Japan. Int. J. Food Microbiol. 93:131-140. [DOI] [PubMed] [Google Scholar]
- 22.Olier, M., D. Garmyn, S. Rousseaux, J.-P. Lemaître, P. Piveteau, and J. Guzzo. 2005. Truncated internalin A and asymptomatic Listeria monocytogenes carriage: in vivo investigation by allelic exchange. Infect. Immun. 73:644-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olier, M., F. Pierre, J. P. Lemaître, C. Divies, A. Rousset, and J. Guzzo. 2002. Assessment of the pathogenic potential of two Listeria monocytogenes human faecal carriage isolates. Microbiology 148:1855-1862. [DOI] [PubMed] [Google Scholar]
- 24.Roche, S. M., P. Gracieux, I. Albert, M. Gouali, C. Jacquet, P. M. V. Martin, and P. Velge. 2003. Experimental validation of low virulence in field strains of Listeria monocytogenes. Infect. Immun. 71:3429-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche, S. M., P. Gracieux, E. Milohanic, I. Albert, I. Virlogeux-Payant, S. Témoin, O. Grépinet, A. Kerouanton, C. Jacquet, P. Cossart, and P. Velge. 2005. Investigation of specific substitutions in virulence genes characterizing phenotypic groups of low-virulence field strains of Listeria monocytogenes. Appl. Environ. Microbiol. 71:6039-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rørvik, L. M., D. A. Caugant, and M. Yndestad. 1995. Contamination pattern of Listeria monocytogenes and other Listeria spp. in a salmon slaughterhouse and smoked salmon processing plant. Int. J. Food Microbiol. 25:19-27. [DOI] [PubMed] [Google Scholar]
- 27.Rousseaux, S., M. Olier, J. P. Lemaître, P. Piveteau, and J. Guzzo. 2004. Use of PCR-restriction fragment length polymorphism of inlA for rapid screening of Listeria monocytogenes strains deficient in the ability to invade Caco-2 cells. Appl. Environ. Microbiol. 70:2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scanga, J. A., A. D. Grona, K. E. Belk, J. N. Sofos, G. R. Bellinger, and G. C. Smith. 2000. Microbiological contamination of raw beef trimmings and ground beef. Meat Sci. 56:145-152. [DOI] [PubMed] [Google Scholar]
- 29.Tappero, J. W., A. Schuchat, K. A. Deaver, L. Mascola, and J. D. Wenger. 1995. Reduction in the incidence of human listeriosis in the United States. Effectiveness of prevention efforts? The Listeriosis Study Group. JAMA 273:1118-1122. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, W., B. M. Jayarao, and S. J. Knabel. 2004. Multi-virulence-locus sequence typing of Listeria monocytogenes. Appl. Environ. Microbiol. 70:913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]