Abstract
Background:
For over two decades, the Nottingham Prognostic Index (NPI) has been used in the United Kingdom to calculate risk scores and inform management about breast cancer patients. It is derived using just three clinical variables – nodal involvement, tumour size and grade. New scientific methods now make cost-effective measurement of many biological characteristics of tumour tissue from breast cancer biopsy samples possible. However, the number of potential explanatory variables to be considered presents a statistical challenge. The aim of this study was to investigate whether in ER+ tamoxifen-treated breast cancer patients, biological variables can add value to NPI predictors, to provide improved prognostic stratification in terms of overall recurrence-free survival (RFS) and also in terms of remaining recurrence free while on tamoxifen treatment (RFoT). A particular goal was to enable the discrimination of patients with a very low risk of recurrence.
Methods:
Tissue samples of 401 cases were analysed by microarray technology, providing biomarker data for 72 variables in total, from AKT, BAD, HER, MTOR, PgR, MAPK and RAS families. Only biomarkers screened as potentially informative (i.e., exhibiting univariate association with recurrence) were offered to the multivariate model. The multiple imputation method was used to deal with missing values, and bootstrap sampling was used to assess internal validity and refine the model.
Results:
Neither the RFS nor RFoT models derived included Grade, but both had better predictive and discrimination ability than NPI. A slight difference was observed between models in terms of biomarkers included, and, in particular, the RFoT model alone included HER2. The estimated 7-year RFS rates in the lowest-risk groups by RFS and RFoT models were 95 and 97%, respectively, whereas the corresponding rate for the lowest-risk group of NPI was 89%.
Conclusion:
The findings demonstrate considerable potential for improved prognostic modelling by incorporation of biological variables into risk prediction. In particular, the ability to identify a low-risk group with minimal risk of recurrence is likely to have clinical appeal. With larger data sets and longer follow-up, this modelling approach has the potential to enhance an understanding of the interplay of biological characteristics, treatment and cancer recurrence.
Keywords: breast cancer, tamoxifen resistance, statistical model, HER2 signalling
Endocrine-targeted therapy remains one of the most successful systemic treatment options for the approximately 80% of patients diagnosed with ER-positive early breast cancer (Miller et al, 2007; Bartlett et al, 2007). However, it has been shown that many patients relapse and die from breast cancer, despite the relative efficacy of current endocrine treatment modalities (Abe et al, 2005). Recent advances, including the third generation aromatase inhibitors, have not dramatically altered this figure (Hughes-Davies et al, 2009). Conversely, a significant proportion of women with ER positive cancer are at a low risk of breast cancer relapse, even when not treated with adjuvant endocrine therapy (Abe et al, 2005).
Currently, treatment selection for breast cancer is guided predominantly by patient prognosis, using classical pathological assessment of tumours to measure risk (Pinder et al, 1995; Elston et al, 1999). Endocrine therapy is offered to the majority of patients with ERα-positive breast cancers, with higher risk patients being more likely to receive aromatase inhibitors and possibly chemotherapy. Two fundamental changes in the understanding of the underlying biology of breast cancers challenge this approach. First, the molecular differences that exist between breast cancers (Perou et al, 2000; Pollack et al, 2002; Desmedt et al, 2004) support treating different molecular subtypes on the basis of their biology and pathology rather than on pathology alone. Second, clear evidence that molecular subtypes of cancer respond differently to different therapeutic options challenges the ‘one size fits all’ approach to chemotherapy in cancer (Hayes et al, 2007; Bartlett et al, 2008, 2009a, 2009b, 2010; Pritchard et al, 2008; Ellis et al, 2009).
Several different approaches have been applied to the subclassification of breast cancers on the basis of genomic (Pollack et al, 2002), transcriptomic (Perou et al, 2000) and immunohistochemical (El Rehim et al, 2004) techniques. No clear evidence has yet emerged as to the superiority of one approach over another. We are pursuing a functional approach for the stratification of breast cancers, seeking to ‘translate’ current knowledge of drug resistance pathways to the identification of subgroups with low, moderate and high risk of relapse after treatment with particular therapeutic agents.
The Nottingham Prognostic Index (NPI) combines information on nodal status, tumour grade and tumour size in untreated breast cancer patients by means of a Cox regression model, to produce an estimate of the risk of cancer recurrence that can be used to classify patients into risk groups (Haybittle et al, 1982) for treatment selection. This model has been widely validated and is now central to the risk stratification of patients with breast cancer across the United Kingdom. However, on the basis of analyses of nearly 10 000 patients, it has been concluded that NPI is not capable of identifying a low enough risk group to warrant a recommendation of no treatment (Balslev et al, 1994). Therefore, there is a need for additional prognostic factors to improve the precision of prediction.
Similar models, notably ‘Adjuvant! Online’, were derived in an analogous manner and are used across Europe and the United States. These models, particularly Adjuvant! Online, have sought to adapt to modern practice by integrating ERα with clinical trial data to select appropriate therapies for patients. However, such approaches tend to select only the single most powerful predictive biomarker for inclusion within the model.
We have, over the past few years, carefully explored the role of a large number of candidate predictive biomarkers in a selected cohort of tamoxifen-treated ERα-positive breast cancer patients (Kirkegaard et al, 2005, 2007; Tovey et al, 2005, 2006b; Naresh et al, 2006; Cannings et al, 2007; McGlynn et al, 2009). In so doing, we have mapped the expression of markers that appear independently predictive of response to tamoxifen (Kirkegaard et al, 2005, 2007; Tovey et al, 2005), including AIB1, HER2 and AKT. In this way, we have identified a novel predictive biomarker panel with the potential to improve selection of patients with ERα-positive breast cancers who are likely to respond well to tamoxifen and, potentially, to other endocrine therapies.
In this study, we explore the potential of combining biomarker data and clinical variables to develop an enhanced prognostic index for breast cancer recurrence.
Materials and methods
Patients
Study subjects comprised 401 ER-positive patients diagnosed between 1983 and 1999 at the Glasgow Royal Infirmary (McGlynn et al, 2009). The median follow-up time was 6.16 years and all patients received tamoxifen with a median treatment duration of 5 years. All patients were treated by surgery with curative intent and received tamoxifen after surgery; 73 (18%) were aged under 50 years at diagnosis. With regard to other adjuvant treatments, 74 (28%) patients received radiotherapy only, 61 (25%) received chemotherapy only and 40 (10%) received both (chemotherapy information was unknown for three patients, one of whom received radiotherapy and is counted among the 74).
By the end of follow-up, there had been 74 deaths among the 112 recurrences, and 84 of the recurrences occurred while the patient was still receiving tamoxifen treatment.
Variables
Data from previous analyses, quality assured by dual scoring (Kirkegaard et al, 2005, 2006, 2007, 2008; Tovey et al, 2005, 2006a, 2006b; Cannings et al, 2007), were considered for inclusion in the model (72 variables relating to 41 biomarkers). Nuclear, cytoplasmic and membrane expressions were scored according to the observed cellular distribution of markers and were analysed separately. Membrane expression was analysed for p118ERα, p167ERα, EGFr, HER2, phosphoHER2, HER3 (m), HER4-ICD (intracellular domain) and HER4 ECD (extracellular domain). Cytoplasmic expression was analysed for ERα, ERβ, p118ERα, p167ERα, phosphoHER2, HER3, HER4-ICD, HER4 ECD, hRAS, nRAS, kRAS, RAF1, p259-RAF1, p338-RAF1, rKip, TES, AKT1, AKT2, AKT3, panAKT, p473AKT, p308AKT, mTOR, phospho-mTOR, p389-p70S6k, Tace, Tacep, MAPK, phosphoMAPK, PTEN, Bcl2, Bax, Bad, p112-Bad and Bcl-xl. Nuclear expression was analysed for ERα, ERβ, PgR, p118ERα, p167ERα, phosphoHER2, HER3, HER4-ICD, HER4 ECD, hRAS, nRAS, kRAS, RAF1, p259-RAF1, p338-RAF1, rKip, TES, AKT1, panAKT, p473AKT, p308AKT, MAPK, phosphoMAPK, PTEN and AIB1. In addition, gene amplification and copy number for HER2 and AIB1 and TUNEL analysis of apoptosis were analysed. Four clinical variables were also offered to the model, namely, nodal status, grade (Bloom and Richardson), tumour size and age.
Outcomes studied
The primary outcome was recurrence-free survival (RFS), with secondary outcomes being overall survival (OS) and remaining recurrence free while on tamoxifen treatment (RFoT), as previously defined (Kirkegaard et al, 2005; Tovey et al, 2005, 2006a, 2006b). Separate models were developed for RFS and RFoT. Kaplan–Meier (K–M) curves are presented for RFS and RFoT in relation to risk groups obtained from the models developed for these end points, and also for OS in relation to the patient risk groupings obtained from the RFS and RFoT models.
Statistical modelling
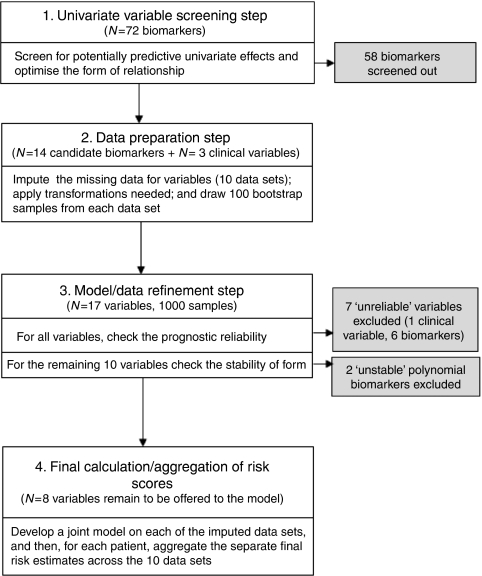
Regression risk modelling techniques perform best when there are relatively large numbers of events and comprehensive data for all biomarkers (Peduzzi et al, 1995). However, the number of events in this cohort is not large (particularly for recurrence on tamoxifen). By their nature, biomarkers are prone to missing values, and it tends to be that the distribution of biomarker expression is positively skewed (and hence relationship with recurrence likely to be nonlinear) (Royston and Sauerbrei, 2008). To address these challenges, we developed a four step modelling approach (Figure 1).
Figure 1.
Process of development of RFS model.
Step 1: Screening and choice of risk function
Our screening process had two inter-linked aims: (i) to select variables to be offered as candidate variables to the multivariate model, those demonstrating univariate association with outcome (recurrence or recurrence while on tamoxifen); and (ii) to identify the best form of association with recurrence (linear, polynomial, threshold or non-ordinal). To avoid screening of variables that are potentially important in the final multivariate model, the screening P-value threshold was set to the equivalent of P=0.1 in a standard univariate Cox model (i.e., P-value <0.1 in fractional polynomial (FP) (explained in the next paragraph); <0.005 in the minimum P-value method; or <0.025 in ‘non-ordinal quartile dichotomisation’ methods.)
For each biomarker in turn, we first applied second-degree FP (FP2) regression to detect polynomial or linear associations. FP1 functions are power transformations modelling Xp rather than the variable X (where P= −2, −1, 0, 0.5, 1, 2, 3), whereas the FP2 form is an extension to β1 Xp1 + β2 Xp2 (Royston and Altman, 1994). The simpler FP1 or linear form was selected if it provided an adequate fit. For any variable not included by the FP method, the existence of a threshold effect (in which expression at or above a specified level predicts outcome) was checked by a minimum P-value method (Clark et al, 1993). Any variable remaining unselected was then checked for non-ordinal effects by comparing cases with expression of the biomarker ranging between two neighbouring quartiles vs the remaining cases; or middle two quartile ranges vs the remaining; or first and third quartile ranges vs the remaining (four comparisons for each biomarker).
Step 2: Data imputation and selection of bootstrap samples
For all candidate variables, we imputed missing data using MICE (multivariate imputations by chained equations), a probability-based simulation technique that takes into account imputation uncertainty (Schafer, 1999). This is an iterative process in which missing data for a variable are estimated using its imputation model and, in turn, these data are used in the estimation of missing data for other variables. In accordance with usual practice, we imputed 10 values for each missing value, thus creating 10 imputed data sets. Any transformations needed to achieve optimum form, as identified during screening in step 1, were then applied to relevant variables in each of the 10 data sets. For subsequent checking of stability/reliability, 100 bootstrap samples were drawn from each imputed data set, resulting in 1000 ‘sample’ data sets.
Step 3: Refinement of model to eliminate unreliable and unstable predictors
A stable effect/form was defined as one occurring in at least 50% of the 1000 sample data sets. First, we checked stability of threshold/non-ordinal effects. Stable threshold/non-ordinal variables, variables screened in step 1 as having linear/polynomial association, and clinical variables were then subjected to predictive model fitting using backward elimination, with the threshold for removing variables P=0.05. This was undertaken separately for each of the 1000 sample data sets. An MFP (multivariate fractional polynomial) multivariate modelling approach was used, which, after fitting of linear factors, ascertains whether the model fit could be improved by using a polynomial form for any of the linear variables (Royston and Sauerbrei, 2008). For classification as unreliable variables, the inclusion frequency of each variable across all 1000 models was checked, and if less than 50%, the variable was deemed ‘unreliable’, as per Sauerbrei's algorithm (Sauerbrei and Schumacher, 1992), and excluded. Furthermore, for continuous biomarkers, if the form of risk function was unstable across bootstrap sample models (as defined above), the variable was dropped.
Step 4: Aggregation of results
Using only the ‘stable’ and ‘reliable’ variables identified in the previous step, a final model was then fitted to each of the 10 imputed data sets. Applying Rubin's rule, coefficients for these 10 models were then averaged across models, and standard errors were combined (Rubin, 1976), and an aggregate risk score was obtained for each patient by averaging his/her risk scores across the models obtained for each of the 10 imputed data sets.
For the RFS model, four equally sized risk groups were created by setting the cutoff points at three-quartiles of the distribution of the corresponding aggregate patient risk scores. Given that the RFoT model was based on fewer events, three risk groups were created by applying two tertile cutoff points to the distribution of corresponding aggregate patient risk scores.
Model performance
The final aggregated model(s) for RFS and RFoT were compared with NPI in terms of its discrimination (C-index) and ability to predict disease relapse (Nagelkerke R2) (Harrell et al, 1996). The C-index is a generalisation of the area under the ROC curve and quantifies the ability to distinguish low- and high-risk patients. This statistic varies between 0.5 (no better than chance) and 1, with values near 1 indicating high discrimination power. The Nagelkerke R2 varies between 0 and 1, which indicates, respectively, very poor and very high predictive ability (Harrell et al, 1996). Kaplan–Meier survival curves have been plotted to allow a visual comparison of event-free survival curves within risk groups for the models being compared.
We also assessed the extent to which each biomarker model classified patients into more appropriate risk groups compared with NPI (Pencina et al, 2008). For recurrence-free patients and in those in whom disease recurred, the method separately considers the joint distribution of patients into risk groups by the standard and new models being compared, quantifying ‘improvement’ in risk group classifications; for recurrence cases, the new model classifies them as higher risk, and for recurrence-free cases, as lower risk, compared with the standard model. As our RFS model classified patients into four equally sized risk groups, a fair application of this method required a comparable division of NPI risk scores (splits at quartiles of NPI risk scores were therefore used, i.e., 3.3, 4.2 and 4.8). A similar approach was used for the RFoT model, using tertile-split patients.
For both models, the actuarial event-free rate in the lowest risk group is reported for up to 3, 5, 7 and 10 years of follow-up. For comparisons of model ability to identify low-risk patients, event-free rates at 7 years are used, because follow-up data to 10 years are as yet sparse.
Software
Analyses were performed using SPSS (V.13) (SPSS, Chicago, IL, USA) and R software using MFP (Ambler and Benner, 2008), Maxstat (Horton, 2007), MICE (Van Buuren and Oudshoorn, 2007), Mitools (Lumley, 2008), Hmisc (Harrell, 2008b) and Design libraries (Harrell, 2008a).
Results
RFS modelling
Table 1 shows the 14 biological variables selected by univariate screening (step 1); for nine variables, the relationship with recurrence was linear, for two the best functional form was expressed by an FP2 model and a reciprocal square transformation (FP1) was used for nuclear PTEN. For nuclear phospho-MAPK (pMAPK), a threshold effect was identified (using a histoscore of 104) and for nuclear AKT1 (AKT1), a non-ordinal association was detected (the group with value between the second and third quartile, differing in recurrence from the rest). The frequency of missing values for candidate variables ranged between 1.2 and 11%, with the average missing rate being 5.2%. In all, 262 patients (65% of cases) had complete data on all selected biomarkers and clinical variables.
Table 1. Univariate screening step for RFS model: variables and form of risk function selected.
| Variable | Form of risk function | Number (%) of cases with available data | Univariate association with recurrence P-value |
|---|---|---|---|
| Tissue marker variables | |||
| Nuclear staining for AKT1 histoscore | Non-ordinal | 396 (99) | 0.003 |
| Cytoplasmic staining for AKT2 histoscore | Linear | 387 (97) | 0.06 |
| mTOR histoscore | Linear | 379 (95) | 0.06 |
| Phospho mTOR histoscore | Linear | 390 (97) | 0.02 |
| PTEN nuclear histoscore | Polynomial (FP1) | 373 (93) | 0.02 |
| Phospho-MAPK nuclear IHC histoscore | Threshold (Optimal split=104) | 381 (92) | 0.003 |
| Phospho Raf (ser338) nuclear histoscore | Linear | 357 (89) | 0.002 |
| Phospho Raf (ser338) cytoplasmic histoscore | Linear | 357 (89) | 0.01 |
| Mapk p42/44 cytoplasmic histoscore | Linear | 376 (94) | 0.01 |
| Cytoplasmic KRAS histoscore | Polynomial (FP2) | 387 (97) | <0.001 |
| PgR nuclear histoscore | Linear | 387 (97) | 0.007 |
| Tunel data | Linear | 362 (90) | 0.07 |
| Phospho HER2 nuclear histoscore | Linear | 376 (94) | 0.07 |
| Nuclear RKIP histoscore | Polynomial (FP2) | 387 (97) | <0.001 |
| Clinical variables | |||
| Pathological tumour size | Linear | 379 (95) | <0.001 |
| Bloom and Richardson Grade | Linear | 390 (97) | <0.001 |
| Nodal status | Linear | 368 (92) | <0.001 |
The inclusion frequency for all 17 variables offered to multivariate RFS models is given in the first column in Table 2. The final multivariate model for RFS retained six biomarkers and two clinical variables as shown in the first panel of Table 2, together with aggregated hazard ratios (as described in Materials and Methods section). It can be seen that the stability of threshold effect for pMAPK and the non-ordinal effect for AKT1 were confirmed across bootstrap samples. Kaplan–Meier curves for RFS are presented in Figure 2A, using standard NPI risk groups, and in Figure 2B using the four risk groups derived from our RFS biomarker model. The main difference between the two plots is that the lowest risk group using the RFS model displays less recurrence than the lowest risk NPI group.
Table 2. Multifactorial RFS and RFoT models; relative frequency of covariate inclusion (in 1000 bootstrap samples).
|
RFS model (112 events) Median follow-up=6.2 (IQR 4.4–8.8) years
|
RFoT model (84 events) Median follow-up=5.0 (IQR 4.0–6.0) years
|
|||||
|---|---|---|---|---|---|---|
| Variable | HRa (95% CI) | P-value | Inclusion frequency (%) | HR (95% CI) | P-value | Inclusion frequency (%) |
| Nodal status | 1.82 (1.38, 2.40) | <0.001 | 98.0 | 2.17 (1.57, 2.99) | <0.001 | 100 |
| Tumour Size (cm) | 1.21 (1.10, 1.31) | 0.001 | 95.2 | 1.20 (1.10, 1.30) | 0.001 | 87.0 |
| Cytoplasmic kRASb | 6.05 (2.23, 16.44) | <0.001 | 81.6 | b | 66.0 | |
| Tunel | 1.49 (1.23, 1.81) | <0.001 | 85.1 | c | ||
| Nuclear Akt1 | 0.54 (0.36, 0.82) | <0.001 | 92.3 | c | ||
| Phospho mTOR | 0.33 (0.19, 0.59) | <0.001 | 79.1 | 0.55 (0.33, 0.94) | 0.03 | 72.0 |
| Phospho Raf (ser338) cytoplasmic | 2.12 (1.07, 4.02) | 0.03 | 70.8 | a | 14.2 | |
| Phospho MAPK nuclear | 2.80 (1.72, 4.57) | <0.001 | 79.0 | c | ||
| PgR nuclear | a | 44.0 | a | 15.4 | ||
| PTEN Nuclear | b | 59.5 | b | 85.0 | ||
| Nuclear rKIP | b | 55.5 | a | 13.5 | ||
| Phospho Raf (ser338) nuclear | a | 15.6 | 2.43 (1.16, 5.13) | 0.02 | 59.4 | |
| Grade | a | 22.0 | a | 10.3 | ||
| mTOR | a | 18.4 | a | 12.6 | ||
| Mapk p42/44 cytoplasmic | a | 8.6 | a | 12.0 | ||
| Cytoplasmic AKT2 | a | 10.5 | c | |||
| Phospho HER2 nuclear | a | 10.0 | c | |||
| HER2 | c | 1.43 (1.04, 2.00) | 0.03 | 57.0 | ||
| Nuclear kRAS | c | a | 47.9 | |||
| Tescy | c | a | 44.0 | |||
| H4jrme | c | a | 6.2 | |||
| Nuclear Mapk | c | a | 12.0 | |||
| Bcl2 | c | a | 20.7 | |||
| Tace | c | a | 31.8 | |||
| Tacep | c | a | 16.5 | |||
Abbreviations: CI=confidence interval; HR=hazard ratio; IQR=inter quartile range; RFS=recurrence-free survival; RfoT=recurrence free while on tamoxifen treatment.
Key: a: Excluded as ‘unreliable’ – inclusion frequency was < 50% samples; b: excluded as ‘unstable’ – form not apparent in ⩾50% samples; c: screened out.
For biomarkers with linear or polynomial effect, reported HR shows the amount of increase in risk of recurrence per 100 unit changes in the independent variable.
Before applying cubic transformation to cytoplasmic kRAS, variable was divided by 100.
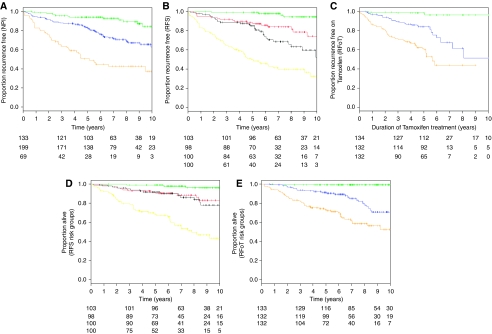
Figure 2.
Kaplan–Meier curves for the following: RFS by NPI grouping (A top left); RFS by RFS biomarker model grouping (B top middle); RFoT by RFoT biomarker model grouping (C top right); OS by RFS biomarker model grouping (D bottom left); and OS by RFoT biomarker model grouping (E bottom right).
Modelling RFoT
Three patients with missing values on duration of tamoxifen treatment could not be included in the analysis. Table 2 (right-hand panel) presents the results for RFoT modelling. Univariate screening with respect to RFoT selected 17 candidate variables, the inclusion frequencies of which are reported. Only five of these were included in the final model, and their estimated hazard ratios are reported. Figure 2C shows K–M curves for risk groups derived from our RFoT model, and it can be seen that there is very little recurrence in the lowest risk group.
Overall survival by risk groupings of RFS and RFoT models
Kaplan–Meier curves for OS, using the RFS and RFoT model risk groupings, are presented in Figures 2D and E, respectively. These suggest that the RFS and RFoT models can discriminate patients with a low and high risk of death.
Performance of models
The RFS biomarker model had a higher discrimination ability than NPI (C-index 79 vs 72% for NPI). The corresponding figures for the RFoT model were 78 and 75%, respectively. There was a similar finding for predictive ability (R2: 27 vs 14% for RFS and 19 vs 17% for RFoT).
Estimated 7-year RFS in the lowest risk group was 95% for the RFS biomarker model (four-group stratification), whereas it was 89% for NPI (standard three groups). The corresponding rates at 10 years were 95 and 79%, respectively.
Recurrence free while on tamoxifen treatment rates were compared at 5 years, because only a small proportion of patients received tamoxifen for more than 5 years. Estimated 5-year RFoT in the lowest risk group was 97% for the biomarker model (also three groups) and 94% for NPI (standard grouping).
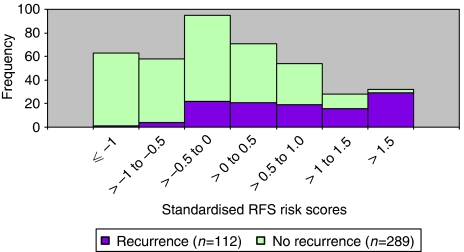
Furthermore, as shown in Figure 3, out of 63 patients with standardised risk scores of one or less, only a single patient recurred giving 7-year RFS of 98%, whereas out of 32 patients with risk scores exceeding 1.5, 29 patients recurred, giving a 7-year RFS of only 14%.
Figure 3.
Distribution of the estimated standardised risk scores for recurrence, with indication within each bar of the number of recurrences observed.
If we compare risk group classifications overall, for our RFS model compared with NPI, then of 112 recurrent cases, 34 (30%) were more appropriately classified by RFS (i.e., to a higher risk group), whereas 18 (16%) were assigned to a less appropriate group (lower), giving a net gain in classification appropriateness of 14% by RFS (P<0.01). For the 289 recurrence-free cases, the corresponding changes in classification were 90 (31%) and 78 (27%), giving a net gain in classification appropriateness of 4% (P=0.17). For the RFoT model, the net gains in classification appropriateness in recurred and non-recurred subgroups were 7.1 and 1.3%, respectively (P>0.05 for both).
Discussion
Endocrine therapy, using either tamoxifen or aromatase inhibitors, remains the most successful systemic treatment of early breast cancer. Significant improvements in recurrence-free and OS are achieved by treating women with hormone receptor-positive disease with ER-targeted therapies for 5–10 years (Abe et al, 2005). However, many women do not require endocrine therapy, achieving sufficient disease control from surgery and local radiotherapy (Abe et al, 2005). A further group may derive minimal additional benefit over that achieved with tamoxifen treatment if treated with aromatase inhibitors and/or chemotherapy (Abe et al, 2005; Miller et al, 2007; Hughes-Davies et al, 2009). The challenge is to devise prospective diagnostic approaches to stratify women for appropriate adjuvant management, including identification of those women who require no adjuvant hormonal or chemotherapy.
Using retrospective statistical modelling of molecular analysis of intracellular signalling pathways, we developed an algorithm that allows the calculation of risk of recurrence for early breast cancers treated with tamoxifen. Individual risk scores were calculated using a simple panel of six immunohistochemical markers (in addition to tumour size and nodal status), and when patients were stratified by risk into four quartiles, marked differences in group relapse rates were observed.
Among patients in the lowest risk group by the RFS model, the estimated 7-year RFS rate was 95%, whereas in the highest risk group, it was only 40% (Figure 2). When we moved the cutoffs to create risk groups that mirrored the numbers of patients in NPI risk groups for our cohort (133 lowest-risk, 199 intermediate-risk and 69 highest-risk group), the estimated 7-year RFS remained well separated, at 95% (95% CI: 91, 99%) and 34% (95% CI: 22, 46%) in the lowest and highest risk groups, respectively.
Low-risk patients could potentially avoid systemic treatment, perhaps those with risk scores no greater than one, with a 98% RFS rate at 7 years. Conversely, higher risk patients might well be candidates for additional treatment, including chemotherapy or other adjuvant treatment options. The application of individual risk scores such as those that have been derived in our models might become a strong driver for the implementation of biological risk prediction.
The advantage of our biomarker model in the stratification of a group with a low risk of recurrence seems to increase with duration of follow up: the RFS rate at 5-year follow-up was 98% (vs 94% by NPI grouping) and at 7 years was 95% (vs 89%). This is similar to the prediction achieved by complex multigene PCR-based assay systems (Paik et al, 2004). However, an important difference is that immunohistochemical assays are more readily applied to routine pathological assessments than complex multigene panels, and are also potentially significantly more cost-effective (Paik et al, 2004).
The model developed provides a significant improvement over conventional prognostic models such as NPI. The limitations of NPI have been recognised for some time and novel modelling approaches, including Adjuvant ! Online, have sought to incorporate biological (ERα) and clinical risk markers. However, such biological modelling is incomplete and further attempts at refining the integration of biological and clinical risk markers are required. One of the limitations of the current approach is the use of a tamoxifen-treated cohort; hence, a further analysis of untreated patient cohorts is required to fully validate the model. Use of historical cohorts is often criticised because improvements in screening, surgery and radiotherapy have improved prognosis in recent years, and this might affect prognosis differentially by patient factors. However, there is a conflict between the use of cohorts of patients who have received first-line treatment reflecting contemporary practice in surgical and radiotherapy techniques, but which are relatively recent cases and will thus have a short follow-up, and historical data sets with a longer follow-up. Neither approach truly investigates the natural history of the disease, and both approaches accept the interpretative compromises that are integral to the study population used.
Although the NPI model is very useful, it was developed using a limited range of risk factors. New biological developments allow us to measure functional features of tumours, so that we have a rich array of biomarkers with potential relevance to cancer progression. To circumvent the risk of an overfitted model, which can arise when there are excess potential explanatory variables, as is often the case with biomarkers, we have used bootstrap sampling to refine the models by excluding variables with an unstable form or those that are unreliably included as necessary for prediction. Recent methodological developments also allow modelling gains through detection of an optimum form of association, and provide powerful multiple imputation methods to salvage as much predictive information as possible from cases with missing data. Our biomarker-based predictive tool has the potential for future application in the selection of patients for conservative vs aggressive adjuvant treatment. In addition, the results and models reported here can contribute to the generation of hypotheses with regard to the mechanisms that might underlie the differences in recurrences observed.
In the case of the RFoT model, there was a smaller number of events in the analysis (84 vs 112); hence, the power was lower compared with the RFS model. This, together with the very limited number of patients with tamoxifen treatment exceeding 8 years, and the potential bias inherent in decisions to proceed or not with tamoxifen therapy (biased censoring), signifies that, at this stage, caution is required if interpreting the model beyond 5 years. Although we applied stringent checks on internal validity of models, the lower power of the model might explain some differences in biomarker selection, compared with other published research. Of variables previously identified as predicting recurrence while on tamoxifen (AIB1, HER2 and AKT) (Kirkegaard et al, 2005, 2007; Tovey et al, 2005), only HER2 was included in our RFoT multivariate model. This may reflect the fact that HER2 remains the dominant driver in endocrine resistance, and may also reflect the relatively small number of events (84).
One of the interesting differences between the RFS and RFoT models developed on the basis of our data is the inclusion of HER2 as a risk factor only in the latter model. This may reflect the relationship between HER2 overexpression and increased risk of early relapse, or a specific interaction with tamoxifen therapy (resistance). Alternatively, the greater power of the RFS analysis (112 events relative to 84 for RFoT) might have enabled the identification/retention of some other variable(s), which together could provide a better predictive information than HER2, and retained these in the RFS model in place of HER2. Perhaps the RFoT model, without this additional power, had to manage with one variable, which was HER2. Further work using larger sample sizes will be required before the explanation can be clarified.
Several different approaches have been taken for the development of ‘risk’ signatures in early breast cancer. The Mammostrat (Ring et al, 2006) panel is based on a functional expression array analysis of different pathways involved in breast cancer recurrence in the absence of adjuvant treatment, which uses five immunohistochemical markers to generate a risk score. The Oncotype Dx test (Paik et al, 2004) and MammaPrint are multigene signature-seeking tests, derived in a similar manner and aiming to predict outcome during tamoxifen therapy. Further studies on this marker panel suggest that it may be broadly prognostic, rather than predictive. Our approach has been to use functional markers of key molecular pathways of tamoxifen resistance to seek to identify a panel that can select patients who may either derive sufficient benefit from treatment with tamoxifen alone, or for whom withdrawal of adjuvant therapy, which is moderately toxic, may pose minimal risk. As with all approaches, this has limitations and therefore future analyses should explore additional markers, including perhaps markers of proliferation in addition to those suggested above. A further validation of our current approach is also required and during such a process, direct comparison with similar panels, such as Mammostrat, Oncotype Dx and MammaPrint, would be of value.
NPI is the recognised tool for risk prediction in the United Kingdom. In this study, we showed that TMA variables can add value to clinical predictors. Our model demonstrated significantly better risk group classification performance than NPI, particularly for patients who will go on to experience recurrence. In particular, the lowest risk group identified, comprising a quarter of all patients, showed a high recurrence-free rate (95% at 7 years). This has clinical potential, in that it suggests that such patients might be spared additional treatments without undue risk of recurrence.
References
- Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, Nomura Y, Sakai K, Sugimachi K, Tominaga T, Uchino J, Yoshida M, Haybittle JL, Davies C, Harvey VJ, Holdaway TM, Kay RG, Mason BH, Forbes JF, Wilcken N, Gnant M, Jakesz R, Ploner M, Yosef HMA, Focan C, Lobelle JP, Peek U, Oates GD, Powell J, Durand M, Mauriac L, Di Leo A, Dolci S, Piccart MJ, Masood MB, Parker D, Price JJ, Hupperets PSGJ, Jackson S, Ragaz J, Berry D, Broadwater G, Cirrincione C, Muss H, Norton L, Weiss RB, Abu-Zahra HT, Portnoj SM, Baum M, Cuzick J, Houghton J, Riley D, Gordon NH, Davis HL, Beatrice A, Mihura J, Naja A, Lehingue Y, Romestaing P, Dubois JB, Delozier T, Mace-Lesec’h J, Rambert P, Andrysek O, Barkmanova J, Owen JR, Meier P, Howell A, Ribeiro GC, Swindell R, Alison R, Boreham J, Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Harwood C, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Mead G, Peto R, Wang Y, Albano J, de Oliveira CF, Gervasio H, Gordilho J, Johansen H, Mouridsen HT, Gelman RS, Harris JR, Henderson IC, Shapiro CL, Andersen KW, Axelsson CK, Blichert-Toft M, Moller S, Mouridsen HT, Overgaard J, Overgaard M, Rose C, Cartensen B, Palshof T, Trampisch HJ, Dalesio O, de Vries EGE, Rodenhuis S, van Tinteren H, Comis RL, Davidson NE, Gray R, Robert N, Sledge G, Tormey DC, Wood W, Cameron D, Chetty U, Forrest P, Jack W, Rossbach J, Klijn JGM, Treurniet-Donker AD, van Putten WLJ, Costa A, Veronesi U, Bartelink H, Duchateau L, Legrand C, Sylvester R, van der Hage JA, van de Velde CJH, Cunningham MP, Catalano R, Creech RH, Bonneterre J, Fargeot P, Fumoleau P, Kerbrat P, Namer M, Jonat W, Kaufmann M, Schumacher M, von Minckwitz G, Bastert G, Rauschecker H, Sauer R, Sauerbrei W, Schauer A, Schumacher M, de Schryver A, Vakaet L, Belfiglio M, Nicolucci A, Pellegrini F, Sacco M, Valentini M, McArdle CS, Smith DC, Galligioni E, Boccardo F, Rubagotti A, Dent DM, Gudgeon CA, Hacking A, Erazo A, Medina JY, Izuo M, Morishita Y, Takei H, Fentiman IS, Hayward JL, Rubens RD, Skilton D, Graeff H, Janicke F, Meisner C, Scheurlen H, Kaufmann M, von Fournier D, Dafni U, Fountzilas G, Klefstrom P, Blomqvist C, Saarto T, Margreiter R, Asselain B, Salmon RJ, Vilcoq JR, Arriagada R, Hill C, Laplanche A, Le MG, Spielmann M, Bruzzi P, Montanaro E, Rosso R, Sertoli MR, Venturini M, Amadori D, Benraadt J, Kooi M, van de Velde AO, Van Dongen JA, Vermorken JB, Castiglione M, Cavalli F, Coates A, Collins J, Forbes J, Gelber RD, Goldhirsch A, Lindtner J, Price KN, Rudenstam CM, Senn HJ, Bliss JM, Chilvers CED, Coombes RC, Hall E, Marty M, Borovik R, Brufman G, Hayat H, Robinson E, Wigler N, Bonadonna G, Camerini T, De Palo G, del Vecchio M, Formelli F, Valagussa P, Martoni A, Pannuti F, Cocconi G, Colozza A, Camisa R, Aogi K, Takashima S, Abe O, Ikeda T, Inokuchi K, Kikuchi K, Sawa K, Sonoo H, Korzeniowski S, Skolyszewski J, Ogawa M, Yamashita J, Bonte J (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–171715894097 [Google Scholar]
- Ambler G, Benner A (2008) mfp: Multivariable Fractional Polynomials. http://stat.ethz.ch/CRAN/
- Balslev I, Axelsson CK, Zedeler K, Rasmussen BB, Carstensen B, Mouridsen HT (1994) The Nottingham Prognostic Index applied to 9,149 patients from the studies of the Danish Breast Cancer Cooperative Group (DBCG). Breast Cancer Res Treat 32: 281–290 [DOI] [PubMed] [Google Scholar]
- Bartlett JMS, Campbell FM, Ibrahim M, Thomas J, Wenyck I, Ellis IO, Kay E, Connolly Y, O’Grady A, Cunningham P, Barnett S, Starczynski J, Miller K (2009a) A UK NEQAS ring study evaluating observer variation in the diagnosis of HER2 amplification using the Kreatech (TM) HER2 FISH probe. Cancer Res 69: 206S [Google Scholar]
- Bartlett JMS, Ellis IO, Dowsett M, Mallon EA, Cameron DA, Johnston S, Hall E, A’Hern R, Peckitt C, Bliss JM, Johnson L, Barrett-Lee P, Ellis P (2007) Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor (ER) negative, but with grade in those with ER-positive early-stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol 25: 4423–4430 [DOI] [PubMed] [Google Scholar]
- Bartlett JMS, Munro AF, Cameron DA, Thomas JS, Prescott RJ, Twelves C (2008) Type I receptor tyrosine kinase profiles identify patients with enhanced benefit from anthracyclines in the BR9601 adjuvant breast cancer chemotherapy trial. J Clin Oncol 26: 5027–5035 [DOI] [PubMed] [Google Scholar]
- Bartlett JM, Munro AF, Dunn JA, McConkey C, Jordan S, Twelves CJ, Cameron DA, Thomas J, Campbell FM, Rea DW, Provenzano E, Caldas C, Pharoah P, Hiller L, Earl H, Poole CJ (2010) Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol 11: 266–274 [DOI] [PubMed] [Google Scholar]
- Bartlett JMS, Thomas JS, Chetty U, Seitz RS, Ross DT, Ring BZ, Pedersen HC, Beck RA, Campbell FM, Jack W, Kerr G, Mckay L, Kunkler IH, Edinburgh BU (2009b) Mammostrat (R) as a tool to stratify patients at risk of recurrence during endocrine therapy. Cancer Res 69: 212S–213S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannings E, Kirkegaard T, Tovey SM, Dunne B, Cooke TG, Bartlett JMS (2007) Bad expression predicts outcome in patients treated with tamoxifen. Breast Cancer Res Treat 102: 173–179 [DOI] [PubMed] [Google Scholar]
- Clark GM, Wenger CR, Beardslee S, Owens MA, Pounds G, Oldaker T, Vendely P, Pandian MR, Harrington D, McGuire WL (1993) How to integrate steroid hormone receptor, flow cytometric, and other prognostic information in regard to primary breast cancer. Cancer 71: 2157–2162 [DOI] [PubMed] [Google Scholar]
- Desmedt C, Larsimont D, Paesmans M, Leroy JY, Fox S, Leek R, Durbecq V, Kohlik M, Ferrara C, Rouas G, Harris AL, Piccart M, Sotiriou C (2004) Molecular classification of breast carcinomas by immunohistochemistry (IHC) using tissue microarrays (TMA): new subtypes with clinical relevance? Breast Cancer Res Treat 88: S25 [Google Scholar]
- El Rehim DMA, Ball G, Pinder S, Ellis IO (2004) Molecular classification of breast carcinoma based on the protein expression immunoprofiles. J Pathol 204: 3A [Google Scholar]
- Ellis P, Barrett-Lee P, Johnson L, Cameron D, Wardley A, O’Reilly S, Verrill M, Smith I, Yarnold J, Coleman R, Earl H, Canney P, Twelves C, Poole C, Bloomfield D, Hopwood P, Johnston S, Dowsett M, Bartlett JMS, Ellis I, Peckitt C, Hall E, Bliss JM (2009) Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet 373: 1681–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston CW, Ellis IO, Pinder SE (1999) Pathological prognostic factors in breast cancer. Crit Rev Oncol-Hematol 31: 209–223 [DOI] [PubMed] [Google Scholar]
- Harrell FE (2008a) Design: Design Package. http://stat.ethz.ch/CRAN/
- Harrell FE (2008b) Hmisc: Harrell Miscellaneous. http://stat.ethz.ch/CRAN/
- Harrell FE, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387 [DOI] [PubMed] [Google Scholar]
- Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, Nicholson RI, Griffiths K (1982) A prognostic index in primary breast cancer. Br J Cancer 45: 361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, Broadwater G, Goldstein LJ, Martino S, Ingle JN, Henderson IC, Norton L, Winer EP, Hudis CA, Ellis MJ, Berry DA (2007) HER2 and response to paclitaxel in node-positive breast cancer. New Engl J Med 357: 1496–1506 [DOI] [PubMed] [Google Scholar]
- Horton T (2007) maxstat: Maximally Selected Rank Statistics. http://stat.ethz.ch/CRAN/
- Hughes-Davies L, Caldas C, Wishart GC (2009) Tamoxifen: the drug that came in from the cold. Br J Cancer 101: 875–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JMS (2006) Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology 48: 787–794 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JMS (2007) Amplified in breast cancer 1 in human epidermal growth factor receptor-positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13: 1405–1411 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Naresh A, Sabine VS, Tovey SM, Edwards J, Dunne B, Cooke TG, Jones FE, Bartlett JMS (2008) Expression of tumor necrosis factor alpha converting enzyme in endocrine cancers. Am J Clin Pathol 129: 735–743 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JMS (2005) AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 207: 139–146 [DOI] [PubMed] [Google Scholar]
- Lumley T (2008) mitools: Tools for multiple imputation of missing data. http://stat.ethz.ch/CRAN/
- McGlynn LM, Kirkegaard T, Edwards J, Tovey S, Cameron D, Twelves C, Bartlett JMS, Cooke TG (2009) Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients. Clin Cancer Res 15: 1487–1495 [DOI] [PubMed] [Google Scholar]
- Miller WR, Bartlett JMS, Canney P, Verrill M (2007) Hormonal therapy for postmenopausal breast cancer: the science of sequencing. Breast Cancer Res Treat 103: 149–160 [DOI] [PubMed] [Google Scholar]
- Naresh A, Long WW, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JMS, Jones FE (2006) The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res 66: 6412–6420 [DOI] [PubMed] [Google Scholar]
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New Engl J Med 351: 2817–2826 [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48: 1503–1510 [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino Sr RB, D’Agostino Jr RB, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172 [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, Van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406: 747–752 [DOI] [PubMed] [Google Scholar]
- Pinder SE, Ellis IO, Elston CW (1995) Prognostic factors in primary breast carcinoma. J Clin Pathol 48: 981–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack JR, Sorlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE, Tibshirani R, Botstein D, Borresen-Dale AL, Brown PO (2002) Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci USA 99: 12963–12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard KI, Messersmith H, Elavathil L, Trudeau M, O’Malley F, Dhesy-Thind B (2008) HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 26: 736–744 [DOI] [PubMed] [Google Scholar]
- Ring BZ, Seitz RS, Beck R, Shasteen WJ, Tarr SM, Cheang MCU, Yoder BJ, Budd GT, Nielsen TO, Hicks DG, Estopinal NC, Ross DT (2006) Novel prognostic immunohistochemical biomarker panel for estrogen receptor-positive breast cancer. J Clin Oncol 24: 3039–3047 [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG (1994) Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling (with discussion). Appl Statist 43: 429–467 [Google Scholar]
- Royston P, Sauerbrei W (2008) Multivariable Model Building A pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. John Wiley: Chichester [Google Scholar]
- Rubin DB (1976) Inferences and missing data. Biometrika 63: 581–590 [Google Scholar]
- Sauerbrei W, Schumacher M (1992) A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- Schafer JL (1999) Multiple imputation: a primer. Stat Methods Med Res 8: 3–15 [DOI] [PubMed] [Google Scholar]
- Tovey SM, Dunne B, Witton CJ, Cooke TG, Bartlett JMS (2006a) HER4 in breast cancer: comparison of antibodies against intra- and extra-cellular domains of HER4. Breast Cancer Res 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey SM, Dunne B, Witton CJ, Forsyth A, Cooke TG, Bartlett JMS (2005) Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin Cancer Res 11: 4835–4842 [DOI] [PubMed] [Google Scholar]
- Tovey SM, Reeves JR, Stanton P, Ozanne BW, Bartlett JMS, Cooke TG (2006b) Low expression of HER2 protein in breast cancer is biologically significant. J Pathol 210: 358–362 [DOI] [PubMed] [Google Scholar]
- Van Buuren S, Oudshoorn CGM (2007) Mice: Multivariate Imputation by Chained Equations. http://stat.ethz.ch/CRAN/