Abstract
Thromboxane A2 receptor (TPr) stimulation induces cellular hypertrophy in vascular smooth muscle cells (VSMCs); however, regulation of VSMC hypertrophy remains poorly understood. Here we show that TPr stimulation activates AMP-activated kinase (AMPK), which in turn limits TPr-induced protein synthesis in VSMCs. Exposure of cultured VSMCs to either TPr agonists, IBOP and U46619, or exogenous hydrogen peroxide (H2O2) caused time- and dose-dependent AMPK activation, as evidenced by increased phosphorylation of both AMPK-Thr172 and acetyl-coenzyme A carboxylase–Ser79, a downstream enzyme of AMPK, whereas SQ29548, a selective TPr antagonist, significantly attenuated TPr-enhanced AMPK activation. In parallel, both IBOP and U46619 significantly increased the production of reactive oxygen species such as H2O2. Furthermore, adenoviral overexpression of catalase (an H2O2 scavenger) abolished, whereas superoxide dismutase (which catalyzes H2O2 formation) enhanced, IBOP-induced AMPK activation, suggesting that TPr-activated AMPK was mediated by H2O2. Consistently, exposure of VSMCs to either TPr agonists or exogenous H2O2 dose-dependently increased the phosphorylation of LKB1 (at serines 428 and 307), an AMPK kinase, as well as coimmunoprecipitation of AMPK with LKB1. In addition, direct mutagenesis of either Ser428 or Ser307 of LKB1 into alanine, like the kinase-dead LKB1 mutant, abolished both TPr-stimulated AMPK activation and coimmunoprecipitation. Finally, genetic inhibition of AMPK significantly accentuated IBOP-enhanced protein synthesis, whereas adenoviral overexpression of constitutively active AMPK abolished IBOP-enhance protein synthesis in VSMCs. We conclude that TPr stimulation triggers reactive oxygen species–mediated LKB1-dependent AMPK activation, which in return inhibits cellular protein synthesis in VSMCs.
Keywords: thromboxane receptor, AMPK, oxidative stress, vascular smooth muscle cells
AMP-activated protein kinase (AMPK) is a well-conserved eukaryotic protein kinase that is a sensor for changes in cellular energy state.1–3 AMPK activity is stimulated by an increase in the intracellular AMP-to-ATP ratios in response to stresses such as exercise,4 hypoxia,5 oxidative stress,6 and glucose deprivation.7 The activation of AMPK turns on catabolic pathways that produce ATP and turns off anabolic pathways that consume ATP.1,8 The activation of AMPK leads to the phosphorylation of a number of proteins, resulting in increased glucose uptake and metabolism and fatty acid oxidation, and simultaneously results in inhibition of hepatic lipogenesis, cholesterol synthesis, and glucose production.1,9,10 Because AMPK activation could have beneficial metabolic consequences for diabetic patients, AMPK has emerged as a potential target for the treatment of obesity and type 2 diabetes.3 Activation of AMPK requires the phosphorylation of Thr172 in the activation loop of the α subunit by at least 2 upstream kinases,11 LKB112–14 and Ca2+/calmodulin-dependent kinase kinase (CaMKK)-β.15–17
There is overwhelming evidence that excessive production of reactive oxygen species (ROS) causes oxidative damage to macromolecules of a host cell, which play an important role in the etiology of many disease processes, including cancer, atherosclerosis, and diabetes.18 Griendling et al have found that smooth muscle cells exposed to angiotensin II exhibit increased superoxide generation via NADH/NADPH oxidase-like enzymatic activity.19 This enzymatic system now appears to be involved in a number of “maladaptive” characteristics of atherosclerosis, such as smooth muscle cell hypertrophy,19,20 diabetic retinopathy,21 platelet-derived growth factor–induced cell proliferation,19,22 and impaired NO bioactivity.21,23 Other sources of ROS in the vasculature may include xanthine oxidase, mitochondrion, NO synthase, and P450 enzymes.24 One emerging concept is that ROS-mediated signaling is not restricted to pathologic events. Indeed, angiotensin II and platelet-derived growth factor are important mediators of vascular signals that, in part, depend on ROS as mediators of signal transduction.19,20,22 Recent evidence from our group25–27 suggests that both hypoxia-reoxygenation and metformin requires peroxynitrite (ONOO−) as a signaling molecule to activate AMPK in endothelial cells. In addition, AMPK is activated by exogenous hydrogen peroxide (H2O2).6,28
Thromboxane (Tx)A2 is a product of arachidonic acid through the cyclooxygenase pathway and is synthesized after activation of a variety of cells, including platelets, vascular smooth muscle cells (VSMCs), endothelial cells, and macro-phages. TxA2 exerts potent biological activity, causing platelet aggregation and secretion, vasoconstriction, and mitogenesis and stimulating hypertrophy in VSMCs.29–32 These biological effects are the consequence of the interaction of TxA2 with membrane receptors (TxA2 receptors [TPrs]), which belong to the heptahelical superfamily of G protein–coupled receptors.33 Although not completely understood, there is evidence that TxA2-induced hypertrophy and proliferation appear to involve the activation of mitogen-activated protein kinase and p70 S6 kinase.34,35 Interestingly, TxA2 has been found to promote the formation of superoxide anions in pulmonary artery and cultured cells.36,37 However, the physiological role of TPr-derived superoxide anions remains unknown.
Therefore, we hypothesize that AMPK may respond to TxA2-induced ROS production. The aim of the present study was to elucidate the mechanisms by which TPr triggers AMPK activation and the physiological functions of AMPK in cultured rat VSMCs.
Methods and Materials
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org. Briefly, rat VSMCs were cultured from rat thoracic aortas. VSMCs were stimulated by the TxA2 mimetic IBOP or U46619. Phosphorylations of LKB1, AMPK, and acetyl-coenzyme A carboxylase (ACC) after stimulation were detected using Western blotting, and intracellular ROS production was monitored by 2′,7′-dichlorodihydrofluorescein (DCF). [3H]Leucine incorporation was performed to investigate the effects of AMPK on IBOP-induced protein synthesis. After infection by adenoviral vectors Ad-AMPK-CA, Ad-AMPK-DN, or AD–green fluorescent protein (Ad-GFP), VSMCs were treated with IBOP for 5 or 48 hours, and then the protein synthesis was assessed by l-[4,5-3H]leucine (1 µCi/mL) incorporation.
Results
Thromboxane Mimetics Activate AMPK in Rat VSMCs
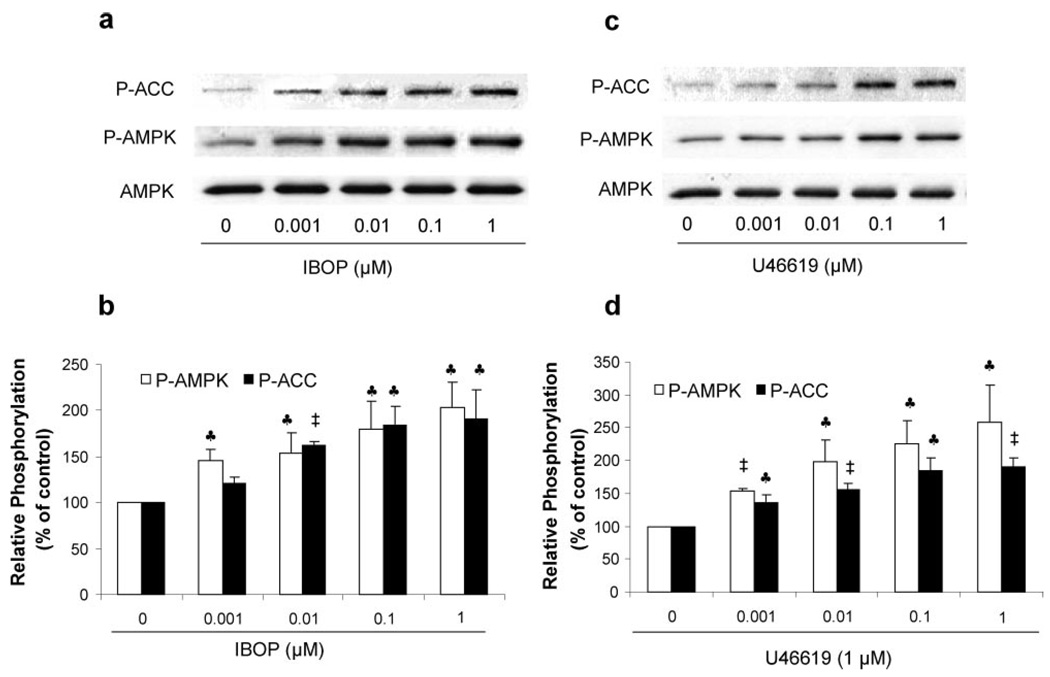
Because activation of AMPK requires the phosphorylation of Thr172 in the activation loop of α1 and α2 subunits, AMPK activity was determined in Western blots by monitoring both Thr172 phosphorylation of AMPK and its best-characterized downstream kinase, ACC phosphorylation at Ser79. As shown in Figure 1a and 1b, exposure of VSMCs to IBOP (0.001 to 1 µmol/L), a TxA2 mimetic, for 10 minutes dose-dependently increased the phosphorylation of both AMPK-Thr172 and ACC-Ser79, implying that IBOP activated AMPK in VSMCs. We also included 2 other structurally related TxA2 mimetics, U46619 and carboxy-TxA2. As depicted in Figure 1c and 1d, exposure of VSMCs to U46619 for 10 minutes, similar to IBOP, dose-dependently increased both AMPK-Thr172 and ACC-Ser79. Exposure of VSMCs to carboxy-TxA2 also increased the phosphorylation of both AMPK-Thr172 and ACC-Ser79 in a dose-dependent manner (Figure Ia in the online data supplement). Furthermore, concentrations as low as 0.1 µmol/L for both IBOP and U46619, which are pathologically relevant,38 were found to increase AMPK-Thr172 by at least 2-fold, thereby reaching levels similar to that caused by AICAR (1 mmol/L, 1 hour) (supplemental Figure Ib).
Figure 1.
TxA2 mimetics activate AMPK in cultured VSMCs. Confluent VSMCs were treated with the TxA2 mimetics IBOP and U46619 at the concentrations indicated for 10 minutes. Both phosphorylated AMPK-Thr172 and ACC-Ser79 were detected in Western blots by using specific antibodies (a and c). Exposure of VSMCs to IBOP (b) and U46619 (d) increased both AMPK-Thr172 and ACC-Ser79 in a dose-dependent manner. Data are means±SEM (b, n=4; d, n=3). ♣P< 0.05; ‡P<0.01, treated vs untreated control cells.
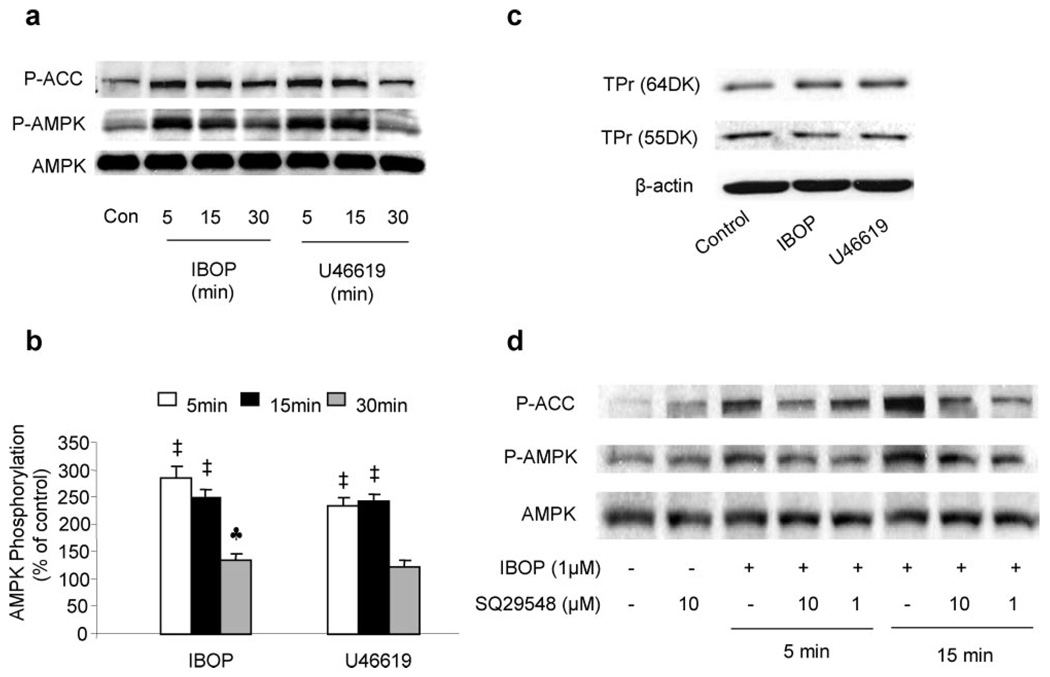
The activation of AMPK by a TxA2 mimetic was also time-dependent. Peak phosphorylation of AMPK was reached between 5 and 15 minutes after stimulation with either IBOP (1 µmol/L) or U46619 (1 µmol/L) (Figure 2a and 2b). The phosphorylation of both AMPK and ACC started to decline at 30 minutes (Figure 2a and 2b) and remained elevated at 5 hours (supplemental Figure IIa) after treatment with either IBOP or U46619. In contrast, no change in the expression of AMPK α subunits was observed in VSMCs exposed to the TxA2 mimetic IBOP up to 96 hours (supplemental Figure IIb), suggesting that altered AMPK-Thr172 phosphorylation by TxA2 mimetics was not because of increased expression of AMPK.
Figure 2.
TxA2 mimetics activate AMPK via TPr in VSMCs. TPr activation causes a time-dependent AMPK activation in VSMCs (a and b). VSMCs were treated with either IBOP (1 µmol/L) or U46619 (1 µmol/L) for 5, 15, and 30 minutes. Data are means±SEM (n=4). ♣P<0.05, ‡P<0.01, treated vs control cells. VSMCs were treated with either IBOP (1 µmol/L) or U46619 (1 µmol/L) for 24 hours (c). TPr was detected at 55 and 64 kDa (Cayman’s TPr polyclonal antibody detects the TPr receptor at 55 and 64 kDa according to the production information) via Western blotting (d). The TPr antagonist SQ29548 abolishes TPr-induced AMPK activation. Confluent VSMCs were preincubated with or without the TPr antagonist SQ29548 at 1 µmol/L or 10 µmol/L for 30 minutes before being exposed to IBOP (1 µmol/L) for either 5 or 15 minutes. The blot is representative of 3 blots from 3 individual experiments.
TPr-Dependent AMPK Activation
We first determined whether TPr agonists altered TPr in VSMCs. TPr expression was determined by Western blotting using a specific antibody. As shown in Figure 2c, exposure of VSMCs to either IBOP or U46619 for 24 hours did not alter the expression of TPr.
We further determined whether the activation of AMPK caused by TxA2 mimetics was mediated by TPr. To this end, SQ29548, a potent TPr antagonist, was preincubated with VSMCs before the addition of a TPr mimetic. As depicted in Figure 2d, SQ29548 (1 or 10 µmol/L), which did not alter the basal level of AMPK-Thr172, markedly attenuated IBOP-enhanced AMPK-Thr172 phosphorylation. Similarly, S18886, a structurally unrelated TPr antagonist, like SQ29548, also blunted IBOP-induced AMPK-Thr172 (supplemental Figure IIc). Taken together, these results strongly suggest that AMPK activation is TPr-dependent.
TPr Increases ROS Generation in VSMCs
There is evidence that TPr activation promotes superoxide production in both pig pulmonary artery36 and corpus cavernosal smooth muscle cells.37 We next tested whether TPr increased ROS production in cultured aortic VSMCs. As shown in Figure 3a, treatment of VSMCs with either IBOP (1 µmol/L) or U46619 (1 µmol/L) markedly increased ROS release 10 minutes after incubation, as detected by H2O2-sensitive DCF fluorescence.
Figure 3.
TxA2-induced AMPK activation is mediated by ROS production. TPr stimulation increases ROS production in VSMCs (a). ROS were determined by measuring DCF after incubation with either IBOP (1 µmol/L) or U46619 (1 µmol/L) for 10 minutes. Data are means±SEM (a, n=4 [♣P<0.05]; b, n=3 [♣P<0.05]). Infection of VSMCs with adenoviruses encoding catalase or SOD1 increased the expression of both catalase and SOD1 in VSMCs, respectively (b). The blot is representative of at least 3 blots of 3 independent experiments. Adenoviral overexpression of catalase increased catalase activity in VSMCs (b). Catalase activity was examined after 48 hours of infection with adenoviral GFP or catalase. Catalase activity is shown in nanomoles per minute per milliliter (means±SEM, n=3). ‡P<0.01 catalase vs GFP controls. Adenoviral overexpression of catalase suppresses IBOP-induced (1 µmol/L, 10 minutes) AMPK activation, whereas overexpression of SOD1 (Cu/Zn SOD) increases AMPK activation (c). The blot is representative of 3 blots from 3 individual experiments. Exogenous H2O2 at concentrations indicated for 10 minutes increases AMPK activation in VSMCs (d). Data are means±SEM (n=3). ♣P<0.05; ‡P<0.01, treated vs control cells.
TPr-Induced AMPK Activation Is H2O2-Dependent
We next determined whether TPr-enhanced H2O2 production contributes to TPr-dependent AMPK activation in VSMCs. To this end, catalase, which can detoxify H2O2, was overexpressed by adenoviruses encoding catalase. Adenoviral infection of catalase in VSMCs greatly increased catalase expression and catalase activity (Figure 3b). As expected, adenoviral overexpression of catalase abolished IBOP-enhanced phosphorylation of both AMPK Thr172 and ACC Ser79 (Figure 3c). On the other hand, adenoviral overexpression of superoxide dismutase (SOD)1 increased SOD1 expression (Figure 3b), which presumably results in enhanced H2O2 production, significantly increased IBOP-enhanced phosphorylation of both AMPK and ACC (Figure 3c). Because SOD1 enhanced, whereas catalase suppressed, the effects of IBOP on AMPK, these results imply that IBOP-activated AMPK is H2O2-dependent.
Activation of AMPK by Exogenous H2O2 in VSMCs
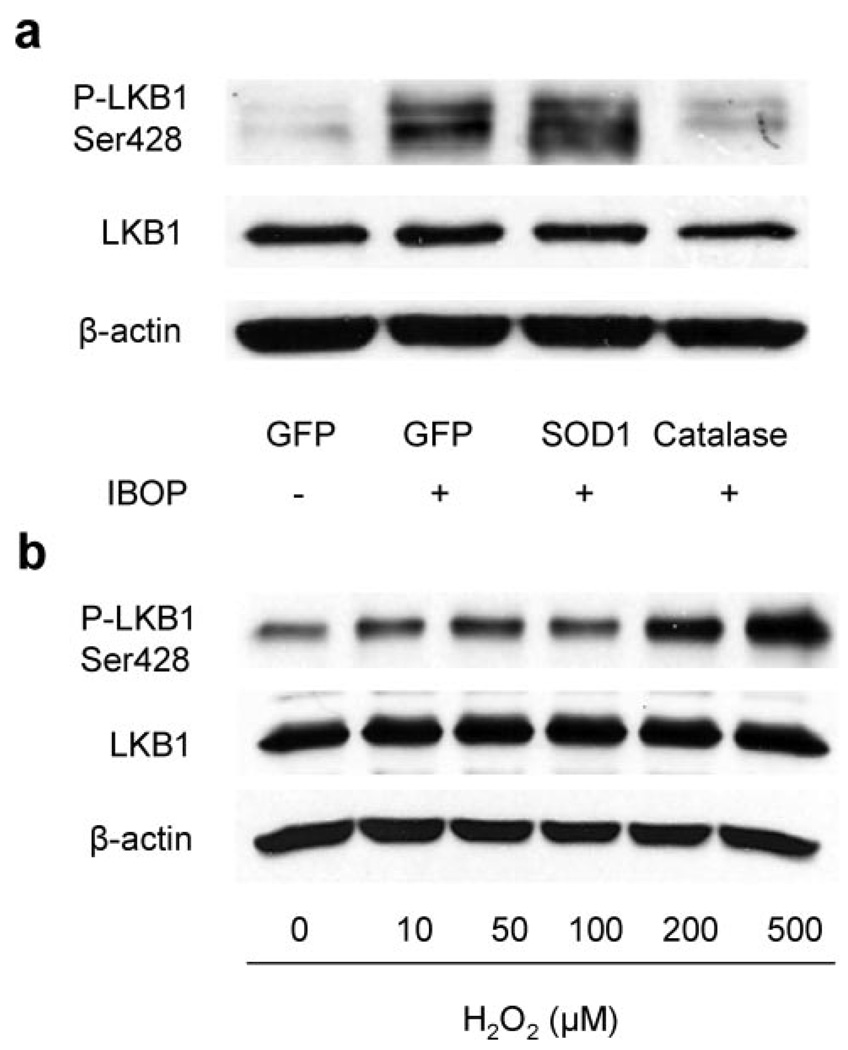
Earlier studies have demonstrated that exogenous H2O2 activates AMPK6,28; therefore, we next investigated whether H2O2 could activate AMPK in VSMCs. As depicted in Figure 3d, exposure of VSMCs to H2O2 increased the phosphorylation of AMPK-Thr172 and ACC-Ser79 in a dose-dependent manner.
TPr-Induced AMPK Activation Is AMP-Independent
AMPK is a stress-activated protein kinase that works as a metabolic sensor of cellular AMP/ATP levels. We next assessed whether AMPK activation by TPr was attributable to alterations of intracellular AMP, ADP, and ATP levels in VSMCs. As shown in supplemental Figure IIIa, neither IBOP nor U46619 treatments (1 µmol/L, 10 minutes, respectively) changed the cellular levels of AMP, ADP, and ATP. Furthermore, the ratios of AMP-to-ATP remained unchanged in VSMCs exposed to either IBOP or U46619 (supplemental Figure IIIb). These results suggest that TPr-activated AMPK is independent of AMP/ATP ratios in VSMCs.
TPr-Induced AMPK Activation Is LKB1-Dependent
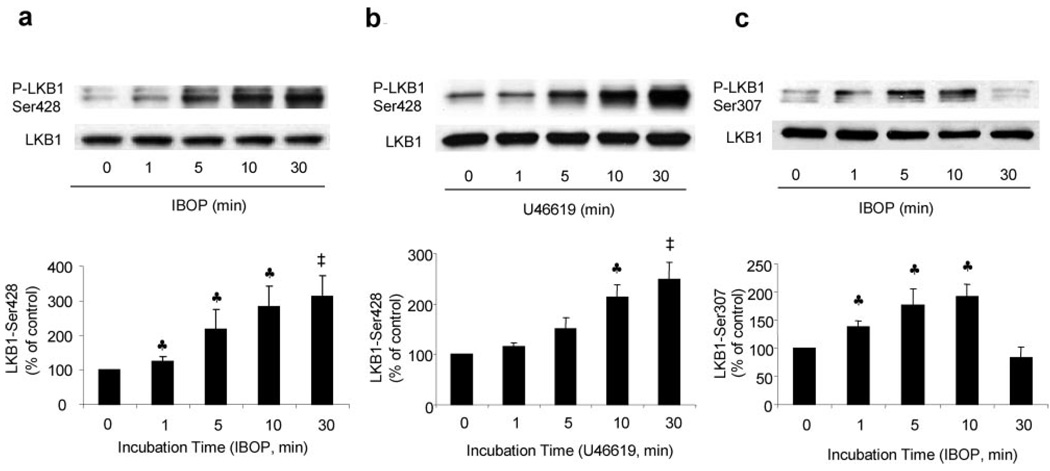
There is evidence that the tumor suppressor LKB1 acts as a major upstream kinase for AMPK. We next investigated whether LKB1 was required for TxA2-enhanced activation of AMPK. As shown in Figure 4a and 4b, exposure of VSMCs to either IBOP (1 µmol/L) or U46619 (1 µmol/L) time-dependently increased the phosphorylation of LKB1 at Ser428, a phosphorylation site that may play a crucial role in regulating AMPK activation.39 IBOP treatment significantly increased LKB1-Ser428 phosphorylation as early as 1 minute following exposure. IBOP also increased LKB1-Ser307 phosphorylation (Figure 4c). In parallel with AMPK-Thr172 phosphorylation, LKB1-Ser307 phosphorylation reached a peak at 10 minutes and then returned to basal levels within 30 minutes.
Figure 4.
TPr stimulation enhances LKB1 phosphorylation in cultured VSMCs. Time-dependent effects of IBOP (1 µm) or U46619 (1 µmol/L) on LKB1-Ser428 phosphorylation in VSMCs (a and b). Data as means±SEM (a, n=5; b, n=3). ♣P<0.05; ‡P<0.01, treated vs untreated cells. Time-dependent effects of IBOP (1 µm) on LKB1-Ser307 phosphorylation in VSMCs (c). Data are means±SEM (n=3). ♣P<0.05, treated vs untreated cells.
As seen in AMPK-Thr172 phosphorylation, overexpression of SOD1 significantly enhanced the phosphorylation of LKB1-Ser428, whereas overexpression of catalase blunted IBOP-induced LKB1-Ser428 phosphorylation (Figure 5a). In addition, exposure of VSMCs to exogenous H2O2 for 10 minutes also increased LKB1 Ser428 phosphorylation (Figure 5b). Taken together, these results suggest that TPr, via H2O2, increases LKB1 phosphorylation at both Ser428 and Ser307.
Figure 5.
TPr-induced LKB1-Ser428 phosphorylation is mediated by ROS production. Effect of catalase and SOD1 overexpression on IBOP-induced (1 µmol/L, 10 minutes) LKB1-Ser428 phosphorylation (a). The blot is representative of 3 blots from 3 independent assays. Exogenous H2O2 dose-dependently increases LKB1 phosphorylation at the 10-minute time point (b). The blot is representative of 3 blots from 3 independent assays.
Phosphorylation of Both Ser428 and Ser307 of LKB1 Is Required for TPr-Stimulated AMPK Activation
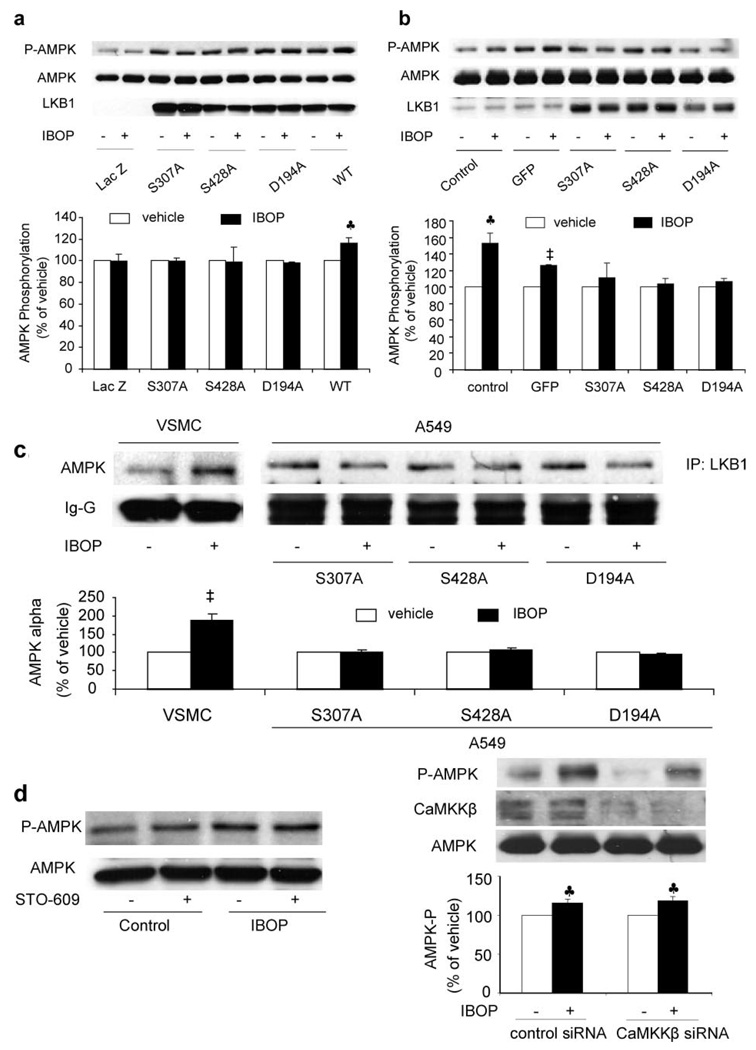
Our previous studies have demonstrated that Ser428 of LKB1 is required for ONOO−-enhanced AMPK activation.39 Ser307 of LKB1 is also required for LKB1-dependent AMPK activation (Z. Xie, Y. Dong, J. Zhang, R. Scholz, D. Neumann, M.-H. Zov, unpublished data, 2007.). We next determined whether the phosphorylation of LKB1 at Ser307 and Ser428 is required for IBOP-induced AMPK activation. As shown in Figure 6a, IBOP did not activate AMPK either in LKB1-deficient A549 cells or A549 cells transfected with the LKB1 mutants of D194A, S307A, or S428A but increased AMPK-Thr172 in A549 transfected with wild-type LKB1, implying that LKB1 is required for IBOP-induced AMPK activation. Furthermore, direct mutagenesis of either S307A or S428A, like the kinase-dead mutants (D194A), also abolished the effect of IBOP on AMPK in VSMCs (Figure 6b), suggesting that the phosphorylation of LKB1 at Ser307 and Ser428 was required for IBOP-enhanced AMPK phosphorylation.
Figure 6.
TPr-induced AMPK activation requires LKB1. Activation of AMPK by IBOP is LKB1-dependent (a). LKB1-deficient A549 cells were transfected for 24 hours with LKB1 wild-type (WT) or LKB1 mutants (S307A, S428A, and D194A). The plasmid encoding LacZ was used as a control. Transfected cells were stimulated with 1 µmol/L IBOP or with vehicle for 10 minutes. Data are means±SEM (n=3). ♣P<0.05, treated vs untreated cells. Adenoviral overexpression of LKB1 mutants abolishes IBOP-induced AMPK phosphorylation in VSMCs (b). VSMCs were infected with adenoviruses encoding LKB1 mutants D194A, S307A, and S428A, respectively. Cells were stimulated with 1 µmol/L IBOP for 10 minutes. Data are means±SEM (n=3). ♣P<0.05; ‡P<0.01, treated vs untreated cells. IBOP enhances the coimmunoprecipitation of LKB1 and AMPK in VSMCs but not in A549 cells transfected with LKB1 mutants (c). LKB1 was immunoprecipitated after IBOP treatment (1 µmol/L, 10 minutes) from VSMCs or A549 cells transfected with S307A, S428A, or D194A mutant LKB1 plasmids, and AMPK was then detected in Western blots. Data are means±SEM (n=3). ‡P<0.01, treated vs untreated cells. Effect of CaMKKβ on TPr-induced AMPK activation (d). VSMCs were preincubated for 6 hours with 1 µg/mL (2.6 µmol/L) STO-609 or transfected by CaMKKβ small interfering RNA to knockdown CaMKKβ expression; then the cells were treated with 1 µmol/L IBOP for 10 minutes. The blot is representative of 3 blots obtained from 3 independent experiments. Data are means±SEM (n=3). ♣P<0.05, treated vs untreated cells.
TPr Increases the Association of AMPK With Its Upstream Kinase LKB1
We have reported previously that ONOO− activates AMPK by increasing the association of LKB1 with AMPK.39 Therefore, we next investigated whether TPr increases the interactions between AMPK and LKB1. LKB1 was first immunoprecipitated and then Western blotted for AMPK or vice versa. As shown in Figure 6c, IBOP significantly increased the coimmunoprecipitation of LKB1 with AMPK-α in VSMCs. However, overexpression of LKB1-S307A, LKB1-S428A, or LKB1-D194A abolished IBOP-enhanced coimmunoprecipitation of LKB1 with AMPK.
We next determined whether TPr agonists alter LKB1 activity by measuring LKB1 activity after treatment with IBOP or U46619. Neither IBOP nor U46619 altered LKB1 activity in VSMCs (supplemental Figure IV). These results suggest that TPr stimulation activated AMPK by increasing the association of AMPK with LKB1, which is an upstream kinase of AMPK, in VSMCs.
TPr-Induced AMPK Is Independent of CaMKK
Recent studies reveal that CaMKKβ serve as AMPK kinases. 15–17 We next determined whether CaMKKβ involved in the AMPK activation induced by TPr. Pharmacologic inhibition with the CaMKK inhibitor STO-609 (1 µmol/L) did not alter IBOP-induced AMPK phosphorylation in VSMCs (Figure 6d). Transfection of CaMKKβ-specific small interfering RNA but not scrambled small interfering RNA, which largely reduced the levels of CaMKKβ in VSMCs, significantly reduced the basal levels of AMPK-Thr172. However, inhibition of CaMKKβ with CaMKKβ-specific small interfering RNA did not affect IBOP-induced AMPK-Thr172 phosphorylation in VSMCs (Figure 6d). Taken to-gether, these data suggest that activation of AMPK by TPr may be independent of CaMKKβ.
AMPK Inhibition Accentuates IBOP-Induced Protein Synthesis in VSMCs
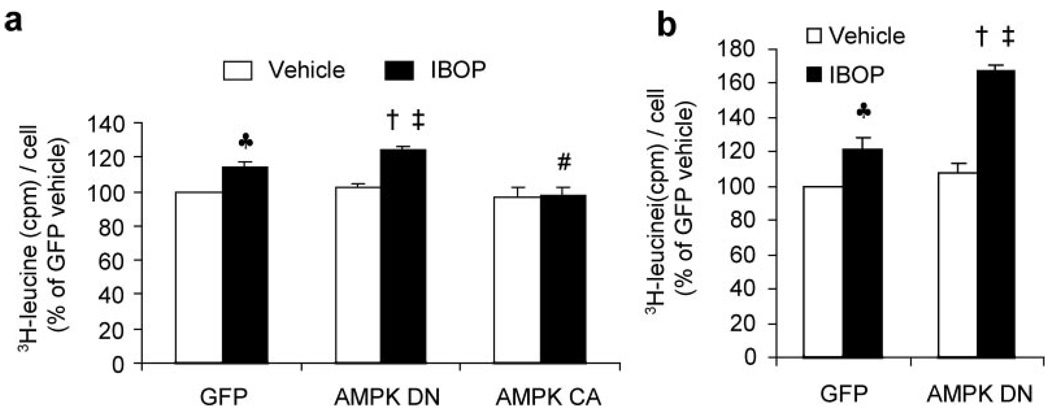
Exposure of VSMCs to IBOP for 5 hours increased the phosphorylation of both AMPK at Thr172 and ACC at Ser79 (supplemental Figure IIa), implying that IBOP activated AMPK in VSMCs. Because earlier studies31,40 have shown that TPr stimulation induces hypertrophy in VSMCs, we first determined the role of AMPK activation in TPr-enhanced protein synthesis by assessing [3H]leucine incorporation in the VSMCs infected with adenoviruses encoded with either dominate-negative AMPK mutants (AMPK-DN) or constitutively active AMPK mutants (AMPK-CA) or GFP (supplemental Figure V) for 48 hours following 5 hours of IBOP treatment. Because IBOP significantly increased VSMC apoptosis (data not shown), VSMC protein synthesis was assessed by increased [3H]leucine (cmp) incorporation per cell. As expected, exposure of VSMCs to IBOP (1 µmol/L) for 5 hours significantly increased [3H]leucine incorporation per cell in GFP-infected VSMCs (Figure 7a), confirming that IBOP significantly increases protein synthesis in VSMCs. Importantly, inhibition of AMPK by overexpression of AMPK-DN (loss-of-function) further enhanced IBOP-induced protein synthesis (Figure 7a). On the other hand, overexpression of AMPK-CA (gain-of-function) abolished IBOP-induced protein synthesis (Figure 7a), suggesting that AMPK activation inhibited IBOP-induced protein synthesis in VSMCs.
Figure 7.
Activation of AMPK by IBOP limits cellular protein synthesis in VSMCs. a, AMPK-dependent inhibition of protein synthesis in 5-hour IBOP-treated VSMCs. Forty-eight hours after having being infected with GFP, AMPK-DN, or AMPK-CA adenoviral vectors, VSMCs were treated for 5 hours with IBOP (1 µmol/L) or vehicle. Protein synthesis was assayed by [3H]leucine incorporation, as described in Materials and Methods. At same time, the cell number was counted using a hemocytometer. VSMC protein synthesis was calculated by dividing the total [3H]leucine (cmp) by the number of cells in each well and the vehicle treated GFP was used as 100%. Two-way ANOVA indicated significant effect of IBOP (P<0.01) for leucine incorporation and effect of AMPK mutation (P<0.01) for leucine incorporation. Interaction effect between IBOP and AMPK mutation approached significance (P<0.05). Data are means±SEM (n=4). ♣P<0.01, GFP vs GFP plus IBOP; ‡P<0.01, AMPK-DN vs AMPK-DN plus IBOP; †P<0.05, AMPK-DN plus IBOP vs GFP plus IBOP; #P<0.05, AMPK-CA plus IBOP vs GFP plus IBOP. b, Inhibition of AMPK by AMPK-DN enhanced IBOP-induced protein synthesis in VSMCs. Confluent VSMCs infected with adenovirus of either GFP or AMPK-DN for 48 hours were treated for 48 hours with IBOP (1 µmol/L) or vehicle. Two-way ANOVA indicated significant effect of IBOP treatment (P<0.01) and AMPK-DN transfection (P<0.01) for leucine incorporation. Interaction effect between IBOP and AMPK-DN transfection approached significance (P<0.01) for leucine incorporation. Data are means±SEM (n=3). ♣P<0.01, GFP vs GFP plus IBOP; ‡P<0.01, AMPK-DN vs AMPK-DN plus IBOP; †P<0.01, AMPK-DN plus IBOP vs GFP plus IBOP.
We next assayed the long term effects of AMPK inhibition on IBOP-increased protein synthesis. As expected, IBOP significantly increased protein synthesis in VSMCs at 48 hours. Inhibition of AMPK by AMPK-DN overexpression significantly enhanced IBOP-induced protein synthesis in VSMCs (by 2-way ANOVA; Figure 7b). Taken together, these results suggest that AMPK inhibition enhances IBOP-induced VSMC protein synthesis.
Discussion
AMPK is a serine/threonine protein kinase and a member of the Snf1/AMPK protein kinase family. It is known that AMPK activity is stimulated by an increase in the intracellular AMP-to-ATP ratios in response to stresses such as exercise, hypoxia, oxidant stress, and glucose deprivation. Here we demonstrate TPr-dependent AMPK activation. Exposure of VSMCs to TPr stimulation caused dose- and time-dependent activation of AMPK. In parallel, both IBOP and U46619 increased the production of ROS, as detected by DCF. Furthermore, exogenous H2O2 increased the phosphorylation of AMPK, as seen in NIH 3T3 cells and rat VSMCs,6,28 as well as LKB1 phosphorylation. Moreover, inhibition of H2O2 production by overexpression of catalase (promoting the conversion of H2O2 to water and molecular oxygen) attenuated IBOP-induced AMPK activation, whereas the overexpression of SOD1 further enhanced AMPK activation. In parallel, IBOP-enhanced phosphorylation of LKB1 at Ser428 was largely diminished by overexpression of catalase but further enhanced by overexpression of SOD1. Therefore, it is suggested that H2O2 mainly contributes to enhanced activation of AMPK by LKB1. How ROS such as H2O2 increase LKB1 phosphorylation, however, is unknown and is under investigation in this laboratory.
Another important finding in the present study is that both Ser428 and Ser307 of LKB1 were required for both TPr- and H2O2-stimulated AMPK activation. The key evidence can be summarized as follows. First, both IBOP and U46619 increased the phosphorylation of LKB1 at Ser428 and Ser307, and according to a previous study in this laboratory,39 the phosphorylation site Ser428 of LKB1 may play a crucial role in regulating AMPK activation. Second, IBOP did not induce AMPK activation in LKB1-deficient A549 cells, whereas IBOP increased AMPK Thr172 phosphorylation in VSMCs. These results suggest that activation of AMPK by TxA2 is dependent on LKB1. Third, mutation of Ser307 (S307A) or Ser428 (S428A) in VSMCs and A549 cells abolished IBOP-enhanced AMPK activation, implying important roles of Ser307 and Ser428 phosphorylation in the regulation of AMPK. These results are further corroborated by the fact that neither IBOP nor U46619 treatments changed the cellular AMP, ADP, and ATP levels or the AMP/ATP ratios, indicating that activation of AMPK by TPr stimulation is likely AMP-independent. Despite many studies reporting that AMPK cascades respond mainly to the intracellular AMP/ATP ratio,1,2,8,9 recent studies suggest that AMPK can also be activated by a second mechanism without a change in AMP or the AMP/ATP ratio.26,41 Finally, IBOP increased the coimmunoprecipitation of LKB1 with AMPK, despite the fact that activity of LKB1 was not increased by TPr stimulation. Our previous studies also showed that AMPK coimmunoprecipitates with LKB1,27,39 suggesting that AMPK associates with LKB1 and that this association may be involved in AMPK activation. The relative contribution of these sites and the upstream kinase(s) responsible for LKB1 phosphorylation, however, remains to be identified. The phosphorylation of Ser307 site seems to parallel that of AMPK better than the Ser 428 site because it peaks and then decreases after 30 minutes. Further studies are warranted.
In the present study, we provide evidence that TxA2 is a potent stimulator of ROS and that activation of AMPK by TPr-derived ROS inhibited VSMC protein synthesis. This finding is consistent with recent reports showing that the TXA2 analogue, U46619, promotes the formation of superoxide in intact pulmonary arteries and in pulmonary artery and corpus cavernosal smooth muscle cells.36,37 TPr expression and serum levels of multiple TPr ligands are elevated, both locally and systemically, in patients with several vascular and thrombotic diseases, including ischemia, angioplasty, unstable angina, myocardial infarction, and reocclusion after coronary thrombolysis.8,30,38,42 TPr density is increased in atherosclerotic coronary arteries and in vessels with severe intimal hyperplasia.38 Because there is overwhelming evidence that ROS play a causal role in the development of cardiovascular diseases and diabetes, TPr-stimulated ROS production might contribute to the excessive oxidant stress observed in these diseases, and ROS might serve as the common pathway for TPr-induced vascular pathways. In-deed, we found that TPr stimulation increased both superoxide and ONOO−, decreased NO bioactivity, and increased protein tyrosine nitration in cultured endothelial cells (M.Z., J.X., M.G., Z.X., M.– H.Z., unpublished data, 2007). Therefore, excessive production of ROS might be a common pathway for TPr-induced vasculopathy.
The function of AMPK activation is still not fully understood. Here we have preliminary evidence that inhibition of AMPK accentuated IBOP-induced protein synthesis. It is known that AMPK activation suppresses protein synthesis and prevents cardiac myocyte hypertrophy by regulation of the eEF2 kinase/eEF2 axis and/or TSC2-mTOR-P70S6 pathways. 43–45 How AMPK suppresses TPr-induced protein synthesis in VSMCs remains unclear. Further study is warranted to elucidate the molecular mechanisms underlying AMPK-mediated inhibition of protein synthesis.
In conclusion, we have provided evidence that TPr stimulation increases ROS, which mediates AMPK activation in VSMCs. In addition, we demonstrate that TPr-activated AMPK is LKB1-dependent, which requires the phosphorylation of both Ser307 and Ser428 of LKB1 resulting in association of AMPK with LKB1. Finally, we have provided evidence that AMPK activation may serve as an endogenous inhibitor for IBOP-induced cellular protein synthesis in VSMCs. Thus, AMPK may be a therapeutic target in preventing cardiovascular diseases such as diabetes and atherosclerosis.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by NIH grants HL079584, HL080499, and HL074399; a grant-in-aid from the Juvenile Diabetes Research foundation; a Research Award from Oklahoma Center for the Advancement of Science and Technology; a research award from the American Diabetes Association; and funds from the Travis Endowed Chair of the University of Oklahoma Health Science Center (all to M. -H.Z.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van DB, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 3.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 5.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo KS, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem Biophys Res Commun. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 7.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(pt 3):533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson WA, Hawley SA, Hardie DG. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol. 1996;6:1426–1434. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG. The AMP-activated protein kinase pathway - new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 10.Hardie DG. New roles for the LKB1->AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 12.Hawley S, Boudeau J, Reid J, Mustard K, Udd L, Makela T, Alessi D, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. Inaugural Article: the tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LGD, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 17.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angioten-sin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 20.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 21.Ellis EA, Guberski DL, Hutson B, Grant MB. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric Oxide. 2002;6:295–304. doi: 10.1006/niox.2001.0419. [DOI] [PubMed] [Google Scholar]
- 22.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 23.Thomas SR, Chen K, Keaney JF., Jr Oxidative stress and endothelial nitric oxide bioactivity. Antioxid Redox Signal. 2003;5:181–194. doi: 10.1089/152308603764816541. [DOI] [PubMed] [Google Scholar]
- 24.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 25.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 26.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells: role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 27.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, IV, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo: role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 28.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 29.Dogne JM, de LX, Delarge J, David JL, Masereel B. New trends in thromboxane and prostacyclin modulators. Curr Med Chem. 2000;7:609–628. doi: 10.2174/0929867003374868. [DOI] [PubMed] [Google Scholar]
- 30.Ogletree ML. Overview of physiological and pathophysiological effects of thromboxane A2. Fed Proc. 1987;46:133–138. [PubMed] [Google Scholar]
- 31.Ali S, Davis MG, Becker MW, Dorn GW. Thromboxane A2 stimulates vascular smooth muscle hypertrophy by up-regulating the synthesis and release of endogenous basic fibroblast growth factor. J Biol Chem. 1993;268:17397–17403. [PubMed] [Google Scholar]
- 32.Hanasaki K, Nakano T, Arita H. Receptor-mediated mitogenic effect of thromboxane A2 in vascular smooth muscle cells. Biochem Pharmacol. 1990;40:2535–2542. doi: 10.1016/0006-2952(90)90096-4. [DOI] [PubMed] [Google Scholar]
- 33.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 34.Morinelli TA, Zhang LM, Newman WH, Meier KE. Thromboxane A2/prostaglandin H2-stimulated mitogenesis of coronary artery smooth muscle cells involves activation of mitogen-activated protein kinase and S6 kinase. J Biol Chem. 1994;269:5693–5698. [PubMed] [Google Scholar]
- 35.Miggin SM, Kinsella BT. Thromboxane A2 receptor mediated activation of the mitogen activated protein kinase cascades in human uterine smooth muscle cells. Biochim Biophys Acta. 2001;1539:147–162. doi: 10.1016/s0167-4889(01)00103-3. [DOI] [PubMed] [Google Scholar]
- 36.Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits superoxide formation and gp91phox expression induced by the thromboxane A2 analogue U46619, 8-isoprostane F2alpha, prostaglandin F2alpha, cytokines and endotoxin in the pig pulmonary artery. Br J Pharmacol. 2004;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int. 2005;96:423–427. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- 38.Katugampola SD, Davenport AP. Thromboxane receptor density is increased in human cardiovascular disease with evidence for inhibition at therapeutic concentrations by the AT(1) receptor antagonist losartan. Br J Pharmacol. 2001;134:1385–1392. doi: 10.1038/sj.bjp.0704416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C{zeta} by per-oxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 40.Dorn GW, Becker MW, Davis MG. Dissociation of the contractile and hypertrophic effects of vasoconstrictor prostanoids in vascular smooth muscle. J Biol Chem. 1992;267:24897–24905. [PubMed] [Google Scholar]
- 41.Toyoda T, Hayashi T, Miyamoto L, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Inoue G, Otaka A, Sato K, Fushiki T, Nakao K. Possible involvement of the {alpha}1 isoform of 5′ AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E166–E173. doi: 10.1152/ajpendo.00487.2003. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. N Engl J Med. 1986;315:983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 43.Chan AY, Dyck JR. Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy. Can J Physiol Pharmacol. 2005;83:24–28. doi: 10.1139/y04-107. [DOI] [PubMed] [Google Scholar]
- 44.Chan AYM, Soltys CL, Young ME, Proud CG, Dyck JRB. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 45.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation - AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol (Lond) 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.