Abstract
Platelet-activating factor (PAF) has been implicated as a mediator of inflammation, allergy, shock, and thrombosis. A specific degradative enzyme, PAF acetylhydrolase (EC 3.1.1.47), is found in plasma and could regulate the concentration of PAF in blood. In plasma, 70% of the PAF acetylhydrolase is found with low density lipoprotein (LDL), and the remainder is in high density lipoprotein (HDL). In previous studies we found that with subsaturating concentrations of PAF the activity in LDL seemed to be the relevant one; e.g., depletion of LDL slowed degradation of PAF, while removal of HDL accelerated the degradation slightly. We have pursued this observation by using plasma from humans with lipoprotein mutations. In abetalipoproteinemia, all of the PAF acetylhydrolase activity was in HDL, whereas in Tangier disease all of the activity was in LDL. In both conditions the total activity measured in an optimized assay was normal or increased. However, when we measured the t1/2 of PAF in plasma, we found that it was prolonged in subjects with abetalipoproteinemia compared to normal controls. Conversely, the t1/2 in Tangier plasma was shortened. We next demonstrated that the PAF acetylhydrolase in HDL was recognized by an antibody to the enzyme purified from LDL, establishing that the enzyme in the two particles is the same protein. Finally, we inactivated the PAF acetylhydrolase in isolated lipoprotein particles and then reconstituted them with enzyme from the opposite particle. The reconstituted particles were used to measure the t1/2 of PAF, and we again found that the LDL particle was more efficient. We conclude that the lipoprotein environment of the PAF acetylhydrolase markedly influences its catalytic behavior. This may be important in pathophysiology and will complicate attempts to assess the role of this enzyme in such circumstances.
Full text
PDF

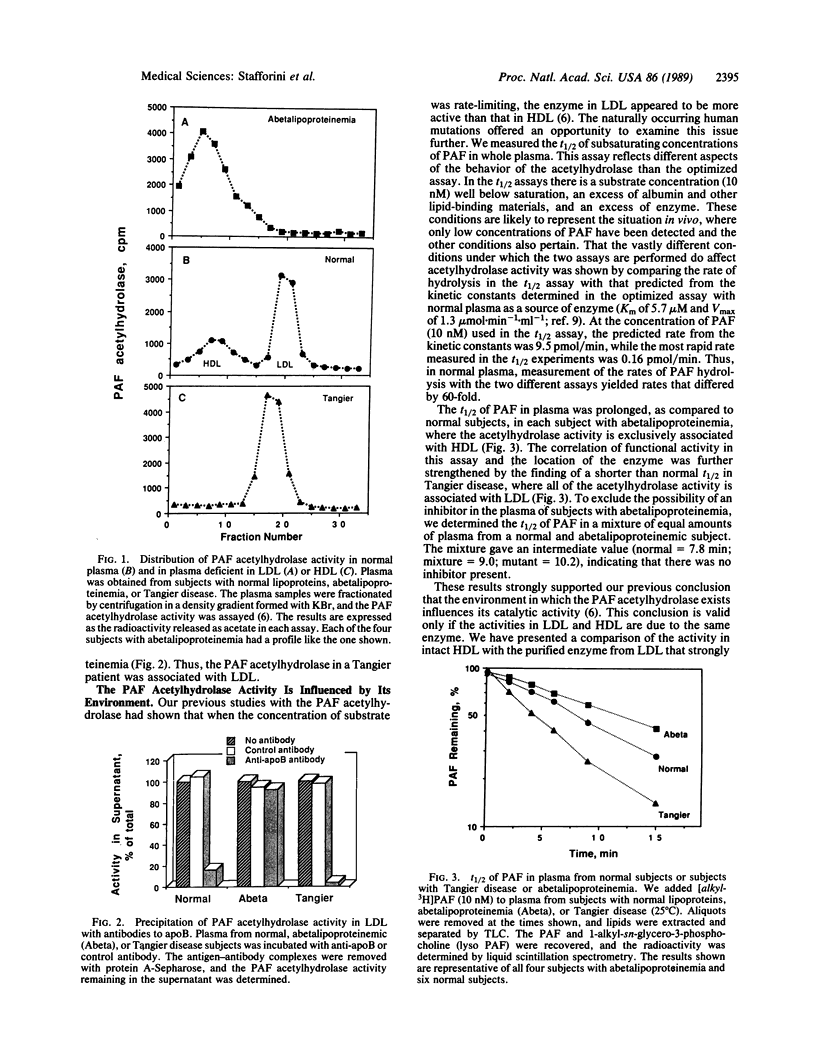
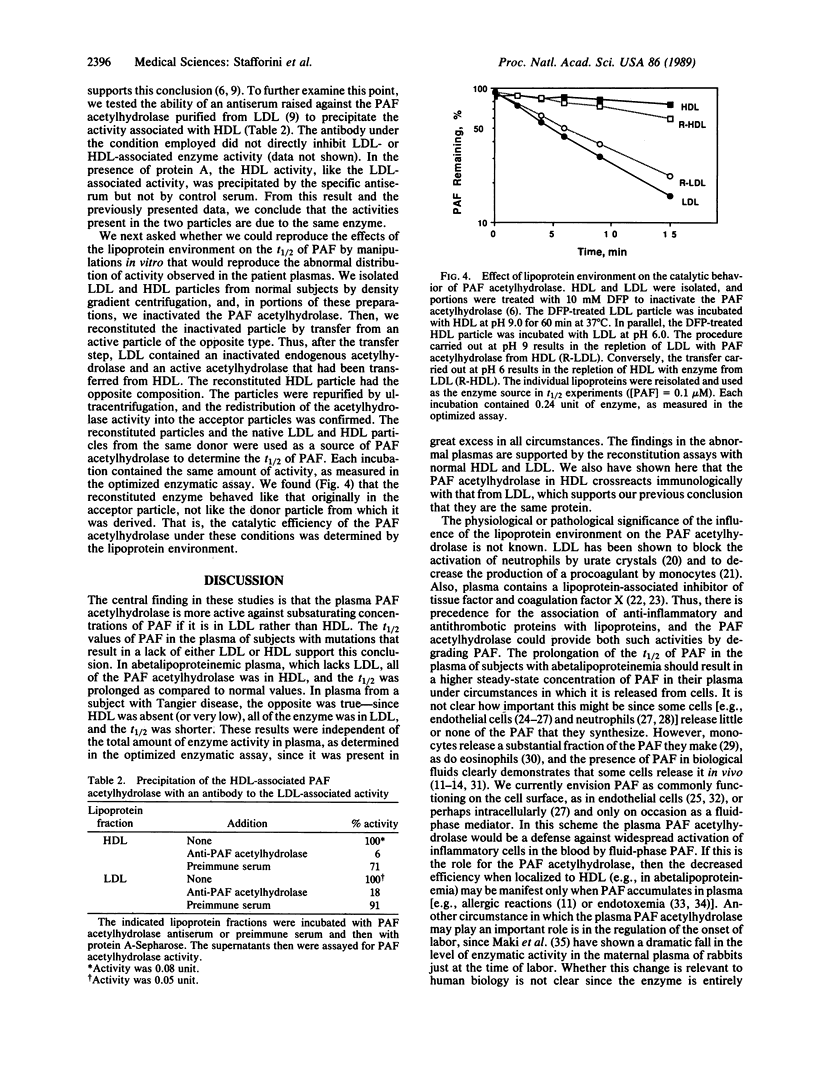

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Johnston J. M. Identification of phospholipid platelet-activating factor (1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human amniotic fluid and urine. Biochem Biophys Res Commun. 1983 May 31;113(1):51–58. doi: 10.1016/0006-291x(83)90430-8. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Hall M. N., Cress E. A., Snyder F. Inactivation of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by a plasma acetylhydrolase: higher activities in hypertensive rats. Biochem Biophys Res Commun. 1983 Jun 15;113(2):666–671. doi: 10.1016/0006-291x(83)91778-3. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Lee T., Fitzgerald V., Snyder F. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid). J Biol Chem. 1981 Jan 10;256(1):175–178. [PubMed] [Google Scholar]
- Broze G. J., Jr, Miletich J. P. Characterization of the inhibition of tissue factor in serum. Blood. 1987 Jan;69(1):150–155. [PubMed] [Google Scholar]
- Broze G. J., Jr, Warren L. A., Novotny W. F., Higuchi D. A., Girard J. J., Miletich J. P. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988 Feb;71(2):335–343. [PubMed] [Google Scholar]
- Caramelo C., Fernández-Gallardo S., Marín-Cao D., Iñarrea P., Santos J. C., López-Novoa J. M., Sanchez Crespo M. Presence of platelet-activating factor in blood from humans and experimental animals. Its absence in anephric individuals. Biochem Biophys Res Commun. 1984 May 16;120(3):789–796. doi: 10.1016/s0006-291x(84)80176-x. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Fernández-Gallardo S., Santos J. C., Iñarrea P., Sánchez-Crespo M., López-Novoa J. M., Hernando L. Increased levels of platelet-activating factor in blood from patients with cirrhosis of the liver. Eur J Clin Invest. 1987 Feb;17(1):7–11. doi: 10.1111/j.1365-2362.1987.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Chang S. W., Feddersen C. O., Henson P. M., Voelkel N. F. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- Elstad M. R., Prescott S. M., McIntyre T. M., Zimmerman G. A. Synthesis and release of platelet-activating factor by stimulated human mononuclear phagocytes. J Immunol. 1988 Mar 1;140(5):1618–1624. [PubMed] [Google Scholar]
- Farr R. S., Wardlow M. L., Cox C. P., Meng K. E., Greene D. E. Human serum acid-labile factor is an acylhydrolase that inactivates platelet-activating factor. Fed Proc. 1983 Nov;42(14):3120–3122. [PubMed] [Google Scholar]
- Grandel K. E., Farr R. S., Wanderer A. A., Eisenstadt T. C., Wasserman S. I. Association of platelet-activating factor with primary acquired cold urticaria. N Engl J Med. 1985 Aug 15;313(7):405–409. doi: 10.1056/NEJM198508153130702. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Alam S. S., Alam N. A. Lipoprotein lipase and hepatic lipase activity after heparin administration in abetalipoproteinemia and hypobetalipoproteinemia. Metabolism. 1983 Sep;32(9):869–873. doi: 10.1016/0026-0495(83)90199-3. [DOI] [PubMed] [Google Scholar]
- Ito S., Camussi G., Tetta C., Milgrom F., Andres G. Hyperacute renal allograft rejection in the rabbit. The role of platelet-activating factor and of cationic proteins derived from polymorphonuclear leukocytes and from platelets. Lab Invest. 1984 Aug;51(2):148–161. [PubMed] [Google Scholar]
- Lee T., Lenihan D. J., Malone B., Roddy L. L., Wasserman S. I. Increased biosynthesis of platelet-activating factor in activated human eosinophils. J Biol Chem. 1984 May 10;259(9):5526–5530. [PubMed] [Google Scholar]
- Lewis M. S., Whatley R. E., Cain P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest. 1988 Dec;82(6):2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. M., Henson P. M. The intracellular retention of newly synthesized platelet-activating factor. J Immunol. 1986 Oct 15;137(8):2653–2661. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- Maki N., Hoffman D. R., Johnston J. M. Platelet-activating factor acetylhydrolase activity in maternal, fetal, and newborn rabbit plasma during pregnancy and lactation. Proc Natl Acad Sci U S A. 1988 Feb;85(3):728–732. doi: 10.1073/pnas.85.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Prescott S. M. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. W., O'Flaherty J. T., Wykle R. L. Biosynthesis of platelet activating factor in rabbit polymorphonuclear neutrophils. J Biol Chem. 1983 May 25;258(10):6213–6218. [PubMed] [Google Scholar]
- Ostermann G., Kertscher H. P., Winkler L., Schlag B., Rühling K., Till U. The role of lipoproteins in the degradation of platelet-activating factor. Thromb Res. 1986 Nov 1;44(3):303–314. doi: 10.1016/0049-3848(86)90005-8. [DOI] [PubMed] [Google Scholar]
- Pritchard P. H., Chonn A., Yeung C. C. The degradation of platelet-activating factor in the plasma of a patient with familial high density lipoprotein deficiency (Tangier disease). Blood. 1985 Dec;66(6):1476–1478. [PubMed] [Google Scholar]
- Schwartz B. S., Levy G. A., Curtiss L. K., Fair D. S., Edgington T. S. Plasma lipoprotein induction and suppression of the generation of cellular procoagulant activity in vitro: two procoagulant activities are produced by peripheral blood mononuclear cells. J Clin Invest. 1981 Jun;67(6):1650–1658. doi: 10.1172/JCI110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson J. H., Prescott S. M., McIntyre T. M., Zimmerman G. A. Production of platelet-activating factor by stimulated human polymorphonuclear leukocytes. Correlation of synthesis with release, functional events, and leukotriene B4 metabolism. J Immunol. 1987 Jun 1;138(11):3918–3926. [PubMed] [Google Scholar]
- Snyder F. Chemical and biochemical aspects of platelet activating factor: a novel class of acetylated ether-linked choline-phospholipids. Med Res Rev. 1985 Jan-Mar;5(1):107–140. doi: 10.1002/med.2610050105. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. Membrane lipid composition and cellular function. J Lipid Res. 1985 Sep;26(9):1015–1035. [PubMed] [Google Scholar]
- Stafforini D. M., McIntyre T. M., Carter M. E., Prescott S. M. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J Biol Chem. 1987 Mar 25;262(9):4215–4222. [PubMed] [Google Scholar]
- Stafforini D. M., Prescott S. M., McIntyre T. M. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J Biol Chem. 1987 Mar 25;262(9):4223–4230. [PubMed] [Google Scholar]
- Terkeltaub R., Curtiss L. K., Tenner A. J., Ginsberg M. H. Lipoproteins containing apoprotein B are a major regulator of neutrophil responses to monosodium urate crystals. J Clin Invest. 1984 Jun;73(6):1719–1730. doi: 10.1172/JCI111380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Wardlow M. L., Cox C. P., Meng K. E., Greene D. E., Farr R. S. Substrate specificity and partial characterization of the PAF-acylhydrolase in human serum that rapidly inactivates platelet-activating factor. J Immunol. 1986 May 1;136(9):3441–3446. [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]